Foundational Engineering of Artificial Blood Vessels’ Biomechanics: The Impact of Wavy Geometric Designs
Abstract
1. Introduction
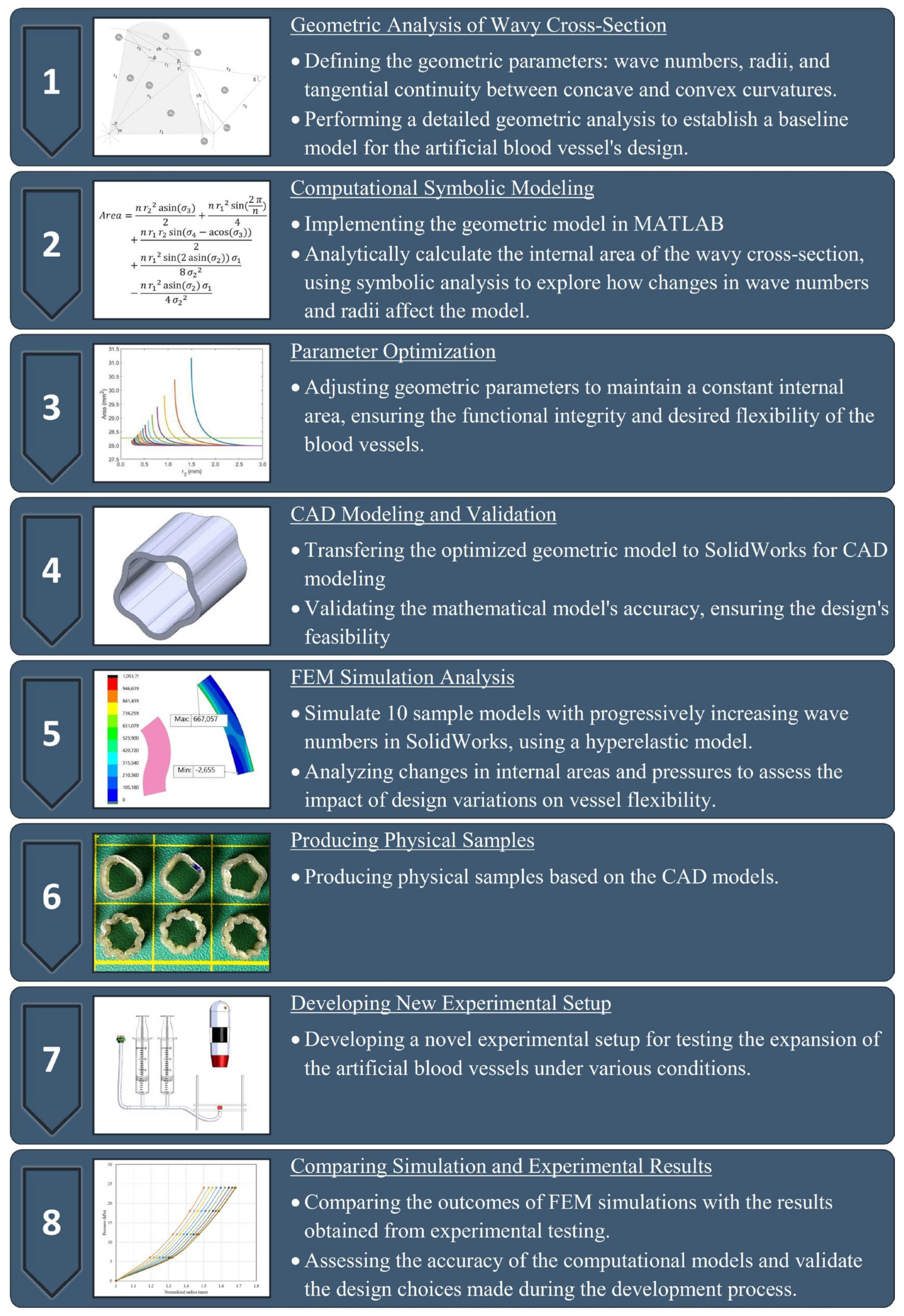
2. Materials and Method
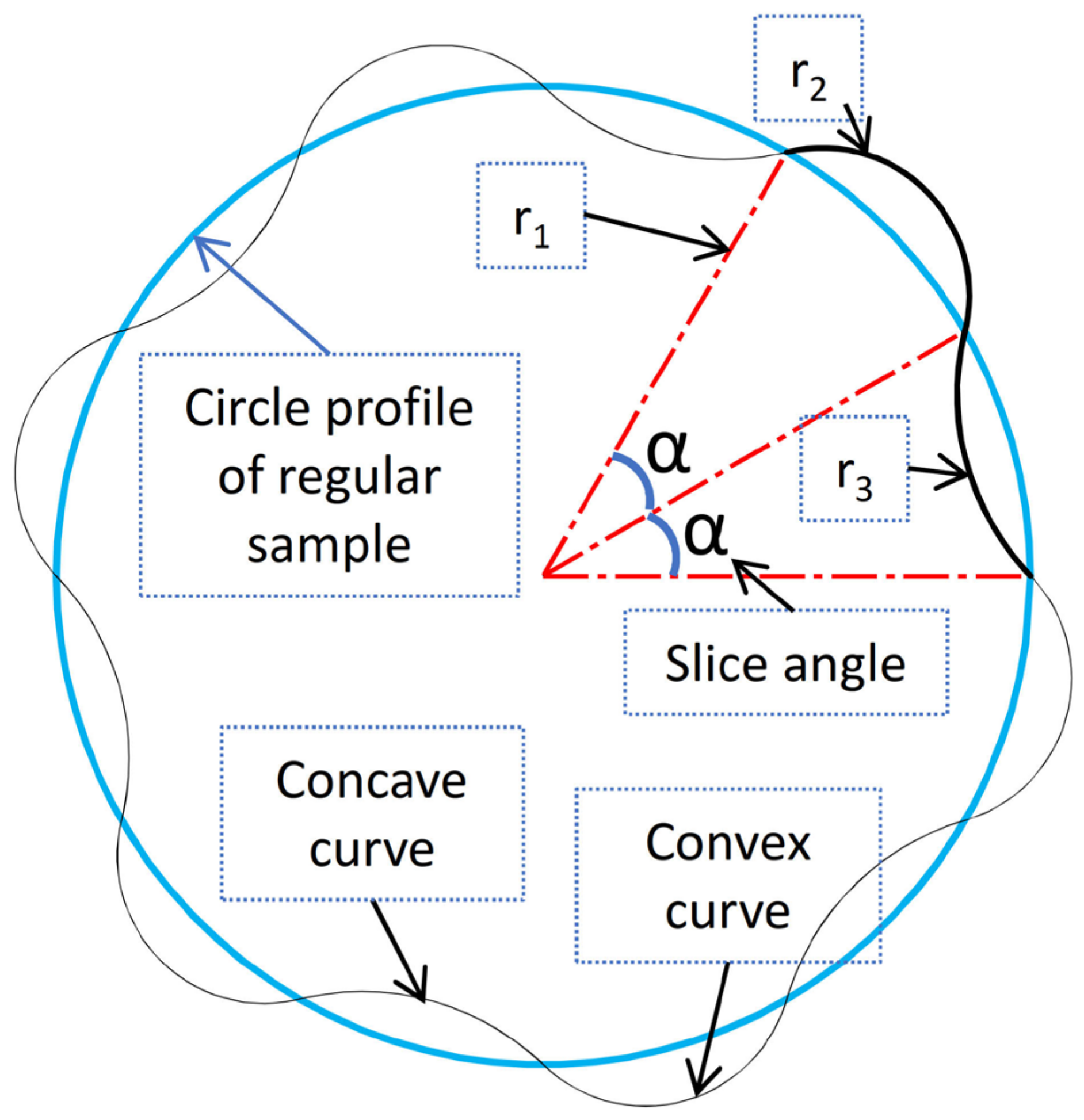
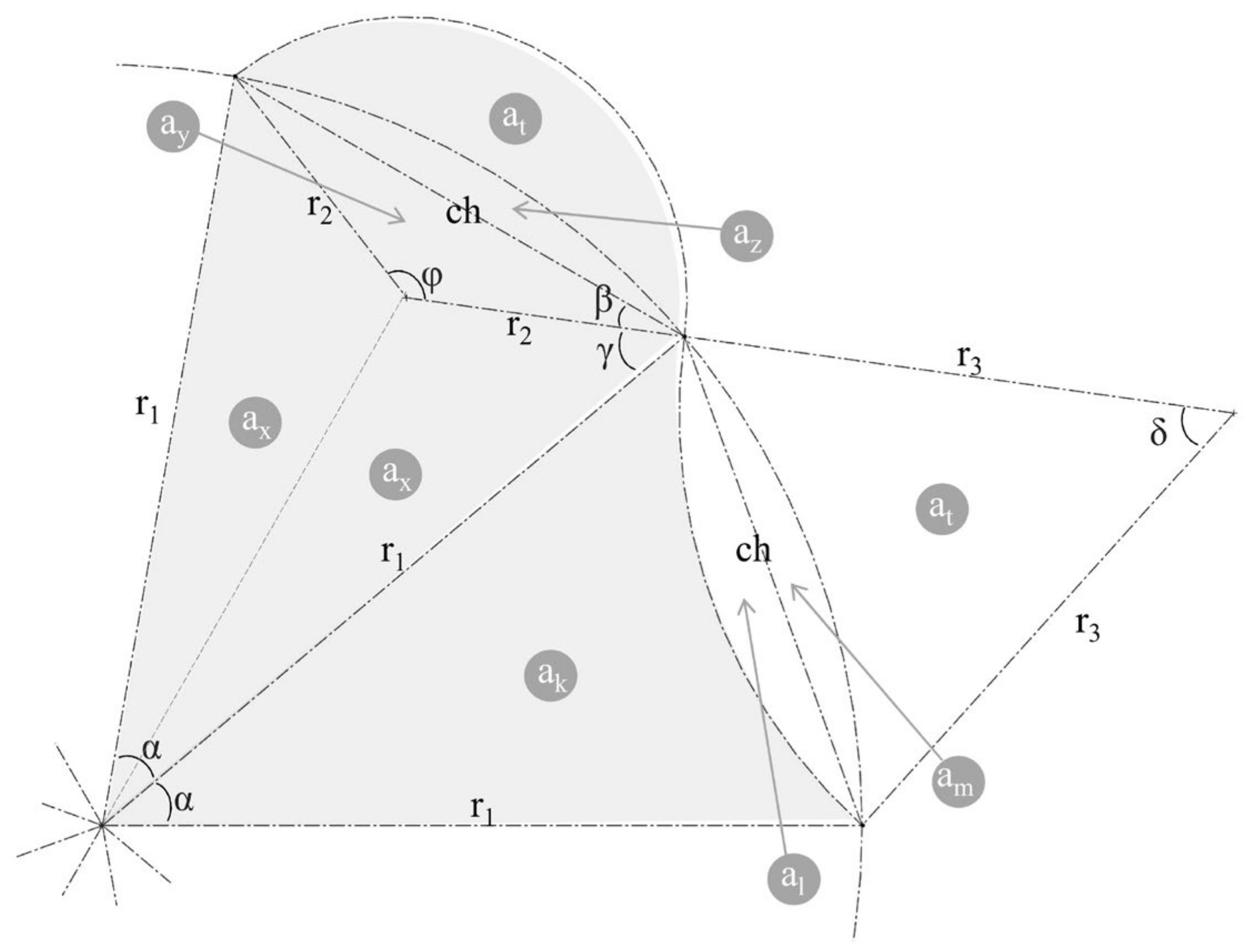
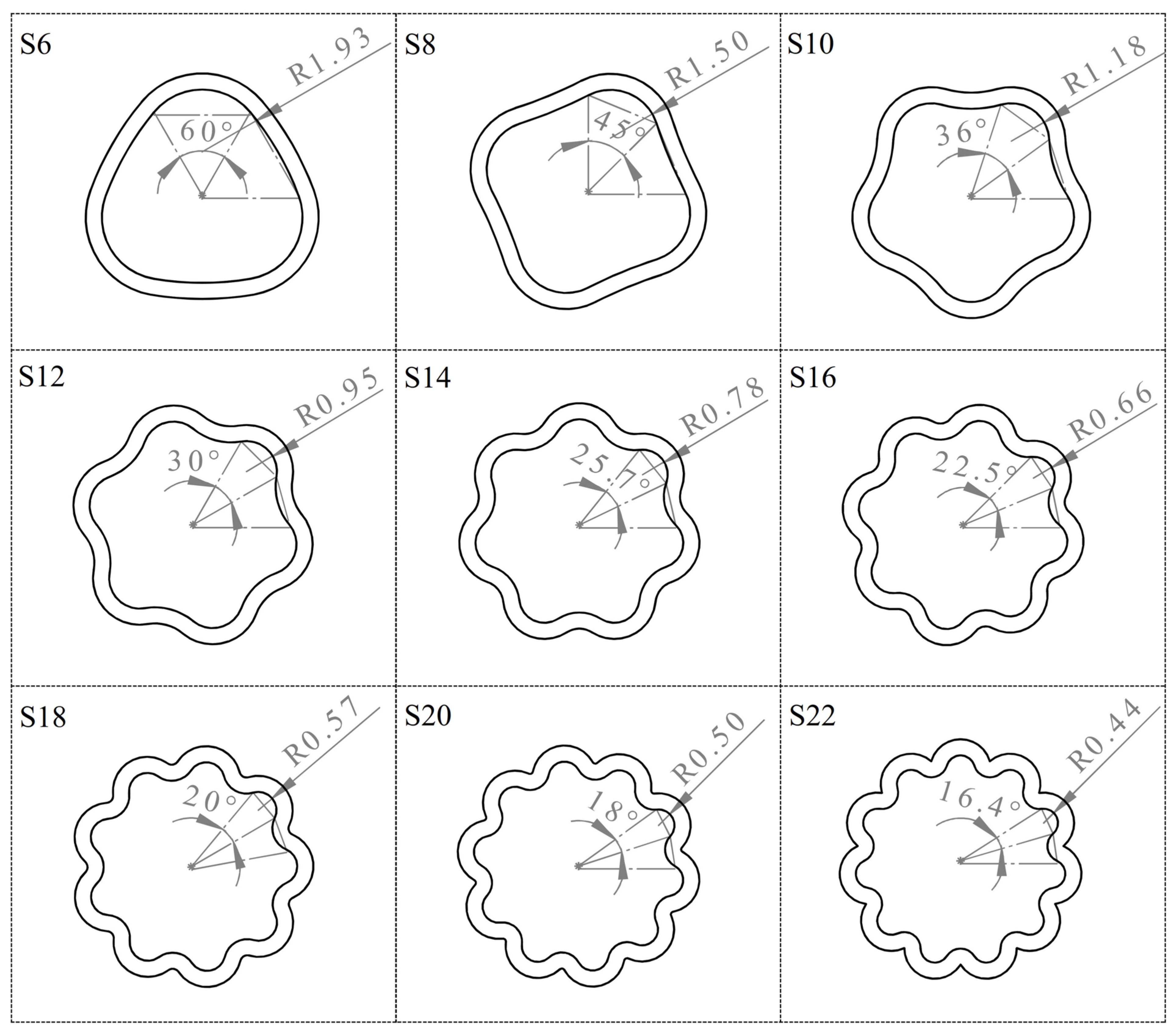
2.1. Geometric Analysis of the Wavy Cross-Section and Sample Design
2.2. Computational Symbolic Modeling
2.3. Parameter Optimization
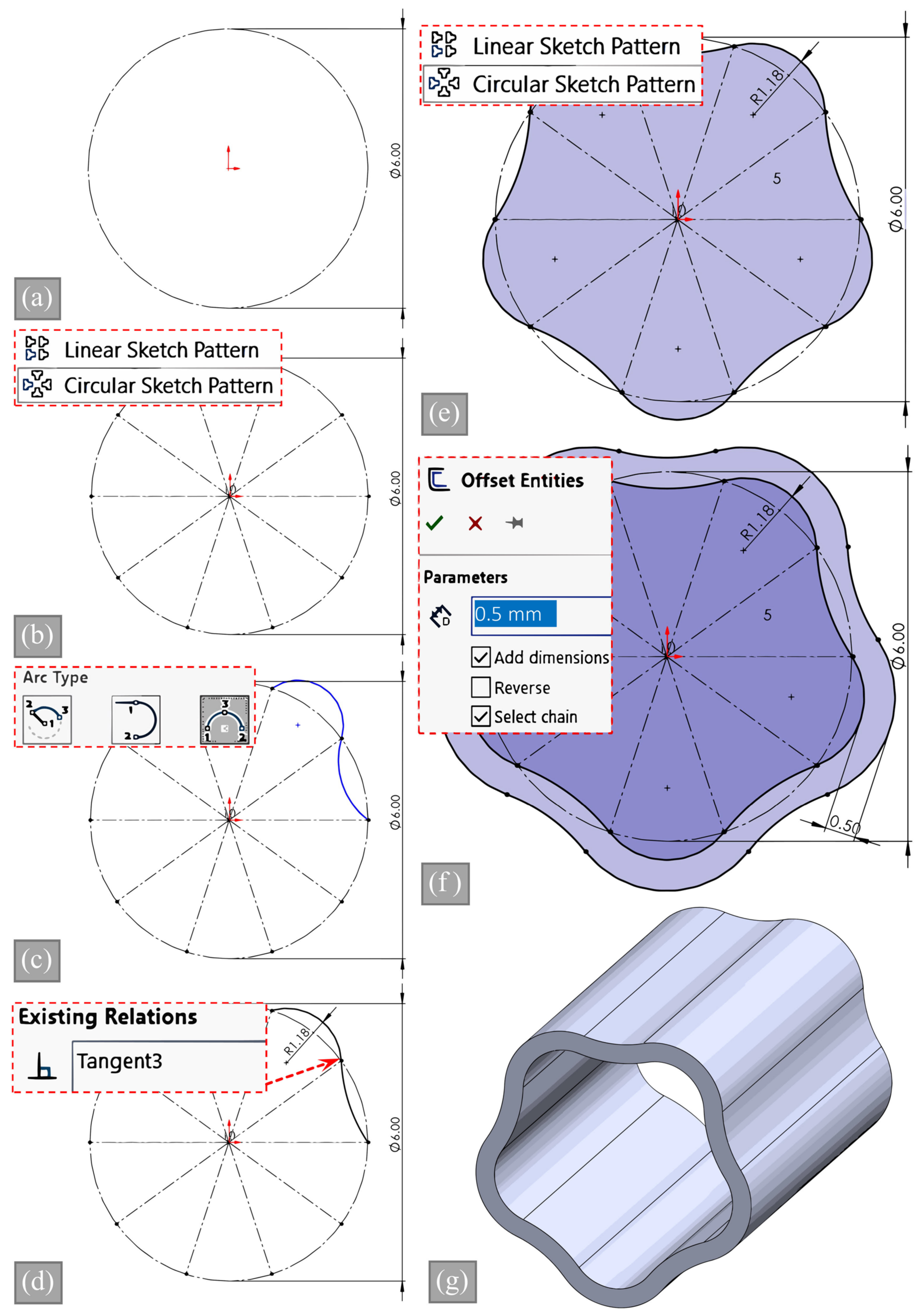
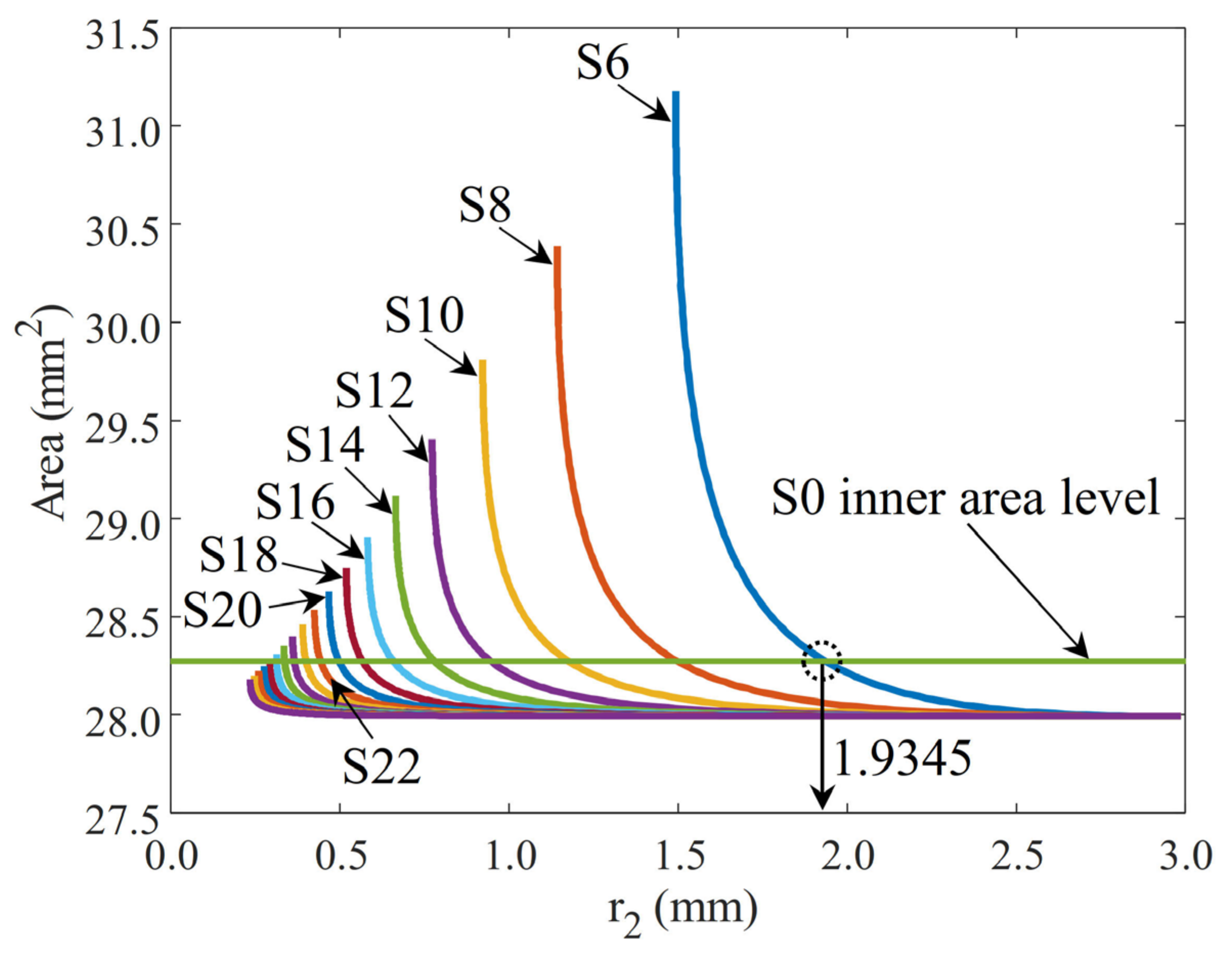
2.4. CAD Modeling and Validation of Inner Area
2.5. FEM Simulation Analysis
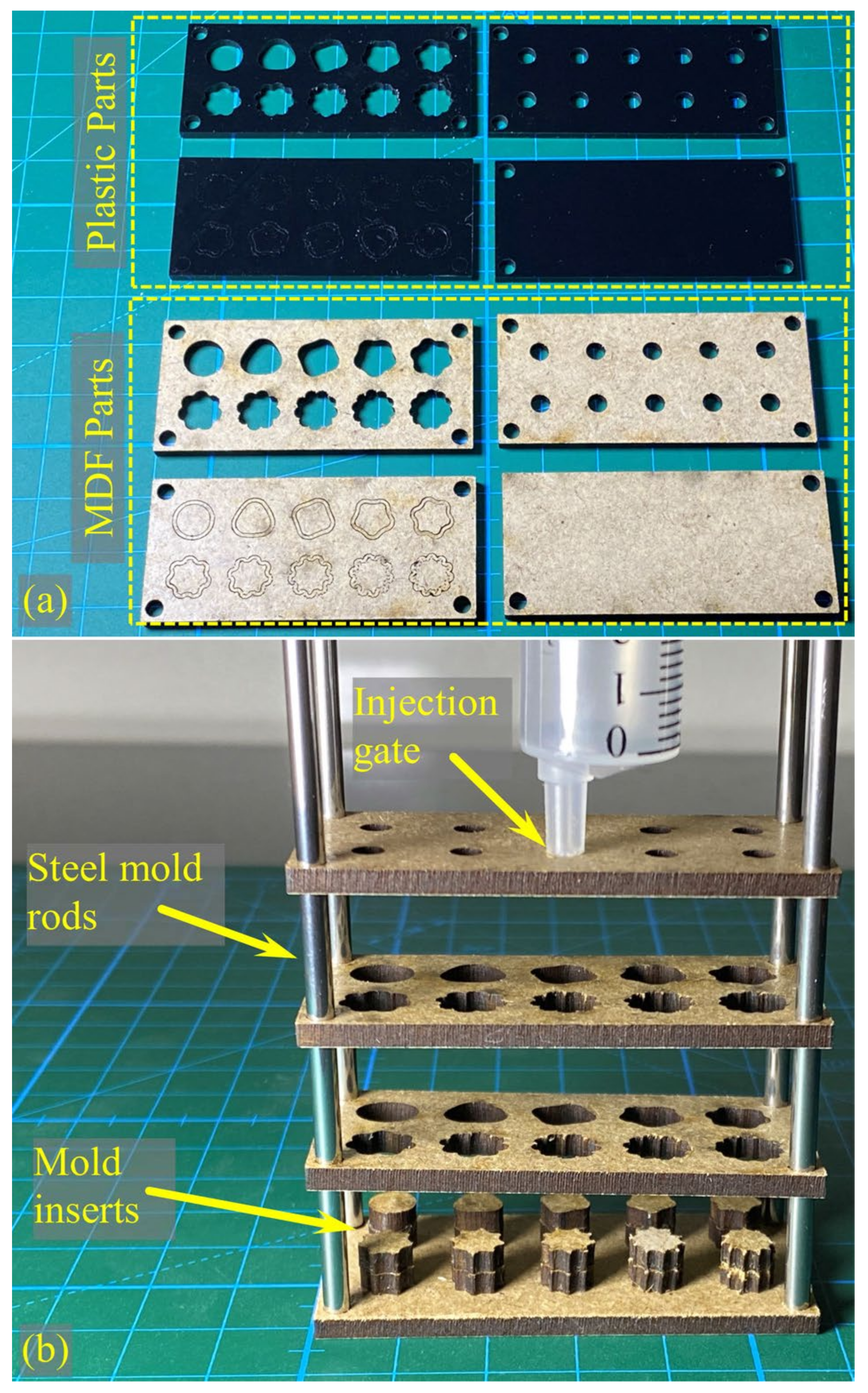
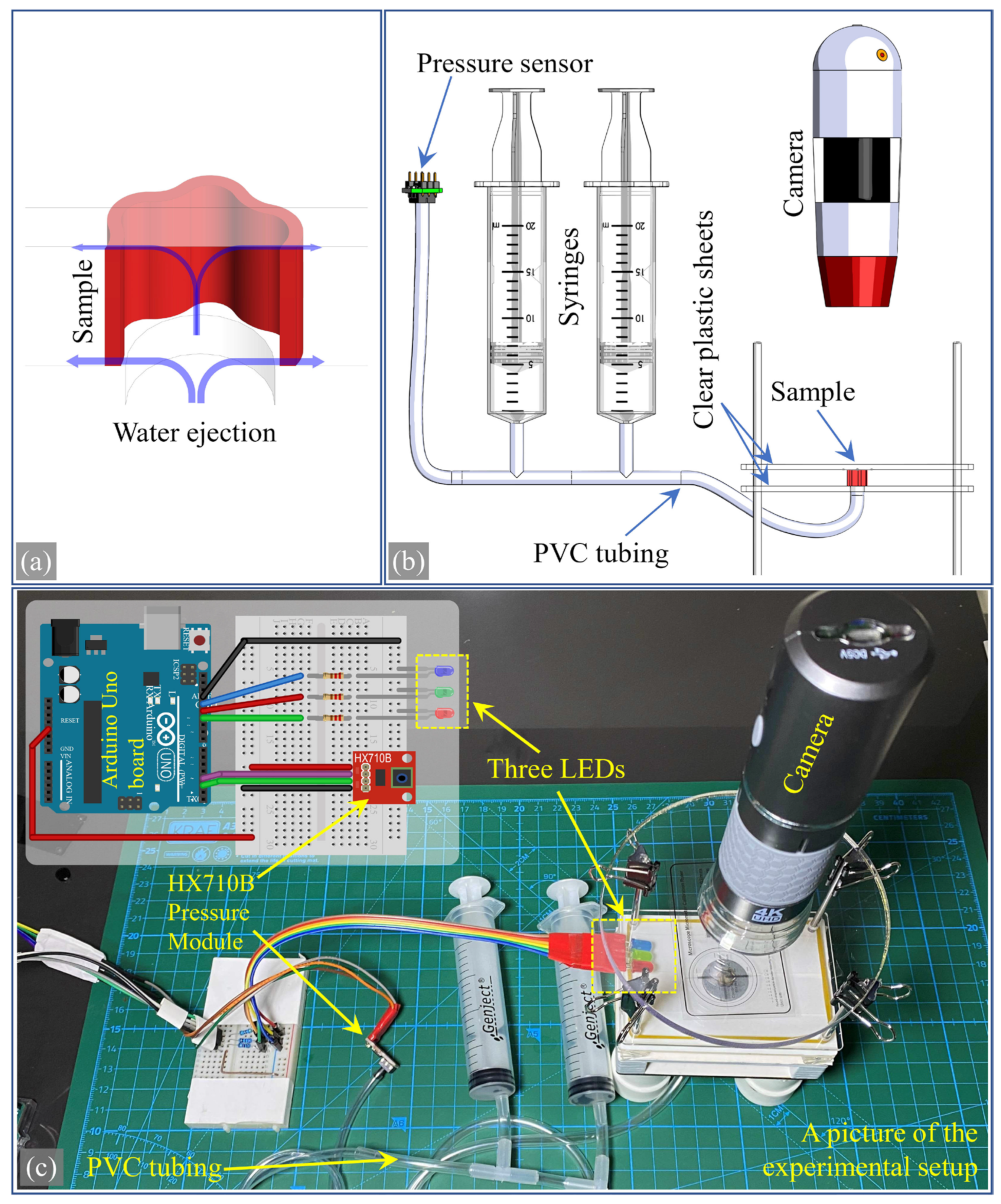
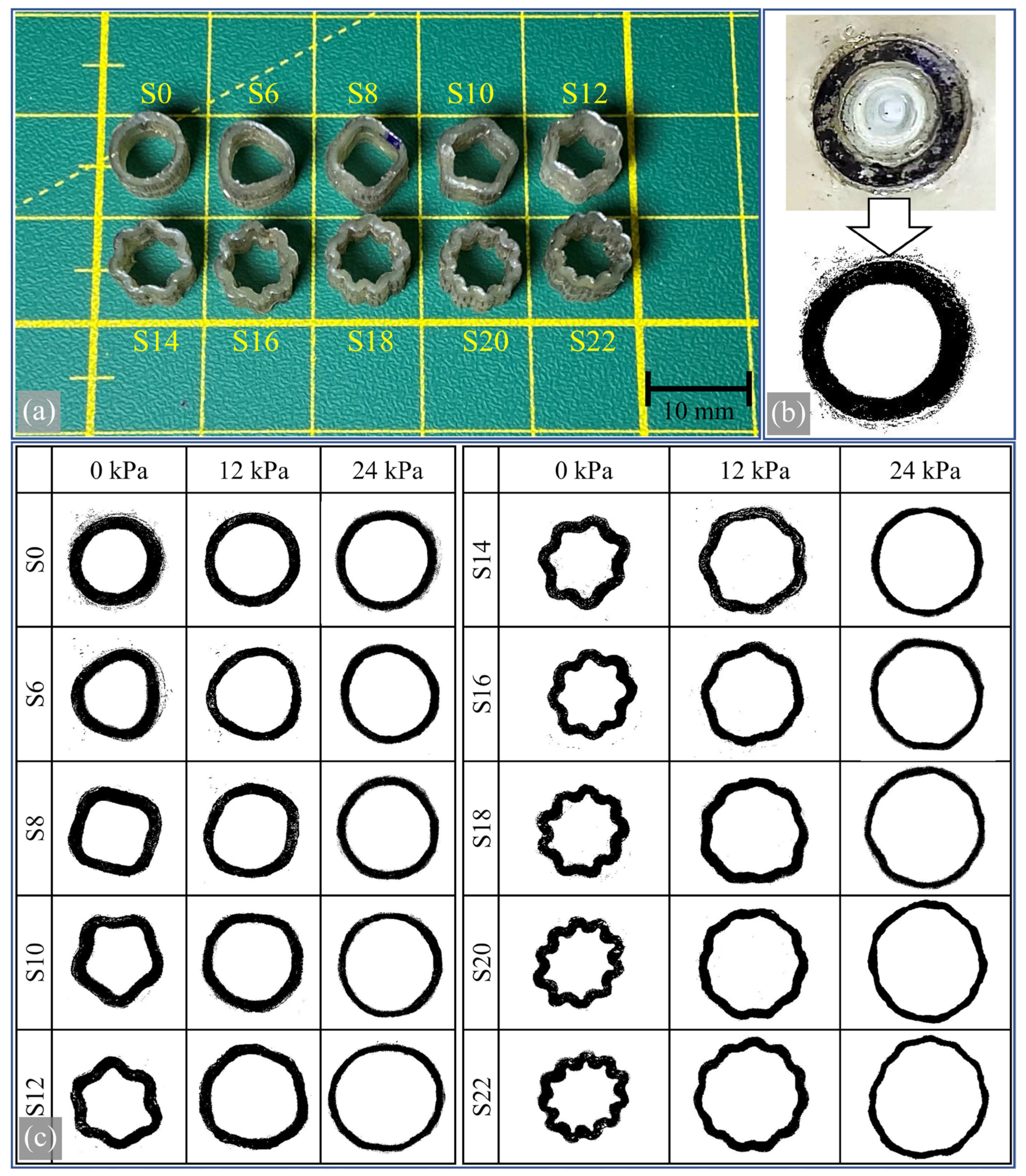
2.6. Producing Physical Samples
2.7. Developing a New Experimental Setup
2.8. Comparing Simulation and Experimental Results
3. Results
3.1. Mathematical Modeling of Vessel Sections
3.2. Matching the Area of Samples
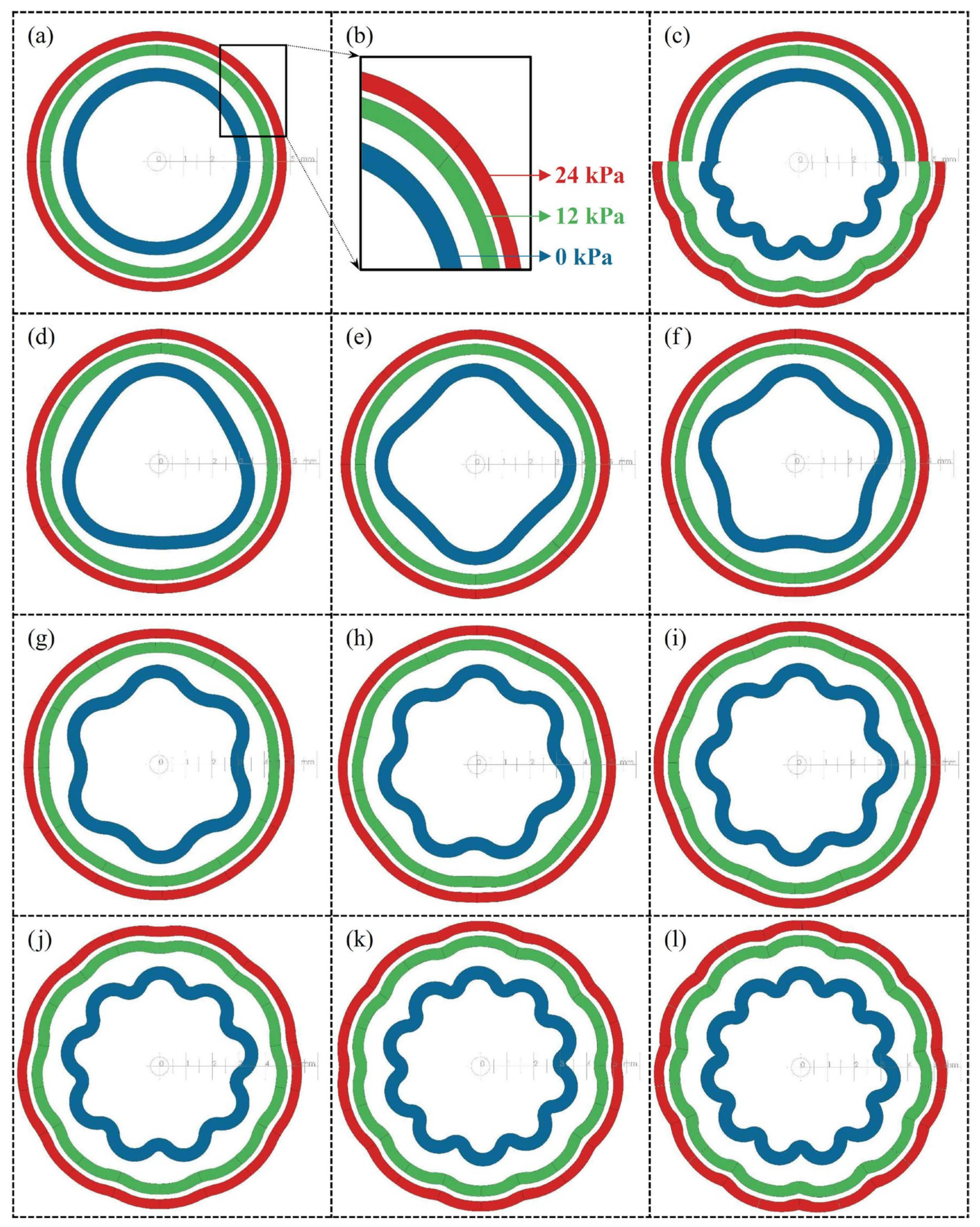
3.3. Simulation Results and Hyperelastic Properties
3.4. Experimental Results and Their Comparison with Simulation Results
4. Conclusions
- The creation of a wavy blood vessel cross-section required the investigation of numerous geometric parameters. A geometric approach incorporating both concave and convex curves was successfully created and modeled using symbolic mathematical tools in a computational environment.
- This analytical model was validated using CAD software to ensure that the internal area of the vessel cross-section (representing the lumen) remained constant across all samples as the number of waves increased. Additionally, technical drawings of the specimens, which are critical for repeatability, were generated for different wave numbers.
- A total of ten CAD models were subjected to internal pressure using a hyperelastic material model within a finite element method (FEM) module to investigate the expansion of the internal areas under varying pressure conditions.
- A unique experimental setup was developed to observe the expansion of the internal areas and the expansion of the waves under pressure. The results showed that the vessels’ flexibility effectively increased with the number of waves. For example, a sample with 22 folds had 45% more area under 24 kPa pressure than a sample with no waves. However, it was also found that increased wave numbers resulted in undesirably high-pressure gradients at higher pressures.
- A notable correlation was observed between experimental and FEM simulation results. The discrepancy was minimal, with a maximum difference of 19.88% decreasing to a mere 3.84% among sample types. This degree of precision is a significant achievement for a model exhibiting hyperelastic nonlinear behavior.
- The expansion of the internal area was found to be modeled by a simple linear equation, which provides a practical calculation method that researchers can use during the design phase. This equation demonstrates the contribution of increasing wave numbers to the expansion of the internal area.
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Townsend, N.; Kazakiewicz, D.; Wright, F.L.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Nag, A.; Pathan, M.M.; Dev, S. Analyzing the impact of feature selection on the accuracy of heart disease prediction. Health Anal. 2022, 2, 100060. [Google Scholar] [CrossRef]
- Auciello, O. Novel Biocompatible Ultrananocrystalline Diamond Coating Technology for a New Generation of Medical Implants, Devices, and Scaffolds for Developmental Biology. Biomater. Med. Appl. 2018, 1, 1–11. [Google Scholar] [CrossRef]
- Hoch, E.; Tovar, G.E.; Borchers, K. Bioprinting of artificial blood vessels: Current approaches towards a demanding goal. Eur. J. Cardio-Thorac. Surg. 2014, 46, 767–778. [Google Scholar] [CrossRef]
- Reinfeldt, S.; Taghavi, H.; Eeg-Olofsson, M.; Hakansson, B. New developments in bone-conduction hearing implants: A review. Med. Devices Evid. Res. 2015, 8, 79–93. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Y.; Li, Q.; Turng, L.-S. Artificial small-diameter blood vessels: Materials, fabrication, surface modification, mechanical properties, and bioactive functionalities. J. Mater. Chem. B 2020, 8, 1801–1822. [Google Scholar] [CrossRef]
- Camasão, D.; Mantovani, D. The mechanical characterization of blood vessels and their substitutes in the continuous quest for physiological-relevant performances. A critical review. Mater. Today Bio 2021, 10, 100106. [Google Scholar] [CrossRef]
- Gholipour, A.; Ghayesh, M.H.; Zander, A.; Mahajan, R. Three-dimensional biomechanics of coronary arteries. Int. J. Eng. Sci. 2018, 130, 93–114. [Google Scholar] [CrossRef]
- Gasser, T.C.; Ogden, R.W.; Holzapfel, G.A. Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J. R. Soc. Interface 2006, 3, 15–35. [Google Scholar] [CrossRef]
- Silver, F.H.; Snowhill, P.B.; Foran, D.J. Mechanical behavior of vessel wall: A comparative study of aorta, vena cava, and carotid artery. Ann. Biomed. Eng. 2003, 31, 793–803. [Google Scholar] [CrossRef]
- Schiavone, A.; Zhao, L. A computational study of stent performance by considering vessel anisotropy and residual stresses. Mater. Sci. Eng. C 2016, 62, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Nolan, D.; Gower, A.; Destrade, M.; Ogden, R.; McGarry, J. A robust anisotropic hyperelastic formulation for the modelling of soft tissue. J. Mech. Behav. Biomed. Mater. 2014, 39, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Niklason, L.E.; Lawson, J.H. Bioengineered human blood vessels. Science 2020, 370, eaaw8682. [Google Scholar] [CrossRef] [PubMed]
- Nemeno-Guanzon, J.G.; Lee, S.; Berg, J.R.; Jo, Y.H.; Yeo, J.E.; Nam, B.M.; Koh, Y.-G.; Lee, J.I. Trends in tissue engineering for blood vessels. J. Biomed. Biotechnol. 2012, 2012, 956345. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, G.; Uslu, E. Tek ve çift katmanlı yapay damar konfigürasyonlarının Holzapfel-Gasser-Ogden hiperelastik modeli ile mekanik uyumluluk analizi. Niğde Ömer Halisdemir Üniversitesi Mühendislik Bilim. Derg. 2022, 11, 1196–1205. [Google Scholar] [CrossRef]
- Mondy, W.L.; Cameron, D.; Timmermans, J.-P.; De Clerck, N.; Sasov, A.; Casteleyn, C.; A Piegl, L. Computer-aided design of microvasculature systems for use in vascular scaffold production. Biofabrication 2009, 1, 035002. [Google Scholar] [CrossRef]
- Simsek, F.G.; Kwon, Y.W. Investigation of material modeling in fluid–structure interaction analysis of an idealized three-layered abdominal aorta: Aneurysm initiation and fully developed aneurysms. J. Biol. Phys. 2015, 41, 173–201. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, Q.; Yu, D.; Shao, Y.; Wang, Z.; Liu, X.; Wang, W.; Chang, L.; Ma, T.; Mok, H.; et al. 3D-bioprinted vascular scaffold with tunable mechanical properties for simulating and promoting neo-vascularization. Smart Mater. Med. 2022, 3, 199–208. [Google Scholar] [CrossRef]
- Lopes, D.; Puga, H.; Teixeira, J.; Teixeira, S. Influence of arterial mechanical properties on carotid blood flow: Comparison of CFD and FSI studies. Int. J. Mech. Sci. 2019, 160, 209–218. [Google Scholar] [CrossRef]
- Updegrove, A.; Wilson, N.M.; Merkow, J.; Lan, H.; Marsden, A.L.; Shadden, S.C. SimVascular: An Open Source Pipeline for Cardiovascular Simulation. Ann. Biomed. Eng. 2017, 45, 525–541. [Google Scholar] [CrossRef]
- Maas, S.A.; Ellis, B.J.; Ateshian, G.A.; Weiss, J.A. FEBio: Finite elements for biomechanics. J. Biomech. Eng. 2012, 134, 011005. [Google Scholar] [CrossRef] [PubMed]
- Welp, C.; Siebers, S.; Ermert, H.; Werner, J. Investigation of the influence of blood flow rate on large vessel cooling in hepatic radiofrequency ablation. Biomed. Tech. 2006, 51, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Wenliao, C.; Jie, Z.; Xiaoliang, S.; Kun, W.; Guofeng, W.; Jian, Z.; Shuxiang, L.; Han, S. Material selection, theoretical design and biomimetic function of artificial periosteum. Chin. J. Tissue Eng. Res. 2021, 25, 600. [Google Scholar] [CrossRef]
- Roina, Y.; Auber, F.; Hocquet, D.; Herlem, G. ePTFE-based biomedical devices: An overview of surgical efficiency. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 302–320. [Google Scholar] [CrossRef]
- Peck, M.; Gebhart, D.; Dusserre, N.; McAllister, T.N.; L’heureux, N. The evolution of vascular tissue engineering and current state of the art. Cells Tissues Organs 2011, 195, 144–158. [Google Scholar] [CrossRef]
- Mezzetto, L.; Scorsone, L.; Silingardi, R.; Gennai, S.; Leone, N.; Piffaretti, G.; Veraldi, G.F. Early and Long-term Results of ePTFE (Gore TAG®) versus Dacron (Relay Plus® Bolton) Grafts in Thoracic Endovascular Aneurysm Repair. Ann. Vasc. Surg. 2020, 71, 419–427. [Google Scholar] [CrossRef]
- Sanchez, L.A.; Gupta, S.K.; Veith, F.J.; Goldsmith, J.; Lyon, R.T.; Wengerter, K.R.; Panetta, T.F.; Marin, M.L.; Cynamon, J.; Berdejo, G.; et al. A ten-year experience with one hundred fifty failing or threatened vein and polytetrafluoroethylene arterial bypass grafts. J. Vasc. Surg. 1991, 14, 729–738. [Google Scholar] [CrossRef][Green Version]
- Van Damme, H.; Deprez, M.; Creemers, E.; Limet, R. Intrinsic structural failure of polyester (dacron) vascular grafts. A general review. Acta Chir. Belg. 2005, 105, 249–255. [Google Scholar] [CrossRef]
- Noorani, A.; Ng, C.; Gopalan, D.; Dunning, J. Haemoptysis from a dacron graft aneurysm 21 years post repair of coarctation of the aorta. Interact. Cardiovasc. Thorac. Surg. 2011, 13, 91–93. [Google Scholar] [CrossRef][Green Version]
- Nagano, N.; Cartier, R.; Zigras, T.; Mongrain, R.; Leask, R.L. Mechanical properties and microscopic findings of a Dacron graft explanted 27 years after coarctation repair. J. Thorac. Cardiovasc. Surg. 2007, 134, 1577–1578. [Google Scholar] [CrossRef]
- Ali, A.A.M.; Sharma, P.; Rege, R.N.; Rajesh, S.; Nadhamuni, K. Dacron graft aneurysm with dissection. Indian J. Radiol. Imaging 2016, 26, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Jing, X.; Li, Z.; Lin, Y.; Thomson, J.A.; Turng, L. Fabrication and modification of wavy multicomponent vascular grafts with biomimetic mechanical properties, antithrombogenicity, and enhanced endothelial cell affinity. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2397–2408. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Napiwocki, B.; Xu, Y.; Zhang, J.; Zhang, X.; Wang, X.; Crone, W.C.; Li, Q.; Turng, L.-S. Wavy small-diameter vascular graft made of eggshell membrane and thermoplastic polyurethane. Mater. Sci. Eng. C 2020, 107, 110311. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.-Y.; Jing, X.; Yu, E.; Wang, X.; Li, Q.; Turng, L.-S. Manipulating the structure and mechanical properties of thermoplastic polyurethane/polycaprolactone hybrid small diameter vascular scaffolds fabricated via electrospinning using an assembled rotating collector. J. Mech. Behav. Biomed. Mater. 2018, 78, 433–441. [Google Scholar] [CrossRef]
- Yu, E.; Mi, H.; Zhang, J.; Thomson, J.A.; Turng, L. Development of biomimetic thermoplastic polyurethane/fibroin small-diameter vascular grafts via a novel electrospinning approach. J. Biomed. Mater. Res. Part A 2018, 106, 985–996. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Y.; Lin, Y.-J.; Yilmaz, G.; Zhang, J.; Schmidt, G.; Li, Q.; Thomson, J.A.; Turng, L.-S. Biologically Functionalized Expanded Polytetrafluoroethylene Blood Vessel Grafts. Biomacromolecules 2020, 21, 3807–3816. [Google Scholar] [CrossRef]
- Gopesh, T.; Friend, J. Facile Analytical Extraction of the Hyperelastic Constants for the Two-Parameter Mooney–Rivlin Model from Experiments on Soft Polymers. Soft Robot. 2021, 8, 365–370. [Google Scholar] [CrossRef]
- Kim, B.; Lee, S.B.; Lee, J.; Cho, S.; Park, H.; Yeom, S.; Park, S.H. A comparison among Neo-Hookean model, Mooney-Rivlin model, and Ogden model for chloroprene rubber. Int. J. Precis. Eng. Manuf. 2012, 13, 759–764. [Google Scholar] [CrossRef]


| Sample Name | Slice Numbers | r1 (mm) |
|---|---|---|
| S0 | 0 | 3.0000 |
| S6 | 6 | 2.9850 |
| S8 | 8 | 2.9850 |
| S10 | 10 | 2.9850 |
| S12 | 12 | 2.9850 |
| S14 | 14 | 2.9850 |
| S16 | 16 | 2.9850 |
| S18 | 18 | 2.9850 |
| S20 | 20 | 2.9850 |
| S22 | 22 | 2.9850 |
| Property | Value | Unit |
|---|---|---|
| Poisson’s Ratio | 0.49 | N/A |
| First Material Constant (A) | 36,958 | N/m2 |
| Second Material Constant (B) | −8783 | N/m2 |
| Third Material Constant (C) | −5907 | N/m2 |
| Fourth Material Constant (D) | 42,613 | N/m2 |
| Fifth Material Constant (E) | 2737 | N/m2 |
| Mass Density | 1130 | kg/m3 |
| Sample Name | Slice Numbers | Slice Angle (deg) | r1 (mm) | r2 (mm) | r3 (mm) | Area (mm2) |
|---|---|---|---|---|---|---|
| S0 | 0 | NA | 3.0000 | NaN | NaN | 28.274 * |
| S6 | 6 | 60.00 | 2.985 | 1.934 | 9.034 ** | 28.274 |
| S8 | 8 | 45.00 | 2.985 | 1.497 | 13.842 | 28.274 |
| S10 | 10 | 36.00 | 2.985 | 1.176 | 3.413 | 28.274 |
| S12 | 12 | 30.00 | 2.985 | 0.945 | 1.841 | 28.274 |
| S14 | 14 | 25.71 | 2.985 | 0.779 | 1.228 | 28.274 |
| S16 | 16 | 22.50 | 2.985 | 0.658 | 0.909 | 28.274 |
| S18 | 18 | 20.00 | 2.985 | 0.566 | 0.718 | 28.274 |
| S20 | 20 | 18.00 | 2.985 | 0.497 | 0.592 | 28.274 |
| S22 | 22 | 16.36 | 2.985 | 0.443 | 0.505 | 28.274 |
| S0 | S6 | S8 | S10 | S12 | S14 | S16 | S18 | S20 | S22 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Experimental areas (mm2) | 53.18 | 57.00 | 60.65 | 64.53 | 65.85 | 68.18 | 70.49 | 73.53 | 75.10 | 77.12 |
| Standard deviation | 0.78 | 1.97 | 2.31 | 0.86 | 0.95 | 3.07 | 0.90 | 0.95 | 2.78 | 1.61 |
| Simulation areas (mm2) | 63.76 | 66.00 | 67.84 | 69.90 | 72.12 | 74.20 | 76.32 | 78.12 | 79.60 | 80.08 |
| Error % | 19.88 | 15.79 | 11.85 | 8.33 | 9.53 | 8.84 | 8.27 | 6.25 | 5.99 | 3.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, G. Foundational Engineering of Artificial Blood Vessels’ Biomechanics: The Impact of Wavy Geometric Designs. Biomimetics 2024, 9, 546. https://doi.org/10.3390/biomimetics9090546
Yilmaz G. Foundational Engineering of Artificial Blood Vessels’ Biomechanics: The Impact of Wavy Geometric Designs. Biomimetics. 2024; 9(9):546. https://doi.org/10.3390/biomimetics9090546
Chicago/Turabian StyleYilmaz, Galip. 2024. "Foundational Engineering of Artificial Blood Vessels’ Biomechanics: The Impact of Wavy Geometric Designs" Biomimetics 9, no. 9: 546. https://doi.org/10.3390/biomimetics9090546
APA StyleYilmaz, G. (2024). Foundational Engineering of Artificial Blood Vessels’ Biomechanics: The Impact of Wavy Geometric Designs. Biomimetics, 9(9), 546. https://doi.org/10.3390/biomimetics9090546






