Preclinical Evaluation of a Novel PSMA-Targeted Agent 68Ga-NOTA-GC-PSMA for Prostate Cancer Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Radioactive Synthesis and Purification of Probes
2.2. In Vitro Stability Study
2.3. Determination of Lipid Water Partition Coefficient
2.4. Cell Lines with Culture Conditions
2.5. Tumor Model
2.6. Analysis of the Affinity of NOTA-GC-PSMA to PSMA Through Surface Plasmon Resonance (SPR) Binding Assays
2.7. Cellular Uptake and Blocking
2.8. Radiotoxicity
2.9. Biodistribution and Pharmacokinetic Studies
2.10. PET Imaging
2.11. Autoradiography, H&E Staining, and Immunohistochemistry
2.12. Graphical and Statistical Analysis
3. Results
3.1. Radioactive Labeling and Quality Control of Probe
3.2. Cellular Uptake and Blocking
3.3. In Vivo Radiotoxicity Study
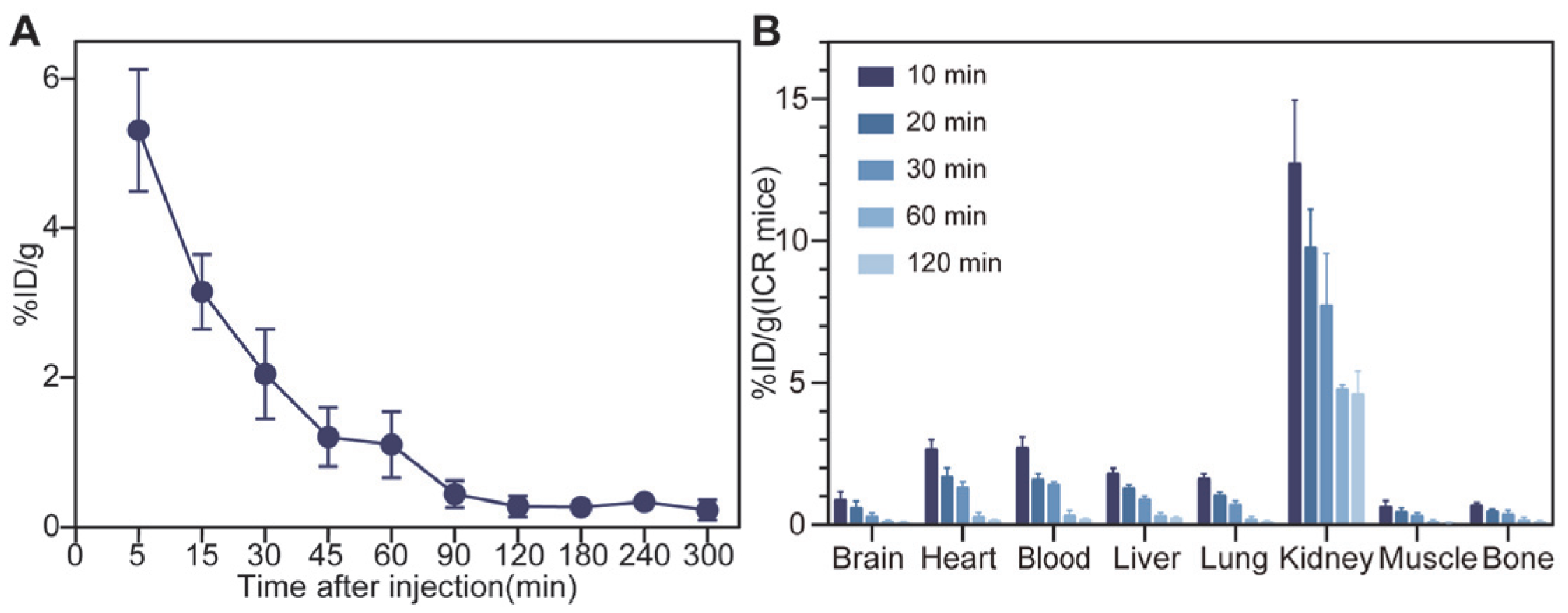
3.4. In Vivo Pharmacokinetics and Biodistribution Studies
3.5. PET Imaging
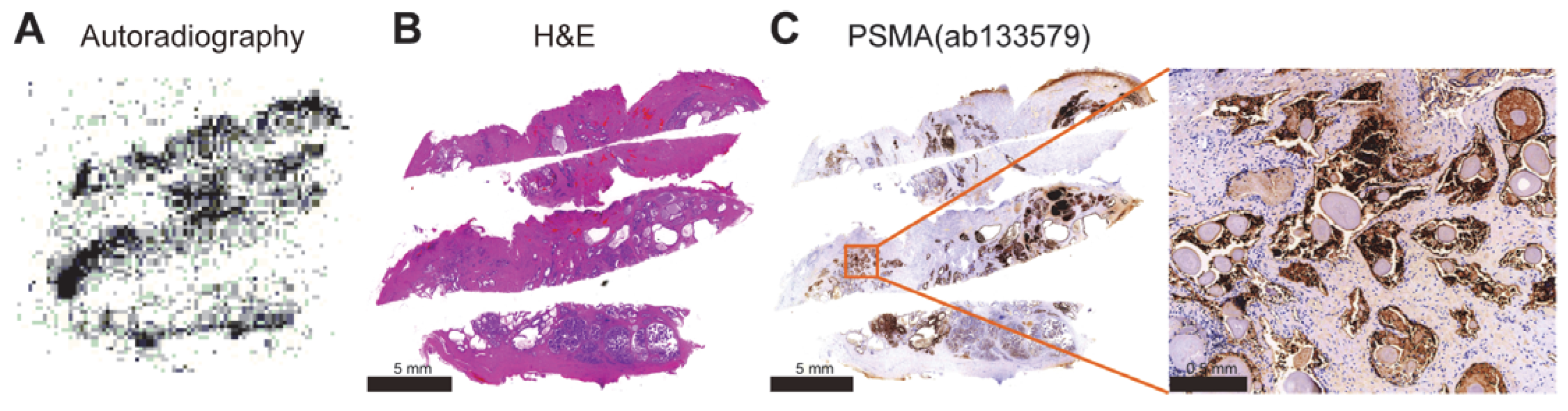
3.6. Autoradiography
4. Discussion
5. Conclusions
6. Associated Content
Supporting Information
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA-A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Survival Rates for Prostate Cancer. Available online: https://www.cancer.org/cancer/types/prostate-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 25 February 2025).
- Mammatas, L.H.; Verheul, H.M.; Hendrikse, N.H.; Yaqub, M.; Lammertsma, A.A.; Menke-van der Houven van Oordt, C.W. Molecular imaging of targeted therapies with positron emission tomography: The visualization of personalized cancer care. Cell. Oncol. 2015, 38, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Mankoff, D.A. A Definition of Molecular Imaging. J. Nucl. Med. 2007, 48, 18N–21N. [Google Scholar] [PubMed]
- Rowe, S.P.; Pomper, M.G. Molecular imaging in oncology: Current impact and future directions. CA-A Cancer J. Clin. 2022, 72, 333–352. [Google Scholar] [CrossRef]
- Cohen, D.; Hazut Krauthammer, S.; Fahoum, I.; Kesler, M.; Even-Sapir, E. PET radiotracers for whole-body in vivo molecular imaging of prostatic neuroendocrine malignancies. Eur. Radiol. 2023, 33, 6502–6512. [Google Scholar] [CrossRef]
- Ray Banerjee, S.; Pullambhatla, M.; Foss, C.A.; Falk, A.; Byun, Y.; Nimmagadda, S.; Mease, R.C.; Pomper, M.G. Effect of Chelators on the Pharmacokinetics of 99mTc-Labeled Imaging Agents for the Prostate-Specific Membrane Antigen (PSMA). J. Med. Chem. 2013, 56, 6108–6121. [Google Scholar] [CrossRef]
- Hicks, R.J.; Hofman, M.S. Is there still a role for SPECT–CT in oncology in the PET–CT era? Nat. Rev. Clin. Oncol. 2012, 9, 712–720. [Google Scholar] [CrossRef]
- Crișan, G.; Moldovean-Cioroianu, N.S.; Timaru, D.-G.; Andrieș, G.; Căinap, C.; Chiș, V. Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. Int. J. Mol. Sci. 2022, 23, 23. [Google Scholar] [CrossRef]
- Jones, W.; Griffiths, K.; Barata, P.C.; Paller, C.J. PSMA Theranostics: Review of the Current Status of PSMA-Targeted Imaging and Radioligand Therapy. Cancers 2020, 12, 1367. [Google Scholar] [CrossRef]
- Hennrich, U.; Eder, M. [68Ga]Ga-PSMA-11: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging of Prostate Cancer. Pharmaceuticals 2021, 14, 713. [Google Scholar] [CrossRef]
- Liu, C.; Liu, T.; Zhang, N.; Liu, Y.; Li, N.; Du, P.; Yang, Y.; Liu, M.; Gong, K.; Yang, X.; et al. 68Ga-PSMA-617 PET/CT: A promising new technique for predicting risk stratification and metastatic risk of prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Hetzheim, H.; Kratochwil, C.; Benesova, M.; Eder, M.; Neels, O.C.; Eisenhut, M.; Kübler, W.; Holland-Letz, T.; Giesel, F.L.; et al. The Theranostic PSMA Ligand PSMA-617 in the Diagnosis of Prostate Cancer by PET/CT: Biodistribution in Humans, Radiation Dosimetry, and First Evaluation of Tumor Lesions. J. Nucl. Med. 2015, 56, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Ora, M.; Saini, V.K.; Dixit, M.; Singh, U.P.; Gambhir, S. An Analysis of the Diagnostic Performance of Tc-99m PSMA SSPECT/CT in Biochemically Recurrent Prostate Cancer Compared with Ga-68 PSMA PET/CT: A Single-center, Prospective Study. Indian J. Nucl. Med. 2024, 39, 170–176. [Google Scholar] [CrossRef]
- Brunello, S.; Salvarese, N.; Carpanese, D.; Gobbi, C.; Melendez-Alafort, L.; Bolzati, C. A Review on the Current State and Future Perspectives of [99mTc]Tc-Housed PSMA-i in Prostate Cancer. Molecules 2022, 27, 2617. [Google Scholar] [CrossRef] [PubMed]
- Berliner, C.; Steinhelfer, L.; Chantadisai, M.; Kroenke, M.; Koehler, D.; Pose, R.; Bannas, P.; Knipper, S.; Eiber, M.; Maurer, T. Delayed Imaging Improves Lesion Detectability in [99mTc]Tc-PSMA-I&S SPECT/CT in Recurrent Prostate Cancer. J. Nucl. Med. 2023, 64, 1036–1042. [Google Scholar] [CrossRef]
- Albalooshi, B.; Al Sharhan, M.; Bagheri, F.; Miyanath, S.; Ray, B.; Muhasin, M.; Zakavi, S.R. Direct comparison of 99mTc-PSMA SPECT/CT and 68Ga-PSMA PET/CT in patients with prostate cancer. Asia Ocean. J. Nucl. Med. Biol. 2020, 8, 1–7. [Google Scholar] [CrossRef]
- Kristiansson, A.; Vilhelmsson Timmermand, O.; Altai, M.; Strand, J.; Strand, S.E.; Åkerström, B.; Örbom, A. Hematological Toxicity in Mice after High Activity Injections of (177)Lu-PSMA-617. Pharmaceutics 2022, 14, 731. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Z.; Li, T.; Wei, Y.; Yu, M.; Ye, L.; Cai, Y.; Yang, S.; Zhang, Y.; Shi, Y.; et al. Head-to-head comparison of 99mTc-PSMA and 99mTc-MDP SPECT/CT in diagnosing prostate cancer bone metastasis: A prospective, comparative imaging trial. Sci. Rep. 2022, 12, 15993. [Google Scholar] [CrossRef]
- Vargas-Ahumada, J.E.; González-Rueda, S.D.; Sinisterra-Solís, F.A.; Casanova-Triviño, P.; Pitalúa-Cortés, Q.; Soldevilla-Gallardo, I.; Scavuzzo, A.; Jimenez-Ríos, M.A.; García-Pérez, F.O. Diagnostic Performance of 99mTc-iPSMA SPECT/CT in the Initial Staging of Patients with Unfavorable Intermediate-, High-, and Very High-Risk Prostate Cancer: A Comparative Analysis with 18F-PSMA-1007 PET/CT. Cancers 2023, 15, 5824. [Google Scholar] [CrossRef]
- Wang, Q.; Ketteler, S.; Bagheri, S.; Ebrahimifard, A.; Luster, M.; Librizzi, D.; Yousefi, B.H. Diagnostic efficacy of [99mTc]Tc-PSMA SPECT/CT for prostate cancer: A meta-analysis. BMC Cancer 2024, 24, 982. [Google Scholar] [CrossRef]
- Eder, M.; Schäfer, M.; Bauder-Wüst, U.; Hull, W.E.; Wängler, C.; Mier, W.; Haberkorn, U.; Eisenhut, M. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconj. Chem. 2012, 23, 688–697. [Google Scholar] [CrossRef]
- Benešová, M.; Schäfer, M.; Bauder-Wüst, U.; Afshar-Oromieh, A.; Kratochwil, C.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2015, 56, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Baranski, A.-C.; Schäfer, M.; Bauder-Wüst, U.; Wacker, A.; Schmidt, J.; Liolios, C.; Mier, W.; Haberkorn, U.; Eisenhut, M.; Kopka, K.; et al. Improving the Imaging Contrast of 68Ga-PSMA-11 by Targeted Linker Design: Charged Spacer Moieties Enhance the Pharmacokinetic Properties. Bioconj. Chem. 2017, 28, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Calais, J.; Ceci, F.; Cho, S.Y.; Fanti, S.; Giesel, F.L.; Goffin, K.; et al. PSMA PET/CT: Joint EANM procedure guideline/SNMMI procedure standard for prostate cancer imaging 2.0. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Maurer, T.; Eiber, M.; Schwaiger, M.; Gschwend, J.E. Current use of PSMA–PET in prostate cancer management. Nat. Rev. Urol. 2016, 13, 226–235. [Google Scholar] [CrossRef]
- Retter, A.; Gong, F.; Syer, T.; Singh, S.; Adeleke, S.; Punwani, S. Emerging methods for prostate cancer imaging: Evaluating cancer structure and metabolic alterations more clearly. Mol. Oncol. 2021, 15, 2565–2579. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer—2020 update. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Farolfi, A.; Calderoni, L.; Mattana, F.; Mei, R.; Telo, S.; Fanti, S.; Castellucci, P. Current and Emerging Clinical Applications of PSMA PET Diagnostic Imaging for Prostate Cancer. J. Nucl. Med. 2021, 62, 596–604. [Google Scholar] [CrossRef]
- Ha, H.; Kwon, H.; Lim, T.; Jang, J.; Park, S.-K.; Byun, Y. Inhibitors of prostate-specific membrane antigen in the diagnosis and therapy of metastatic prostate cancer–a review of patent literature. Expert Opin. Ther. Pat. 2021, 31, 525–547. [Google Scholar] [CrossRef]
- Kwon, H.; Son, S.-H.; Byun, Y. Prostate-Specific Membrane Antigen (PSMA)-Targeted Radionuclide Probes for Imaging and Therapy of Prostate Cancer. Asian J. Org. Chem. 2019, 8, 1588–1600. [Google Scholar] [CrossRef]
- Mosayebnia, M.; Hajimahdi, Z.; Beiki, D.; Rezaeianpour, M.; Hajiramezanali, M.; Geramifar, P.; sabzevari, O.; Amini, M.; Hatamabadi, D.; Shahhosseini, S. Design, synthesis, radiolabeling and biological evaluation of new urea-based peptides targeting prostate specific membrane antigen. Bioorg. Chem. 2020, 99, 103743. [Google Scholar] [CrossRef] [PubMed]
- El Fakiri, M.; Geis, N.M.; Ayada, N.; Eder, M.; Eder, A.C. PSMA-Targeting Radiopharmaceuticals for Prostate Cancer Therapy: Recent Developments and Future Perspectives. Cancers 2021, 13, 3967. [Google Scholar] [CrossRef]
- Kopka, K.; Benešová, M.; Bařinka, C.; Haberkorn, U.; Babich, J. Glu-Ureido-Based Inhibitors of Prostate-Specific Membrane Antigen: Lessons Learned During the Development of a Novel Class of Low-Molecular-Weight Theranostic Radiotracers. J. Nucl. Med. 2017, 58, 17s–26s. [Google Scholar] [CrossRef] [PubMed]
- Benešová, M.; Bauder-Wüst, U.; Schäfer, M.; Klika, K.D.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Linker Modification Strategies To Control the Prostate-Specific Membrane Antigen (PSMA)-Targeting and Pharmacokinetic Properties of DOTA-Conjugated PSMA Inhibitors. J. Med. Chem. 2016, 59, 1761–1775. [Google Scholar] [CrossRef]
- Kim, S.B.; Song, I.H.; Song, Y.S.; Lee, B.C.; Gupta, A.; Lee, J.S.; Park, H.S.; Kim, S.E. Biodistribution and internal radiation dosimetry of a companion diagnostic radiopharmaceutical, [68Ga]PSMA-11, in subcutaneous prostate cancer xenograft model mice. Sci. Rep. 2021, 11, 15263. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Xiong, Y.; Zhang, L.; Wang, B.; Liu, Y.; Cui, M. Design and Characterization of Squaramic Acid-Based Prostate-Specific Membrane Antigen Inhibitors for Prostate Cancer. J. Med. Chem. 2023, 66, 6889–6904. [Google Scholar] [CrossRef]
- Maurer, T.; Gschwend, J.E.; Rauscher, I.; Souvatzoglou, M.; Haller, B.; Weirich, G.; Wester, H.-J.; Heck, M.; Kübler, H.; Beer, A.J. Diagnostic efficacy of 68gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J. Urol. 2016, 195, 1436–1443. [Google Scholar] [CrossRef]
- Hofström, C.; Orlova, A.; Altai, M.; Wångsell, F.; Gräslund, T.; Tolmachev, V. Use of a HEHEHE purification tag instead of a hexahistidine tag improves biodistribution of affibody molecules site-specifically labeled with 99mTc, 111In, and 125I. J. Med. Chem. 2011, 54, 3817–3826. [Google Scholar] [CrossRef]
- Boschi, S.; Lee, J.T.; Beykan, S.; Slavik, R.; Wei, L.; Spick, C.; Eberlein, U.; Buck, A.K.; Lodi, F.; Cicoria, G.; et al. Synthesis and preclinical evaluation of an Al18F radiofluorinated GLU-UREA-LYS(AHX)-HBED-CC PSMA ligand. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2122–2130. [Google Scholar] [CrossRef]
- Huang, S.S.; Wang, X.; Zhang, Y.; Doke, A.; DiFilippo, F.P.; Heston, W.D. Improving the biodistribution of PSMA-targeting tracers with a highly negatively charged linker. Prostate 2014, 74, 702–713. [Google Scholar] [CrossRef]
- Kuo, H.-T.; Pan, J.; Zhang, Z.; Lau, J.; Merkens, H.; Zhang, C.; Colpo, N.; Lin, K.-S.; Bénard, F. Effects of Linker Modification on Tumor-to-Kidney Contrast of 68Ga-Labeled PSMA-Targeted Imaging Probes. Mol. Pharm. 2018, 15, 3502–3511. [Google Scholar] [CrossRef] [PubMed]
- Liolios, C.; Schäfer, M.; Haberkorn, U.; Eder, M.; Kopka, K. Novel Bispecific PSMA/GRPr Targeting Radioligands with Optimized Pharmacokinetics for Improved PET Imaging of Prostate Cancer. Bioconj. Chem. 2016, 27, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Kasina, S.; Sanderson, J.A.; Fitzner, J.N.; Srinivasan, A.; Rao, T.N.; Hobson, L.J.; Reno, J.M.; Axworthy, D.B.; Beaumier, P.L.; Fritzberg, A.R. Simplified preformed chelate protein radiolabeling with technetium-99m mercaptoacetamidoadipoylglycylglycine (N3S-adipate). Bioconj. Chem. 1998, 9, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J. A Review of 99mTc-labeled Tumor Metabolic Imaging Agents. Mini-Rev. Med. Chem. 2022, 22, 1586–1596. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Luo, Y.; Hua, Y.; Shen, Q.; Chen, L.; Xu, Y.; Fu, H.; Yu, C. Preclinical Evaluation of a Novel PSMA-Targeted Agent 68Ga-NOTA-GC-PSMA for Prostate Cancer Imaging. Tomography 2025, 11, 29. https://doi.org/10.3390/tomography11030029
Li W, Luo Y, Hua Y, Shen Q, Chen L, Xu Y, Fu H, Yu C. Preclinical Evaluation of a Novel PSMA-Targeted Agent 68Ga-NOTA-GC-PSMA for Prostate Cancer Imaging. Tomography. 2025; 11(3):29. https://doi.org/10.3390/tomography11030029
Chicago/Turabian StyleLi, Wenjin, Yihui Luo, Yuqi Hua, Qiaoling Shen, Liping Chen, Yu Xu, Haitian Fu, and Chunjing Yu. 2025. "Preclinical Evaluation of a Novel PSMA-Targeted Agent 68Ga-NOTA-GC-PSMA for Prostate Cancer Imaging" Tomography 11, no. 3: 29. https://doi.org/10.3390/tomography11030029
APA StyleLi, W., Luo, Y., Hua, Y., Shen, Q., Chen, L., Xu, Y., Fu, H., & Yu, C. (2025). Preclinical Evaluation of a Novel PSMA-Targeted Agent 68Ga-NOTA-GC-PSMA for Prostate Cancer Imaging. Tomography, 11(3), 29. https://doi.org/10.3390/tomography11030029




