Diagnostic Value of Contrast-Enhanced Dual-Energy Computed Tomography in the Pancreatic Parenchymal and Delayed Phases for Pancreatic Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dual-Energy Computed Tomography (CT) Data Acquisition
2.3. Quantitative Image Analysis
2.4. Qualitative Image Analysis
2.5. Statistical Analyses
3. Results
3.1. Study Population
3.2. Quantitative Parameters
3.3. Visual Evaluation of Tumor and Vascular Invasion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA A Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Blouhos, K.; Boulas, K.A.; Tsalis, K.; Hatzigeorgiadis, A. The isoattenuating pancreatic adenocarcinoma: Review of the literature and critical analysis. Surg. Oncol. 2015, 24, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Lee, J.M.; Cho, J.Y.; Lee, K.B.; Kim, J.E.; Moon, S.K.; Kim, S.J.; Baek, J.H.; Kim, S.H.; Kim, S.H.; et al. Small (≤20 mm) Pancreatic Adenocarcinomas: Analysis of Enhancement Patterns and Secondary Signs with Multiphasic Multidetector CT. Radiology 2011, 259, 442–452. [Google Scholar] [CrossRef]
- Gonda, T.A.; Farrell, J.; Wallace, M.; Khanna, L.; Janec, E.; Kwon, R.; Saunders, M.; Siddiqui, U.D.; Brand, R.; Simeone, D.M. Standardization of EUS imaging and reporting in high-risk individuals of pancreatic adenocarcinoma: Consensus statement of the Pancreatic Cancer Early Detection Consortium. Gastrointest. Endosc. 2022, 95, 723–732.e7. [Google Scholar] [CrossRef]
- Parker, R.A.; Zhou, Y.; Puttock, E.J.; Chen, W.; Lustigova, E.; Wu, B.U. Early features of pancreatic cancer on magnetic resonance imaging (MRI): A case-control study. Abdom. Radiol. 2024, 49, 1489–1501. [Google Scholar] [CrossRef]
- Ichikawa, T.; Motosugi, U.; Morisaka, H.; Sano, K.; Ali, M.; Araki, T. Volumetric low-tube-voltage CT imaging for evaluating hypervascular hepatocellular carcinoma; effects on radiation exposure, image quality, and diagnostic performance. Jpn. J. Radiol. 2013, 31, 521–529. [Google Scholar] [CrossRef]
- Oda, S.; Utsunomiya, D.; Funama, Y.; Awai, K.; Katahira, K.; Nakaura, T.; Yanaga, Y.; Namimoto, T.; Yamashita, Y. A low tube voltage technique reduces the radiation dose at retrospective ECG-gated cardiac computed tomography for anatomical and functional analyses. Abdom. Radiol. 2011, 18, 991–999. [Google Scholar] [CrossRef]
- Liang, H.; Du, S.; Yan, G.; Zhou, Y.; Yang, T.; Zhang, Z.; Luo, C.; Liao, H.; Li, Y. Dual-energy CT of the pancreas: Comparison between virtual non-contrast images and true non-contrast images in the detection of pancreatic lesion. Abdom. Radiol. 2023, 48, 2596–2603. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Takai, Y.; Asano, M.; Yamada, N.; Seko, T.; Kawai, N.; Kaga, T.; Miyoshi, T.; Hyodo, F.; Kato, H.; et al. Comparison of image quality and pancreatic ductal adenocarcinoma conspicuity between the low-kVp and dual-energy CT reconstructed with deep-learning image reconstruction algorithm. Eur. J. Radiol. 2023, 159, 110685. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, Y.; Fukukura, Y.; Kumagae, Y.; Ejima, F.; Yamagishi, R.; Nakamura, S.; Kamizono, J.; Kurahara, H.; Hashimoto, S.; Yoshiura, T. Value of Dual-Energy Computed Tomography for Detecting Small Pancreatic Ductal Adenocarcinoma. Pancreas 2022, 51, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.; Toepker, M.; Ba-Ssalamah, A.; Schestak, C.; Dutschke, A.; Schindl, M.; Wressnegger, A.; Ringl, H.; Apfaltrer, P. Objective and subjective comparison of virtual monoenergetic vs. polychromatic images in patients with pancreatic ductal adenocarcinoma. Eur. Radiol. 2019, 29, 3617–3625. [Google Scholar] [CrossRef]
- Nagayama, Y.; Tanoue, S.; Inoue, T.; Oda, S.; Nakaura, T.; Utsunomiya, D.; Yamashita, Y. Dual-layer spectral CT improves image quality of multiphasic pancreas CT in patients with pancreatic ductal adenocarcinoma. Eur. Radiol. 2020, 30, 394–403. [Google Scholar] [CrossRef]
- Fukukura, Y.; Kumagae, Y.; Fujisaki, Y.; Yamagishi, R.; Nakamura, S.; Kamizono, J.; Nakajo, M.; Kamimura, K.; Nagano, H.; Takumi, K.; et al. Adding Delayed Phase Images to Dual-Phase Contrast-Enhanced CT Increases Sensitivity for Small Pancreatic Ductal Adenocarcinoma. Am. J. Roentgenol. 2021, 217, 888–897. [Google Scholar] [CrossRef]
- Vauthey, J.-N.; Dixon, E. AHPBA/SSO/SSAT Consensus Conference on Resectable and Borderline Resectable Pancreatic Cancer: Rationale and Overview of the Conference. Ann. Surg. Oncol. 2009, 16, 1725–1726. [Google Scholar] [CrossRef]
- Noda, Y.; Tochigi, T.; Parakh, A.; Joseph, E.; Hahn, P.F.; Kambadakone, A. Low keV portal venous phase as a surrogate for pancreatic phase in a pancreatic protocol dual-energy CT: Feasibility, image quality, and lesion conspicuity. Eur. Radiol. 2021, 31, 6898–6908. [Google Scholar] [CrossRef]
- McNamara, M.M.; Little, M.D.; Alexander, L.F.; Van Carroll, L.; Mark Beasley, T.; Morgan, D.E. Multireader evaluation of lesion conspicuity in small pancreatic adenocarcinomas: Complimentary value of iodine material density and low keV simulated monoenergetic images using multiphasic rapid kVp-switching dual energy CT. Abdom. Imaging 2015, 40, 1230–1240. [Google Scholar] [CrossRef]
- Bellini, D.; Gupta, S.; Ramirez-Giraldo, J.C.; Fu, W.; Stinnett, S.S.; Patel, B.; Mileto, A.; Marin, D. Use of a Noise Optimized Monoenergetic Algorithm for Patient-Size Independent Selection of an Optimal Energy Level During Dual-Energy CT of the Pancreas. J. Comput. Assist. Tomogr. 2017, 41, 39–47. [Google Scholar] [CrossRef]
- Patel, B.N.; Thomas, J.V.; Lockhart, M.E.; Berland, L.L.; Morgan, D.E. Single-source dual-energy spectral multidetector CT of pancreatic adenocarcinoma: Optimization of energy level viewing significantly increases lesion contrast. Clin. Radiol. 2013, 68, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.; Camlidag, I.; Nural, M.S. Lower energy levels and iodine-based material decomposition images increase pancreatic ductal adenocarcinoma conspicuity on rapid kV-switching dual-energy CT. Abdom. Radiol. 2019, 44, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Boicean, A.; Prisca, D.; Bratu, D.G.; Bacila, C.I.; Tanasescu, C.; Chicea, R.; Fleaca, S.R.; Birsan, S.A.; Ichim, C.; Mohor, C.I.; et al. Uncommon Presentation of Gastric Duplication Cyst with Left-Sided Portal Hypertension: A Case Report and Literature Review. Diagnostics 2024, 14, 675. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Masamune, A.; Hanada, K.; Maguchi, H.; Shimizu, Y.; Ueki, T.; Hasebe, O.; Ohtsuka, T.; Nakamura, M.; Takenaka, M.; et al. Multicenter study of early pancreatic cancer in Japan. Pancreatol. Off. J. Int. Assoc. Pancreatol. 2018, 18, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.; Muthusamy, V.R.; McGrath, C.M.; Sepulveda, A.R.; Das, A.; Messersmith, W.; Kochman, M.L.; Shah, J. AGA white paper: Optimizing endoscopic ultrasound–guided tissue acquisition and future directions. Clin. Gastroenterol. Hepatol. 2018, 16, 318–327. [Google Scholar] [CrossRef]
- Kurita, Y.; Kuwahara, T.; Hara, K.; Mizuno, N.; Okuno, N.; Matsumoto, S.; Obata, M.; Koda, H.; Tajika, M.; Shimizu, Y.; et al. Features of chronic pancreatitis by endoscopic ultrasound influence the diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration of small pancreatic lesions. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2020, 32, 399–408. [Google Scholar] [CrossRef]
- Kitano, M.; Yoshida, T.; Itonaga, M.; Tamura, T.; Hatamaru, K.; Yamashita, Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J. Gastroenterol. 2019, 54, 19–32. [Google Scholar] [CrossRef]
- Lao, Y.; David, J.; Fan, Z.; Bian, S.; Shiu, A.; Chang, E.L.; Sheng, K.; Yang, W.; Tuli, R. Quantifying vascular invasion in pancreatic cancer-a contrast CT based method for surgical resectability evaluation. Phys. Med. Biol. 2020, 65, 105012. [Google Scholar] [CrossRef]
- Yang, R.; Lu, M.; Qian, X.; Chen, J.; Li, L.; Wang, J.; Zhang, Y. Diagnostic accuracy of EUS and CT of vascular invasion in pancreatic cancer: A systematic review. J. Cancer Res. Clin. Oncol. 2014, 140, 2077–2086. [Google Scholar] [CrossRef]
- Jajodia, A.; Wang, A.; Alabousi, M.; Wilks, C.; Kulkarni, A.; van der Pol, C.B. MRI vs. CT for pancreatic adenocarcinoma vascular invasion: Comparative diagnostic test accuracy systematic review and meta-analysis. Eur. Radiol. 2023, 33, 6883–6891. [Google Scholar] [CrossRef]
- Rassouli, N.; Chalian, H.; Rajiah, P.; Dhanantwari, A.; Landeras, L. Assessment of 70-keV virtual monoenergetic spectral images in abdominal CT imaging: A comparison study to conventional polychromatic 120-kVp images. Abdom. Radiol. 2017, 42, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.; Le, O.; Balachandran, A.; Fox, P.; Paulson, E.; Tamm, E. Quantitative and Qualitative Comparison of Single-Source Dual-Energy Computed Tomography and 120-kVp Computed Tomography for the Assessment of Pancreatic Ductal Adenocarcinoma. J. Comput. Assist. Tomogr. 2015, 39, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Frellesen, C.; Fessler, F.; Hardie, A.D.; Wichmann, J.L.; Cecco, C.N.D.; Schoepf, U.J.; Kerl, J.M.; Schulz, B.; Hammerstingl, R.; Vogl, T.J.; et al. Dual-energy CT of the pancreas: Improved carcinoma-to-pancreas contrast with a noise-optimized monoenergetic reconstruction algorithm. Eur. J. Radiol. 2015, 84, 2052–2058. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; Galdiero, R.; Maggialetti, N.; Silvestro, L.; De Bellis, M.; Di Girolamo, E.; Grazzini, G.; Chiti, G.; et al. Risk Assessment and Pancreatic Cancer: Diagnostic Management and Artificial Intelligence. Cancers 2023, 15, 351. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, T.; Hara, K.; Mizuno, N.; Haba, S.; Okuno, N.; Koda, H.; Miyano, A.; Fumihara, D. Current status of artificial intelligence analysis for endoscopic ultrasonography. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2021, 33, 298–305. [Google Scholar] [CrossRef]
- Kuwahara, T.; Hara, K.; Mizuno, N.; Haba, S.; Okuno, N.; Kuraishi, Y.; Fumihara, D.; Yanaidani, T.; Ishikawa, S.; Yasuda, T.; et al. Artificial intelligence using deep learning analysis of endoscopic ultrasonography images for the differential diagnosis of pancreatic masses. Endoscopy 2023, 55, 140–149. [Google Scholar] [CrossRef]
- Tong, T.; Gu, J.; Xu, D.; Song, L.; Zhao, Q.; Cheng, F.; Yuan, Z.; Tian, S.; Yang, X.; Tian, J.; et al. Deep learning radiomics based on contrast-enhanced ultrasound images for assisted diagnosis of pancreatic ductal adenocarcinoma and chronic pancreatitis. BMC Med. 2022, 20, 74. [Google Scholar] [CrossRef]
- Borsekofsky, S.; Tsuriel, S.; Hagege, R.R.; Hershkovitz, D. Perineural invasion detection in pancreatic ductal adenocarcinoma using artificial intelligence. Sci. Rep. 2023, 13, 13628. [Google Scholar] [CrossRef]
- Parakh, A.; An, C.; Lennartz, S.; Rajiah, P.; Yeh, B.M.; Simeone, F.J.; Sahani, D.V.; Kambadakone, A.R. Recognizing and Minimizing Artifacts at Dual-Energy CT. Radiographics 2021, 41, 509–523. [Google Scholar] [CrossRef]





| Characteristic | n = 25 |
|---|---|
| Age, median (range), years | 73 (45–90) |
| Sex, male (%) | 13 (52.0) |
| Diabetes (%) | 9 (36.0) |
| Smoking (%) | 6 (24.0) |
| Alcohol intake (%) | 12 (48.0) |
| Body mass index, median (range) | 20.1 (16.5–25.8) |
| Tumor size, median (range), mm | 22 (10–70) |
| Tumor location (%) | |
| Head | 11 (44.0) |
| Body | 10 (40.0) |
| Tail | 4 (16.0) |
| 50 KeV CT (Low Kilovoltage) | 70 KeV CT (Conventional Kilovoltage) | p-Value | |
|---|---|---|---|
| Pancreatic parenchymal phase, median (range), HU | |||
| Tumor | 114 (36–222) | 84 (27–139) | <0.001 * |
| Normal pancreatic parenchyma | 271 (179–398) | 152 (103–200) | <0.001 * |
| Delayed phase, median (range), HU | |||
| Tumor | 161 (25–306) | 96 (27–154) | <0.001 * |
| Normal pancreatic parenchyma | 155 (100–269) | 90 (66–138) | <0.001 * |
| Noise, median (range) | |||
| Pancreatic parenchymal phase | 35 (21–43) | 13 (10–17) | <0.001 * |
| Delayed phase | 33 (24–41) | 10 (9–15) | <0.001 * |
| Tumor-to-pancreas contrast, median (range), HU | |||
| Pancreatic parenchymal phase (Total: n = 25) | 133 (78–279) | 68 (33–116) | <0.001 * |
| ≤20 mm (n = 12) | 238 (78–279) | 65 (33–116) | 0.002 * |
| >20 mm (n = 13) | 134 (82–246) | 73 (33–109) | 0.001 * |
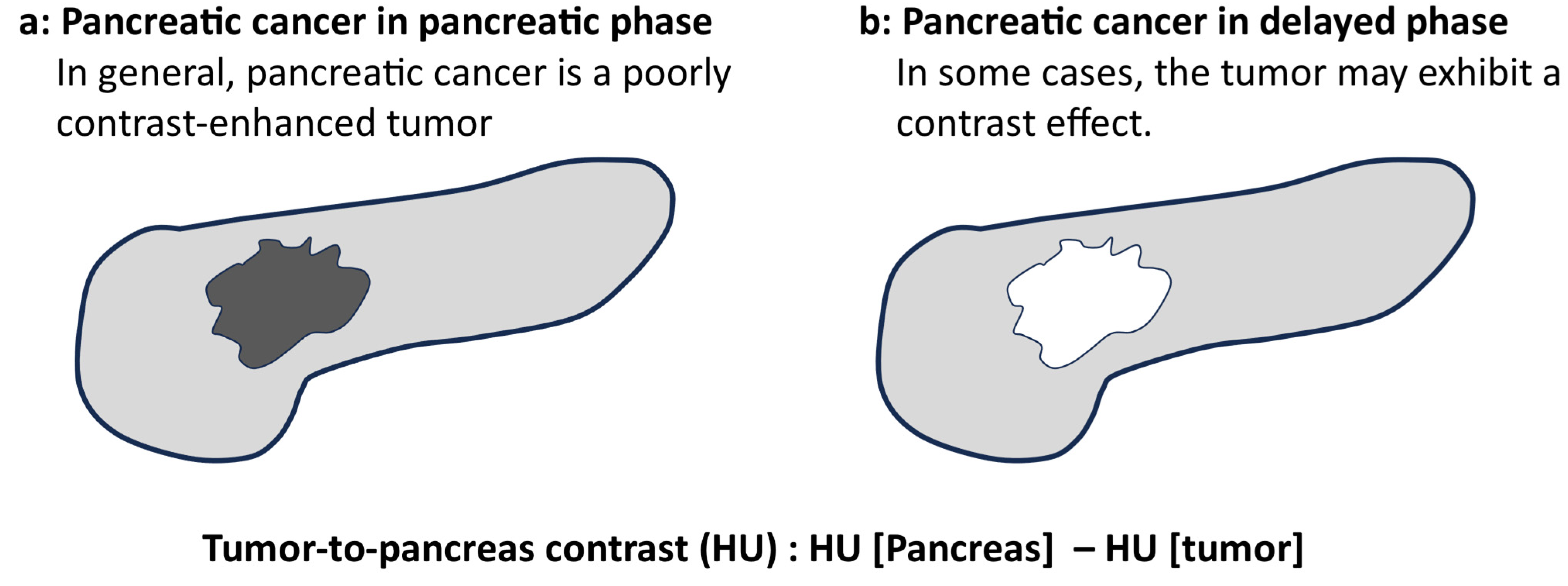
| Delayed phase (Total: n = 25) | −28 (−61–146) | −9 (−26–66) | 0.545 |
| ≤20 mm (n = 12) | −39 (−61–57) | −16.5 (−26–13) | 0.034 * |
| >20 mm (n = 13) | 5 (−41–146) | −1 (−19–66) | 0.208 |
| 50 KeV CT (Low Kilovoltage) | 70 KeV CT (Conventional Kilovoltage) | p | |||
|---|---|---|---|---|---|
| Mean ± SD (Range) | κ | Mean ± SD (Range) | κ | ||
| Low contrast effect of pancreatic parenchymal phase | |||||
| Observer 1 (Y. K.) | 4.72 ± 0.54 (3–5) | 3.96 ± 0.84 (2–5) | <0.001 * | ||
| Observer 2 (D. U.) | 4.72 ± 0.61 (3–5) | 0.65 | 4.20 ± 0.91 (2–5) | 0.49 | <0.001 * |
| Contrast effects of delayed phase | |||||
| Observer 1 (Y. K.) | 3.08 ± 1.08 (1–5) | 2.28 ± 0.84 (1–4) | <0.001 * | ||
| Observer 2 (D. U.) | 3.04 ± 0.84 (2–4) | 0.27 | 2.28 ± 0.54 (2–4) | 0.20 | <0.001 * |
| Vascular invasion | |||||
| Evaluation of arterial infiltration | |||||
| Observer 1 (Y. K.) | 4.56 ± 0.77 (2–5) | 0.53 | 4.04 ± 0.84 (2–5) | 0.49 | <0.001 * |
| Observer 2 (D. U.) | 4.56 ± 0.82 (2–5) | 4.16 ± 0.99 (2–5) | 0.002 * | ||
| Evaluation of venous and portal vascular invasion | |||||
| Observer 1 (Y. K.) | 4.28 ± 0.61 (3–5) | 3.40 ± 1.12 (1–5) | <0.001 * | ||
| Observer 2 (D. U.) | 4.32 ± 0.99 (2–5) | 0.21 | 3.84 ± 1.07(2–5) | 0.19 | 0.005 * |
| Author | Study Design | Patients | CT Scanning Method | Comparison | Sample Size | Conclusions |
|---|---|---|---|---|---|---|
| Bellini, D., 2017, United States [20] | Cohort | Patients with lesions and patients without lesions | Contrast | 40, 50, 60, 70, and 80 KeV | 59 | The maximal contrast-to-noise ratio pancreas occurred at 40 keV |
| Fujisaki, Y., 2022, Japan [13] | Cohort | Patients with pancreatic cancer and patients without pancreatic tumor | Contrast | 40 KeV vs. 120 kVp | 112 | 40 KeV DECT had better sensitivity to small pancreatic cancer |
| Patel, B., 2013, United States [21] | Cohort | Pancreatic cancer | Contrast | 45 KeV vs. 70 KeV | 64 | Significantly increased pancreatic lesion contrast was noted at 45 KeV |
| McNamara, M., 2015, United States [19] | Cohort | Patients with small (<3 cm) pancreatic cancer | Contrast | 52 KeV vs. 70 KeV | 46 | The contrast between tumors and non-tumors was greatest at 52 KeV |
| Aslan, S., 2019, Turkey [22] | Cohort | Pancreatic tumor (cancer, endocrine tumors, other cystic and solid masses) | Contrast | 45 KeV vs. 70 KeV | 90 | The use of low energy levels improves tumor conspicuity |
| Liang, H., 2023, China [11] | Cohort | Pancreatic tumor (cancer, endocrine tumors, other cystic and solid masse | Non-contrast | Non-contrast and virtual non-contrast images obtained from DECT | 106 | Virtual non-contrast images of DECT provide diagnostic image quality and accurate pancreatic lesion detection |
| Noda, Y., 2023, Japan [12] | Cohort | Pancreatic tumor (cancer, endocrine tumors, other cystic and solid masse | Contrast | 40 keV vs. 80 kVp | 111 | 40 keV demonstrated higher SNR and tumor-to-pancreas contrast-to-noise ratio compared to the 80 kVp setting. |
| Kurita, Y., Japan (The present study) | Cohort | Pancreatic cancer | Contrast | 50 KeV vs. 70 KeV | 25 | 50 KeV may clarify the contrast between tumors and non-tumors. A delayed contrast effect was observed in small pancreatic cancers on 50 KeV delayed-phase images |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurita, Y.; Utsunomiya, D.; Kubota, K.; Koyama, S.; Hasegawa, S.; Hosono, K.; Irie, K.; Suzuki, Y.; Maeda, S.; Kobayashi, N.; et al. Diagnostic Value of Contrast-Enhanced Dual-Energy Computed Tomography in the Pancreatic Parenchymal and Delayed Phases for Pancreatic Cancer. Tomography 2024, 10, 1591-1604. https://doi.org/10.3390/tomography10100117
Kurita Y, Utsunomiya D, Kubota K, Koyama S, Hasegawa S, Hosono K, Irie K, Suzuki Y, Maeda S, Kobayashi N, et al. Diagnostic Value of Contrast-Enhanced Dual-Energy Computed Tomography in the Pancreatic Parenchymal and Delayed Phases for Pancreatic Cancer. Tomography. 2024; 10(10):1591-1604. https://doi.org/10.3390/tomography10100117
Chicago/Turabian StyleKurita, Yusuke, Daisuke Utsunomiya, Kensuke Kubota, Shingo Koyama, Sho Hasegawa, Kunihiro Hosono, Kuniyasu Irie, Yuichi Suzuki, Shin Maeda, Noritoshi Kobayashi, and et al. 2024. "Diagnostic Value of Contrast-Enhanced Dual-Energy Computed Tomography in the Pancreatic Parenchymal and Delayed Phases for Pancreatic Cancer" Tomography 10, no. 10: 1591-1604. https://doi.org/10.3390/tomography10100117
APA StyleKurita, Y., Utsunomiya, D., Kubota, K., Koyama, S., Hasegawa, S., Hosono, K., Irie, K., Suzuki, Y., Maeda, S., Kobayashi, N., Ichikawa, Y., Endo, I., & Nakajima, A. (2024). Diagnostic Value of Contrast-Enhanced Dual-Energy Computed Tomography in the Pancreatic Parenchymal and Delayed Phases for Pancreatic Cancer. Tomography, 10(10), 1591-1604. https://doi.org/10.3390/tomography10100117







