Assessment of Psoas Muscle Index in Middle-Aged Type 2 Diabetes Patients: Impact of Insulin Therapy on Sarcopenia
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
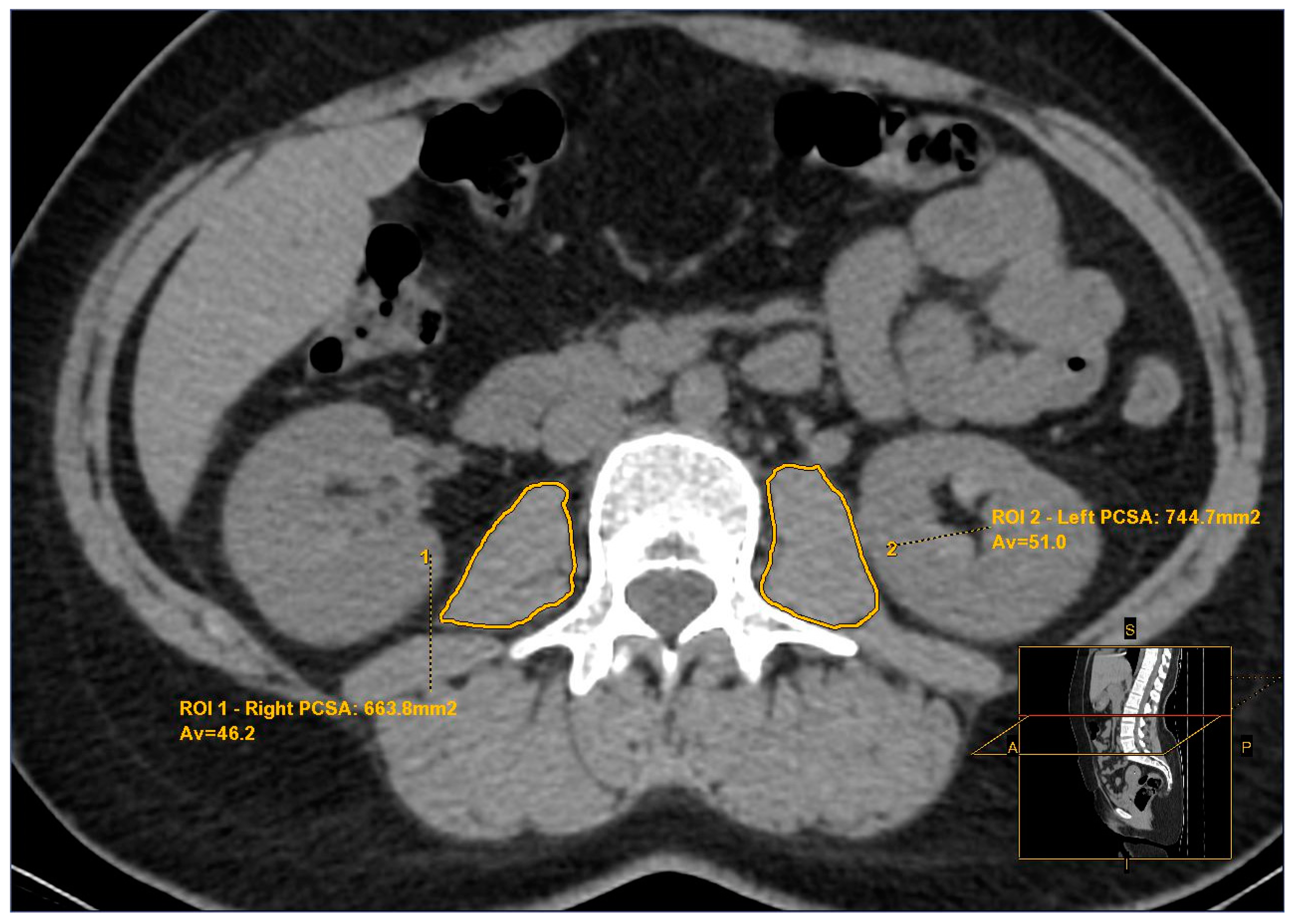
2.2. Psoas Muscle Assessment
2.3. Statistical Analysis
3. Results
4. Discussion
- The retrospective design precluded obtaining data on muscle strength and function, which are essential for a comprehensive definition of sarcopenia.
- Due to the retrospective nature of the study, we were unable to analyze the short- and long-term effects of patient medications.
- The small sample size limits the generalizability of our findings.
- We did not have sufficient information on the physical activity levels of the patients.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, G.H.; Kim, J.H.; Chung, M.S. Changes in weight, body composition, and physical activity among patients with breast cancer under adjuvant chemotherapy. Eur. J. Oncol. Nurs. 2020, 44, 101680. [Google Scholar] [CrossRef]
- Park, S.J.D. Health, Aging, and Body Composition Study: Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: The Health, Aging, and Body Composition Study. Diabetes Care 2007, 30, 1507–1512. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T.J.B. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef]
- Mesinovic, J.; Zengin, A.; De Courten, B.; Ebeling, P.R.; Scott, D.J.D. Sarcopenia and type 2 diabetes mellitus: A bidirectional relationship. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1057–1072. [Google Scholar] [CrossRef]
- Inoue, H.; Morino, K.; Ugi, S.; Tanaka-Mizuno, S.; Fuse, K.; Miyazawa, I.; Kondo, K.; Sato, D.; Ohashi, N.; Ida, S.; et al. Ipragliflozin, a sodium-glucose cotransporter 2 inhibitor, reduces bodyweight and fat mass, but not muscle mass, in Japanese type 2 diabetes patients treated with insulin: A randomized clinical trial. J. Diabetes Investig. 2019, 10, 1012–1021. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M. Sarcopenia: European consensus on definition and diagnosisReport of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Tucker, B.M.; Hsu, F.C.; Register, T.C.; Xu, J.; Smith, S.C.; Murea, M.; Bowden, D.W.; Freedman, B.I.; Lenchik, L. Psoas and paraspinous muscle measurements on computed tomography predict mortality in European Americans with type 2 diabetes mellitus. J. Frailty Aging 2019, 8, 72–78. [Google Scholar] [CrossRef]
- Murea, M.; Lenchik, L.; Register, T.C.; Russell, G.B.; Xu, J.; Smith, S.C.; Bowden, D.W.; Divers, J.; Freedman, B.I. Psoas and paraspinous muscle index as a predictor of mortality in African American men with type 2 diabetes mellitus. J. Diabetes Complicat. 2018, 32, 558–564. [Google Scholar] [CrossRef]
- Ng, T.P.; Feng, L.; Nyunt, M.S.Z.; Feng, L.; Niti, M.; Tan, B.Y.; Chan, G.; Khoo, S.A.; Chan, S.M.; Yap, P.; et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: A randomized controlled trial. Am. J. Med. 2015, 128, 1225–1236.e1221. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Derstine, B.A.; Holcombe, S.A.; Goulson, R.L.; Ross, B.E.; Wang, N.C.; Sullivan, J.A.; Su, G.L.; Wang, S.C. Quantifying sarcopenia reference values using lumbar and thoracic muscle areas in a healthy population. J. Nutr. Heal. Aging 2018, 22, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.I.; Doleman, B.; Scott, S.; Lund, J.N.; Williams, J.P. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Color. Dis. 2015, 17, O20–O26. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Perez, S.L.; Haus, J.M.; Sheean, P.; Patel, B.; Mar, W.; Chaudhry, V.; McKeever, L.; Braunschweig, C. Measuring abdominal circumference and skeletal muscle from a single cross-sectional computed tomography image: A step-by-step guide for clinicians using National Institutes of Health ImageJ. JPEN J. Parenter. Enter. Nutr. 2016, 40, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Amini, B.; Boyle, S.P.; Boutin, R.D.; Lenchik, L. Approaches to assessment of muscle mass and myosteatosis on computed tomography: A systematic review. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Punyanitya, M.; Wang, Z.; Gallagher, D.; St-Onge, M.-P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J. Appl. Physiol. 2004, 97, 2333–2338. [Google Scholar] [CrossRef] [PubMed]
- Durand, F.; Buyse, S.; Francoz, C.; Laouénan, C.; Bruno, O.; Belghiti, J.; Moreau, R.; Vilgrain, V.; Valla, D. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J. Hepatol. 2014, 60, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Ney, M.; Irwin, I.; Ma, M.M.; Gramlich, L.; Bain, V.G.; Esfandiari, N.; Baracos, V.; Montano-Loza, A.J.; Myers, R.P. Severe muscle depletion in patients on the liver transplant wait list: Its prevalence and independent prognostic value. Liver Transplant. 2012, 18, 1209–1216. [Google Scholar] [CrossRef]
- Hawkins, R.B.; Mehaffey, J.H.; Charles, E.J.; Kern, J.A.; Lim, D.S.; Teman, N.R.; Ailawadi, G. Psoas muscle size predicts risk-adjusted outcomes after surgical aortic valve replacement. Ann. Thorac. Surg. 2018, 106, 39–45. [Google Scholar] [CrossRef]
- Sanada, K.; Miyachi, M.; Tanimoto, M.; Yamamoto, K.; Murakami, H.; Okumura, S.; Gando, Y.; Suzuki, K.; Tabata, I.; Higuchi, M. A cross-sectional study of sarcopenia in Japanese men and women: Reference values and association with cardiovascular risk factors. European journal of applied physiology. Eur. J App. Physiol. 2010, 110, 57–65. [Google Scholar] [CrossRef]
- Sugiyama, S.; Jinnouchi, H.; Kurinami, N.; Hieshima, K.; Yoshida, A.; Jinnouchi, K.; Nishimura, H.; Suzuki, T.; Miyamoto, F.; Kajiwara, K.; et al. Dapagliflozin Reduces Fat Mass without Affecting Muscle Mass in Type 2 Diabetes. Journal of atherosclerosis and thrombosis. J. Atheroscler. Thromb. 2018, 25, 467–476. [Google Scholar] [CrossRef]
- Srikanthan, P.; Hevener, A.L.; Karlamangla, A.S. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: Findings from the National Health and Nutrition Examination Survey III. PLoS ONE 2010, 5, e10805. [Google Scholar] [CrossRef] [PubMed]
- Bouchi, R.; Fukuda, T.; Takeuchi, T.; Nakano, Y.; Murakami, M.; Minami, I.; Izumiyama, H.; Hashimoto, K.; Yoshimoto, T.; Ogawa, Y. Insulin treatment attenuates decline of muscle mass in Japanese patients with type 2 diabetes. Calcif. Tissue Int. 2017, 101, 1–8. [Google Scholar] [CrossRef]
- Fujita, S.; Glynn, E.L.; Timmerman, K.L.; Rasmussen, B.B.; Volpi, E.J.D. Supraphysiological hyperinsulinaemia is necessary to stimulate skeletal muscle protein anabolism in older adults: Evidence of a true age-related insulin resistance of muscle protein metabolism. Diabetologia 2009, 52, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Boyko, E.J.; Barrett-Connor, E.; Miljkovic, I.; Hoffman, A.R.; Everson-Rose, S.A.; Lewis, C.E.; Cawthon, P.M.; Strotmeyer, E.S.; Orwoll, E.S.; et al. Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care 2011, 34, 2381–2386. [Google Scholar] [CrossRef]
- Mensink, M.; Hesselink, M.; Russell, A.; Schaart, G.; Sels, J.; Schrauwen, P. Improved skeletal muscle oxidative enzyme activity and restoration of PGC-1α and PPARβ/δ gene expression upon rosiglitazone treatment in obese patients with type 2 diabetes mellitus. Int. J. Obes. 2007, 31, 1302–1310. [Google Scholar] [CrossRef]
- Sugimoto, K.; Ikegami, H.; Takata, Y.; Katsuya, T.; Fukuda, M.; Akasaka, H.; Tabara, Y.; Osawa, H.; Hiromine, Y.; Rakugi, H. Glycemic control and insulin improve muscle mass and gait speed in type 2 diabetes: The MUSCLES-DM study. J. Am. Med. Dir. Assoc. 2021, 22, 834–838.e831. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.-I.; Kanazawa, I.; Sugimoto, T. Reduction in endogenous insulin secretion is a risk factor of sarcopenia in men with type 2 diabetes mellitus. Calcif. Tissue Int. 2015, 97, 385–390. [Google Scholar] [CrossRef]
- Greenberg, R. The Design and Analysis of Clinical Experiments: By Joseph L. Fleiss; Wiley: New York, NY, USA, 1986; p. 432. [Google Scholar]
- Barclay, R.D.; Burd, N.A.; Tyler, C.; Tillin, N.A.; Mackenzie, R.W. The role of the IGF-1 signaling cascade in muscle protein synthesis and anabolic resistance in aging skeletal muscle. Front. Nutr. 2019, 6, 146. [Google Scholar] [CrossRef]
- Cetrone, M.; Mele, A.; Tricarico, D. Effects of the antidiabetic drugs on the age-related atrophy and sarcopenia associated with diabetes type II. Curr. Diabetes Rev. 2014, 10, 231–237. [Google Scholar] [CrossRef]
- Ferrari, U.; Then, C.; Rottenkolber, M.; Selte, C.; Seissler, J.; Conzade, R.; Linkohr, B.; Peters, A.; Drey, M.; Thorand, B. Longitudinal association of type 2 diabetes and insulin therapy with muscle parameters in the KORA-Age study. Acta Diabetol. 2020, 57, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control. Release 2002, 82, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Leenders, M.; Verdijk, L.B.; van der Hoeven, L.; Adam, J.J.; van Kranenburg, J.; Nilwik, R.; van Loon, L.J. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J. Am. Med. Dir. Assoc. 2013, 14, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; de Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; Newman, A.B. Decreased muscle strength and quality in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes 2006, 55, 1813–1818. [Google Scholar] [CrossRef]
- Shishikura, K.; Tanimoto, K.; Sakai, S.; Tanimoto, Y.; Terasaki, J.; Hanafusa, T. Association between skeletal muscle mass and insulin secretion in patients with type 2 diabetes mellitus. Endocr. J. 2014, 61, 281–287. [Google Scholar] [CrossRef]
- Kim, J.S.; Wilson, J.M.; Lee, S.R. Dietary implications on mechanisms of sarcopenia: Roles of protein, amino acids and antioxidants. J. Nutr. Biochem. 2010, 21, 1–13. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Leland Thaete, F.; Simoneau, J.-A.; Kelley, D.E.J.D. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 1997, 46, 1579–1585. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Metter, E.J.; Egan, J.; Golden, S.H.; Ferrucci, L. Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care 2015, 38, 82–90. [Google Scholar] [CrossRef]
- Mavros, Y.; Kay, S.; Anderberg, K.A.; Baker, M.K.; Wang, Y.; Zhao, R.; Meiklejohn, J.; Climstein, M.; O’Sullivan, A.; De Vos, N.; et al. Changes in insulin resistance and HbA1c are related to exercise-mediated changes in body composition in older adults with type 2 diabetes: Interim outcomes from the GREAT2DO trial. Diabetes Care 2013, 36, 2372–2379. [Google Scholar] [CrossRef]
- Fukuoka, Y.; Narita, T.; Fujita, H.; Morii, T.; Sato, T.; Sassa, M.H.; Yamada, Y. Importance of physical evaluation using skeletal muscle mass index and body fat percentage to prevent sarcopenia in elderly Japanese diabetes patients. J. Diabetes Investig. 2019, 10, 322–330. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Tra, Y.; Yeh, H.C.; Egan, J.M.; Ferrucci, L.; Brancati, F.L. Quadriceps strength, quadriceps power, and gait speed in older US adults with diabetes mellitus: Results from the National Health and Nutrition Examination Survey, 1999–2002. J. Am. Geriatr. Soc. 2013, 61, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Prashanthi, P.L.; Ramachandran, R.; Adhilakshmi, A.; Radhan, P.; Sai, V. Standardization of PSOAS muscle index measurements using computed tomography. Int. J. Contemp. Med. Surg. Radiol. 2020, 5, A169–A172. [Google Scholar] [CrossRef]
- Zannoni, S.; Albano, D.; Jannone, M.L.; Messina, C.; Sconfienza, L.M. Correlation between muscle mass and quality around the hip and of psoas muscles at L3 level using unenhanced CT scans. Skelet. Radiol. 2020, 49, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
| Study Group | Control Group d (n = 58) | a,b p Value | c,d p Value | |||
|---|---|---|---|---|---|---|
| Insulin a (n = 52) | OAD b (n = 55) | Total Study Group c (n = 107) | ||||
| Age * (year) (Mean ± SD) | 49.6 ± 9.6 | 48.7 ± 8.5 | 49.1 ± 9.0 | 50.0 ± 9.3 | 0.592 | 0.576 |
| Gender ** (Male/Female), n (%) | 24(46)/ 28(54) | 25(45)/ 30(55) | 49(46)/ 58(54) | 27(47)/ 31(53) | 0.548 | 0.528 |
| Study Group | Control Group d (n = 58) | a,b p Value | a–d p Value | b–d p Value | c,d p Value | |||
|---|---|---|---|---|---|---|---|---|
| Insulin a (n = 52) | OAD b (n = 55) | Total Study Group c (n = 107) | ||||||
| BMI * (kg/m2) (Mean ± SD) | 27.8 ± 3.1 | 27.1 ± 3.3 | 27.4 ± 3.2 | 26.5 ± 3.3 | 0.234 | 0.036 | 0.372 | 0.084 |
| Total PCSA * (mm2) (Mean ± SD) | 1579 ± 278 | 1459 ± 225 | 1517 ± 258 | 1613 ± 307 | 0.015 | 0.544 | 0.003 | 0.035 |
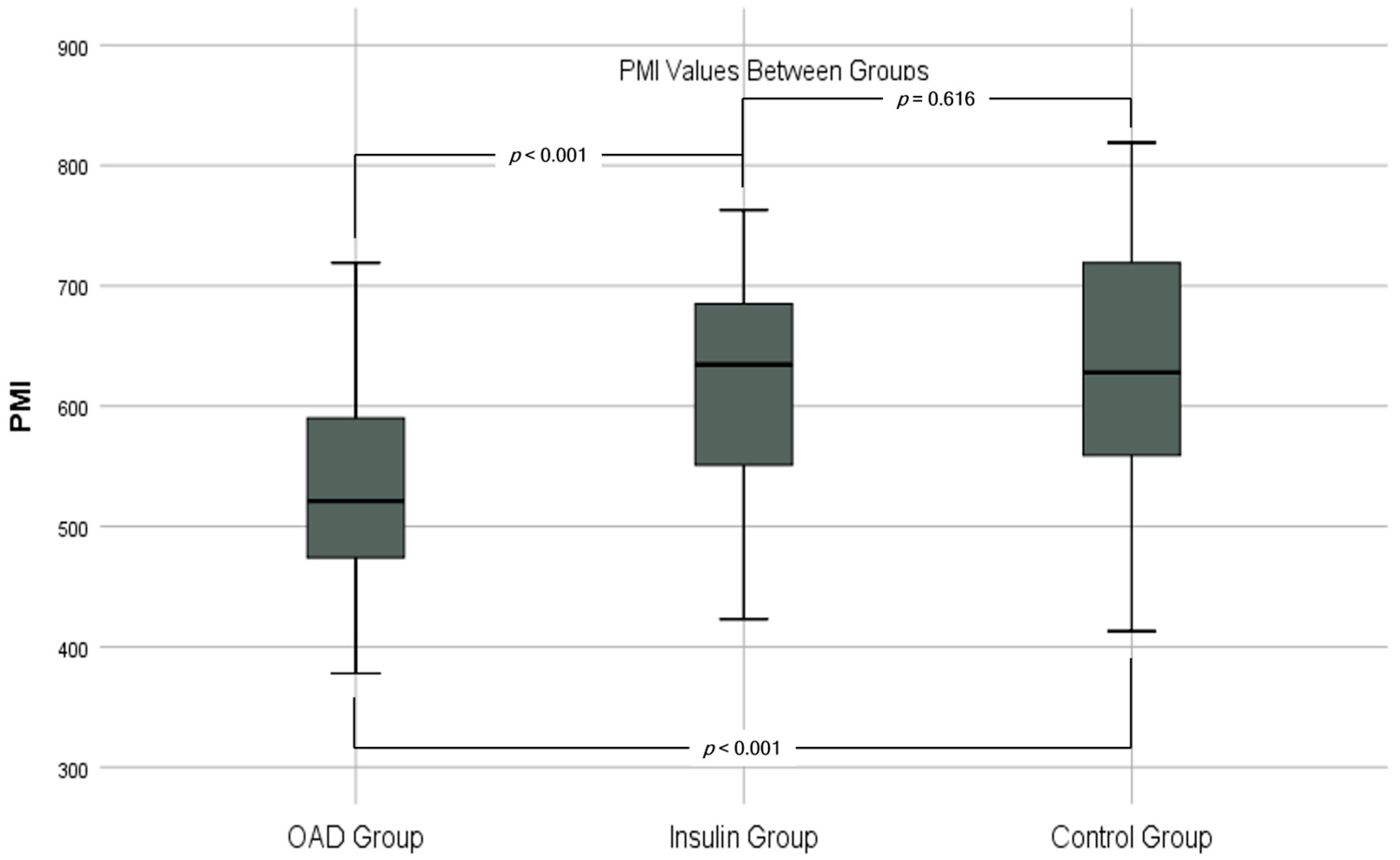
| PMI * (mm2/m2) | 618 ± 95 | 533 ± 85 | 574 ± 99 | 627 ± 103 | <0.001 | 0.616 | <0.001 | 0.001 |
| PMD * | 44.2 ± 4.1 | 43.9 ± 3.9 | 44.0 ± 4.0 | 44.4 ± 3.9 | 0.756 | 0.823 | 0.595 | 0.685 |
| HbA1c * (%) (Mean ± SD) | 7.1 ± 4.2 | 7.1 ± 0.5 | 7.1 ± 0.4 | - | 0.736 | - | - | - |
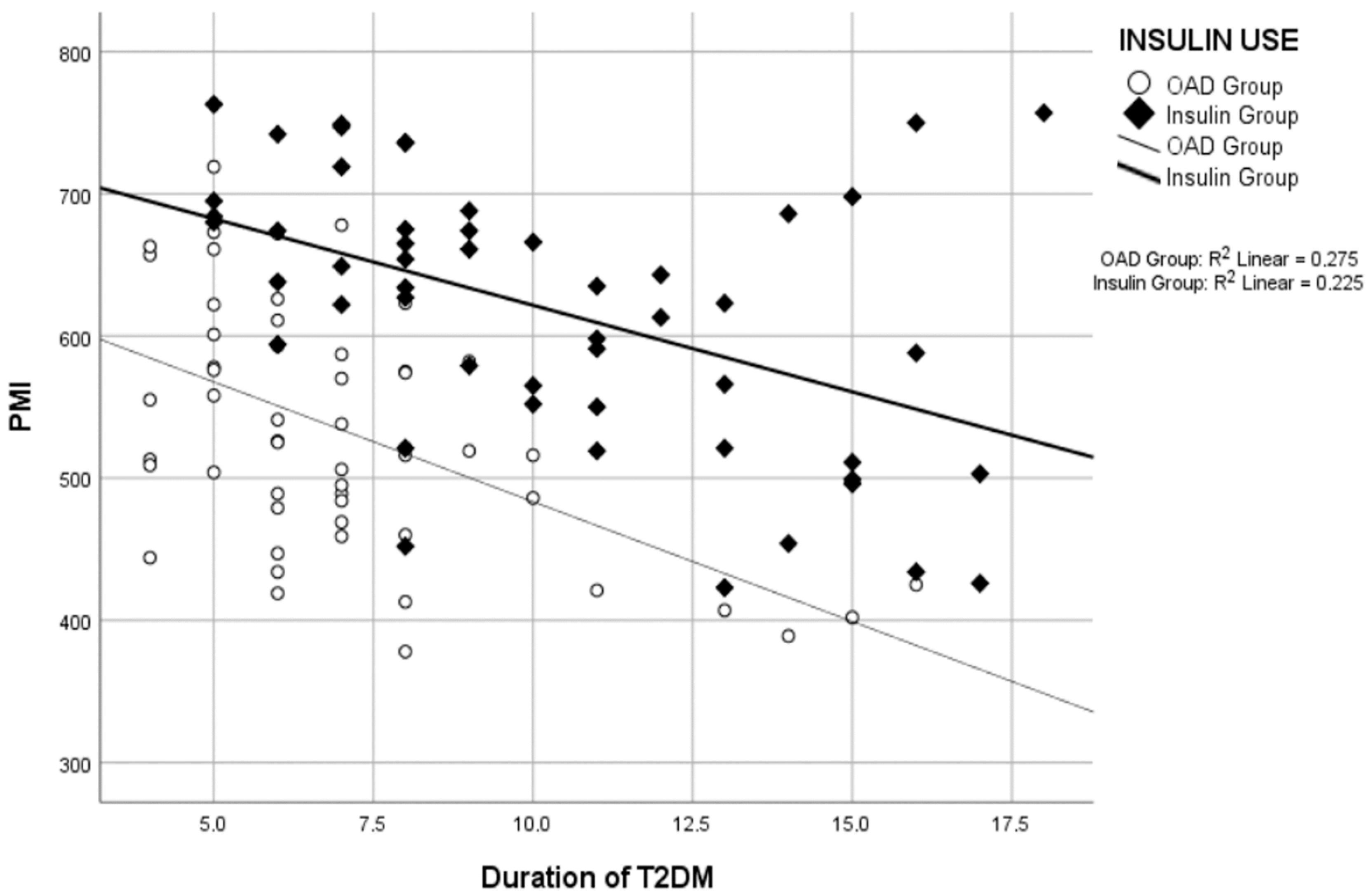
| Duration of T2DM (year) (Mean ± SD) | 10.3 ± 3.7 | 7.1 ± 2.7 | 8.6 ± 3.6 | - | <0.001 | - | - | - |
| Insulin (n = 52) | OAD (n = 55) | Total Study Group (n = 107) | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Duration of T2DM/PMI * | −0.474 | <0.001 | −0.525 | <0.001 | −0.192 | 0.038 |
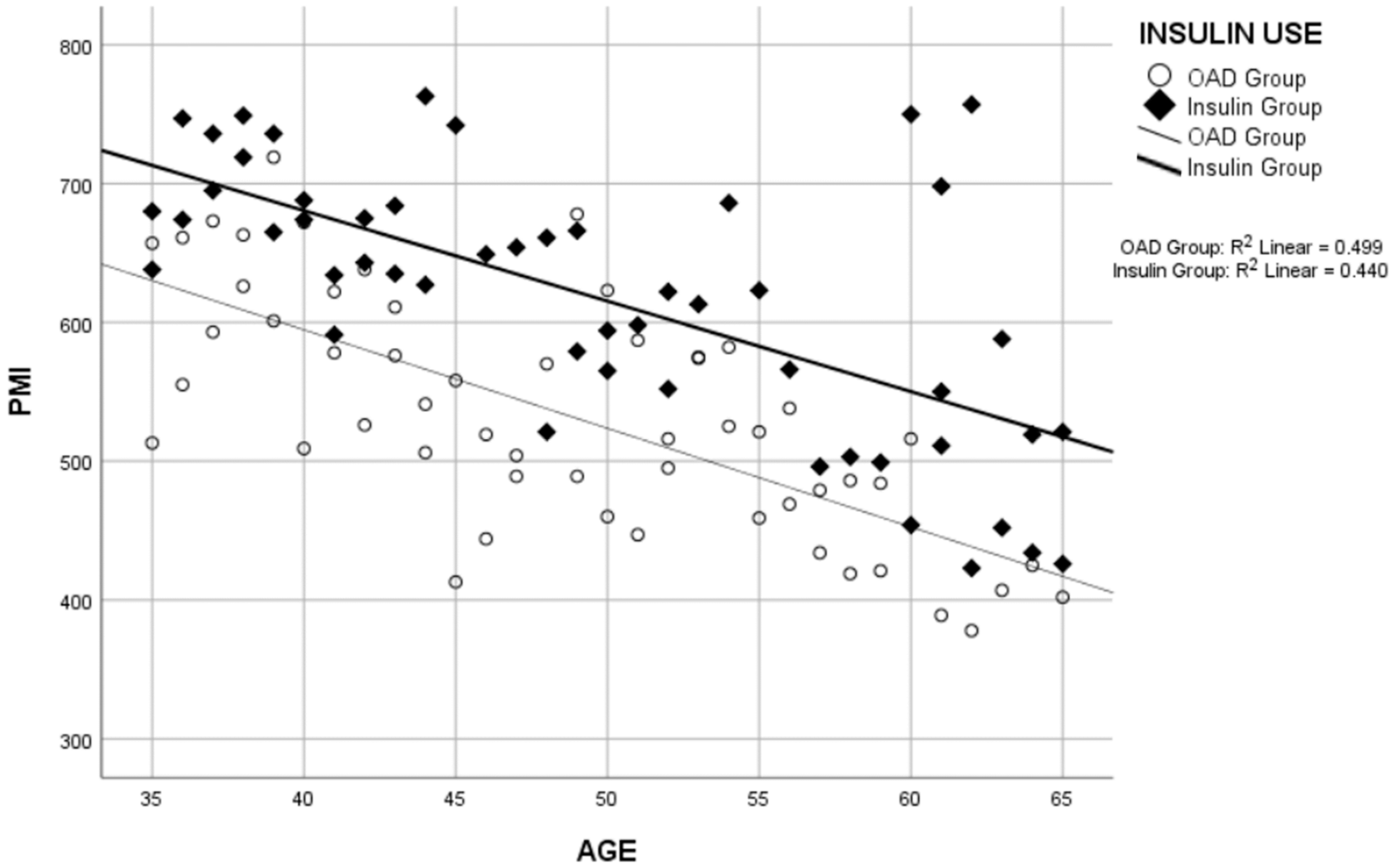
| Age/PMI ** | −0.663 | <0.001 | −0.707 | <0.001 | −0.597 | <0.001 |
| Duration of T2DM/BMI * | 0.233 | 0.096 | 0.779 | <0.001 | 0.332 | <0.001 |
| BMI/PMI * | −0.078 | 0.582 | −0.213 | 0.118 | −0.093 | 0.338 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taskent, I.; Ece, B.; Aydin, S. Assessment of Psoas Muscle Index in Middle-Aged Type 2 Diabetes Patients: Impact of Insulin Therapy on Sarcopenia. Tomography 2024, 10, 1054-1063. https://doi.org/10.3390/tomography10070079
Taskent I, Ece B, Aydin S. Assessment of Psoas Muscle Index in Middle-Aged Type 2 Diabetes Patients: Impact of Insulin Therapy on Sarcopenia. Tomography. 2024; 10(7):1054-1063. https://doi.org/10.3390/tomography10070079
Chicago/Turabian StyleTaskent, Ismail, Bunyamin Ece, and Sonay Aydin. 2024. "Assessment of Psoas Muscle Index in Middle-Aged Type 2 Diabetes Patients: Impact of Insulin Therapy on Sarcopenia" Tomography 10, no. 7: 1054-1063. https://doi.org/10.3390/tomography10070079
APA StyleTaskent, I., Ece, B., & Aydin, S. (2024). Assessment of Psoas Muscle Index in Middle-Aged Type 2 Diabetes Patients: Impact of Insulin Therapy on Sarcopenia. Tomography, 10(7), 1054-1063. https://doi.org/10.3390/tomography10070079







