Differentiation of Hamartomas and Malignant Lung Tumors in Single-Phased Dual-Energy Computed Tomography
Abstract
1. Introduction
2. Methods and Materials
2.1. Patient Population
2.2. Acquisition Parameters
2.3. Dual-Energy Image Analysis
2.4. Reference Standard
2.5. Statistical Analysis
3. Results
3.1. General Analysis
3.2. DECT Image Analysis
3.3. Diagnostic Accuracy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CT | Computed tomography |
| DECT | Dual-energy computed tomography |
| FDG | Fluorodeoxyglucose |
| kV | Kilovolt |
| PET-CT | Positron emission tomography computed tomography |
| ROI | Region of interest |
| ROC | Receiver operating characteristic |
| SECT | Single-energy computed tomography |
| VNC | Virtual non-contrast |
References
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Muller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef]
- Agress, H., Jr.; Cooper, B.Z. Detection of clinically unexpected malignant and premalignant tumors with whole-body FDG PET: Histopathologic comparison. Radiology 2004, 230, 417–422. [Google Scholar] [CrossRef]
- Lee, J.E.; Jeong, W.G.; Kim, Y.-H. Differentiation of primary lung cancer from solitary lung metastasis in patients with colorectal cancer: A retrospective cohort study. World J. Surg. Oncol. 2021, 19, 28. [Google Scholar] [CrossRef]
- Truong, M.T.; Ko, J.P.; Rossi, S.E.; Rossi, I.; Viswanathan, C.; Bruzzi, J.F.; Marom, E.M.; Erasmus, J.J. Update in the evaluation of the solitary pulmonary nodule. Radiographics 2014, 34, 1658–1679. [Google Scholar] [CrossRef] [PubMed]
- Erasmus, J.J.; Connolly, J.E.; McAdams, H.P.; Roggli, V.L. Solitary Pulmonary Nodules: Part I. Morphologic Evaluation for Differentiation of Benign and Malignant Lesions. Radiographics 2000, 20, 43–58. [Google Scholar] [CrossRef]
- Gjevre, J.A.; Myers, J.L.; Prakash, U.B. Pulmonary hamartomas. Mayo Clin. Proc. 1996, 71, 14–20. [Google Scholar] [CrossRef]
- Ekinci, G.H.; Haciomeroglu, O.; Ersev, A.; Alpay, L.; Ozgen, H.; Yilmaz, A. The frequency of lung cancer in patients with pulmonary hamartomas: An evaluation of clinical, radiological, and pathological features and follow-up data of 96 patients with pulmonary hamartomas. Rev. Port. Pneumol. 2017, 23, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Siegelman, S.S.; Khouri, N.F.; Scott, W.W., Jr.; Leo, F.P.; Hamper, U.M.; Fishman, E.K.; Zerhouni, E.A. Pulmonary hamartoma: CT findings. Radiology 1986, 160, 313–317. [Google Scholar] [CrossRef]
- Kishore, M.; Gupta, P.; Preeti; Deepak, D. Pulmonary Hamartoma Mimicking Malignancy: A Cytopathological Diagnosis. J. Clin. Diagn. Res. 2016, 10, ED06–ED07. [Google Scholar] [CrossRef] [PubMed]
- Ganti, S.; Milton, R.; Davidson, L.; Anikin, V. Giant pulmonary hamartoma. J. Cardiothorac. Surg. 2006, 1, 19. [Google Scholar] [CrossRef]
- Potente, G.; Macori, F.; Caimi, M.; Mingazzini, P.; Volpino, P. Noncalcified pulmonary hamartomas: Computed tomography enhancement patterns with histologic correlation. J. Thorac. Imaging 1999, 14, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Hochhegger, B.; Nin, C.S.; Alves, G.R.T.; Hochhegger, D.R.; de Souza, V.V.S.; Watte, G.; Irion, K.L.; Guimarães, M.D.; Marchiori, E. Multidetector Computed Tomography Findings in Pulmonary Hamartomas: A New Fat Detection Threshold. J. Thorac. Imaging 2016, 31, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Hochhegger, B.; Marchiori, E.; dos Reis, D.Q.; Souza, A.S., Jr.; Souza, L.S.; Brum, T.; Irion, K.L. Chemical-shift MRI of pulmonary hamartomas: Initial experience using a modified technique to assess nodule fat. AJR. Am. J. Roentgenol. 2012, 199, W331–W334. [Google Scholar] [CrossRef] [PubMed]
- Hamper, U.M.; Khouri, N.F.; Stitik, F.P.; Siegelman, S.S. Pulmonary hamartoma: Diagnosis by transthoracic needle-aspiration biopsy. Radiology 1985, 155, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Takumi, K.; Nagano, H.; Harasawa, T.; Tabata, K.; Tokunaga, T.; Yoshiura, T. Pulmonary hamartoma: Feasibility of dual-energy CT detection of intranodular fat. Radiol. Case Rep. 2021, 16, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.H.; Young, N.A.; Wilbur, D.C.; Renshaw, A.A.; Mody, D.R. Fine-needle aspiration of pulmonary hamartoma: A common source of false-positive diagnoses in the College of American Pathologists Interlaboratory Comparison Program in Nongynecologic Cytology. Arch. Pathol. Lab. Med. 2005, 129, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Cellina, M.; Cè, M.; Irmici, G.; Ascenti, V.; Khenkina, N.; Toto-Brocchi, M.; Martinenghi, C.; Papa, S.; Carrafiello, G. Artificial Intelligence in Lung Cancer Imaging: Unfolding the Future. Diagnostics 2022, 12, 2644. [Google Scholar] [CrossRef]
- Tatsugami, F.; Higaki, T.; Nakamura, Y.; Honda, Y.; Awai, K. Dual-energy CT: Minimal essentials for radiologists. Jpn. J. Radiol. 2022, 40, 547–559. [Google Scholar] [CrossRef]
- Matsumoto, K.; Jinzaki, M.; Tanami, Y.; Ueno, A.; Yamada, M.; Kuribayashi, S. Virtual monochromatic spectral imaging with fast kilovoltage switching: Improved image quality as compared with that obtained with conventional 120-kVp CT. Radiology 2011, 259, 257–262. [Google Scholar] [CrossRef]
- Cellina, M.; Bausano, M.V.; Pais, D.; Chiarpenello, V.; Costa, M.; Vincenzo, Z.; Cè, M.; Martinenghi, C.; Oliva, G.; Carrafiello, G. Dual-Energy CT Applications in Urological Diseases. Appl. Sci. 2023, 13, 7653. [Google Scholar] [CrossRef]
- Yang, L.; Sun, J.; Li, J.; Peng, Y. Dual-energy spectral CT imaging of pulmonary embolism with Mycoplasma pneumoniae pneumonia in children. La Radiol. Medica 2022, 127, 154–161. [Google Scholar] [CrossRef]
- Alvarez, R.E.; Macovski, A. Energy-selective reconstructions in x-ray computerised tomography. Phys. Med. Biol. 1976, 21, 733. [Google Scholar] [CrossRef]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-counting CT: Technical Principles and Clinical Prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef]
- Goo, H.W.; Goo, J.M. Dual-Energy CT: New Horizon in Medical Imaging. Korean J. Radiol. 2017, 18, 555–569. [Google Scholar] [CrossRef]
- Mileto, A.; Sofue, K.; Marin, D. Imaging the renal lesion with dual-energy multidetector CT and multi-energy applications in clinical practice: What can it truly do for you? Eur. Radiol. 2016, 26, 3677–3690. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Czwikla, R.; Wichmann, J.L.; Albrecht, M.H.; Lenga, L.; Savage, R.H.; Arendt, C.; Hammerstingl, R.; Vogl, T.J.; Kaltenbach, B. Dual-energy CT-based iodine quantification to differentiate abdominal malignant lymphoma from lymph node metastasis. Eur. J. Radiol. 2018, 105, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Weidinger, S.; Czwikla, R.; Kaltenbach, B.; Albrecht, M.H.; Lenga, L.; Vogl, T.J.; Wichmann, J.L. Iodine and Fat Quantification for Differentiation of Adrenal Gland Adenomas From Metastases Using Third-Generation Dual-Source Dual-Energy Computed Tomography. Investig. Radiol. 2018, 53, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Kaltenbach, B.; Wichmann, J.L.; Pfeifer, S.; Albrecht, M.H.; Booz, C.; Lenga, L.; Hammerstingl, R.; D’Angelo, T.; Vogl, T.J.; Martin, S.S. Iodine quantification to distinguish hepatic neuroendocrine tumor metastasis from hepatocellular carcinoma at dual-source dual-energy liver CT. Eur. J. Radiol. 2018, 105, 20–24. [Google Scholar] [CrossRef]
- González-Pérez, V.; Arana, E.; Barrios, M.; Bartrés, A.; Cruz, J.; Montero, R.; González, M.; Deltoro, C.; Martínez-Pérez, E.; De Aguiar-Quevedo, K.; et al. Differentiation of benign and malignant lung lesions: Dual-Energy Computed Tomography findings. Eur. J. Radiol. 2016, 85, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fan, X.; Luo, T.-Y.; Lv, F.-J.; Huang, X.-T. Differentiating malignant and benign necrotic lung lesions using kVp-switching dual-energy spectral computed tomography. BMC Med. Imaging 2021, 21, 81. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Nelson, R.C.; Vernuccio, F.; González, F.; Farjat, A.E.; Patel, B.N.; Samei, E.; Henzler, T.; Schoenberg, S.O.; Marin, D. Virtual Unenhanced Images at Dual-Energy CT: Influence on Renal Lesion Characterization. Radiology 2019, 291, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.; Sauter, A.; Spira, D.; Gatidis, S.; Ketelsen, D.; Heuschmid, M.; Claussen, C.D.; Thomas, C. Tin-filter Enhanced Dual-Energy-CT: Image Quality and Accuracy of CT Numbers in Virtual Noncontrast Imaging. Acad. Radiol. 2013, 20, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Hansel, S.L.; Bruining, D.H.; Fletcher, J.G.; Froemming, A.T.; Barlow, J.M.; Fidler, J.L. CT for Evaluation of Acute Gastrointestinal Bleeding. Radiographics 2018, 38, 1089–1107. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.S.; Schneeweiß, S.; Maurer, M.; Kraus, M.S.; Wichmann, J.L.; Bongers, M.N.; Lescan, M.; Bamberg, F.; Othman, A.E. Virtual non-enhanced dual-energy CT reconstruction may replace true non-enhanced CT scans in the setting of suspected active hemorrhage. Eur. J. Radiol. 2018, 109, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Trapp, F.; Wichmann, J.L.; Albrecht, M.H.; Lenga, L.; Durden, J.; Booz, C.; Vogl, T.J.; D’Angelo, T. Dual-energy CT in early acute pancreatitis: Improved detection using iodine quantification. Eur. Radiol. 2019, 29, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; Radice, D.; Femia, M.; De Marco, P.; Origgi, D.; Preda, L.; Barberis, M.; Vigorito, R.; Mauri, G.; Mauro, A.; et al. Metastatic and non-metastatic lymph nodes: Quantification and different distribution of iodine uptake assessed by dual-energy CT. Eur. Radiol. 2018, 28, 760–769. [Google Scholar] [CrossRef]
- Li, J.; Fang, M.; Wang, R.; Dong, D.; Tian, J.; Liang, P.; Liu, J.; Gao, J. Diagnostic accuracy of dual-energy CT-based nomograms to predict lymph node metastasis in gastric cancer. Eur. Radiol. 2018, 28, 5241–5249. [Google Scholar] [CrossRef]
- Winkelmann, M.T.; Hagen, F.; Artzner, K.; Bongers, M.N.; Artzner, C. Dual-Energy CT for Accurate Discrimination of Intraperitoneal Hematoma and Intestinal Structures. Diagnostics 2022, 12, 2542. [Google Scholar] [CrossRef]
- Hur, B.Y.; Lee, J.M.; Hyunsik, W.; Lee, K.B.; Joo, I.; Han, J.K.; Choi, B.I. Quantification of the Fat Fraction in the Liver Using Dual-Energy Computed Tomography and Multimaterial Decomposition. J. Comput. Assist. Tomogr. 2014, 38, 845–852. [Google Scholar] [CrossRef]
- Gassenmaier, S.; Kähm, K.; Walter, S.S.; Machann, J.; Nikolaou, K.; Bongers, M.N. Quantification of liver and muscular fat using contrast-enhanced Dual Source Dual Energy Computed Tomography compared to an established multi-echo Dixon MRI sequence. Eur. J. Radiol. 2021, 142, 109845. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, G.; Qi, X.; Fan, X.; Wang, L. Quantitative analysis of the dual-energy CT virtual spectral curve for focal liver lesions characterization. Eur. J. Radiol. 2014, 83, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Swensen, S.J.; Brown, L.R.; Colby, T.V.; Weaver, A.L. Pulmonary nodules: CT evaluation of enhancement with iodinated contrast material. Radiology 1995, 194, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, D.; Jirapatnakul, A.; Reeves, A.P.; Farooqi, A.; Zhang, L.; Giunta, S.; Zulueta, J.; Aye, R.; Miller, A.; et al. CT- and computer-based features of small hamartomas. Clin. Imaging 2011, 35, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.T.; Ho, L.M.; Marin, D.; Boll, D.T.; Barnhart, H.X.; Nelson, R.C. Dual-Energy CT for Characterization of Adrenal Nodules: Initial Experience. Am. J. Roentgenol. 2010, 194, 1479–1483. [Google Scholar] [CrossRef]
- Kazimierczak, W.; Kazimierczak, N.; Serafin, Z. Quality of virtual-non-contrast phases derived from arterial and delayed phases of fast-kVp switching dual-energy CT in patients after endovascular aortic repair. Int. J. Cardiovasc. Imaging 2023, 39, 1805–1813. [Google Scholar] [CrossRef]
- Lehti, L.; Söderberg, M.; Höglund, P.; Wassélius, J. Comparing Arterial- and Venous-Phase Acquisition for Optimization of Virtual Noncontrast Images From Dual-Energy Computed Tomography Angiography. J. Comput. Assist. Tomogr. 2019, 43, 770–774. [Google Scholar] [CrossRef]


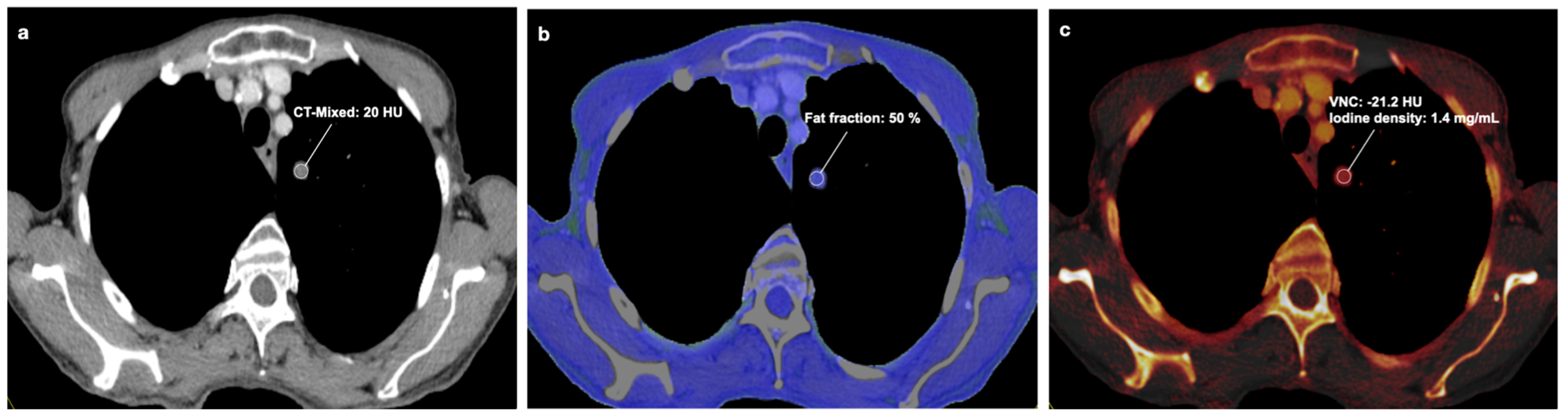

| Variables | /Mean ± SD |
|---|---|
| Age (years) | 65.2 ± 12.1 |
| Male patients (n) | 15 |
| Female patients (n) | 31 |
| Hamartomas (n) | 22 |
| Mean lesion size (mm) | 13.7 ± 7.3 |
| Lesion characteristics | |
| 13 |
| 5 |
| 4 |
| 6 |
| 9 |
| Malignant lung lesions (n) | 25 |
| Mean lesion size (mm) | 16.3 ± 7.5 |
| Lesion characteristics: | |
| 8 |
| 11 |
| 6 |
| 0 |
| Tumor entitiy: | |
| Melanoma (n) | 4 |
| Renal cell carcinoma (n) | 4 |
| Breast cancer (n) | 3 |
| Lung cancer (n) | 3 |
| Colorectal cancer (n) | 2 |
| Oropharyngeal cancer (n) | 2 |
| Prostate cancer (n) | 1 |
| Rhabdomyosarcoma (n) | 1 |
| Ewing-like sarcoma (n) | 1 |
| Soft-tissue sarcoma (n) | 1 |
| Clear cell sarcoma (n) | 1 |
| Gallbladder carcinoma (n) | 1 |
| Appendiceal carcinoma (n) | 1 |
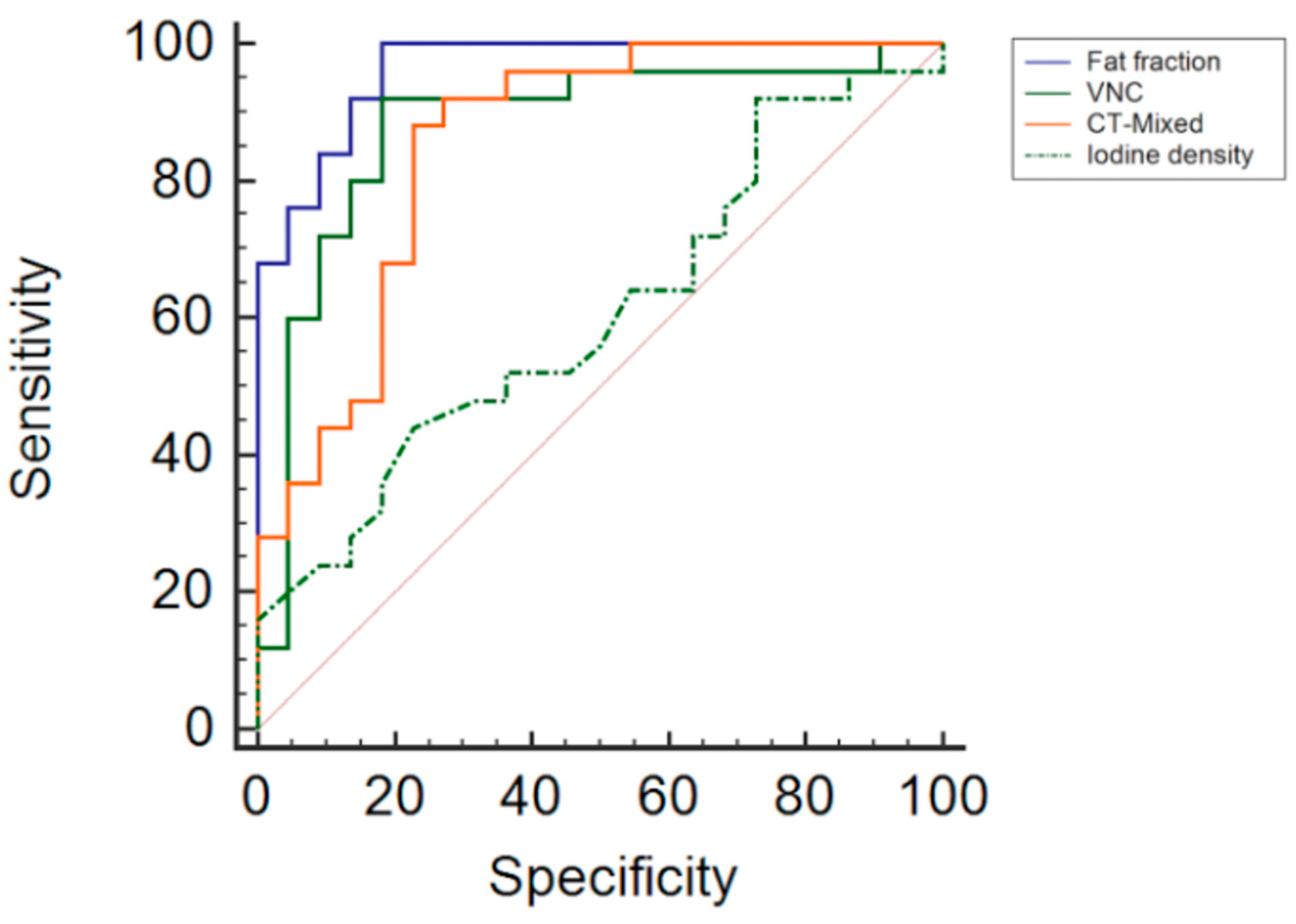
| Fat Fraction | VNC | CT Mixed | Iodine Density | |
|---|---|---|---|---|
| Area under the curve (AUC) | 96.4% | 88% | 85.6% | 60.6% |
| Standard error (AUC) | 0.02 | 0.06 | 0.06 | 0.08 |
| 95% confidence intervalt | 0.86–0.99 | 0.75–0.96 | 0.72–0.94 | 0.45–0.75 |
| Optimal threshold | ≤38% | >−5.6 HU | >29.2 HU | ≤0.87 mg/dL |
| Sensitivity | 100% | 92% | 88% | 44% |
| Specificity | 81.8% | 81.8% | 77% | 77.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winkelmann, M.T.; Gassenmaier, S.; Walter, S.S.; Artzner, C.; Nikolaou, K.; Bongers, M.N. Differentiation of Hamartomas and Malignant Lung Tumors in Single-Phased Dual-Energy Computed Tomography. Tomography 2024, 10, 255-265. https://doi.org/10.3390/tomography10020020
Winkelmann MT, Gassenmaier S, Walter SS, Artzner C, Nikolaou K, Bongers MN. Differentiation of Hamartomas and Malignant Lung Tumors in Single-Phased Dual-Energy Computed Tomography. Tomography. 2024; 10(2):255-265. https://doi.org/10.3390/tomography10020020
Chicago/Turabian StyleWinkelmann, Moritz T., Sebastian Gassenmaier, Sven S. Walter, Christoph Artzner, Konstantin Nikolaou, and Malte N. Bongers. 2024. "Differentiation of Hamartomas and Malignant Lung Tumors in Single-Phased Dual-Energy Computed Tomography" Tomography 10, no. 2: 255-265. https://doi.org/10.3390/tomography10020020
APA StyleWinkelmann, M. T., Gassenmaier, S., Walter, S. S., Artzner, C., Nikolaou, K., & Bongers, M. N. (2024). Differentiation of Hamartomas and Malignant Lung Tumors in Single-Phased Dual-Energy Computed Tomography. Tomography, 10(2), 255-265. https://doi.org/10.3390/tomography10020020







