Intravoxel Incoherent Motion Diffusion-Weighted MRI, Fat Quantification, and Electromyography: Correlation in Polymyositis and Dermatomyositis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MRI Protocols
2.3. Image Analysis
2.4. Electromyography
2.5. Statistical Analysis
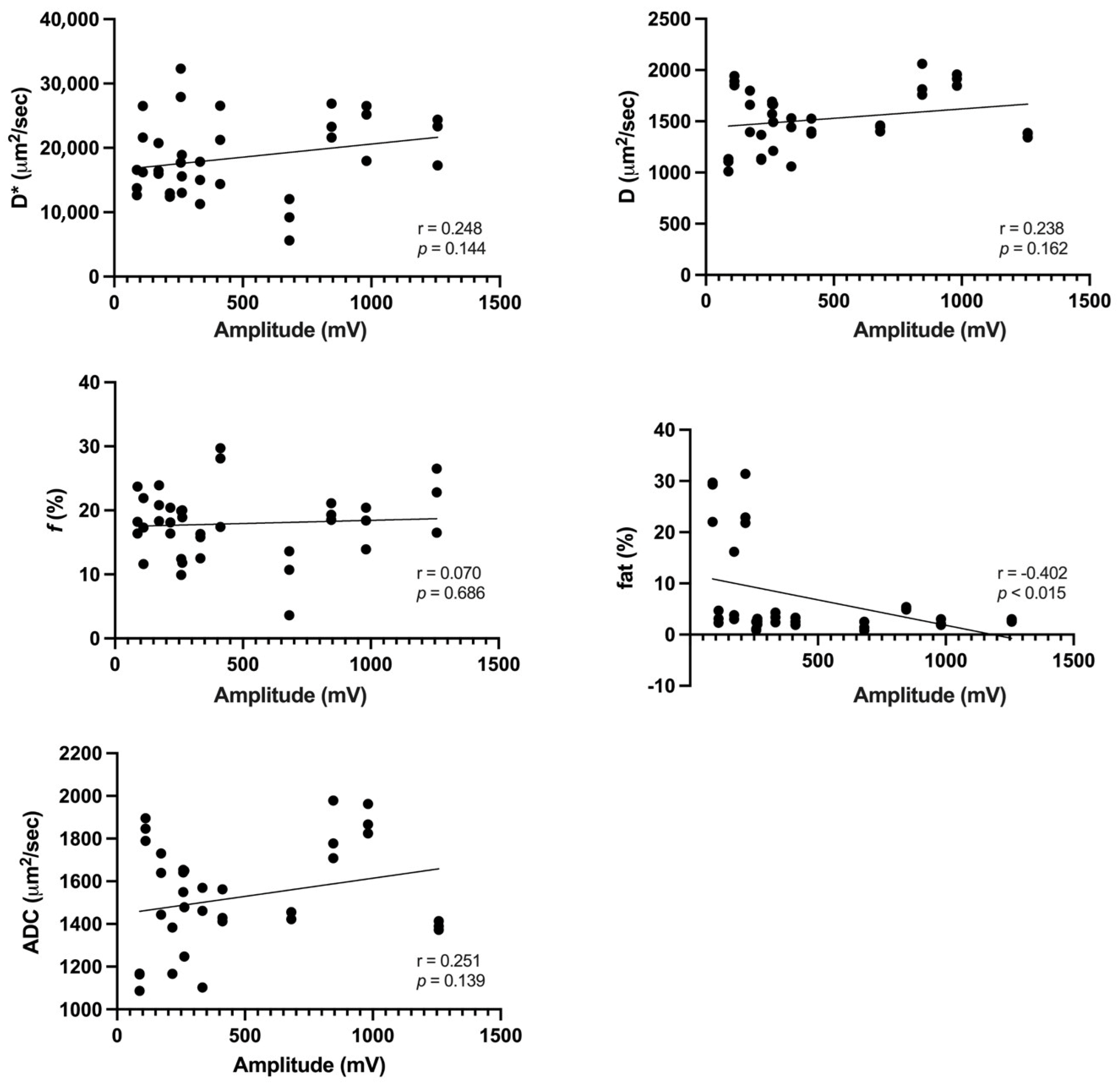
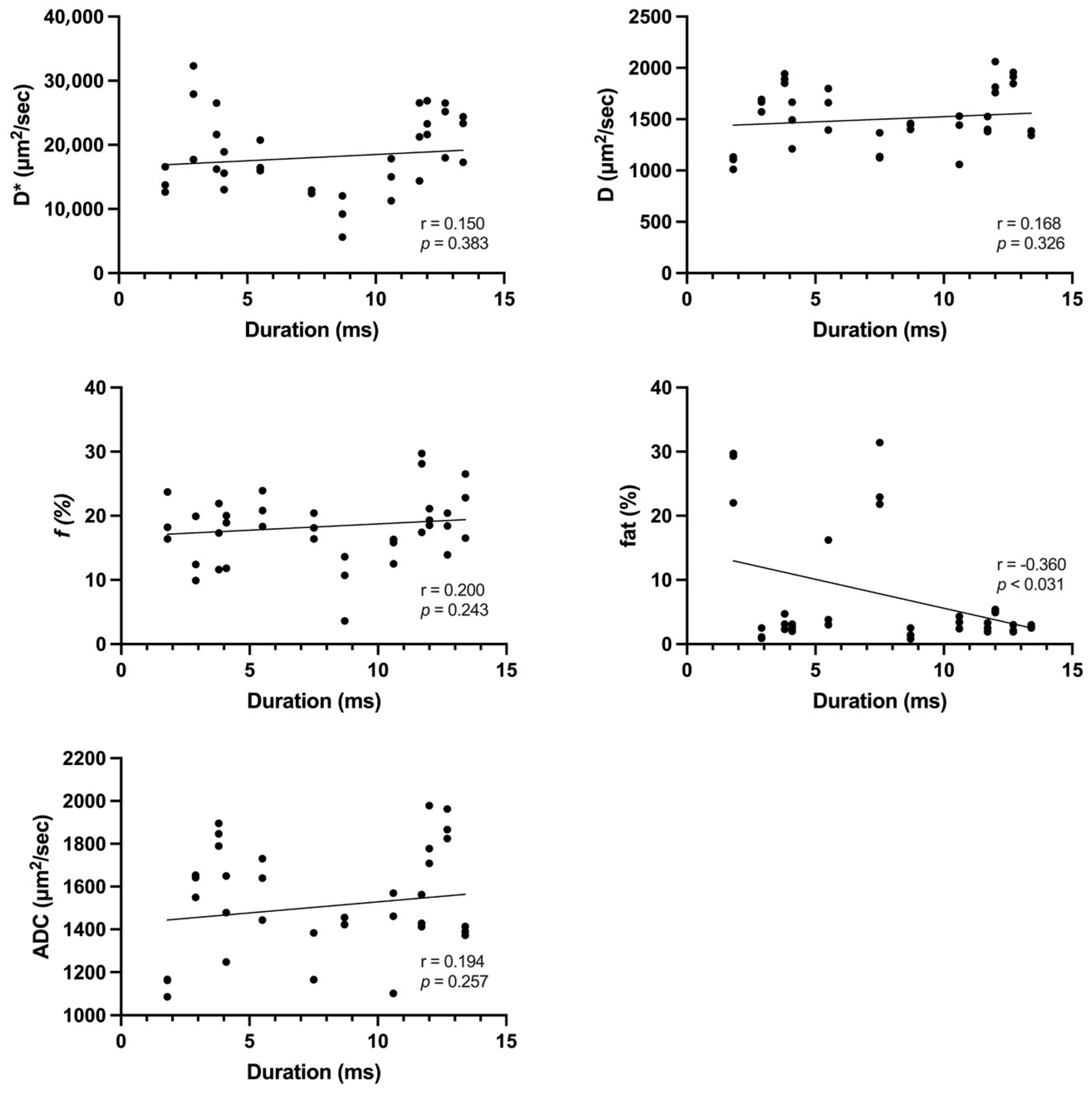
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalakas, M.C. Inflammatory muscle diseases. N. Engl. J. Med. 2015, 372, 1734–1747. [Google Scholar] [CrossRef]
- Bohan, A.; Peter, J.B. Polymyositis and dermatomyositis (second of two parts). N. Engl. J. Med. 1975, 292, 403–407. [Google Scholar] [CrossRef]
- Fraser, D.D.; Frank, J.A.; Dalakas, M.; Miller, F.W.; Hicks, J.E.; Plotz, P. Magnetic resonance imaging in the idiopathic inflammatory myopathies. J. Rheumatol. 1991, 18, 1693–1700. [Google Scholar] [PubMed]
- O’Connell, M.J.; Powell, T.; Brennan, D.; Lynch, T.; McCarthy, C.J.; Eustace, S.J. Whole-body MR imaging in the diagnosis of polymyositis. AJR Am. J. Roentgenol. 2002, 179, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Pitt, A.M.; Fleckenstein, J.L.; Greenlee, R.G., Jr.; Burns, D.K.; Bryan, W.W.; Haller, R. MRI-guided biopsy in inflammatory myopathy: Initial results. Magn. Reson. Imaging 1993, 11, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Grenier, P.; Cabanis, E.; Laval-Jeantet, M. MR imaging of intravoxel incoherent motions: Application to diffusion and perfusion in neurologic disorders. Radiology 1986, 161, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Englund, E.K.; Reiter, D.A.; Shahidi, B.; Sigmund, E.E. Intravoxel Incoherent Motion Magnetic Resonance Imaging in Skeletal Muscle: Review and Future Directions. J. Magn. Reson. Imaging 2022, 55, 988–1012. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D. What can we see with IVIM MRI? Neuroimage 2019, 187, 56–67. [Google Scholar] [CrossRef] [PubMed]
- de Mello, R.; Ma, Y.; Ji, Y.; Du, J.; Chang, E.Y. Quantitative MRI Musculoskeletal Techniques: An Update. AJR Am. J. Roentgenol. 2019, 213, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Burakiewicz, J.; Sinclair, C.D.J.; Fischer, D.; Walter, G.A.; Kan, H.E.; Hollingsworth, K.G. Quantifying fat replacement of muscle by quantitative MRI in muscular dystrophy. J. Neurol. 2017, 264, 2053–2067. [Google Scholar] [CrossRef]
- Gaeta, M.; Scribano, E.; Mileto, A.; Mazziotti, S.; Rodolico, C.; Toscano, A.; Settineri, N.; Ascenti, G.; Blandino, A. Muscle fat fraction in neuromuscular disorders: Dual-echo dual-flip-angle spoiled gradient-recalled MR imaging technique for quantification--a feasibility study. Radiology 2011, 259, 487–494. [Google Scholar] [CrossRef]
- Meyer, H.J.; Emmer, A.; Kornhuber, M.; Surov, A. Associations between apparent diffusion coefficient and electromyography parameters in myositis-A preliminary study. Brain Behav. 2018, 8, e00958. [Google Scholar] [CrossRef] [PubMed]
- Aoki, R.; Kokubun, N.; Komagamine, T.; Shimizu, J.; Nishino, I.; Kurasawa, K.; Hirata, K. Needle electromyography, muscle MRI, and muscle pathology: Correlations in idiopathic inflammatory myopathies. Neurol. Clin. Neurosci. 2020, 8, 28–35. [Google Scholar] [CrossRef]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Aubin, M.L.; Vignaud, J.; Laval-Jeantet, M.; Zhang, X.-Y.; Wang, L.; Zhu, H.-T.; Li, Z.-W.; et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988, 168, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Nickel, M.D.; Kannengiesser, S.A.; Dale, B.M.; Kiefer, B.; Bashir, M.R. Liver fat quantification using a multi-step adaptive fitting approach with multi-echo GRE imaging. Magn. Reson. Med. 2014, 72, 1353–1365. [Google Scholar] [CrossRef]
- Jungmann, P.M.; Pfirrmann, C.; Federau, C. Characterization of lower limb muscle activation patterns during walking and running with Intravoxel Incoherent Motion (IVIM) MR perfusion imaging. Magn. Reson. Imaging 2019, 63, 12–20. [Google Scholar] [CrossRef]
- Nguyen, A.; Ledoux, J.B.; Omoumi, P.; Becce, F.; Forget, J.; Federau, C. Application of intravoxel incoherent motion perfusion imaging to shoulder muscles after a lift-off test of varying duration. NMR Biomed. 2016, 29, 66–73. [Google Scholar] [CrossRef]
- Nguyen, A.; Ledoux, J.B.; Omoumi, P.; Becce, F.; Forget, J.; Federau, C. Selective microvascular muscle perfusion imaging in the shoulder with intravoxel incoherent motion (IVIM). Magn. Reson. Imaging 2017, 35, 91–97. [Google Scholar] [CrossRef]
- Le Bihan, D.; Turner, R. The capillary network: A link between IVIM and classical perfusion. Magn. Reson. Med. 1992, 27, 171–178. [Google Scholar] [CrossRef]
- Dalakas, M.C. Pathophysiology of inflammatory and autoimmune myopathies. Presse Med. 2011, 40, e237–e247. [Google Scholar] [CrossRef]
- Järvinen, T.A.; Järvinen, T.L.; Kääriäinen, M.; Kalimo, H.; Järvinen, M. Muscle injuries: Biology and treatment. Am. J. Sports Med. 2005, 33, 745–764. [Google Scholar] [CrossRef]
- Wiig, H. Pathophysiology of tissue fluid accumulation in inflammation. J. Physiol. 2011, 589, 2945–2953. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gutiérrez, G.; Barbosa López, C.; Navacerrada, F.; Miralles Martínez, A. Use of electromyography in the diagnosis of inflammatory myopathies. Reumatol. Clin. 2012, 8, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Olsen, N.J.; Price, R.R.; Winston, J.A.; Park, J.H. Diffusion-weighted imaging of inflammatory myopathies: Polymyositis and dermatomyositis. J. Magn. Reson. Imaging 2008, 27, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.; Yu, K.; Gao, L.; Zhang, P.; Goerner, F.; Runge, V.M.; Li, X. Diffusion tensor imaging in evaluation of thigh muscles in patients with polymyositis and dermatomyositis. Br. J. Radiol. 2014, 87. [Google Scholar] [CrossRef] [PubMed]
- Sigmund, E.E.; Baete, S.H.; Luo, T.; Patel, K.; Wang, D.; Rossi, I.; Duarte, A.; Bruno, M.; Mossa, D.; Femia, A.; et al. MRI assesment of the thigh musculature in dermatomyositis and healthy subjects using diffusion tensor imaging, intravoxel incoherent motion and dynamic DTI. Eur. Radiol. 2018, 28, 53045315. [Google Scholar] [CrossRef]
- Amato, A.A.; Barohn, R.J. Evaluation and treatment of inflammatory myopathies. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1060–1068. [Google Scholar] [CrossRef]
- Ran, J.; Liu, Y.; Sun, D.; Morelli, J.; Zhang, P.; Wu, G.; Sheng, Y.; Xie, R.; Zhang, X.; Li, X. The diagnostic value of biexponential apparent diffusion coefficients in myopathy. J. Neurol. 2016, 263, 1296–1302. [Google Scholar] [CrossRef]
- Barsotti, S.; Zampa, V.; Talarico, R.; Minichilli, F.; Ortori, S.; Iacopetti, V.; D’ascanio, A.; Tavoni, A.G.; Bombardieri, S.; Mosca, M. Thigh magnetic resonance imaging for the evaluation of disease activity in patients with idiopathic inflammatory myopathies followed in a single center. Muscle Nerve 2016, 54, 666–672. [Google Scholar] [CrossRef]

| No. | Sex | Age | Diagnosis | Disease Duration (Months) * | Time Interval between MRI and EMG (Days) | Fib | PSW |
|---|---|---|---|---|---|---|---|
| 1 | M | 47 | DM | 1 | 1 | + | + |
| 2 | F | 55 | DM | 1 | 4 | + | + |
| 3 | F | 61 | DM | 2 | 9 | - | - |
| 4 | F | 39 | DM | 1 | 7 | + | - |
| 5 | M | 38 | DM | 2 | 9 | - | - |
| 6 | F | 56 | DM | 24 | 2 | + | + |
| 7 | F | 76 | DM | 12 | 7 | + | + |
| 8 | F | 35 | PM | 10 | 1 | + | + |
| 9 | F | 56 | DM | 3 | 5 | - | - |
| 10 | F | 51 | PM | 2 | 6 | + | + |
| 11 | M | 60 | PM | 1 | 8 | + | + |
| 12 | M | 42 | PM | 9 | 9 | + | + |
| Parameter | IVIM-DWI | T1 VIBE Multi-Echo DIXON |
|---|---|---|
| FOV (mm) | 400 × 400 | 400 × 350 |
| Matrix size | 140 × 140 | 160 × 111 |
| TR (ms) | 2900 | 9 |
| TE (ms) | 68 | 1.05, 2.46, 3.69, 4.92, 6.15, 7.38 |
| Flip angle ° | - | 4 |
| Fat suppression | SPAIR | |
| Slice thickness (mm) | 8 | 4 |
| Intersection Gap (mm) | 0.8 | 0 |
| EPI factor | 140 | - |
| Number of excitations | 3 | - |
| Acquisition time (s) | 293 | 26 |
| Parameters | κ (Kappa) | 95% CI |
|---|---|---|
| ADC (μm2/s) | 0.937 | 0.864–0.969 |
| D (μm2/s) | 0.951 | 0.899–0.976 |
| D* (μm2/s) | 0.943 | 0.884–0.972 |
| f (%) | 0.958 | 0.918–0.979 |
| Fat (%) | 0.978 | 0.956–0.989 |
| Parameters | Pathologic Spontaneous Activity | No Pathologic Spontaneous Activity | p Value |
|---|---|---|---|
| D* (μm2/s) | 19,285.18 ± 5615.90 | 15,867.78 ± 7061.79 | 0.147 |
| D (μm2/s) | 1513.44 ± 289.14 | 1553.02 ± 289.00 | 0.724 |
| f (%) | 19.0 ± 4.9 | 14.6 ± 5.3 | 0.027 |
| ADC (μm2/s) | 1515.97 ± 255.62 | 1547.81 ± 250.80 | 0.747 |
| fat (%) | 8.35 ± 10.27 | 3.37 ± 1.69 | 0.160 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Yong, S.Y.; Otgonbaatar, C.; Nam, S.W. Intravoxel Incoherent Motion Diffusion-Weighted MRI, Fat Quantification, and Electromyography: Correlation in Polymyositis and Dermatomyositis. Tomography 2024, 10, 368-377. https://doi.org/10.3390/tomography10030029
Kim H, Yong SY, Otgonbaatar C, Nam SW. Intravoxel Incoherent Motion Diffusion-Weighted MRI, Fat Quantification, and Electromyography: Correlation in Polymyositis and Dermatomyositis. Tomography. 2024; 10(3):368-377. https://doi.org/10.3390/tomography10030029
Chicago/Turabian StyleKim, Hyunjung, Sang Yeol Yong, Chuluunbaatar Otgonbaatar, and Seoung Wan Nam. 2024. "Intravoxel Incoherent Motion Diffusion-Weighted MRI, Fat Quantification, and Electromyography: Correlation in Polymyositis and Dermatomyositis" Tomography 10, no. 3: 368-377. https://doi.org/10.3390/tomography10030029
APA StyleKim, H., Yong, S. Y., Otgonbaatar, C., & Nam, S. W. (2024). Intravoxel Incoherent Motion Diffusion-Weighted MRI, Fat Quantification, and Electromyography: Correlation in Polymyositis and Dermatomyositis. Tomography, 10(3), 368-377. https://doi.org/10.3390/tomography10030029







