A Comprehensive Review on Studying and Developing Guidelines to Standardize the Inspection of Properties and Production Methods for Mycelium-Bound Composites in Bio-Based Building Material Applications
Abstract
1. Introduction
2. Collection of Information
3. Standardization
3.1. Inspection of Properties
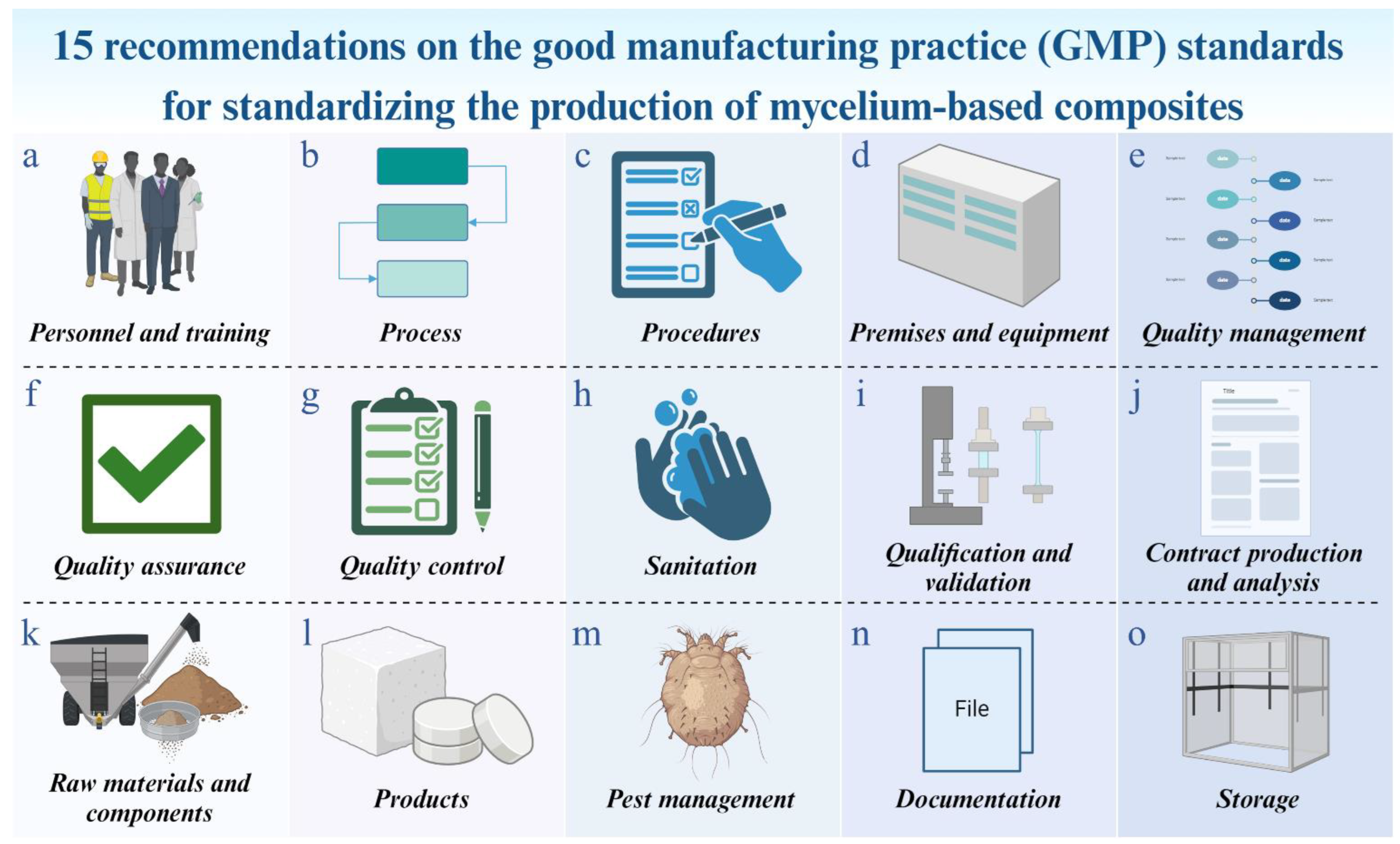
3.2. Production Methods
- Personnel and training (a): People are the most crucial factor in ensuring product quality in the manufacturing process [63]. All personnel involved in production should receive comprehensive training on essential parameters [64,65]. This includes handling mycelium, substrate materials, equipment, and following established procedures that constitute the main manufacturing process for MBCs [10,23,69]. Understanding the mycelium networking process is crucial, as it provides insights into how these composites achieve their unique properties. Knowledge of the substrate composition and its role as a supportive structure is also important, as it affects the adhesive properties of the mycelium [4]. Additionally, optimizing growth conditions through carefully regulating temperature, humidity, aeration, gas exchange, incubation time, light exposure, and nutrient concentrations, can enhance the adhesive qualities of mycelium [23,59]. To improve understanding of the situations that result in optimum strong characteristics, techniques for training, simulating, and machine learning can be applied [4,70]. Responsibilities should be clearly defined, understood, and documented as written job descriptions, with each person assigned tasks based on their capability, knowledge, and experience [63,71]. Additionally, strict adherence to personal hygiene practices, such as handwashing and wearing clean clothing, is essential to prevent contamination throughout the manufacturing process, which should always maintain sterile conditions [63,72]. Importantly, assessing their performance is also essential to raising skill, productivity, and efficiency [65].
- Process (b): In general, using MBCs in building materials and their integration into architecture involves a classification method based on the species of mycelium, substrate combination, supporting structure, and post-treatment processes [4,73]. To ensure consistent production, critical steps in the production process must be identified, and control procedures should be flexible enough to be adjusted as necessary [74]. The production process should be well-defined and documented, detailing each step from the initial process to the final product [65]. For MBC production, this typically includes steps from substrate preparation to final product storage. Comprehensive and standardized processes should be defined for the production of MBCs, including substrate preparation, inoculation, mycelium cultivation, molding, post-processing, until storage [25,59]. Monitoring and controlling process parameters, such as temperature, humidity, aeration and gas exchange, incubation time, and light exposure, are essential to ensure consistency and reproducibility [1,59]. These parameters should be well-documented and regularly checked by personnel. Regular evaluations should be conducted to ensure compliance with practices and organizational requirements, reducing the risk of contamination and ensuring product safety [64]. Additionally, the documentation of all activities and the reporting of deviations when they occur is crucial. This comprehensive approach helps in the early detection of errors and other deviations, reducing potential losses for the manufacturer [64,75,76].
- Procedures (c): A procedure is a set of guidelines designed to achieve consistent results in a critical process or part of a process. Employees in each manufacturing process must adhere to these rules and procedures to ensure smooth operations. These guidelines should be communicated to all employees and followed consistently [65,77]. All procedures must be created and documented to provide clear instructions on what each process must do and how it must meet the required standards. These procedures must be explicitly indicated and consistently followed [78]. Instructions and procedures should be written in clear and unambiguous language, specifically tailored to the facilities provided. Any deviation from standard procedures should be reported immediately and investigated [63,65]. This principle is crucial because if a defect or potentially unsafe output occurs in a lot, there is no need to inspect the entire production. Proper documentation allows staff to quickly identify the source of the problem and address it promptly [79]. For each step in the production process of MBCs, from substrate preparation, inoculation, and growth monitoring to molding and dehydrated processing [25,59,60], procedures should be regularly reviewed and updated to reflect new insights or technological advancements. These procedures are necessary to ensure the composite’s structural integrity and prevent any biological activity that could affect its qualities. Based on their purpose, MBCs might need additional processing, like machining or pressing to obtain the appropriate density or coatings to improve durability or visual appeal. Additionally, optimizing substrate composition and production methodology is important for maximizing the efficiency of MBCs [4].
- Premises and equipment (d): Generally, any building or structure, including any machinery, apparatus, engineering systems, or other objects physically affixed and integrated into it, is referred to as the premises. Machines and other devices used for assistance, prevention, treatment, or measurement are referred to as equipment [64]. Primally, premises must be located, constructed, adapted, designed, and maintained to suit the operations to be carried out. The layout and design of premises should aim to minimize the risk of errors and allow for effective cleaning and maintenance to prevent cross-contamination, and the buildup of dust or dirt, and to avoid any adverse effect on the quality of final products. The layout, ventilation, and water supply must always be in favorable condition [63,64,79,80,81]. Additionally, maintenance and storage areas should be separated from the production area, and storage areas must have sufficient capacity to store products in a well-organized and orderly manner [64]. Meanwhile, manufacturing equipment should be capable of producing materials or products that meet the required quality standards. Equipment must be designed and built to be thoroughly cleaned and sterilized, as well as used efficiently. Surfaces that come into contact with samples should have polished finishes and be smooth to minimize contamination, ease cleaning, and facilitate use. Equipment must withstand repeated, thorough cleaning. All manufacturing equipment must be thoroughly cleaned or sterilized between batches [63,82]. Moreover, all equipment should be properly placed or stored and regularly checked to ensure it is fit for producing consistent results and to prevent various risks [65]. Importantly, all facilities and equipment must have properly documented cleaning processes. Measures to prevent cross-contamination must be in place, along with written instructions for inspections [64,80]. Routine inspections of equipment and machinery, as well as sanitation inspections, must be carried out [83]. With accessible supplies, cleaning and maintenance are easier. Staff should also have adequate facilities and tools to maintain personal hygiene [79]. For the production of MBCs, the manufacturing facility must be designed to support the optimal growth conditions for mycelium and to ensure contamination control. The premises should be clean, and growth conditions must be carefully controlled to enhance the binding qualities of mycelium [4,23]. Equipment used in all stages of production, such as autoclaves, molds, unidirectional press machines, incubators, along with drying machines, must be maintained in good working condition to prevent contamination and ensure reproducibility.
- Quality management (e): The key to creating a successful manufacturing process is first to understand all the basic concepts [79]. Quality management ensures that all operations adhere to GMP guidelines, a fundamental concept accepted across various industries [82]. Implementing a quality management system (QMS) to oversee all aspects of production, ensuring compliance with standards and continuous improvement, is mandatory for many industries, especially those seeking GMP certification [79]. Similarly, ISO 9001:2015 is another essential standard that provides comprehensive requirements related to QMS. Compliance with ISO standards, and regularly setting and reviewing quality objectives to align with production goals and regulatory requirements, demonstrates an industry’s ability to consistently produce high-quality products in line with regulatory requirements, including GMPs [79,84]. In MBC manufacturing, a strong QMS should be implemented to manage the entire production process. This includes QA and control measures to monitor the consistency and performance of the obtained MBCs. Regular quality checks and audits should be conducted to ensure compliance with internal standards as well as any external regulations that may emerge in the future.
- Quality assurance (f): QA is a broad concept encompassing all factors that influence product quality, ensuring that final products meet the required standards. It involves organized arrangements aimed at ensuring products are of the necessary quality for their intended use, incorporating principles such as GMP along with factors like product design and development [63]. In the production of MBCs, QA might face challenges due to batch variability arising from different fungal growth patterns [85]. Moreover, QA still involves systematic activities within a QMS to fulfill product quality requirements [86]. Guidelines adapted from GMP standards can provide a comprehensive framework for QA in MBC production. This includes overseeing production and control operations, implementing necessary controls on starting materials, ensuring correct processing, and checking of the finished product, establishing arrangements for reporting, investigating, and recording deviations, along with conducting regular evaluations of product quality [63]. Implementing these comprehensive QA guidelines, derived from GMP standards, might help ensure consistent production of high-quality MBCs for various applications, particularly in engineering fields where dimensional stability is essential.
- Quality control (g): Producing high-quality MBCs presents challenges due to the variations in fungal species and substrate types used [1,8,36]. Both the choice of fungal species and substrate type, along with manufacturing methods, significantly affect the quality of MBCs. However, the fungal species typically have a greater impact on the final composite properties than the substrate type [1,33]. Therefore, developing appropriate QC measures is essential to address these challenges and ensure consistent product quality [87]. Standardization is crucial for maintaining consistent material properties across different batches and meeting industry standards [88]. Some companies are working to establish standardized methods and QC measures for mycelium production [89]. Adapting guidelines from GMP standards might provide a structured approach for QC in MBC production. Generally, QC involves sampling, specifications, testing, and documentation to ensure that all necessary tests are carried out. It is not limited to laboratory operations but influences many decisions concerning product quality [63]. Additionally, conducting systematic QC activities involves carrying out protocols for maintaining, or storing materials [90]. By adhering to QC guidelines derived from GMP standards, producers may consistently deliver high-quality MBCs that meet the stringent requirements of applications.
- Sanitation (h): Adopting GMP standards might offer a comprehensive framework for sanitation in MBC production. Primarily, the scope of sanitation covers personnel, premises, equipment and apparatuses, production materials and containers, cleaning and disinfection products, and anything that could become a source of contamination to the product. Potential contamination sources should be eliminated through an integrated and comprehensive program of sanitation and hygiene [63,91]. To eliminate potential sources of contamination, an integrated and comprehensive sanitation and hygiene program should be implemented. Areas, surfaces, and equipment involved in MBC manufacturing must be kept clean, as dirt and the microbes it harbors must not come into contact with the products. Several research works on MBC production and other related fields have indicated that these surfaces can be cleaned by applying a cleaning agent, such as 70–75% ethanol, sodium hypochlorite, or hydrogen peroxide, followed by rinsing with autoclaved water [29,59,63,92]. Additionally, UV-C sterilization, which employs light to sterilize surfaces and air by disrupting the Deoxyribonucleic acid (DNA) of microorganisms, serves as another effective option for sterilizing some tools, molds, working surfaces, and air within clean rooms [59,93].
- Qualification and validation (i): The guidelines for qualifying and validating MBCs in engineering offer a systematic approach to ensure production reliability and consistency. Adapting these practices from GMP standards may help establish robust protocols specific to MBC production. Qualification and validation are essential for material QA and processes to meet regulatory requirements and produce consistent products. Generally, the qualification process includes design qualification (DQ), installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ), each requiring detailed documentation and verification [63]. Validation confirms that the production consistently meets predetermined specifications. Documentation, including protocols and test results, is crucial for tracking various activities. Continuous monitoring and periodic revalidation are necessary to maintain compliance and adapt to any changes in regulations or technology [94]. Adhering to these guidelines can achieve high-quality, reliable MBC production, meeting regulatory requirements and benefiting engineering advancements. In this context, all aspects of production, including processes, equipment, materials, and fungal species or strains, should undergo rigorous qualification and validation. This ensures that growth conditions for mycelium, equipment performance, and the consistency of the final composites meet the required standards. Importantly, the validation activities should be documented and reviewed periodically.
- Contract production and analysis (j): MBCs in engineering should be contract manufactured and analyzed under strict quality, safety, and compliance criteria. These comprehensive guidelines might be developed for contract manufacturers and analytical labs by adapting based on GMP standards. Contracts should clearly define roles, responsibilities, and expectations, ensuring facilities and expertise align with requirements. It should be ensured that contract manufacturers have the necessary facilities, equipment, and expertise to meet specified requirements. Protocols for material handling, processing, and storage to prevent contamination and maintain product integrity should be established. Regular audits and inspections of manufacturing facilities verify compliance with quality standards [82]. Analytical methods and specifications for testing [95], covering the physical, mechanical, chemical, and microbiological attributes that are essential for assessing MBC products, should be defined [6,10,12,45,59]. Analytical labs have validated methods and qualified personnel for accurate and reliable analyses. Consistent protocols for sample collection, preparation, and testing should be established. Analytical methods for accuracy, precision, and specificity should be validated before practice use [63,82,95]. By following these guidelines, stakeholders can ensure consistency, quality, and safety in MBC production, supporting the advancement of MBCs in engineering applications.
- Raw materials and components (k): The availability of raw materials and the simplicity of producing materials based on microbes can minimize costs and ensure the reliable manufacturing of high-quality products [8]. Guidelines adapted from GMP standards encompass the selection, handling, storage, and QC of these materials [82]. Raw materials, including fungal mycelium (strains) and substrates like agricultural or forestry wastes, must be sourced from reliable suppliers with documented quality practices. Whenever possible, these materials should be sourced close to the production site to avoid transportation issues [73,96]. Additionally, selecting substrates rich in cellulose, hemicellulose, and lignin, known to enhance adhesion, can improve the binding efficiency of mycelium and the mechanical properties of the obtained MBCs [4,10,23]. Detailed specifications, based on scientific data, should be established for moisture content, particle size, and absence of contaminants. Materials should be stored in dry, temperature-controlled environments to prevent degradation and contamination. Rigorous inspection and assessment, including visual and laboratory testing, are necessary upon receipt to confirm compliance with specifications. Non-conforming materials should be stored separately to prevent accidental use and contamination [82]. By adhering to these guidelines, manufacturers can ensure consistent production of high-quality MBCs, supporting the development of reliable materials for engineering applications.
- Products (l): Developing standards to produce MBCs for engineering applications and adherence to guidelines derived from GMP standards may be crucial. These guidelines ensure the quality and efficacy of the final products [64]. A key challenge is ensuring that biomaterials meet performance standards and industry requirements [97]. Consistent performance, durability, and safety are essential for gaining the trust and confidence of construction professionals and building developers. Creating comprehensive attributes and specifications for MBC materials involves defining parameters such as dimensions, characteristics, strength, safety, and environmental sustainability. Robust QC testing protocols are essential for evaluating the quality and consistency of MBC products. Tests should cover physical, mechanical, chemical, and biological properties, as well as dimensional stability [98]. Stability testing to assess the shelf-life and storage conditions of products is also important, evaluating factors such as temperature, humidity, and light exposure [99]. By adhering to these guidelines adapted from GMP standards, manufacturers can ensure the consistent production of high-quality MBC products that meet regulatory requirements and the needs of engineering applications.
- Pest management (m): Effective pest management is essential, following GMP standards [66]. To maintain the quality of MBCs in engineering applications, these guidelines focus on preventing pest infestations that could compromise the integrity of MBCs. Key aspects include implementing measures to prevent pests from entering the production area, such as sealing entry points and using appropriate pest control methods or pest-resistant materials approved for use in production areas. Regular inspections and monitoring are essential to detect and address pest issues promptly. Non-chemical control methods, such as traps and barriers, should be prioritized, with chemical control methods used as a last resort and in compliance with regulations. All pest management activities should be documented, including records of inspections, treatments, and any corrective actions taken. Additionally, training employees on pest management practices and maintaining cleanliness in production areas are vital components of an effective pest management program for production processes [66,100]. Nonetheless, prior research suggests that guayule resin and various kinds of natural oils can be effective treatments for enhancing pest resistance in MBCs, particularly for improving resistance to termites [55]. Implementing these methods can further improve pest resistance and support the overall integrity of the production process.
- Documentation (n): The main objective of the documentation system is to establish, control, monitor, and record all activities that directly or indirectly impact the quality of products [63]. Comprehensive recording is crucial for QC, especially when developing products for engineering purposes. Guidelines adapted from GMP standards emphasize the importance of comprehensive documentation throughout the production process. This includes detailed records of raw material sourcing, production procedures, QC measures, and storage conditions. All documentation must be accurate, up-to-date, and easily accessible for review [64,101]. Any deviations from standard procedures or quality specifications must be documented, along with corrective and preventive actions taken. Clear and thorough documentation ensures traceability, facilitates QA [63], and supports regulatory compliance in MBC production for future engineering applications. In this case, detailed records should be maintained for each stage of production. This includes documenting substrate and ingredient sources, growth conditions, molding and drying requirements, the post-processing process, testing results, and suitable storage. Proper documentation not only facilitates traceability and accountability but also supports continuous improvement in the production process.
- Storage (o): To ensure the quality of MBCs in engineering applications, stringent storage guidelines are crucial, adapted from GMP standards. These guidelines focus on storing finished MBCs under controlled conditions to prevent degradation and contamination. Maintain a controlled environment with monitored temperature and humidity levels (ideally below 60%, preferably between 30–50%, and at a specified temperature range) to prevent microbial growth [59]. Adequate ventilation is essential to prevent moisture buildup. Implement pest control measures to prevent material damage. Detailed storage records should be maintained, including conditions, monitoring data, and deviations from standard conditions [82]. These guidelines ensure the integrity of composites during storage, contributing to the reliability and effectiveness of the final products.
4. Inspection of Composite Structures
5. Overview of Selected Inspection Standards
6. MBC Applications for Bio-Based Building Materials
7. Challenges and Future Perspectives
7.1. Challenges
- Biological variability: One of the primary challenges in standardizing MBCs is the inherent variability of biological materials [3]. Factors such as fungal species, substrate composition, and growth conditions can significantly influence the properties of MBCs [115], making it difficult to achieve uniformity across different collections and production sites.
- Complexity of material properties: MBCs possess complex properties, including mechanical strength, physical characteristics, and biodegradability, which are influenced by both the internal structure and external morphology [116]. Accurately characterizing and standardizing these multifaceted properties requires sophisticated analytical techniques and interdisciplinary expertise.
- Measurement techniques: The selection and implementation of appropriate measurement techniques for characterizing MBC properties can be challenging [7]. Ensuring that these techniques are accurate, reproducible, and applicable across various scales (from micro to macro) is essential for standardization efforts.
- Economic and technical constraints: Developing and implementing standardized inspection protocols and production methods can be resource-intensive, especially when extended production cycles are required. These constraints pose significant challenges for large-scale production [73]. Small-scale producers and new market entrants may also face economic and technical barriers in adopting these standards.
- Dynamic field of research: The field of MBCs is rapidly evolving, with continuous innovations in production methods and applications. Developing guidelines that remain relevant and adaptable to new advancements poses a significant challenge [116].
- Insect infestation: MBCs, being organic in nature, are susceptible to insect infestations and/or degradation by other pests [117], which can compromise their structural integrity and quality. Developing inspection guidelines to detect and mitigate insect-related issues is essential for maintaining the reliability of MBCs.
- Integration with international building codes: The challenge of integrating MBCs into existing international building codes lies in evaluating and validating them against standards primarily designed for traditional materials like synthetic foams and wood-based composites. Due to the unique properties of MBCs, careful consideration is needed, as they may not align with conventional testing methods.
- Scale-up and manufacturing consistency: Scaling up MBC production from the laboratory to an industrial scale while maintaining consistent quality and properties is a significant challenge. Variability in production processes can result in inconsistent composite performance, making it difficult to establish reliable inspection protocols.
- Environmental and health safety concerns: The biological nature of MBCs raises concerns about potential allergenic or toxic effects, as well as their environmental impact during production and disposal. Developing inspection guidelines that address these concerns requires extensive research and testing, including studies on their effects on plants, soil organisms, and water bodies.
7.2. Future Perspectives
- Interdisciplinary collaboration: Establishing standardized guidelines will benefit from collaboration across disciplines, including biotechnology science, materials science, architecture, engineering, environmental science, chemistry, and multidisciplinary [59]. Such collaborations can pool knowledge and resources to develop comprehensive and robust protocols. Moreover, enhanced cooperation between industries and regulatory bodies could also accelerate the creation of inspection guidelines by merging practical insights with cutting-edge research. These efforts might lead to the development of thorough, evidence-based standards that ensure the safe and effective use of MBCs in building applications.
- Advanced characterization tools and testing technologies: The adoption of advanced characterization tools and techniques, such as real-time growth monitoring and contamination tracking, can enhance the accuracy and reliability of property inspections for MBCs [118]. Additionally, various biotechnological tools, including industrial fermentation, strain improvement, recombinant DNA technology, gene editing, and gene silencing, have recently been experimentally applied in the design and development of MBCs [102]. These tools provide deeper insights into the microstructure and performance of MBCs.
- Development of reference materials: Creating and disseminating reference materials for MBCs will be crucial for standardization [102]. These reference materials can serve as benchmarks for QC and facilitate the comparison of results across different studies and production sites. As research progresses, there is potential to develop specific standards and certifications for MBCs, similar to those for other bio-based materials, particularly in building applications.
- Regulatory frameworks: Establishing clear regulatory frameworks and certification processes for MBCs will support the development and adoption of standardized guidelines. Regulatory bodies can play a key role in defining safety, performance, quality, and environmental standards for MBCs. Importantly, clear regulatory frameworks will provide confidence to manufacturers and consumers, promoting the commercialization of MBCs [119].
- Industry standards: The development of industry standards, led by organizations such as the ISO and ASTM, will provide a formalized approach to the standardization of MBC properties and production methods [6]. These standards can enhance the credibility and acceptance of MBCs in various applications, particularly in building materials, making it easier for companies to develop and market MBCs worldwide.
- Sustainability and market adoption: Standardizing MBC production and inspection protocols will contribute to the sustainability and scalability of these materials. As standardized MBCs become more reliable and consistent, their market adoption is likely to increase, driving further innovation and investment in this field [59,62]. Future guidelines could incorporate sustainability indices, such as carbon footprint and LCA, into the inspection process for MBCs. This would not only ensure material performance but also align with the growing demand for sustainable building practices. Moreover, incorporating sustainability metrics into the guidelines can highlight the environmental benefits of MBCs. Standardized methods for assessing and reporting the ecological impact of MBC production and lifecycle will underscore their value as sustainable alternatives to conventional materials [121]. Developing guidelines that prioritize eco-friendly practices will align with global SDGs, advancing green building technologies, and overarching goals of engineering and sustainability [4,122]. However, these real-world applications will demonstrate the viability and advantages of MBCs.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barta, D.G.; Simion, I.; Tiuc, A.E.; Vasile, O. Mycelium-based composites as a sustainable solution for waste management and circular economy. Materials 2024, 17, 404. [Google Scholar] [CrossRef] [PubMed]
- Alaneme, K.K.; Anaele, J.U.; Oke, T.M.; Kareem, S.A.; Adediran, M.; Ajibuwa, O.A.; Anabaranze, Y.O. Mycelium based composites: A review of their bio-fabrication procedures, material properties and potential for green building and construction applications. Alex. Eng. J. 2023, 83, 234–250. [Google Scholar] [CrossRef]
- Akromah, S.; Chandarana, N.; Eichhorn, S.J. Mycelium composites for sustainable development in developing countries: The case for Africa. Adv. Sustain. Syst. 2024, 8, 2300305. [Google Scholar] [CrossRef]
- Voutetaki, M.E.; Mpalaskas, A.C. Natural fiber-reinforced mycelium composite for innovative and sustainable construction materials. Fibers 2024, 12, 57. [Google Scholar] [CrossRef]
- Yang, L.; Park, D.; Qin, Z. Material function of mycelium-based bio-composite: A review. Front. Mater. 2021, 8, 737377. [Google Scholar] [CrossRef]
- Aiduang, W.; Chanthaluck, A.; Kumla, J.; Jatuwong, K.; Srinuanpan, S.; Waroonkun, T.; Oranratmanee, W.; Lumyong, S.; Suwannarach, N. Amazing fungi for eco-friendly composite materials: A comprehensive review. J. Fungi. 2022, 8, 842. [Google Scholar] [CrossRef] [PubMed]
- Javadian, A.; Le Ferrand, H.; Hebel, D.E.; Saeidi, N. Application of mycelium-bound composite materials in construction industry: A short review. SOJ Mater. Sci. Eng. 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Alemu, D.; Tafesse, M.; Mondal, A.K. Mycelium-based composite: The future sustainable biomaterial. Int. J. Biomater. 2022, 2022, 8401528. [Google Scholar] [CrossRef]
- Appels, F.V.W.; Camere, S.; Montalti, M.; Karana, E.; Jansen, K.M.B.; Dijksterhuis, J.; Krijgsheld, P.; Wosten, H.A.B. Fabrication factors influencing mechanical, moisture and water related properties of mycelium-based composites. Mater. Des. 2019, 161, 64–71. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Brancart, J.; Peeters, E.; Laet, L.D. Mechanical, physical and chemical characterisation of mycelium-based composites with different types of lignocellulosic substrates. PLoS ONE 2019, 7, e0213954. [Google Scholar] [CrossRef]
- Gou, L.; Li, S.; Yin, J.; Li, T.; Liu, X. Morphological and physico-mechanical properties of mycelium biocomposites with natural reinforcement particles. Constr. Build. Mater. 2021, 304, 124656. [Google Scholar] [CrossRef]
- Aiduang, W.; Kumla, J.; Srinuanpan, S.; Thamjaree, W.; Lumyong, S.; Suwannarach, N. Mechanical, physical, and chemical properties of mycelium-based composites produced from various lignocellulosic residues and fungal species. J. Fungi. 2022, 8, 1125. [Google Scholar] [CrossRef] [PubMed]
- Gezer, E.; Uçar, E.; Gümüşkaya, E. Physical and mechanical properties of mycelium-based fiberboards. BioResources 2024, 19, 3421–3435. [Google Scholar] [CrossRef]
- Mbabali, H.; Lubwama, M.; Yiga, V.A.; Were, E.; Kasedde, H. Development of rice husk and sawdust mycelium-based bio-composites: Optimization of mechanical, physical and thermal properties. J. Inst. Eng. D. 2024, 105, 97–117. [Google Scholar] [CrossRef]
- Kohphaisansombat, C.; Jongpipitaporn, Y.; Laoratanakul, P.; Tantipaibulvut, S.; Euanorasetr, J.; Rungjindamai, N.; Chuaseeharonnachaid, C.; Kwantong, P.; Somrithipol, S.; Boonyuen, N. Fabrication of mycelium (oyster mushroom)-based composites derived from spent coffee grounds with pineapple fibre reinforcement. Mycology 2023, 1–18. [Google Scholar] [CrossRef]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered mycelium composite construction materials from fungal biorefineries: A critical review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- Saez, D.; Grizmann, D.; Trautz, M.; Werner, A. Exploring the binding capacity of mycelium and wood-based composites for use in construction. Biomimetics 2022, 7, 78. [Google Scholar] [CrossRef]
- Lingam, D.; Narayan, S.; Mamun, K.; Charan, D. Engineered mycelium-based composite materials: Comprehensive study of various properties and applications. Constr. Build. Mater. 2023, 391, 131841. [Google Scholar] [CrossRef]
- Sağlam, S.S.; Özgünler, S.A. Production of mycelium-based composite materials and evaluation of thermal insulation performance. J. Green Build. 2024, 19, 193–222. [Google Scholar] [CrossRef]
- Früchtl, M.; Senz, A.; Sydow, S.; Frank, J.B.; Hohmann, A.; Albrecht, S.; Fischer, M.; Holland, M.; Wilhelm, F.; Christ, H.A. Sustainable pultruded sandwich profiles with mycelium core. Polymers 2023, 15, 3205. [Google Scholar] [CrossRef]
- Abdelhady, O.; Spyridonos, E.; Dahy, H. Bio-modules: Mycelium-based composites forming a modular interlocking system through a computational design towards sustainable architecture. Designs 2023, 7, 20. [Google Scholar] [CrossRef]
- Kromoser, B.; Preinstorfer, P.; Kollegger, J. Building lightweight structures with carbon-fiber-reinforced polymer-reinforced ultra-high-performance concrete: Research approach, construction materials, and conceptual design of three building components. Struct. Concr. 2019, 20, 730–744. [Google Scholar] [CrossRef]
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Atta, O.M.; Yang, G. Synthesis and applications of fungal mycelium-based advanced functional materials. J. Bioresour. Bioprod. 2021, 6, 1–10. [Google Scholar] [CrossRef]
- Sydor, M.; Bonenberg, A.; Doczekalska, B.; Cofta, G. Mycelium-based composites in art, architecture, and interior design: A review. Polymers 2021, 14, 145. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Van Wylick, A.; Ruytinx, J.; De Laet, L.; Peeters, E. A comprehensive framework for the production of mycelium-based lignocellulosic composites. Sci. Total Environ. 2020, 725, 138431. [Google Scholar] [CrossRef]
- Dessi-Olive, J. Strategies for growing large-scale mycelium structures. Biomimetics 2022, 7, 129. [Google Scholar] [CrossRef]
- Eisner, C. 7 Steps to Properly Conduct a Materials Inspection. Available online: https://www.getmaintainx.com/blog/materials-inspection (accessed on 28 June 2024).
- Aiduang, W.; Jatuwong, K.; Jinanukul, P.; Suwannarach, N.; Kumla, J.; Thamjaree, W.; Teeraphantuvat, T.; Waroonkun, T.; Oranratmanee, R.; Lumyong, S. Sustainable innovation: Fabrication and characterization of mycelium-based green composites for modern interior materials using agro-industrial wastes and different species of fungi. Polymers 2024, 16, 550. [Google Scholar] [CrossRef]
- Teeraphantuvat, T.; Jatuwong, K.; Jinanukul, P.; Thamjaree, W.; Lumyong, S.; Aiduang, W. Improving the physical and mechanical properties of mycelium-based green composites using paper waste. Polymers 2024, 16, 262. [Google Scholar] [CrossRef] [PubMed]
- Picco, C.M.; Suarez, N.E.; Regenhardt, S.A. Exploring the impact of substrate composition and process parameters on biomaterial derived from fungus mycelium (Pleurotus ostreatus) and agricultural wastes. MRS Adv. 2024, 9, 33–38. [Google Scholar] [CrossRef]
- Shakir, M.A.; Ahmad, M.I.; Yusup, Y.; Rafatullah, M. From waste to wealth: Converting rubber wood sawdust into green mycelium-based composite. Biomass Convers Bior. 2023, 1–19. [Google Scholar] [CrossRef]
- Shen, S.C.; Lee, N.A.; Lockett, W.J.; Acuil, A.D.; Gazdus, H.B.; Spitzer, B.N.; Buehler, M.J. Robust myco-composites: A biocomposite platform for versatile hybrid-living materials. Mater. Horiz. 2024, 11, 1689–1703. [Google Scholar] [CrossRef]
- Zimele, Z.; Irbe, I.; Grinins, J.; Bikovens, O.; Verovkins, A.; Bajare, D. Novel mycelium-based biocomposites (Mbb) as building materials. J. Renew. Mater. 2020, 8, 1067–1076. [Google Scholar] [CrossRef]
- Pittau, F.; Carcassi, O.G.; Servalli, M.; Pellegrini, S.; Claude, S. Hygrothermal characterization of bio-based thermal insulation made of fibres from invasive alien lake plants bounded with mycelium. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Berlin, Germany, 2022; Volume 1078, p. 012069. [Google Scholar]
- Aiduang, W.; Suwannarach, N.; Kumla, J.; Thamjaree, W.; Lumyong, S. Valorization of agricultural waste to produce myco-composite materials from mushroom mycelia and their physical properties. Agric. Nat. Resour. 2022, 56, 1083–1090. [Google Scholar]
- Vašatko, H.; Gosch, L.; Jauk, J.; Stavric, M. Basic research of material properties of mycelium-based composites. Biomimetics 2022, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Rigobello, A.; Ayres, P. Compressive behaviour of anisotropic mycelium-based composites. Sci. Rep. 2022, 12, 6846. [Google Scholar] [CrossRef] [PubMed]
- Pohl, C.; Schmidt, B.; Nunez Guitar, T.; Klemm, S.; Gusovius, H.J.; Platzk, S.; Kruggel-Emden, H.; Klunker, A.; Völlmecke, C.; Fleck, C.; et al. Establishment of the basidiomycete Fomes fomentarius for the production of composite materials. Fungal Biol. Biotechnol. 2022, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Răut, I.; Călin, M.; Vuluga, Z.; Oancea, F.; Paceagiu, J.; Radu, N.; Doni, M.; Alexandrescu, E.; Purcar, V.; Gurban, A.M.; et al. Fungal based biopolymer composites for construction materials. Materials 2021, 14, 2906. [Google Scholar] [CrossRef]
- Houette, T.; Maurer, C.; Niewiarowski, R.; Gruber, P. Growth and mechanical characterization of mycelium-based composites towards future bioremediation and food production in the material manufacturing cycle. Biomimetics 2022, 7, 103. [Google Scholar] [CrossRef]
- Elsacker, E.; De Laet, L.; Peeters, E. Functional grading of mycelium materials with inorganic particles: The effect of nanoclay on the biological, chemical and mechanical properties. Biomimetics 2022, 7, 57. [Google Scholar] [CrossRef]
- Cai, J.; Han, J.; Ge, F.; Lin, Y.; Pan, J.; Ren, A. Development of impact-resistant mycelium-based composites (MBCs) with agricultural waste straws. Constr Build Mater. 2023, 389, 131730. [Google Scholar] [CrossRef]
- Özdemir, E.; Saeidi, N.; Javadian, A.; Rossi, A.; Nolte, N.; Ren, S.; Dwan, A.; Acosta, I.; Hebel, D.E.; Wurm, J.; et al. Wood-veneer-reinforced mycelium composites for sustainable building components. Biomimetics 2022, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Elsacker, E.V. Mycelium Matters-an Interdisciplinary Exploration of the Fabrication and Properties of Mycelium-Based Materials. Ph.D. Thesis, Vrije Universiteit Brussel, Brussels, Belgium, 2021. [Google Scholar]
- Kuştaş, S.; Gezer, E.D. Physical and mechanical properties of mycelium-based insulation materials produced from desilicated wheat straws—Part A. BioResources 2024, 19, 1330–1347. [Google Scholar] [CrossRef]
- Liu, R.; Li, X.; Long, L.; Sheng, Y.; Xu, J.; Wang, Y. Improvement of mechanical properties of mycelium/cotton stalk composites by water immersion. Compos. Interfaces 2020, 27, 953–966. [Google Scholar] [CrossRef]
- Sun, W.; Tajvidi, M.; Howell, C.; Hunt, C.G. Insight into mycelium-lignocellulosic bio-composites: Essential factors and properties. Compos.-A Appl. Sci. Manuf. 2022, 161, 107125. [Google Scholar] [CrossRef]
- Carcassi, O.B.; Minotti, P.; Habert, G.; Paoletti, I.; Claude, S.; Pittau, F. Carbon footprint assessment of a novel bio-based composite for building insulation. Sustainability 2022, 14, 1384. [Google Scholar] [CrossRef]
- Rossi, A.; Javadian, A.; Acosta, I.; Özdemir, E.; Nolte, N.; Saeidi, N.; Dwan, A.; Ren, S.; Vries, L.; Hebel, D.; et al. Home: Wood-mycelium composites for CO2-neutral, circular interior construction and fittings. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Berlin, Germany, 2022; Volume 1078, p. 012068. [Google Scholar]
- Alaux, N.; Vašatko, H.; Maierhofer, D.; Saade, M.R.M.; Stavric, M.; Passer, A. Environmental potential of fungal insulation: A prospective life cycle assessment of mycelium-based composites. Int. J. Life Cycle Assess. 2024, 29, 255–272. [Google Scholar] [CrossRef]
- Bagheriehnajjar, G.; Yousefpour, H.; Rahimnejad, M. Environmental impacts of mycelium-based bio-composite construction materials. Int. J. Environ. Sci. Technol. 2024, 21, 5437–5458. [Google Scholar] [CrossRef]
- Stelzer, L.; Hoberg, F.; Bach, V.; Schmidt, B.; Pfeiffer, S.; Meyer, V.; Finkbeiner, M. Life cycle assessment of fungal-based composite bricks. Sustainability 2021, 13, 11573. [Google Scholar] [CrossRef]
- Jones, M.; Bhat, T.; Wang, C.H.; Moinuddin, K.; John, S. Thermal degradation and fire reaction properties of mycelium composites. In Proceedings of the 21st International Conference on Composite Materials, Xi’an, China, 20–25 August 2017; pp. 20–25. [Google Scholar]
- Jones, M.; Bhat, T.; Kandare, E.; Thomas, A.; Joseph, P.; Dekiwadia, C.; Yuen, R.; John, S.; Ma, J.; Wang, C.H. Thermal degradation and fire properties of fungal mycelium and mycelium-biomass composite materials. Sci. Rep. 2018, 8, 17583. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Holt, G.A.; Bajwa, S.G.; Duke, S.E.; McIntyre, G. Enhancement of termite (Reticulitermes flavipes L.) resistance in mycelium reinforced biofiber-composites. Ind. Crops Prod. 2017, 107, 420–426. [Google Scholar] [CrossRef]
- Sisti, L.; Gioia, C.; Totaro, G.; Verstichel, S.; Cartabia, M.; Camere, S.; Celli, A. Valorization of wheat bran agro-industrial byproduct as an upgrading filler for mycelium-based composite materials. Ind. Crops Prod. 2021, 170, 113742. [Google Scholar] [CrossRef]
- Van Wylick, A.; Elsacker, E.; Yap, L.L.; Peeters, E.; De Laet, L. Mycelium composites and their biodegradability: An explorationon the disintegration of mycelium-based materials in soil. Constr. Technol. Archit. 2022, 1, 652–659. [Google Scholar]
- Prajapati, B. Manufacturing Process of Mycelium Packaging Material. Available online: https://www.linkedin.com/pulse/mycelium-packaging-material-natures-gift-sustainable-bhavin-prajapati-pypzf (accessed on 30 June 2024).
- Aiduang, W.; Jatuwong, K.; Luangharn, T.; Jinanukul, P.; Thamjaree, W.; Teeraphantuvat, T.; Waroonkun, T.; Lumyong, S. A review delving into the factors influencing mycelium-based green composites (MBCs) production and their properties for long-term sustainability targets. Biomimetics 2024, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Almpani-Lekka, D.; Pfeiffer, S.; Schmidts, C.; Seo, S.I. A review on architecture with fungal biomaterials: The desired and the feasible. Fungal Biol. Biotechnol. 2021, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Macdonell, J. Good Manufacturing Practices (GMP): Ensuring Quality in Manufacturing Processes. Available online: https://usdm.com/resources/blogs/good-manufacturing-practices-gmp-ensuring-quality-in-manufacturing-processes (accessed on 30 June 2024).
- Mogu. Biofrontiers 2023: Potential of Fungal-Based Materials in Architecture. Available online: https://mogu.bio/biofrontiers-2023-potential-of-fungal-based-materials-in-architecture/ (accessed on 30 June 2024).
- Chaudhari, V.K.; Yadav, V.; Verma, P.K.; Singh, A.K. A review on good manufacturing practice (GMP) for medicinal products. Pharm. Tu. J. 2014, 2, 8–19. [Google Scholar]
- Hole, G.; Hole, A.S.; McFalone-Shaw, I. Digitalization in pharmaceutical industry: What to focus on under the digital implementation process? Int. J. Pharm. X. 2021, 3, 100095. [Google Scholar] [CrossRef]
- Tarlengco, J. GMP: Good Manufacturing Practices. Available online: https://safetyculture.com/topics/gmp/ (accessed on 15 May 2024).
- Groundnut-academy. GMPs for Pest Management. Available online: https://groundnut-academy.uga.edu/topic/gmps-for-pest-management/ (accessed on 15 May 2024).
- Moravek Inc. Understanding GMP Storage Condition Standards. Available online: https://www.moravek.com/understanding-gmp-storage-condition-standards/ (accessed on 17 May 2024).
- BioRender.com. Available online: https://www.biorender.com/ (accessed on 5 June 2024).
- Karana, E.; Blauwhoff, D.; Hultink, E.J.; Camere, S. When the material grows: A case study on designing (with) mycelium-based materials. Int. J. Des. 2018, 12, 119–136. [Google Scholar]
- Yang, L.; Qin, Z. Mycelium-based wood composites for light weight and high strength by experiment and machine learning. Cell Rep. Phys. Sci. 2023, 4, 101424. [Google Scholar] [CrossRef]
- Velagaleti, R.; Burns, P.K.; Gill, M.; Prothro, J. Impact of current good manufacturing practices and emission regulations and guidances on the discharge of pharmaceutical chemicals into the environment from manufacturing, use, and disposal. Environ. Health. Perspect. 2002, 110, 213–220. [Google Scholar] [CrossRef]
- Padilla-Zakour, O.I. Good manufacturing practices. In Microbiologically Safe Foods; Heredia, N., Wesley, I., García, S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 395–414. [Google Scholar]
- Volk, R.; Schröter, M.; Saeidi, N.; Steffl, S.; Javadian, A.; Hebel, D.E.; Schultmann, F. Life cycle assessment of mycelium-based composite materials. Resour. Conserv. Recy. 2024, 205, 107579. [Google Scholar]
- Gad, S.C. Pharmaceutical Manufacturing Handbook: Regulations and Quality; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 311–411. [Google Scholar]
- World Health Organization. Quality Assurance of Pharmaceuticals: A Compendium of Guidelines and Related Materials; Good manufacturing practices and inspection; World Health Organization: Geneva, Switzerland, 2007; Volume 2, pp. 9–212. [Google Scholar]
- World Health Organization. WHO Guidelines on Good Manufacturing Practices (GMP) for Herbal Medicines; World Health Organization: Geneva, Switzerland, 2007; pp. 1–15. [Google Scholar]
- Joseph, D.N. (Ed.) Good Manufacturing Practices for Pharmaceuticals: A Plan for Total Quality Control from Manufacturer to Consumer; CRC Press: Boca Raton, FL, USA, 2000; 752p. [Google Scholar]
- Schaufelberger, D.E.; Koleck, M.P.; Beutler, J.A.; Vatakis, A.M.; Alvarado, A.B.; Andrews, P.; Marzo, L.V.; Muschik, G.M.; Roach, J.; Ross, J.T.; et al. The large-scale isolation of bryostatin 1 from Bugula neritina following current good manufacturing practices. J. Nat. Prod. 1991, 54, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J. The Complete Guide to Good Manufacturing Practices (GMP). Available online: https://qvalon.com/blog/the-complete-guide-to-good-manufacturing-practices-gmp-by-qvalon/ (accessed on 18 May 2024).
- Krekora, M. Contract Manufacturing of Medicines; Kluwer Law International, B.V.: Alphen aan den Rijn, The Netherlands, 2008; 432p. [Google Scholar]
- Sharp, J. Good Pharmaceutical Manufacturing Practice: Rationale and Compliance; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Scheme, Pharmaceutical Inspection Co-Operation. Guide to Good Manufacturing Practice for Medicinal Products Part I. In Pharmaceutical Inspection Convention Pharmaceutical Inspection Co-Operation Scheme; PIC/S Secretariat, Ed.; Annexes PE: Geneva, Switzerland, 2023; pp. 1–54. [Google Scholar]
- Aghayan, H.R.; Arjmand, B.; Burger, S.R. GMP facilities for clinical cell therapy product manufacturing: A brief review of requirements and design considerations. In Perinatal Tissue-Derived Stem Cells: Alternative Sources of Fetal Stem Cells; Arjmand, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 215–227. [Google Scholar]
- Mallick, P. What are the Features of ISO 9001:2015? Available online: https://www.quora.com/What-are-the-quality-management-system-in-ISO-9001-2015 (accessed on 19 May 2024).
- Chulikavit, N.; Huynh, T.; Dekiwadia, C.; Khatibi, A.; Mouritz, A.; Kandare, E. Influence of growth rates, microstructural properties and biochemical composition on the thermal stability of mycelia fungi. Sci. Rep. 2022, 12, 15105. [Google Scholar] [CrossRef] [PubMed]
- Adedayo, O. Understanding QA and QC in Product Development and Delivery: Ensuring Quality throughout the Process. Available online: https://www.linkedin.com/pulse/understanding-qa-qc-product-development-delivery-ensuring (accessed on 19 May 2024).
- Mycelium Team. The Advantages and Applications of Mycelium Technology. Available online: https://mycellium.co/archives/5091 (accessed on 19 May 2024).
- Bayern Innovativ. Sustainable Materials from Mushroom Mycelium. Available online: https://www.bayern-innovativ.de/en/page/sustainable-materials-from-mushroom-mycelium (accessed on 19 May 2024).
- Blogpackers. Mycelium Packaging Material–Nature’s Gift for Sustainable Packaging. Available online: https://blogpackers.com/mycelium-packaging-material-natures-gift-for-sustainable-packaging/ (accessed on 19 May 2024).
- Chakraborty, S. How to Store Quality Control Materials in a Medical Laboratory? Available online: https://www.linkedin.com/pulse/1-how-store-quality-control-materials-medical-dr-sambhu-chakraborty (accessed on 20 May 2024).
- Sharp, J. Quality in the Manufacture of Medicines and Other Healthcare Products; Pharmaceutical Press: London, UK, 2000; 516p. [Google Scholar]
- Jacobs, T.; Signore, A.A. Good Design Practices for GMP Pharmaceutical Facilities; CRC Press: Boca Raton, FL, USA, 2016; 536p. [Google Scholar]
- Pereira, A.R.; Braga, D.F.; Vassal, M.; Gomes, I.B.; Simões, M. Ultraviolet C irradiation: A promising approach for the disinfection of public spaces? Sci. Total Environ. 2023, 879, 163007. [Google Scholar] [CrossRef]
- Choudhary, A. Components of GMP: GMP in Detail. 2024. Available online: https://www.pharmaguideline.com/2023/05/components-of-gmp.html (accessed on 20 May 2024).
- European Medicines Agency. ICH Topic Q 7 Good Manufacturing Practice for Active Pharmaceutical Ingredients. Step 5: Note for Guidance on Good Manufacturing Practice for Active Pharmaceutical Ingredients (CPMP/ICH/4106/00); European Medicines Agency: London, UK, 2000; pp. 1–48. [Google Scholar]
- Terezia. High Quality Raw Materials are the Basis. 2024. Available online: https://www.terezia.eu/en/high-quality-raw-materials-are-the-basis/ (accessed on 20 May 2024).
- Harrington, R.E.; Guda, T.; Lambert, B.; Martin, J. 3.1.4—Sterilization and Disinfection of Biomaterials for Medical Devices; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: New York, NY, USA, 2020; pp. 1431–1446. [Google Scholar]
- Chen, L.; Zhang, Y.; Chen, Z.; Dong, Y.; Jiang, Y.; Hua, J.; Liu, Y.; Osman, A.I.; Farghali, M.; Huang, L.; et al. Biomaterials technology and policies in the building sector: A review. Environ. Chem. Lett. 2024, 22, 715–750. [Google Scholar] [CrossRef]
- Integrated Labs. Shelf-Life Stability Testing with Integrated Labs. 2024. Available online: https://integrated-labs.com/service/shelf-life-stability-testing/ (accessed on 22 May 2024).
- Technews. Integrated Pest Management Programme. 2024. Available online: https://www.dairyknowledge.in/sites/default/files/technews_97_pest_management.pdf (accessed on 22 May 2024).
- Olanrewaju. GMP Documentation and Record-Keeping: Ensuring Quality in Manufacturing. 2024. Available online: https://www.linkedin.com/pulse/gmp-documentation-record-keeping-ensuring-quality-olanrewaju-ochgf (accessed on 24 May 2024).
- Madusanka, C.; Udayanga, D.; Nilmini, R.; Rajapaksha, S.; Hewawasam, C.; Manamgoda, D.; Vasco-Correa, J. A review of recent advances in fungal mycelium based composites. Discov. Mater. 2024, 4, 13. [Google Scholar] [CrossRef]
- Balaeș, T.; Radu, B.M.; Tănase, C. Mycelium-composite materials—A promising alternative to plastics? J. Fungi. 2023, 9, 210. [Google Scholar] [CrossRef]
- Antinori, M.E.; Ceseracciu, L.; Mancini, G.; Heredia-Guerrero, J.A.; Athanassiou, A. Fine-tuning of physicochemical properties and growth dynamics of mycelium-based materials. ACS Appl. Bio Mater. 2020, 3, 1044–1051. [Google Scholar] [CrossRef]
- Charpentier-Alfaro, C.; Benavides-Hernández, J.; Poggerini, M.; Crisci, A.; Mele, G.; Della Rocca, G.; Emiliani, G.; Frascella, A.; Torrigiani, T.; Palanti, S. Wood-decaying fungi: From timber degradation to sustainable insulating biomaterials production. Materials 2023, 16, 3547. [Google Scholar] [CrossRef]
- Bonenberg, A.; Sydor, M.; Cofta, G.; Doczekalska, B.; Grygorowicz-Kosakowska, K. Mycelium-based composite materials: Study of acceptance. Materials 2023, 16, 2164. [Google Scholar] [CrossRef]
- Peng, L.; Yi, J.; Yang, X.; Xie, J.; Chen, C. Development and characterization of mycelium bio-composites by utilization of different agricultural residual byproducts. J. Bioresour. Bioprod. 2023, 8, 78–89. [Google Scholar] [CrossRef]
- Krummenauer, A.; Bergamo, D.; Soares, R.S.; Gomes, V.E.D.O.; Nardelli, V.C. Comparison of dimensional accuracy between a laser scanner and a laser tracker with handheld scan in a laboratory setting. Metrology 2024, 4, 205–226. [Google Scholar] [CrossRef]
- Silva, M.I.; Malitckii, E.; Santos, T.G.; Vilaça, P. Review of conventional and advanced non-destructive testing techniques for detection and characterization of small-scale defects. Prog. Mater. Sci. 2023, 138, 101155. [Google Scholar] [CrossRef]
- Karaca, C.; Karaca, G. Comparison of Fungi with Sclerotia as Sustainable Materials for Product Design. In E3S Web of Conferences; EDP Sciences: Paris, France, 2023; Volume 436, p. 03004. [Google Scholar]
- Turgut, P. Cement composites with limestone dust and different grades of wood sawdust. Build. Environ. 2007, 42, 3801–3807. [Google Scholar] [CrossRef]
- Kiyorndlab. Material Testing Techniques. Available online: https://www.kiyorndlab.com/material-testing-techniques/ (accessed on 2 June 2024).
- Ghazvinian, A. A sustainable alternative to architectural materials: Mycelium-based bio-composites. In Proceedings of the Divergence in Architectural Research; Georgia Tech School of Architecture: Atlanta, GA, USA, 2021; Volume 15, pp. 159–167. [Google Scholar]
- Sreerag, N.K.; Shilpa, V.S. A review on synthesis, properties and applications of mycelium biocomposite. J. Pharm. Innov. 2023, 12, 2200–2207. [Google Scholar]
- Girometta, C.; Picco, A.M.; Baiguera, R.M.; Dondi, D.; Babbini, S.; Cartabia, M.; Pellegrini, M.; Savino, E. Physico-mechanical and thermodynamic properties of mycelium-based biocomposites: A review. Sustainability 2019, 11, 281. [Google Scholar] [CrossRef]
- Shakir, M.A.; Ahmad, M.I. Bioproduct advances: Insight into failure factors in mycelium composite fabrication. Biofuel. Bioprod. Bior. 2024, 18, 1739–1754. [Google Scholar] [CrossRef]
- Peeters, S.S. Assessing Modifications on Mycelium-Based Composites and the Effects on Fungal Degradation and Material Properties. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2023. [Google Scholar]
- Mohseni, A.; Vieira, F.R.; Pecchia, J.A.; Gürsoy, B. Three-dimensional printing of living mycelium-based composites: Material compositions, workflows, and ways to mitigate contamination. Biomimetics 2023, 8, 257. [Google Scholar] [CrossRef]
- Blundell, R. Innovative Mycelium Composites: Pioneering Sustainable Solutions. Available online: https://www.independent.com.mt/articles/2024-06-30/health/Innovative-mycelium-composites-Pioneering-sustainable-solutions-6736262323 (accessed on 3 June 2024).
- Bitting, S.; Derme, T.; Lee, J.; Van Mele, T.; Dillenburger, B.; Block, P. Challenges and opportunities in scaling up architectural applications of mycelium-based materials with digital fabrication. Biomimetics 2022, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Akromah, S.; Chandarana, N.; Rowlandson, J.L.; Eichhorn, S.J. Potential environmental impact of mycelium composites on African communities. Sci. Rep. 2024, 14, 11867. [Google Scholar] [CrossRef]
- Garcia, D.A. Green areas management and bioengineering techniques for improving urban ecological sustainability. Sustain. Cities Soc. 2017, 30, 108–117. [Google Scholar] [CrossRef]


| Symbols and Letters | Description |
|---|---|
| MBCs | Mycelium-bound composites |
| % | Percentage |
| ASTM | American Society for Testing and Materials |
| ISO | International Organization for Standardization |
| JIS | Japanese Industrial Standard |
| EN | European Standards |
| DIN | Deutsches Institut für Normung |
| TIS | Thai Industrial Standards Institute |
| GMPs | Good manufacturing practices |
| SEM | Scanning electron microscopy |
| CT | X-ray computed tomography |
| SDGs | Sustainable Development Goals |
| & | And |
| GFRP | Glass fiber-reinforced plastic |
| LCA | Life cycle assessment |
| ID | Identification |
| BCG | Bio-circular-green economy |
| QMS | Quality management system |
| QA | Quality assurance |
| QC | Quality control |
| UV-C | Ultraviolet clean |
| DNA | Deoxyribonucleic acid |
| DQ | Design qualification |
| IQ | Installation qualification |
| OQ | Operational qualification |
| PQ | Performance qualification |
| TEM | Transmission electron microscopy |
| MIP | Mercury intrusion porosimetry |
| Properties | Testing Standard ID | Standard Title (Description) | Level | Reference |
|---|---|---|---|---|
| Density | ISO 9427 | Wood-based panels—determination of density | Lab-scale | [12,14,28,29] |
| ASTM D1622 | Standard test method for apparent density of rigid cellular plastics | Lab-scale | [30] | |
| JIS A 5908 | Japan Industrial Standard: particleboards | Application-scale | [31] | |
| Moisture content | ISO 16979 | Determination of moisture content | Lab-scale | [14] |
| Water absorption | ASTM D1037 | Standard test methods for evaluating properties of wood-base fiber and particle panel materials | Application-scale | [6,15,29,30,32,33] |
| ASTM C272/272M | Water absorption of core materials | Application-scale | [12,14] | |
| JIS A 5908 | Japan Industrial Standard: particleboards | Application-scale | [31] | |
| ISO 15148 | Hygrothermal performance of building materials and products—determination of water absorption coefficient by partial immersion | Application-scale | [34] | |
| ASTM D570 | Standard test method for water absorption of plastics | Lab-scale | [35] | |
| Thickness of swelling | ASTM D1037 | Standard test methods for evaluating properties of wood-base fiber and particle panel materials | Lab-scale | [33] |
| TIS 876 | Thai Industrial Standard: flat-pressed particleboard | Application-scale | [15] | |
| JIS A 5908 | Japan Industrial Standard: particleboards | Application-scale | [31] | |
| Compression strength | EN 1015 | Methods of test for mortar for masonry—Part 11: determination of flexural and compressive strength of hardened mortar | Application-scale | [36] |
| ASTM D3501 | Standard test methods for wood-based structural panels in compression | Application-scale | [14,37] | |
| DIN 50134 | Testing of metallic materials—compression test of metallic cellular materials | Lab-scale | [38] | |
| ASTM D1621 | Standard test method for compressive properties of rigid cellular plastics | Lab-scale | [30] | |
| ASTM C109 | Standard test method for compressive strength of hydraulic cement mortars | Lab-scale | [15] | |
| ISO 844 | Rigid cellular plastics—determination of compression properties | Lab-scale | [39] | |
| ASTM D2166 | Standard test method for unconfined compressive strength of cohesive soil | Lab-scale | [40] | |
| Tensile strength | ASTM D1037 | Standard test methods for evaluating properties of wood-base fiber and particle panel materials | Application-scale | [32,41] |
| ASTM D 638 | Standard test method for tensile properties of plastics | Lab-scale | [12,28,29] | |
| DIN 53292 | Testing of sandwiches; tensile test perpendicular to the faces | Application-scale | [20] | |
| Impact strength | ASTM D256 | Standard test methods for determining the Izod pendulum impact resistance of plastics | Lab-scale | [12,29] |
| ASTM D7136 | Standard test method for measuring the damage resistance of a fiber-reinforced polymer matrix composite to a drop-weight impact event | Lab-scale | [42] | |
| Modulus of elasticity | ASTM D1037 | Standard test methods for evaluating properties of wood-base fiber and particle panel materials | Application-scale | [37] |
| ASTM D3504 | Standard specification for maleic anhydride | Lab-scale | [14] | |
| ISO 16978 | Wood-based panels—determination of modulus of elasticity in bending and of bending strength | Application-scale | [41] | |
| Flexural/bending strength | JIS A5908 | Japan Industrial Standard: particleboards | Application-scale | [31] |
| ASTM D1037 | Standard test methods for evaluating properties of wood-base fiber and particle panel materials | Application-scale | [36,40,43] | |
| ASTM C78 | Standard test method for flexural strength of concrete (using simple beam with third-point loading) | Lab-scale | [15] | |
| ISO 16978 | Wood-based panels—determination of modulus of elasticity in bending and of bending strength | Application-scale | [41,44] | |
| ASTM D790 | Standard test methods for flexural properties of unreinforced and reinforced plastics and electrical insulating materials | Lab-scale | [12,28,29] | |
| DIN 52186 | Testing of wood; bending test | Lab-scale | [33] | |
| DIN 53293 | Testing of sandwiches; bending test | Application-scale | [20] | |
| Internal bonding | JIS A 5908 | Japan Industrial Standard: particleboards | Application-scale | [31] |
| EN 319:1993 | Particleboards and fiberboards. Determination of tensile strength perpendicular to the plane of the board. | Application-scale | [41,45] | |
| ASTM D1037 | Standard test methods for evaluating properties of wood-base fiber and particle panel materials | Lab-scale | [46,47] | |
| Shear strength | DIN-EN 12090 | Thermal insulating products for building applications. Determination of shear behavior. | Application-scale | [17] |
| DIN EN 205 | Adhesives—Wood adhesives for non-structural applications—Determination of tensile shear strength of lap joints | Application-scale | [43] | |
| Thermal Insulation property | ISO 22007-2 | Plastics—Determination of thermal conductivity and thermal diffusivity | Lab-scale | [48] |
| ASTM C1113 | Standard test method for thermal conductivity of refractories by hot wire (platinum resistance thermometer technique) | Lab-scale | [14] | |
| ISO 12664 | Thermal performance of building materials and products—Determination of thermal resistance by means of guarded hot plate and heat flow meter methods—Dry and moist products of medium and low thermal resistance | Application-scale | [34] | |
| Sound insulation absorption | ASTM E1050 | Standard test method for impedance and absorption of acoustical materials using a tube, two microphones and a digital frequency analysis system | Application-scale | [15] |
| DIN 4109:1989 | Sound insulation in buildings; construction examples and calculation methods | Application-scale | [49] | |
| Environmental assessment | DIN EN 15978 | Sustainability of construction works—Assessment of environmental performance of buildings—Calculation method | Application-scale | [49] |
| DIN EN 15804 | Sustainability of construction works—Environmental product declarations-Core rules for the product category of construction products | Application-scale | [50] | |
| ISO 14040/14044 | Environmental management—Life cycle assessment—Requirements and guidelines | Application-scale | [20,51,52] | |
| Flammability | ISO 5660-1 | Reaction-to-fire tests—Heat release, smoke production and mass loss rate | Lab-scale | [15] |
| ISO 5660-1 | Reaction-to-fire tests—Heat release, smoke production and mass loss rate | Lab-scale | [53] | |
| ASTM D7309 | Standard test method for determining flammability characteristics of plastics and other solid materials using microscale combustion calorimetry | Lab-scale | [54] | |
| Termite resistance | ASTM D3345-08 | Standard test method for laboratory evaluation of wood and other cellulosic materials for resistance to termites | Lab-scale | [55] |
| Biodegradability | EN 13432:2000 | Requirements for packaging recoverable through composting and biodegradation. Test scheme and evaluation criteria for the final acceptance of packaging | Lab-scale | [56] |
| ISO 16929:2021 | Plastics—Determination of the degree of disintegration of plastic materials under defined composting conditions in a pilot-scale test | Pilot-scale | [56] | |
| ISO 20200:2015 | Plastics—Determination of the degree of disintegration of plastic materials under simulated composting conditions in a laboratory-scale test | Lab-scale | [57] | |
| ISO 846/2000 | Plastics—Evaluation of the action of microorganisms | Lab-scale | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aiduang, W.; Jinanukul, P.; Thamjaree, W.; Kiatsiriroat, T.; Waroonkun, T.; Lumyong, S. A Comprehensive Review on Studying and Developing Guidelines to Standardize the Inspection of Properties and Production Methods for Mycelium-Bound Composites in Bio-Based Building Material Applications. Biomimetics 2024, 9, 549. https://doi.org/10.3390/biomimetics9090549
Aiduang W, Jinanukul P, Thamjaree W, Kiatsiriroat T, Waroonkun T, Lumyong S. A Comprehensive Review on Studying and Developing Guidelines to Standardize the Inspection of Properties and Production Methods for Mycelium-Bound Composites in Bio-Based Building Material Applications. Biomimetics. 2024; 9(9):549. https://doi.org/10.3390/biomimetics9090549
Chicago/Turabian StyleAiduang, Worawoot, Praween Jinanukul, Wandee Thamjaree, Tanongkiat Kiatsiriroat, Tanut Waroonkun, and Saisamorn Lumyong. 2024. "A Comprehensive Review on Studying and Developing Guidelines to Standardize the Inspection of Properties and Production Methods for Mycelium-Bound Composites in Bio-Based Building Material Applications" Biomimetics 9, no. 9: 549. https://doi.org/10.3390/biomimetics9090549
APA StyleAiduang, W., Jinanukul, P., Thamjaree, W., Kiatsiriroat, T., Waroonkun, T., & Lumyong, S. (2024). A Comprehensive Review on Studying and Developing Guidelines to Standardize the Inspection of Properties and Production Methods for Mycelium-Bound Composites in Bio-Based Building Material Applications. Biomimetics, 9(9), 549. https://doi.org/10.3390/biomimetics9090549






