A Systematic Review of Human Amnion Enhanced Cartilage Regeneration in Full-Thickness Cartilage Defects
Abstract
1. Introduction
2. Materials and Methods
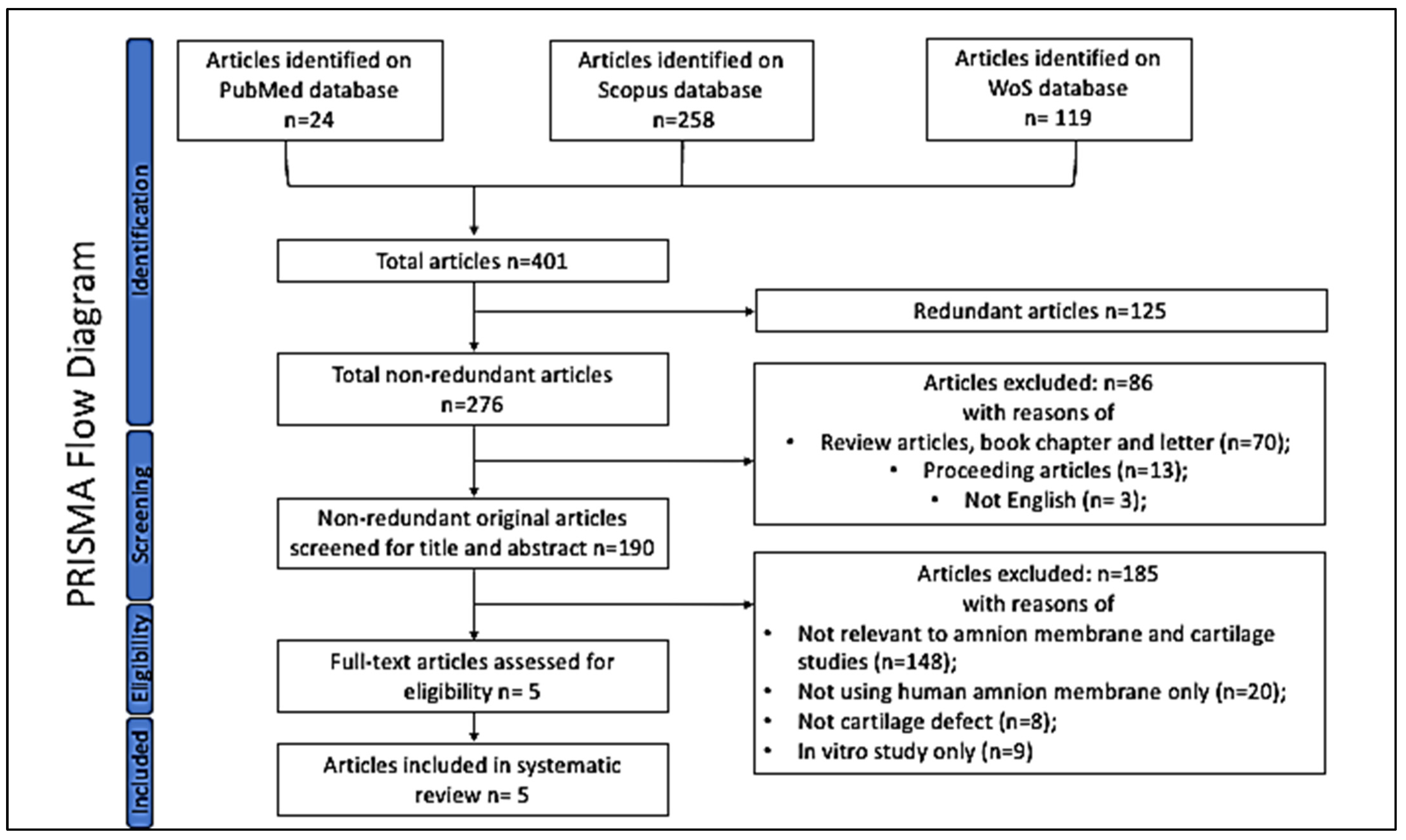
2.1. Search Strategy
2.2. Selection Criteria
2.3. Search Outcomes
3. Results
3.1. Use of HAM to Enhance Cartilage Regeneration for Repairing a Full-Thickness Cartilage Defect
3.2. Isolation of and Cultivation of MSCs
3.3. Surgical Procedure and Creation of Osteochondral Defects
3.4. Histological Examination
4. Discussion
4.1. HAM Scaffolds Enhancing Osteochondral Repair and Cartilage Regeneration
4.2. Bioactive Component of HAM Promoting Tissue Repair and Regeneration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katagiri, H.; Mendes, L.F.; Luyten, F.P. Definition of a critical size osteochondral knee defect and its negative effect on the surounding articular cartilage in the rat. Osteoarthr. Cartil. 2017, 25, 1531–1540. [Google Scholar] [CrossRef]
- Khoo, S.S.; Mansor, A.; Ahmad, T.S. Answers and additional information for orthopaedic clinical quiz. Malays. Orthop. J. 2020, 14, 108–109. [Google Scholar]
- Tabet, S.K.; Kimmerling, K.A.; Hale, G.J.; Munson, N.R.; Mowry, K.C. Hypothermically Stored Amniotic Membrane for the Treatment of Cartilage Lesions: A Single-Arm Prospective Study with 2-Year Follow-Up. Cartilage 2022, 13, 19476035211072213. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xi, L.; Yu, M.; Fan, Z.; Wang, W.; Ju, A.; Liang, Z.; Zhou, G.; Ren, W. Regeneration of articular cartilage defects: Therapeutic strategies and perspectives. J. Tissue Eng. 2023, 14, 20417314231164765. [Google Scholar] [CrossRef] [PubMed]
- Brovold, M.; Almeida, J.I.; Pla-Palacín, I.; Sainz-Arnal, P.; Sánchez-Romero, N.; Rivas, J.J.; Almeida, H.; Dachary, P.R.; Serrano-Aulló, T.; Soker, S.; et al. Naturally-derived biomaterials for tissue engineering applications. Adv. Exp. Med. Biol. 2018, 1077, 421–449. [Google Scholar] [PubMed]
- Malhotra, C.; Jain, A.K. Human amniotic membrane transplantation: Different modalities of its use in ophthalmology. World J. Transpl. 2014, 4, 111–121. [Google Scholar] [CrossRef]
- Salimbeigi, G.; Vrana, N.E.; Ghaemmaghami, A.M.; Huri, P.Y.; McGuinness, G.B. Basement membrane properties and their recapitulation in organ-on-chip applications. Mater. Today Bio 2022, 23, 100301. [Google Scholar] [CrossRef]
- McInnes, A.D.; Moser, M.A.J.; Chen, X. Preparation and use of decellularized extracellular matrix for tissue engineering. J. Funct. Biomater. 2022, 13, 240. [Google Scholar] [CrossRef]
- Jay, R.M.; Huish, J.P.; Wray, J.H. Amniotic membrane in clinical medicine: History, current status, and future use. In Extracellular Matrix-Derived Implants in Clinical Medicine; Woodhead Publishing: Sawston, UK, 2016; pp. 151–176. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar]
- Jin, C.Z.; Park, S.R.; Choi, B.H.; Lee, K.Y.; Kang, C.K.; Min, B.H. Human amniotic membrane as a delivery matrix for articular cartilage repair. Tissue Eng. 2007, 13, 693–702. [Google Scholar] [CrossRef]
- Liu, P.F.; Guo, L.; Zhao, D.W.; Zhang, Z.J.; Kang, K.; Zhu, R.P.; Yuan, X.L. Study of human acellular amniotic membrane loading bone marrow MSCs in repair of articular cartilage defect in rabbits. Genet. Mol. Res. 2014, 13, 7992–8001. [Google Scholar] [CrossRef]
- You, Q.; Liu, Z.; Zhang, J.; Shen, M.; Li, Y.; Jin, Y.; Liu, Y. Human amniotic mesenchymal stem cell sheets encapsulating cartilage particles facilitate repair of rabbit osteochondral defects. Am. J. Sports Med. 2020, 48, 599–611. [Google Scholar] [CrossRef]
- Zou, G.; Zhang, J.; Yang, Q.; Wang, X.; Sun, P. Human acellular amniotic membrane scaffolds encapsulating juvenile cartilage fragments accelerate the repair of rabbit osteochondral defects. Stem Cells Int. 2022, 3967722. [Google Scholar]
- Jun, Z.; Yuping, W.; Yanran, H.; Ziming, L.; Yuwan, L.; Xizhong, Z.; Zhilin, W.; Xiaoji, L. Human acellular amniotic membrane scaffolds encapsulating juvenile cartilage fragments accelerate the repair of rabbit osteochondral defects. Bone Jt. Res. 2022, 11, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Daou, F.; Cochis, A.; Leigheb, M.; Rimondini, L. Current advances in the regeneration of degenerated articular cartilage: A literature review on tissue engineering and its recent clinical translation. Materials 2021, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Mosaddad, S.A.; Hussain, A.; Tebyaniyan, H. Exploring the use of animal models in craniofacial regenerative medicine: A narrative review. Tissue Eng. Part. B Rev. 2024, 30, 29–59. [Google Scholar] [CrossRef] [PubMed]
- Ribitsch, I.; Baptista, P.M.; Lange-Consiglio, A.; Melotti, L.; Patruno, M.; Jenner, F.; Schnabl-Feichter, E.; Dutton, L.C.; Connolly, D.J.; van Steenbeek, F.G.; et al. Large animal models in regenerative medicine and tissue engineering: To do or not to do. Front. Bioeng. Biotechnol. 2020, 13, 972. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, D.A.; Wu, D.; He, F.; Wang, H.; Huang, L.; Shi, D.; Liu, Q.; Ni, N.; Pakvasa, M.; et al. Applications of biocompatible scaffold materials in stem cell-based cartilage tissue engineering. Front. Bioeng. Biotechnol. 2021, 25, 603444. [Google Scholar] [CrossRef] [PubMed]
- Alkaya, D.; Gurcan, C.; Kilic, P.; Yilmazer, A.; Gurman, G. Where is human-based cellular pharmaceutical R&D taking us in cartilage regeneration? Biotechnol. J. 2020, 10, 161. [Google Scholar]
- Fénelon, M.; Catros, S.; Meyer, C.; Fricain, J.C.; Obert, L.; Auber, F.; Louvrier, A.; Gindraux, F. Applications of Human Amniotic Membrane for Tissue Engineering. Membranes 2021, 11, 387. [Google Scholar] [CrossRef]
- Choi, Y.S.; Park, Y.B.; Ha, C.W.; Kim, J.A.; Heo, J.C.; Han, W.J.; Oh, S.Y.; Choi, S.J. Different characteristics of mesenchymal stem cells isolated from different layers of full term placenta. PLoS ONE 2017, 12, e0172642. [Google Scholar] [CrossRef]
- Baghaei, K.; Hashemi, S.M.; Tokhanbigli, S.; Rad, A.A.; Assadzadeh-Aghdaei, H.; Sharifi, A.; Zali, M.R. Isolation, differentiation, and characterization of mesenchymal stem cells from human bone marrow. GHFBB 2017, 10, 208–213. [Google Scholar]
- Liu, S.; Yang, W.; Li, Y.; Sun, C. Fetal bovine serum, an important factor affecting the reproducibility of cell experiments. Sci. Rep. 2023, 13, 1942. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.-S.; El-Ashram, S.; Luo, D.-Z.; Luo, H.-N.; Wang, B.-Y.; Chen, S.-F.; Bai, Y.-S.; Chen, Z.-S.; Liu, C.-Y.; Ji, H.-Q. A comparative study of biological characteristics and transcriptome profiles of mesenchymal stem cells from different canine tissues. Int. J. Mol. Sci. 2019, 20, 1485. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Díaz-Prado, S.; Rendal-Vázquez, M.E.; Muiños-López, E.; Hermida-Gómez, T.; Rodríguez-Cabarcos, M.; Fuentes-Boquete, I.; de Toro, F.J.; Blanco, F.J. Potential use of the human amniotic membrane as a scaffold in human articular cartilage repair. Cell Tissue Bank. 2010, 11, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Meinert, M.; Eriksen, G.V.; Petersen, A.C.; Helmig, R.B. Proteoglycans and hyaluronan in human fetal membranes. Am. J. Obs. Gynecol. 2001, 184, 679–685. [Google Scholar] [CrossRef]
- Levingstone, T.J.; Moran, C.; Almeida, H.V.; Kelly, D.J.; O’Brien, F.J. Layer-specific stem cell differentiation in tri-layered tissue engineering biomaterials: Towards development of a single-stage cell-based approach for osteochondral defect repair. Mater. Today Bio 2021, 12, 100173. [Google Scholar] [CrossRef] [PubMed]
- Gurina, T.S.; Simms, L. Histology, Staining. [Updated 1 May 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557663/ (accessed on 15 March 2024).
- Popa, E.; Rodrigues, M.; Coutinho, D.; Oliveira, M.; Mano, J.F.; Reis, R.L.; Gomes, M. Cryopreservation of cell laden natural origin hydrogels for cartilage regeneration strategies. J. Soft Matter 2012, 9, 875–885. [Google Scholar] [CrossRef]
- Qiao, Z.; Xin, M.; Wang, L.; Li, H.; Wang, C.; Wang, L.; Tang, T.; Zhu, B.; Huang, G.; Wang, Y.; et al. Proteoglycan 4 predicts tribological properties of repaired cartilage tissue. Theranostics 2020, 10, 2538–2552. [Google Scholar] [CrossRef]
- Mainil-Varlet, P.; Van, D.B.; Nesic, D.; Knutsen, G.; Kandel, R.; Roberts, S. A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am. J. Sports Med. 2010, 38, 880–890. [Google Scholar] [CrossRef]
- Gullberg, S.; Šimaiová, V.; Holovská, K.; Luptakova, L.; Koľvek, F.; Varga, M.; Petrovova, E. Histological Scoring Systems in the Cartilage Repair of Sheep. Folia Vet. 2019, 63, 15–26. [Google Scholar] [CrossRef]
- Moody, H.R.; Heard, B.J.; Frank, C.B.; Shrive, N.G.; Oloyede, A.O. Investigating the potential value of individual parameters of histological grading systems in a sheep model of cartilage damage: The Modified Mankin method. J. Anat. 2012, 221, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, L.; Zhang, C.; Hu, J.; Chen, J.; Du, W.; Liu, F.; Ren, W.; Wang, J.; Quan, R. Feasibility of repairing full-thickness skin defects by iPSC-derived epithelial stem cells seeded on a human acellular amniotic membrane. Stem Cell Res. Ther. 2019, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Do Ki, K.; Lee, M.K.; So, J.W.; Chung, S.K.; Kang, J. Comparison of cytokine expression and ultrastructural alterations in fresh-frozen and dried electron beam-irradiated human amniotic membrane and chorion. Cell Tissue Bank. 2019, 20, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Bernabé-García, Á.; Liarte, S.; Rodríguez-Valiente, M.; Nicolás, F.J. Chronic wound healing by amniotic membrane: TGF-β and EGF signaling modulation in re-epithelialization. Front. Bioeng. Biotechnol. 2021, 9, 689328. [Google Scholar]
- Bhatnagar, P.; Law, J.X.; Ng, S.F. Delivery systems for platelet derived growth factors in wound healing: A review of recent developments and global patent landscape. J. Drug Deliv. Sci. Technol. 2022, 71, 103270. [Google Scholar] [CrossRef]
- Fernández-Guarino, M.; Hernández-Bule, M.L.; Bacci, S. Cellular and Molecular Processes in Wound Healing. Biomedicines 2023, 11, 2526. [Google Scholar] [CrossRef]
| Database | Search Strategy |
|---|---|
| Web of Science | TI (((((((((TI = (Full-thickness cartilage defect)) OR TI = (Cartilage)) OR TI = (Hyaline cartilage)) OR TI = (Articular cartilage)) OR TI = (Osteochondral defect)) OR TI = (Chondral lesion)) OR TI = (Chondral defect)) OR TI = (Articular cartilage defect)) OR TI = (Articular cartilage lesions)) OR TI = (Knee chondral injury) AND ((((((((((TI = (Human amnion)) OR TI = (HAM)) OR TI = (AM)) OR TI = (Amniotic membrane)) OR TI = (Amnion membrane)) OR TI = (Placenta membrane)) OR TI = (Fetus-membrane)) OR TI = (Amniotic tissue)) OR TI = (Placental-driven biomaterials)) OR TI = (Freeze-dried HAM)) OR TI = (Air-dried HAM) AND ((((((TI = (Cartilage regeneration)) OR TI = (Fibrocartilage formation)) OR TI = (Chondrogenesis)) OR TI = (Cartilage tissue repair)) OR TI = (Articular cartilage regeneration)) OR TI = (Hyaline cartilage formation)) OR TI = (Articular cartilage repair) AB (((((((((AB = (full-thickness cartilage defect)) OR AB = (cartilage)) OR AB = (hyaline cartilage)) OR AB = (articular cartilage)) OR AB = (osteochondral defect)) OR AB = (chondral lesion)) OR AB = (chondral defect)) OR AB = (articular cartilage defect)) OR AB = (articular cartilage lesions)) OR AB = (knee chondral injury) AND (((((((((((AB = (human amnion)) OR AB = (HAM))) OR AB = (AM)) OR AB = (amniotic membrane)) OR AB = (amnion membrane)) OR AB = (placenta membrane)) OR AB = (fetus-membrane)) OR AB = (amniotic tissue)) OR AB = (placental-driven biomaterials)) OR AB = (freeze-dried HAM)) OR AB = (air-dried HAM) AND ((((((AB = (cartilage regeneration)) OR AB = (fibrocartilage formation)) OR AB = (chondrogenesis)) OR AB = (cartilage tissue repair)) OR AB = (articular cartilage regeneration)) OR AB = (hyaline cartilage formation)) OR AB = (articular cartilage repair) |
| Scopus | ((TITLE-ABS-KEY (full AND thickness AND cartilage AND defect) OR TITLE-ABS-KEY (cartilage) OR TITLE-ABS-KEY (hyaline AND cartilage) OR TITLE-ABS-KEY (articular AND cartilage) OR TITLE-ABS-KEY (osteochondral AND defect) OR TITLE-ABS-KEY (chondral AND lesion) OR TITLE-ABS-KEY (chondral AND defect) OR TITLE-ABS-KEY (articular AND cartilage AND defect) OR TITLE-ABS-KEY (articular AND cartilage AND lesions) OR TITLE-ABS-KEY (knee AND chondral AND injury))) AND ((TITLE-ABS-KEY (ham) OR TITLE-ABS-KEY (am) OR TITLE-ABS-KEY (amniotic AND membrane) OR TITLE-ABS-KEY (amnion AND membrane) OR TITLE-ABS-KEY (placenta AND membrane) OR TITLE-ABS-KEY (fetus-membrane) OR TITLE-ABS-KEY (amniotic AND tissue) OR TITLE-ABS-KEY (placental-driven AND biomaterials) OR TITLE-ABS-KEY (freeze-dried AND ham) OR TITLE-ABS-KEY (air-dried AND ham))) AND ((TITLE-ABS-KEY (cartilage AND regeneration) OR TITLE-ABS-KEY (fibrocartilage AND formation) OR TITLE-ABS-KEY (chondrogenesis) OR TITLE-ABS-KEY (cartilage AND tissue AND repair) OR TITLE-ABS-KEY (articular AND cartilage AND regeneration) OR TITLE-ABS-KEY (hyaline AND cartilage AND formation) OR TITLE-ABS-KEY (articular AND cartilage AND repair))) |
| PubMed | Search: “full-thickness cartilage defect”[Title/Abstract] OR “Cartilage”[Title/Abstract] OR “hyaline cartilage”[Title/Abstract] OR “articular cartilage”[Title/Abstract] OR “osteochondral defect”[Title/Abstract] OR “chondral lesion”[Title/Abstract] OR “chondral defect”[Title/Abstract] OR “articular cartilage defect”[Title/Abstract] OR “articular cartilage lesions”[Title/Abstract] OR “knee chondral injury”[Title/Abstract] AND “human amnion”[Title/Abstract] OR “HAM”[Title/Abstract] OR “AM”[Title/Abstract] OR “amniotic membrane”[Title/Abstract] OR “amnion membrane”[Title/Abstract] OR “placenta membrane”[Title/Abstract] OR “Fetus-membrane”[Title/Abstract] OR “amniotic tissue”[Title/Abstract] OR (“Placental-driven”[All Fields] AND “biomaterials”[Title/Abstract]) OR (“Freeze-dried”[All Fields] AND “HAM”[Title/Abstract]) OR (“Air-dried”[All Fields] AND “HAM”[Title/Abstract]) AND “cartilage regeneration”[Title/Abstract] OR “fibrocartilage formation”[Title/Abstract] OR “Chondrogenesis”[Title/Abstract] OR “cartilage tissue repair”[Title/Abstract] OR “articular cartilage regeneration”[Title/Abstract] OR “hyaline cartilage formation”[Title/Abstract] OR “articular cartilage repair”[Title/Abstract] |
| References | Article Title | Journal Details | HAM Used |
|---|---|---|---|
| [11] | Human amniotic membrane as a delivery matrix for articular cartilage repair | Tissue Engineering, Vol. 13, Issue 4, Pages 693–702. | the epithelial side of intact HAM (IHE), basement side of denuded HAM (DHB), and stromal side of denuded HAM (DHS). |
| [12] | Study of human acellular amniotic membrane loading bone marrow MSCs in repair of articular cartilage defect in rabbits | Genetics and Molecular Research, Vol. 13, Issue 3, Pages 7992–8001. | human acellular amniotic membrane |
| [13] | Human amniotic mesenchymal stem cell sheets encapsulating cartilage particles facilitate repair of rabbit osteochondral defects | American Journal of Sports Medicine, Vol. 48, Issue 3, Pages 599–611. | hAMSC sheets were constructed with passage 3 hAMSCs. |
| [14] | hAMSC sheet promotes repair of rabbit osteochondral defects | Stem Cells International, 3967722 | chondrogenically induced hAMSC sheet |
| [15] | Human acellular amniotic membrane scaffolds encapsulating juvenile cartilage fragments accelerate the repair of rabbit osteochondral defects | Bone & Joint Research, Vol. 11, Issue 6, Pages 349–361. | HAAM scaffold |
| References | HAM Origin | HAM Preservation Methods | HAM Deposition | HAM Transplantation | HAM Uses |
|---|---|---|---|---|---|
| [11] | Human | Fresh De-epithelialized | On the defect | Rabbit | Seeded with cells |
| [12] | Human | Fresh | On the defect | Rabbit | Seeded with cells |
| [13] | Human | Fresh | Into the defect | Rabbit | Sheets seeded with cells |
| [14] | Human | Fresh | Into the defect | Rabbit | Sheets seeded with cells |
| [15] | Human | Decellularized | Into the defect | Rabbit | Scaffold seeded with cells |
| References | Culture Condition | Isolation Method | Generation Used | Characterization |
|---|---|---|---|---|
| [11] | LG-DMEM/F12 medium containing 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, 1% L-glutamine, and 1% nonessential amino acids | Articular cartilages were harvested from the knee joints of three 2-week-old New Zealand white rabbits | P3 | Osteogenic differentiation (alizalin red staining) and chondrogenic differentiation (alcian blue staining) |
| [12] | - | BMSCs were isolated using the whole bone marrow culture method from rabbit | P3 | Cell morphology, adhesion to plastic and superficial markers expression with flow cytometry |
| [13] | - | hAMSCs were isolated from the amnion of human placentas from 8 full-term births within 6 h. | P3 | Chondrogenic differentiation |
| [14] | LG-DMEM/F12 medium containing 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, 1% L-glutamine, 1% nonessential amino acids, and 5 ng/mL basic fibroblast growth factor PeproTech | hAMSCs were isolated from the amnion of human placentas | P3 | Osteogenic differentiation (alzalin red staining) and chondrogenic differentiation (alcian blue staining) |
| [15] | LG-DMEM/F12 medium containing 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, 1% L-glutamine, and 1% nonessential amino acids was used to culture the hAMSCs. | hAMSCs were isolated from the amnions of human placentas | P3 | Osteogenic differentiation (alzalin red staining) and chondrogenic differentiation (alcian blue staining) |
| References | Animals (No. Per Condition | Model: Defect Localization and Size | Treatments | Evaluation Methodology | Results |
|---|---|---|---|---|---|
| [11] | Rabbit (n = 12) | Femoral condyle (5 mm) | Group A: null Group B: control—covered with denuded HAM Group B: denuded HAM + seeded cells | Histology | The defect area was barely filled in the defect-only group or filled with immature cartilage tissue in the denuded HAM group. The defect area was completely filled with fully mature cartilage in the DHS group at 8 weeks. |
| [12] | Rabbit (n = 24) | Bilateral femoral condyle (Diameter 4 mm × depth 3 mm) | Group A: HAAM + BMSCS Group B: HAAM | Microscopic observation Histology | The repair effect in group A was better than in group B, suggesting that in vitro-amplified BMSC had a dominant effect on cartilage defect repair. |
| [13] | Rabbit (n = 24) | Osteochondral defects (Diameter 3.5 mm × depth 3 mm) | Group A: Fibrin glue Group B: hAMSC sheet Group C: cartilage particles Group D: hAMSC sheet/cartilage particles | Macroscopic observation Histology | In the hAMSC sheet/cartilage particle group, the defect area was almost completely filled with a white semitranslucent tissue at 3 months postoperatively, and the boundary connected to the normal cartilage tissue had almost completely disappeared. |
| [14] | Rabbit (n = 15) | Osteochondral defects (Diameter 3.5 mm × depth 3 mm) | Group A: control Group B: noninduced cell sheet Group C: chondrogenically induced cell sheet | Histology | Chondrogenically induced cell sheet group had the better repair effect than the noninduced cell sheet group and the control group. |
| [15] | Rabbit (n = 20) | Osteochondral defects (Diameter 3.5 mm × depth 3 mm) | Group A: control Group B: HAAM scaffold Group C: JCFs Group D: HAAM + JCFs | Histology | HAAM + JCFs has newly formed cartilage fillings in the defects and also found smooth and continuous cartilage. |
| References | Specimen Preparation | Sagittal Sections | Staining | Scoring | Subject to |
|---|---|---|---|---|---|
| [11] | 10% buffered formalin for 48 h and embedded in paraffin | 4 μm | Masson’s trichrome and hematoxylin and eosin (H&E) staining. | ICRS scores | Immunohistochemical staining of Col-II |
| [12] | Not mentioned | Not mentioned | H&E | Wakitani scoring method | Not mentioned |
| [13] | Fixed in 4% paraformaldehyde | 5 μm | H&E, and Safranin-O/Fast-green (SO/FG) | O’Driscoll histological grading scale | Immunohistochemical staining of Col-II |
| [14] | 4% paraformaldehyde for 24 h and decalcified for approximate 2 weeks. Then, they were embedded in paraffin for histological sectioning | 5 μm | H&E, toluidine Blue (TB), Safranin-O/Fast-green (SO/FG) | O’Driscoll histological grading scale and the modified Mankin’s histological scoring | Immunohistochemical staining of Col-I and Col-II |
| [15] | fixed in 4% paraformaldehyde for 24 h, decalcified for two weeks, and embedded in paraffin for routine histological sectioning | 5 μm | H&E, Safranin O/Fast Green (SO/FG), and toluidine blue (TB) | ICRS II scores | Immunohistochemical staining of Col-II |
| Advantages | Disadvantages |
|---|---|
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Halim, N.F.A.; Ab Aziz, A.; Tan, S.-L.; Selvaratnam, V.; Kamarul, T. A Systematic Review of Human Amnion Enhanced Cartilage Regeneration in Full-Thickness Cartilage Defects. Biomimetics 2024, 9, 383. https://doi.org/10.3390/biomimetics9070383
Abd Halim NFA, Ab Aziz A, Tan S-L, Selvaratnam V, Kamarul T. A Systematic Review of Human Amnion Enhanced Cartilage Regeneration in Full-Thickness Cartilage Defects. Biomimetics. 2024; 9(7):383. https://doi.org/10.3390/biomimetics9070383
Chicago/Turabian StyleAbd Halim, Nur Farah Anis, Atiqah Ab Aziz, Sik-Loo Tan, Veenesh Selvaratnam, and Tunku Kamarul. 2024. "A Systematic Review of Human Amnion Enhanced Cartilage Regeneration in Full-Thickness Cartilage Defects" Biomimetics 9, no. 7: 383. https://doi.org/10.3390/biomimetics9070383
APA StyleAbd Halim, N. F. A., Ab Aziz, A., Tan, S.-L., Selvaratnam, V., & Kamarul, T. (2024). A Systematic Review of Human Amnion Enhanced Cartilage Regeneration in Full-Thickness Cartilage Defects. Biomimetics, 9(7), 383. https://doi.org/10.3390/biomimetics9070383







