Biological Surface Layer Formation on Bioceramic Particles for Protein Adsorption
Abstract
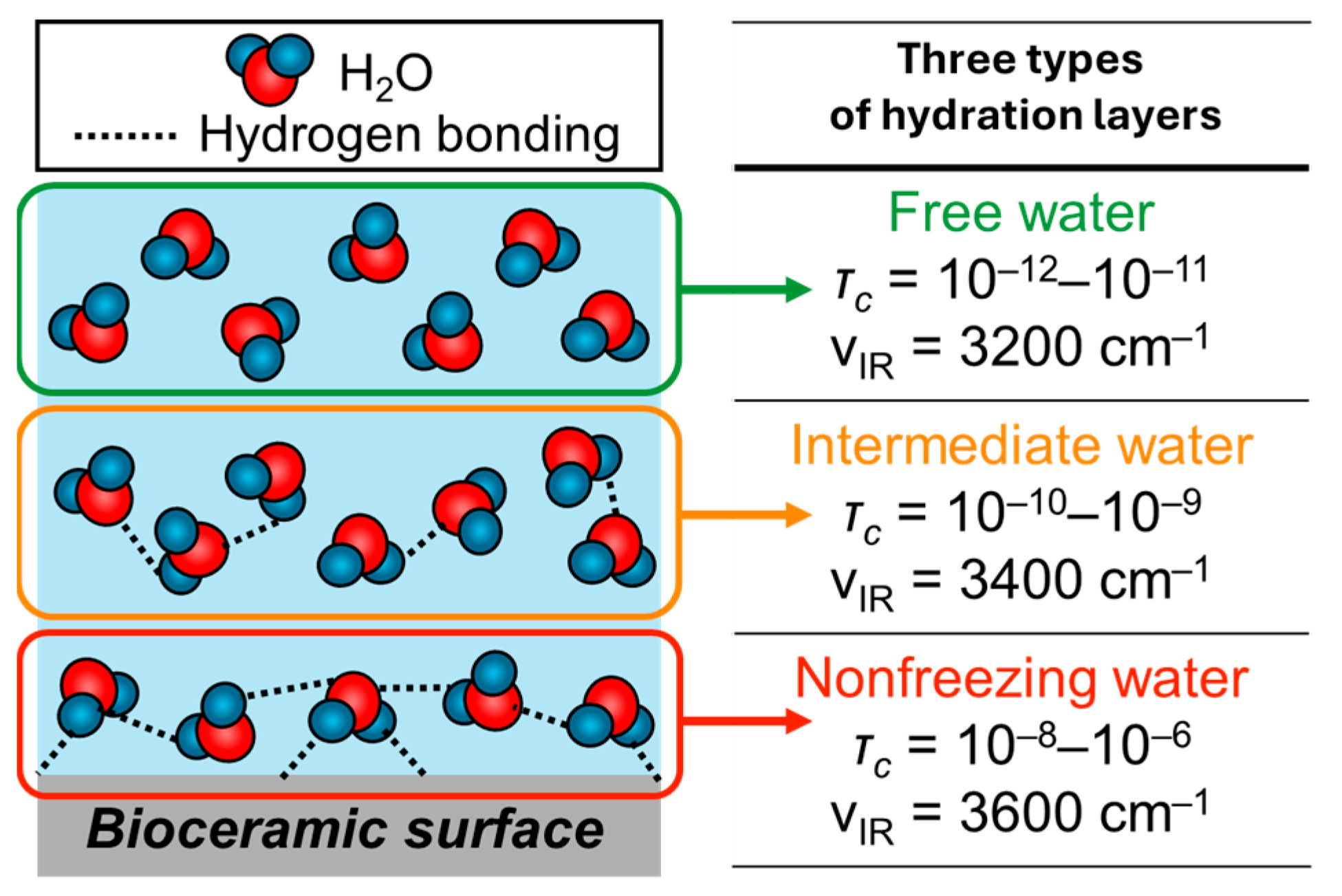
1. Introduction
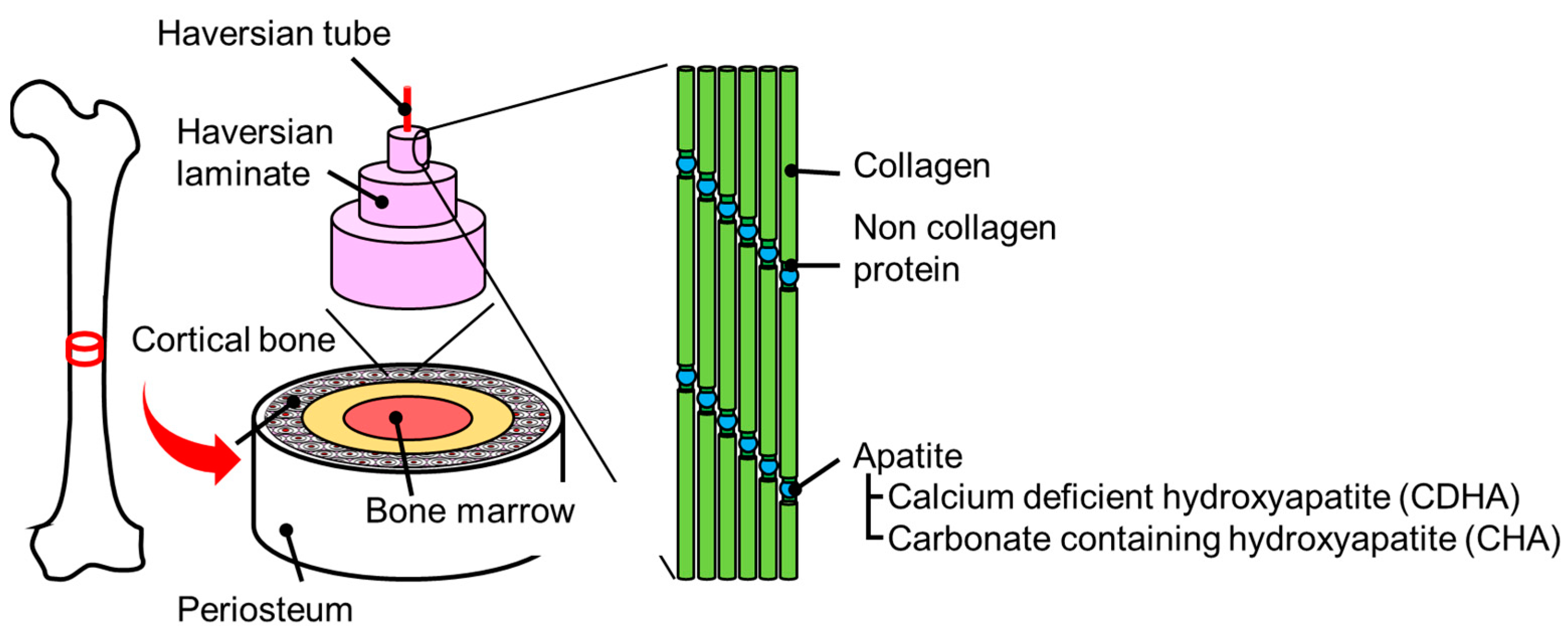
2. Protein Immobilization Phenomena on Biological Bone
3. Bioceramic Particles and Surface Function
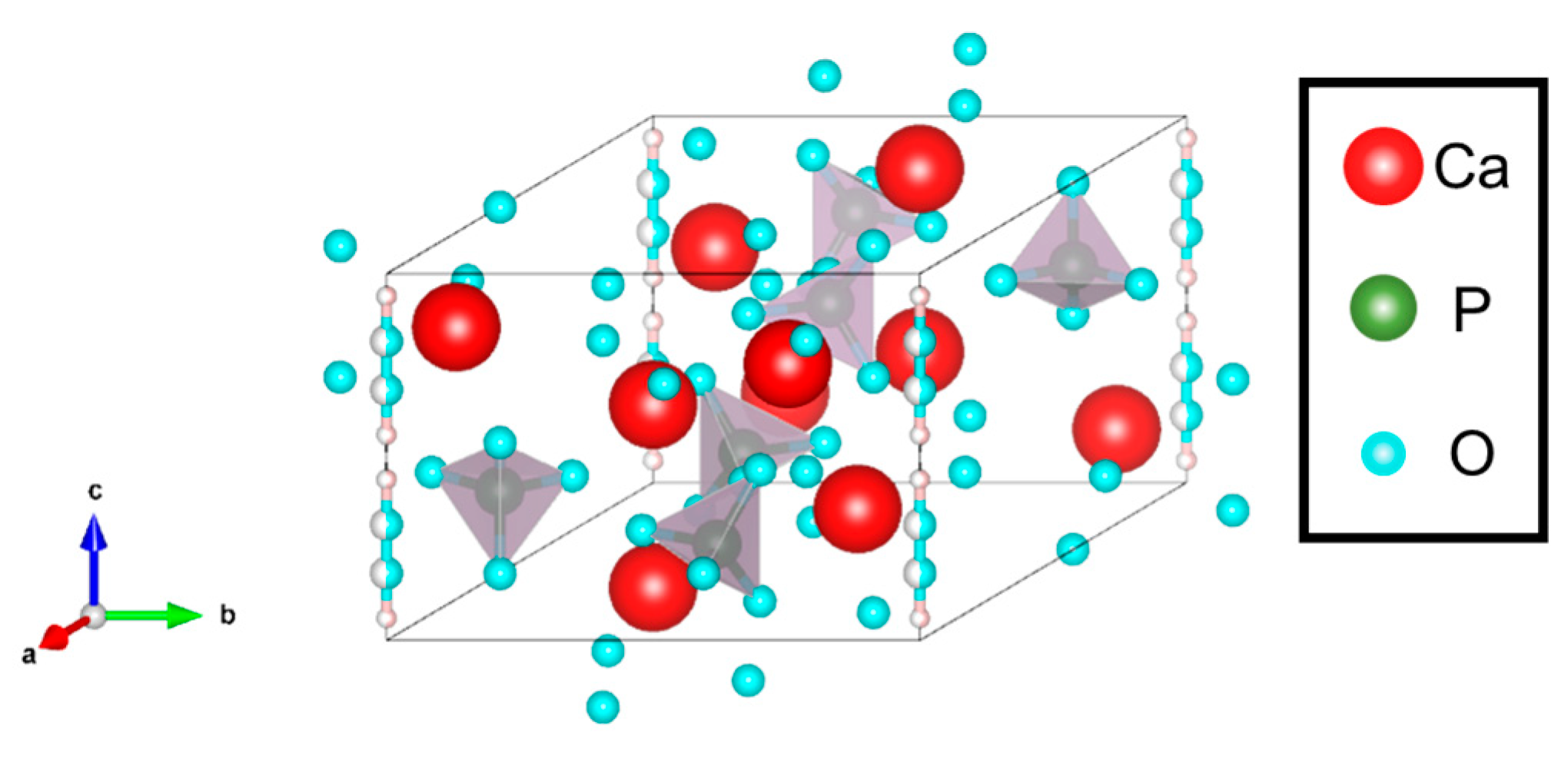
3.1. Hydroxyapatite Particles
3.1.1. Features
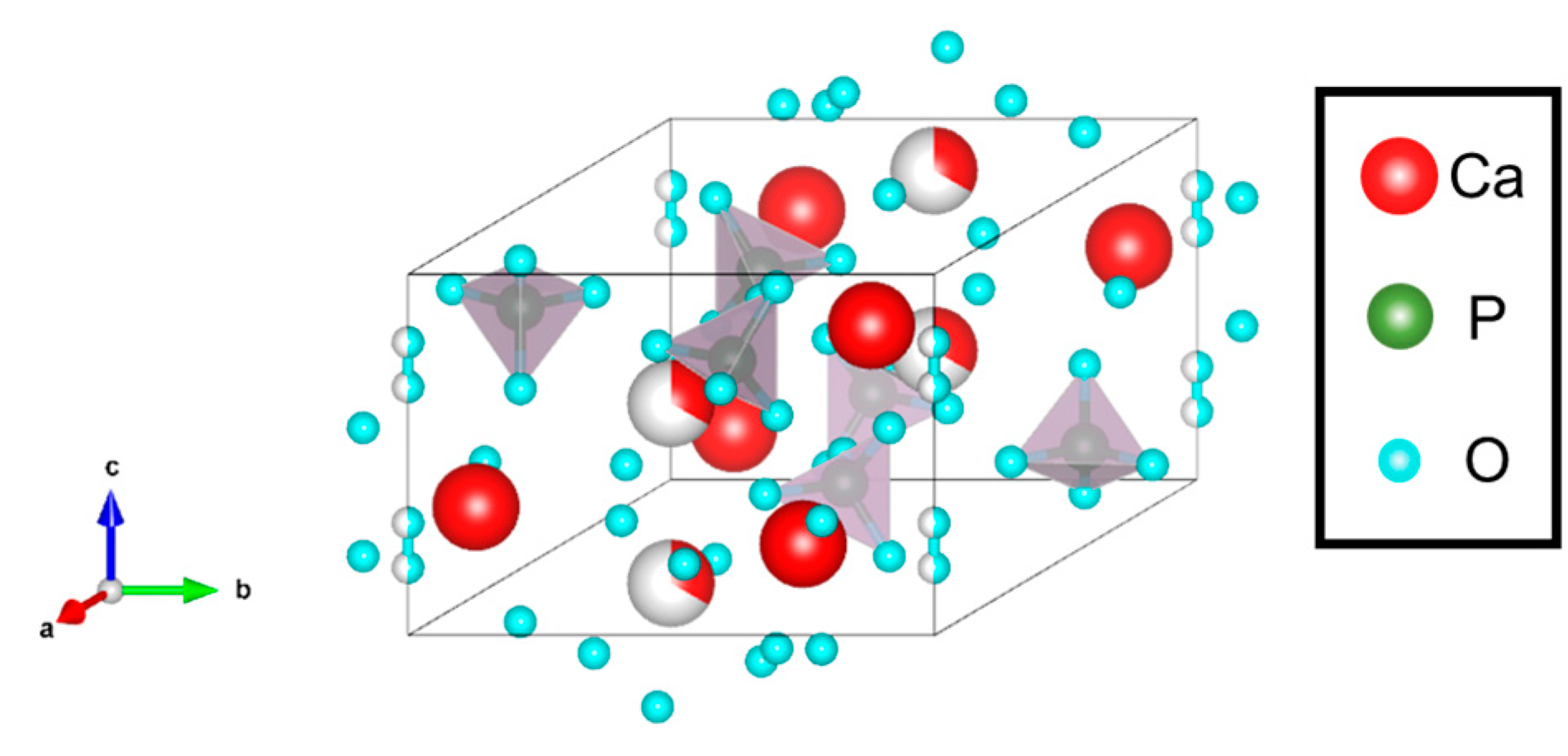
3.1.2. Ca-Deficient Hydroxyapatite
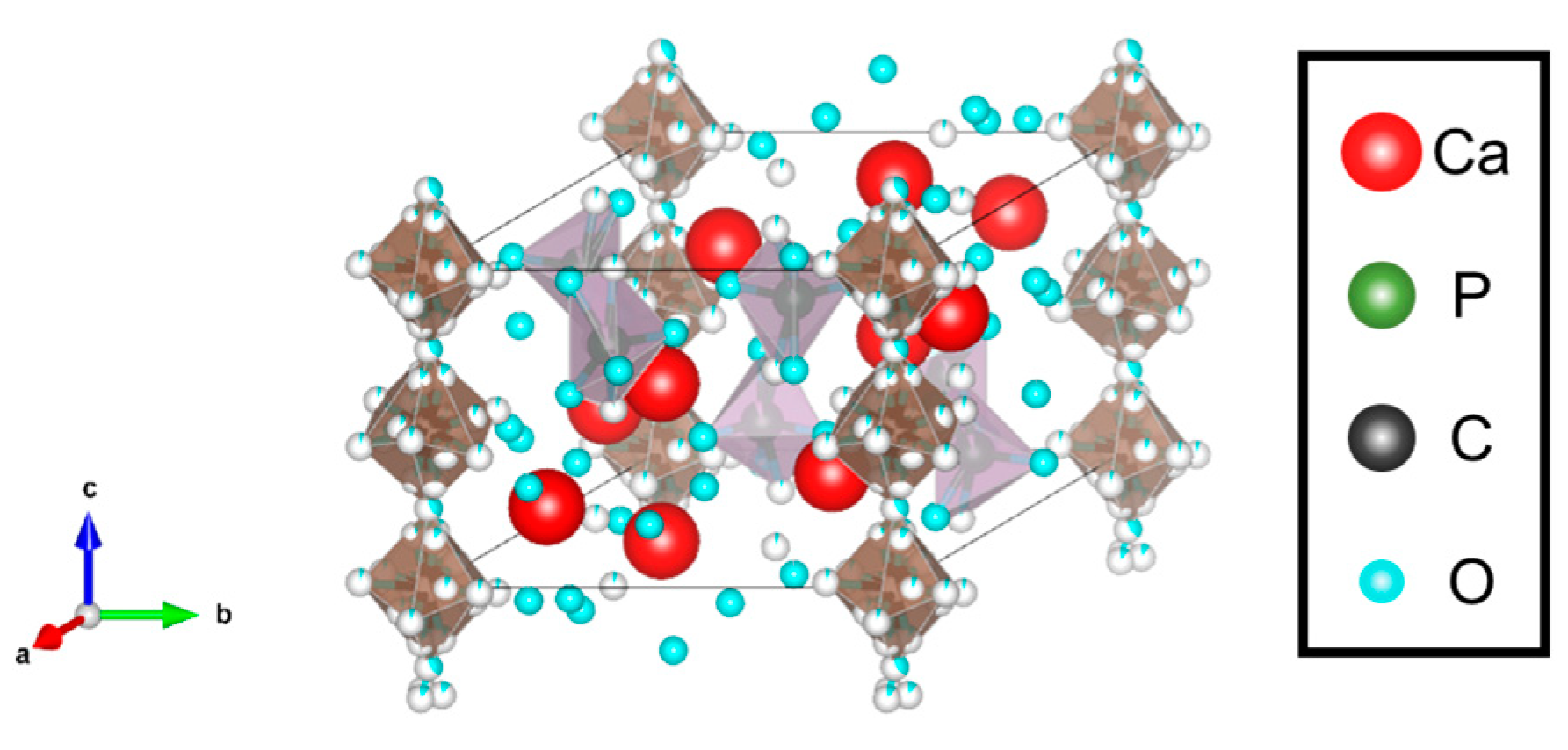
3.1.3. Carbonate-Substituted Hydroxyapatite
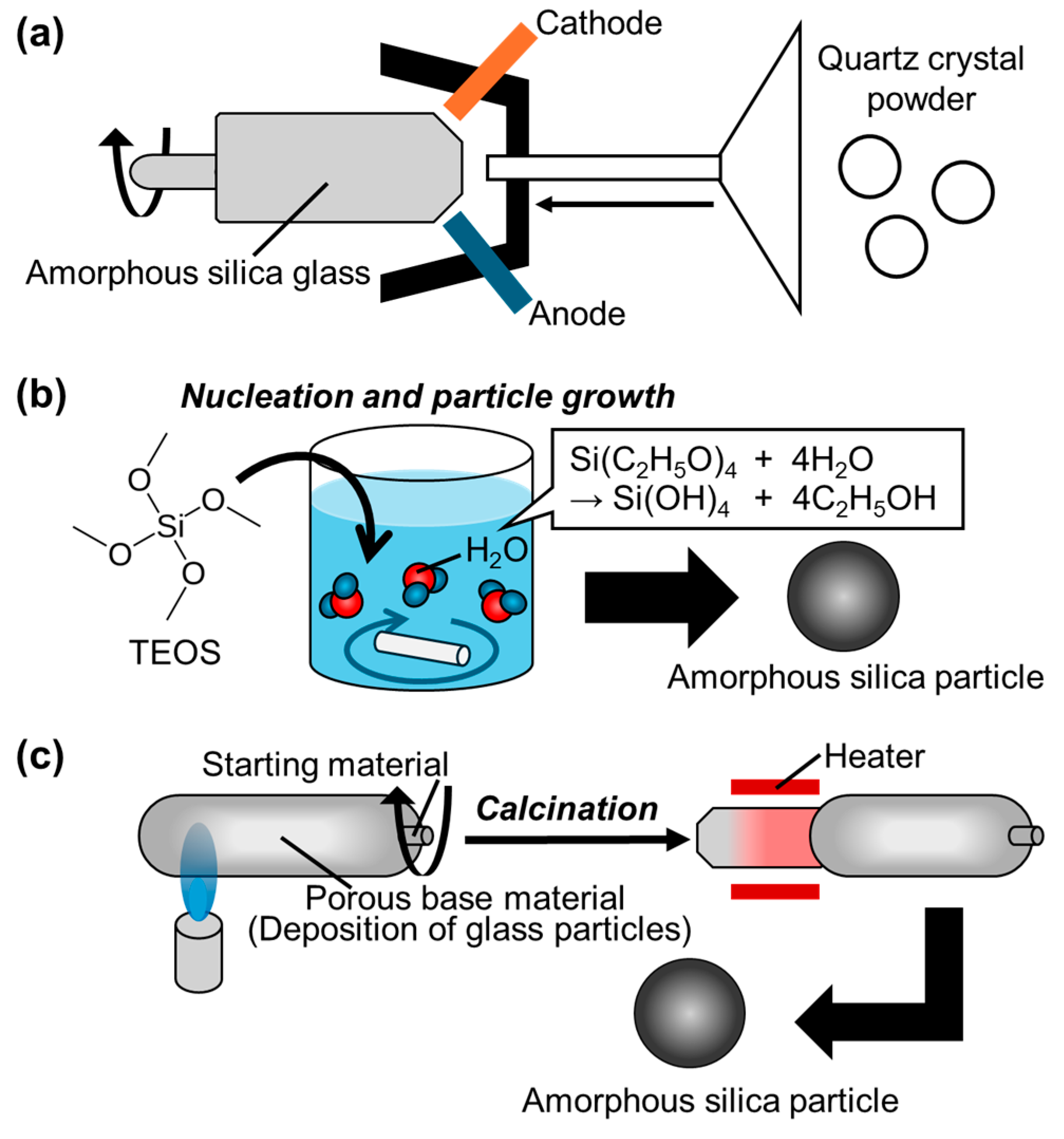
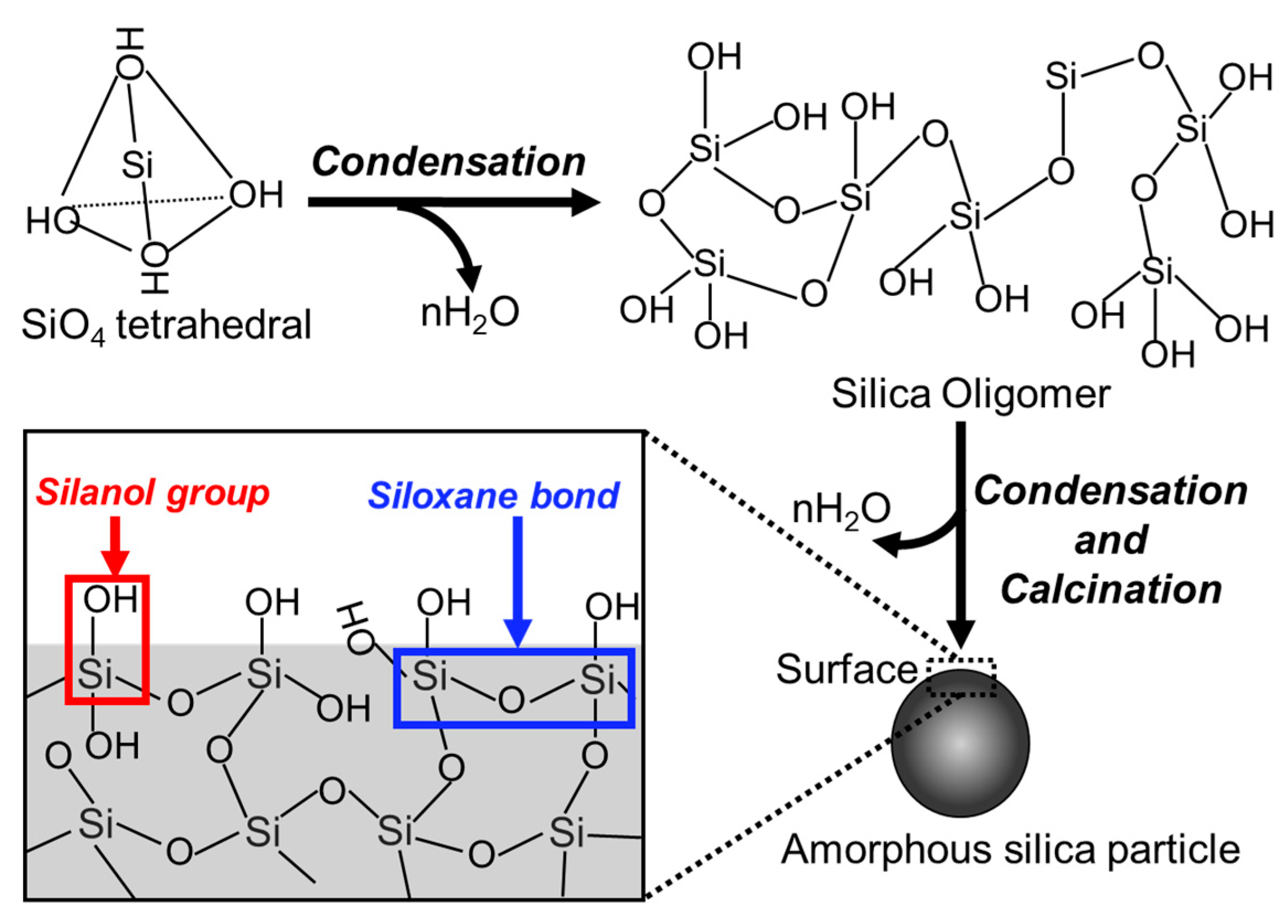
3.2. Amorphous Silica Particles
3.2.1. Features
Structure and Surfaces
3.2.2. Biofunctionalization
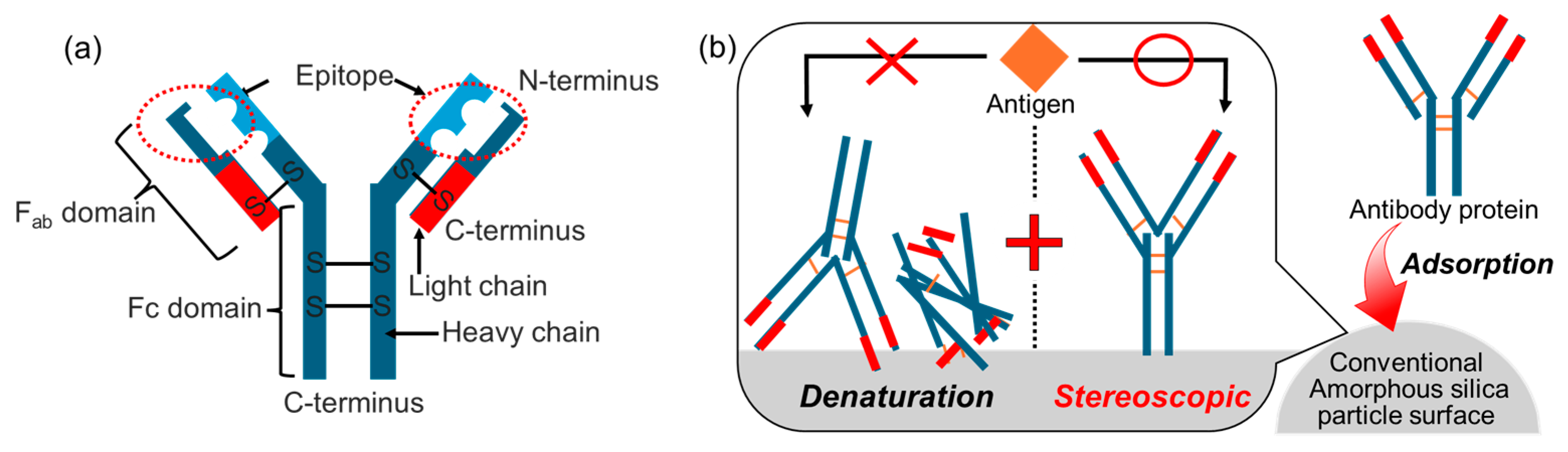
3.2.3. Application for Antibody Protein Immobilization
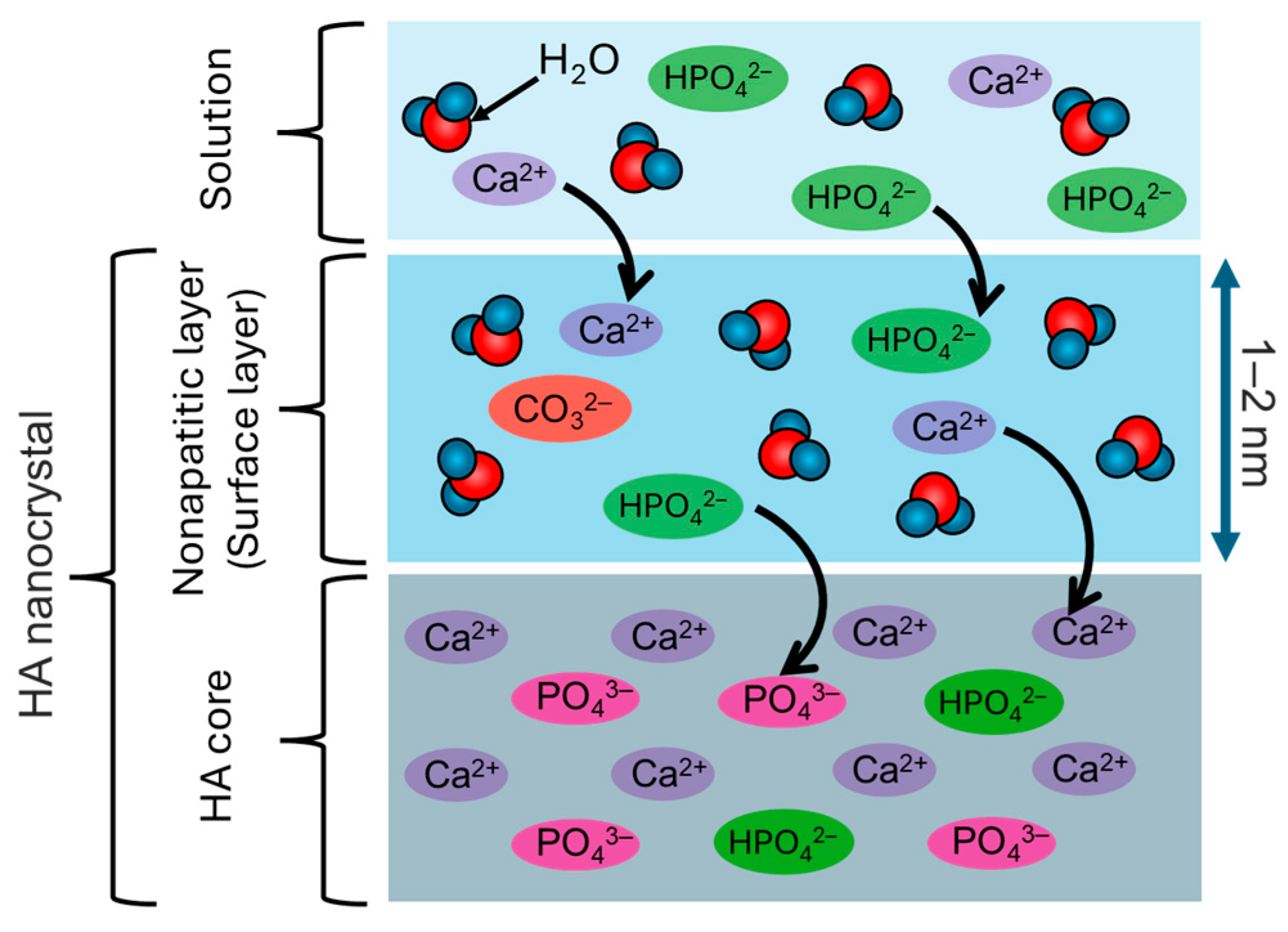
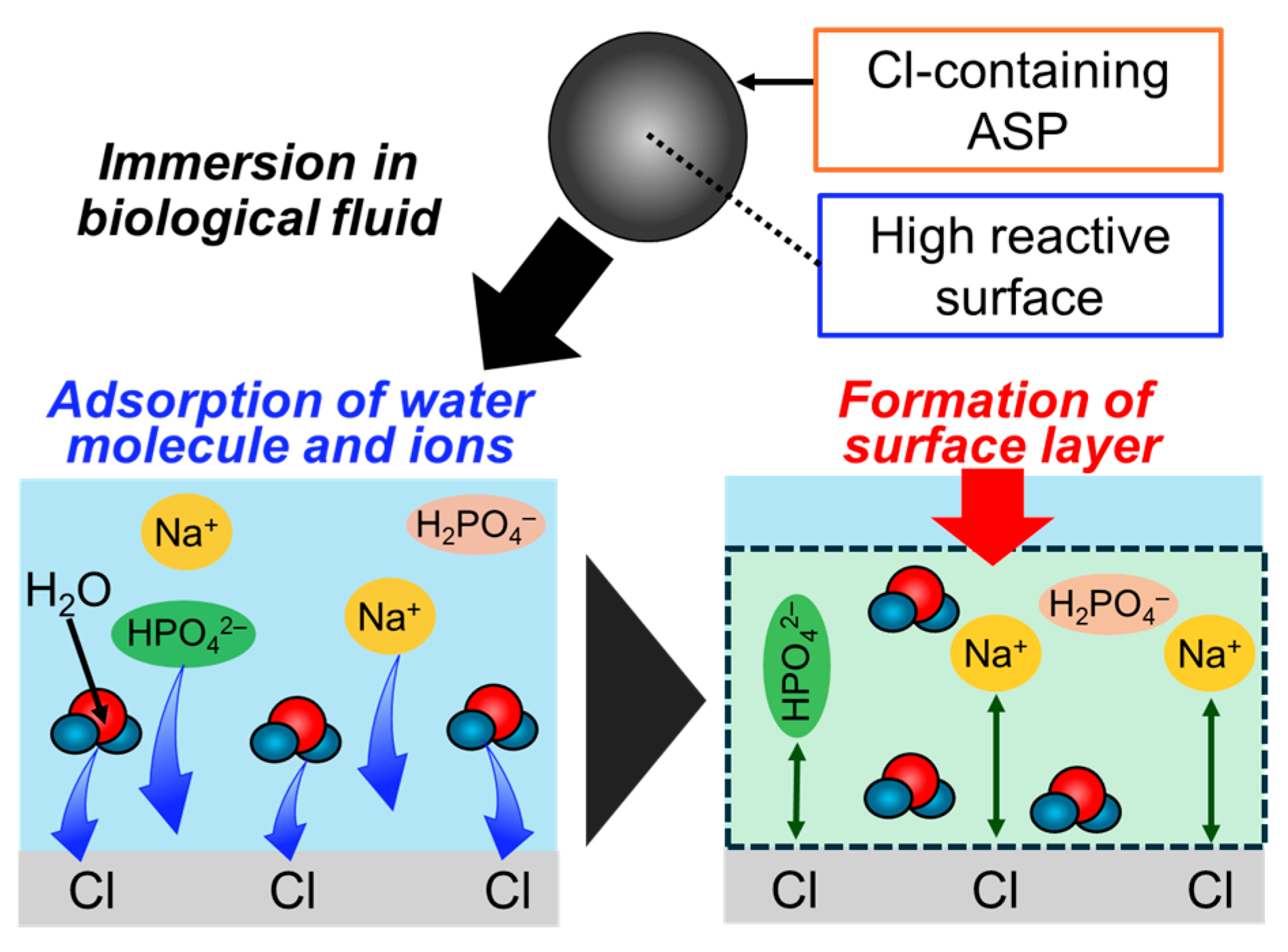
4. The Surface Layer Formed on the Bioceramic Particles
4.1. Formation of the Surface Layer in Biological Fluids
4.2. Formation of the Surface Layer in Biological Fluids
4.3. Formation of the Non-Apatitic Layer and Protein Immobilization Ability
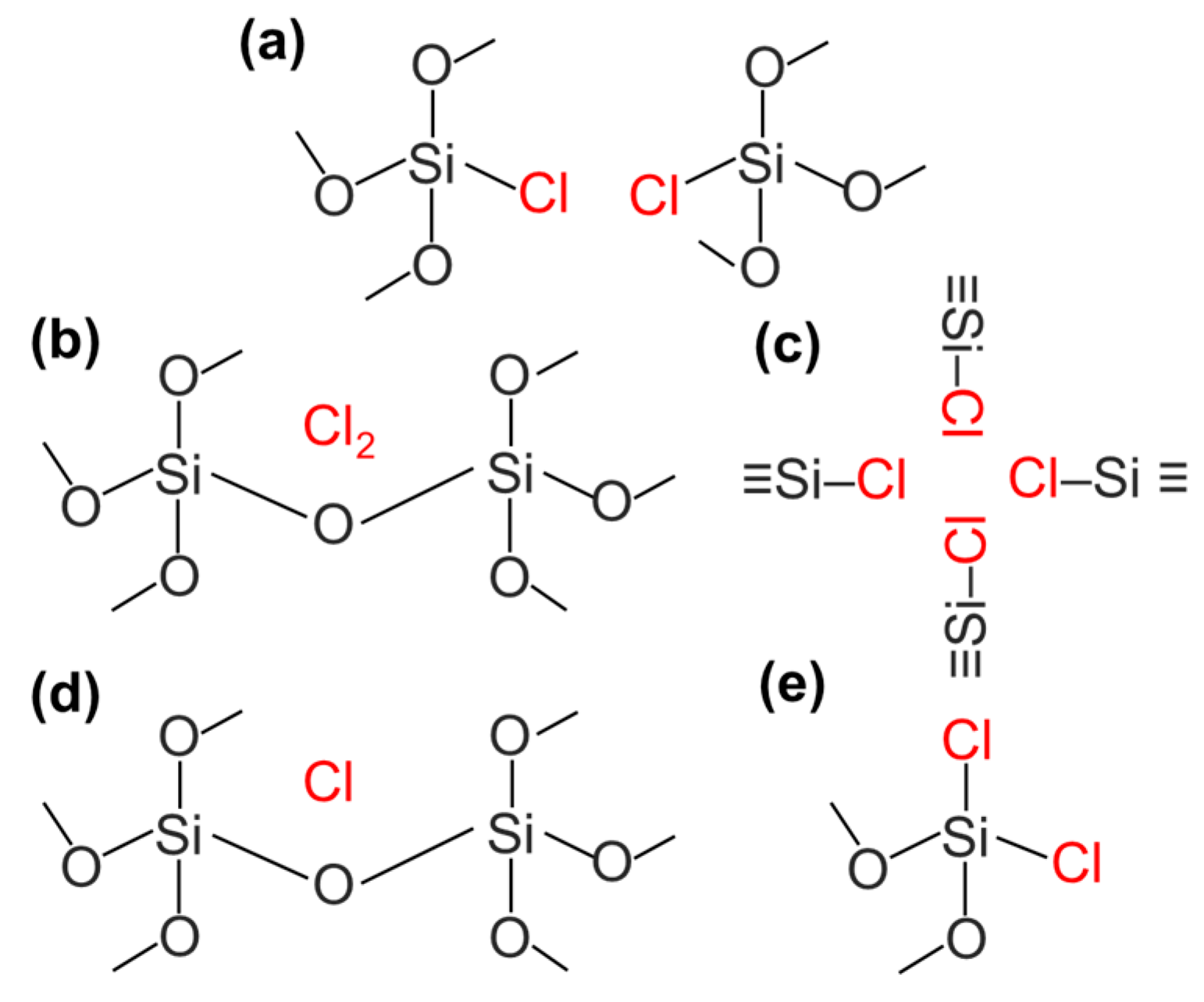
4.4. Amorphous Silica Particles for the New Layer Formation
SiCl4 + 3SiO2 ⇆ 4SiO1.5Cl
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.-Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone Structure and Formation: A New Perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Sasaki, S. Non-Collagenous Proteins in Bone. J. Bone Miner. Metab. 1981, 4, 323–326. [Google Scholar]
- Chai, Y.; Zhou, Y.; Tagaya, M. Rubbing-Assisted Approach for Fabricating Oriented Nanobiomaterials. Micromachines 2022, 13, 1358. [Google Scholar] [CrossRef] [PubMed]
- Kay, M.I.; Young, R.A.; Posner, A.S. Crystal Structure of Hydroxyapatite. Nature 1964, 204, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted Hydroxyapatites for Bone Regeneration: A Review of Current Trends. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T. Theory of Chromatography on Hydroxyapatite Columns with Small Loads. J. Chromatogr. A 1978, 157, 7–42. [Google Scholar] [CrossRef]
- Brown, W.E.; Eidelman, N.; Tomazic, B. Octacalcium Phosphate as a Precursor in Biomineral Formation. Adv. Dent. Res. 1987, 1, 306–313. [Google Scholar] [CrossRef]
- Shinto, Y.; Uchida, A.; Korkusuz, F.; Araki, N.; Ono, K. Calcium Hydroxyapatite Ceramic Used as a Delivery System for Antibiotics. J. Bone Joint Surg. Br. 1992, 74-B, 600–604. [Google Scholar] [CrossRef]
- Otsuka, M.; Matsuda, Y.; Yu, D.; Wong, J.; Fox, J.L.; Higuchi, W.I. A Novel Skeletal Drug Delivery System for Anti-Bacterial Drugs Using Self-Setting Hydroxyapatite Cement. Chem. Pharm. Bull. 1990, 38, 3500–3502. [Google Scholar] [CrossRef]
- Zapanta LeGeros, R. Apatites in Biological Systems. Prog. Cryst. Growth Charact. 1981, 4, 1–45. [Google Scholar] [CrossRef]
- Ishikawa, K.; Ducheyne, P.; Radin, S. Determination of the Ca/P Ratio in Calcium-Deficient Hydroxyapatite Using X-ray Diffraction Analysis. J. Mater. Sci. Mater. Med. 1993, 4, 165–168. [Google Scholar] [CrossRef]
- Dorozhkina, E.I.; Dorozhkin, S.V. Mechanism of the Solid-State Transformation of a Calcium-Deficient Hydroxyapatite (CDHA) into Biphasic Calcium Phosphate (BCP) at Elevated Temperatures. Chem. Mater. 2002, 14, 4267–4272. [Google Scholar] [CrossRef]
- Shen, Y.; Semon, J.A.; Brow, R.K. Conversion of Borate Glass Microspheres to Hollow Biphasic Calcium Phosphate Ceramic Microspheres for Bone Defects. J. Am. Ceram. Soc. 2022, 105, 7211–7227. [Google Scholar] [CrossRef]
- Camire’, C.L.; Jegou Saint-Jean, S.; Hansen, S.; McCarthy, I.; Lidgren, L. Hydration Characteristics of Alpha-Tricalcium Phosphates: Comparison of Preparation Routes. J. Appl. Biomater. Biomech. 2005, 3, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-Tricalcium Phosphate for Bone Substitution: Synthesis and Properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Carrodeguas, R.G.; De Aza, S. α-Tricalcium Phosphate: Synthesis, Properties and Biomedical Applications. Acta Biomater. 2011, 7, 3536–3546. [Google Scholar] [CrossRef] [PubMed]
- LIU, T.; CHEN, S.; LIU, D.; LIOU, S. On the Study of BSA-Loaded Calcium-Deficient Hydroxyapatite Nano-Carriers for Controlled Drug Delivery. J. Control. Release 2005, 107, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Al-Kattan, A.; Girod-Fullana, S.; Charvillat, C.; Ternet-Fontebasso, H.; Dufour, P.; Dexpert-Ghys, J.; Santran, V.; Bordère, J.; Pipy, B.; Bernad, J.; et al. Biomimetic Nanocrystalline Apatites: Emerging Perspectives in Cancer Diagnosis and Treatment. Int. J. Pharm. 2012, 423, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Hayashi, K. Carbonate Apatite Artificial Bone. Sci. Technol. Adv. Mater. 2021, 22, 683–694. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Ripamonti, A.; Roveri, N.; Romanello, M.; Noris Suarez, K.; Moro, L. Chemical and Structural Characterization of the Mineral Phase from Cortical and Trabecular Bone. J. Inorg. Biochem. 1997, 68, 45–51. [Google Scholar] [CrossRef]
- Driessens, F.C.M. The Mineral in Bone, Dentin and Tooth Enamel. Bull. Sociétés Chim. Belg. 2010, 89, 663–689. [Google Scholar] [CrossRef]
- Young, R.A.; Bartlett, M.L.; Spooner, S.; Mackie, P.E.; Bonel, G. Reversible High Temperature Exchange of Carbonate and Hydroxyl Ions in Tooth Enamel and Synthetic Hydroxyapatite. J. Biol. Phys. 1981, 9, 1–26. [Google Scholar] [CrossRef]
- Young, R.A. Biological Apatite vs Hydroxyapatite at the Atomic Level. Clin. Orthop. Relat. Res. 1975, 113, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Bel Hadj Yahia, F.; Jemal, M. Synthesis, Structural Analysis and Thermochemistry of B-Type Carbonate Apatites. Thermochim. Acta 2010, 505, 22–32. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Trautz, O.R.; Klein, E.; LeGeros, J.P. Two Types of Carbonate Substitution in the Apatite Structure. Experientia 1969, 25, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Combes, C.; Cazalbou, S.; Rey, C. Apatite Biominerals. Minerals 2016, 6, 34. [Google Scholar] [CrossRef]
- Kataoka, T.; Samitsu, S.; Okuda, M.; Kawagoe, D.; Tagaya, M. Highly Luminescent Hydroxyapatite Nanoparticles Hybridized with Citric Acid for Their Bifunctional Cell-Labeling and Cytostatic Suppression Properties. ACS Appl. Nano Mater. 2020, 3, 241–256. [Google Scholar] [CrossRef]
- Kataoka, T.; Hashimoto, T.; Shi, W.; Tagaya, M. Investigation of the Surface Elution Mechanism of Citric Acid-Coordinated Hydroxyapatite Nanoparticles in Biological Solutions. Ind. Eng. Chem. Res. 2022, 61, 10915–10921. [Google Scholar] [CrossRef]
- Heyde, M.; Shaikhutdinov, S.; Freund, H.J. Two-Dimensional Silica: Crystalline and Vitreous. Chem. Phys. Lett. 2012, 550, 1–7. [Google Scholar] [CrossRef]
- Weissenrieder, J.; Kaya, S.; Lu, J.L.; Gao, H.J.; Shaikhutdinov, S.; Freund, H.J.; Sierka, M.; Todorova, T.K.; Sauer, J. Atomic Structure of a Thin Silica Film on a Mo(112) Substrate: A Two-Dimensional Network of SiO4 Tetrahedra. Phys. Rev. Lett. 2005, 95, 076103. [Google Scholar] [CrossRef]
- Cho, W.S.; Choi, M.; Han, B.S.; Cho, M.; Oh, J.H.; Park, K.; Kim, S.J.; Kim, S.H.; Jeong, J. Inflammatory Mediators Induced by Intratracheal Instillation of Ultrafine Amorphous Silica Particles. Toxicol. Lett. 2007, 175, 24–33. [Google Scholar] [CrossRef]
- Boissière, C.; Kümmel, M.; Persin, M.; Larbot, A.; Prouzet, E. Spherical MSU-1 Mesoporous Silica Particles Tuned for HPLC. Adv. Funtional Mater. 2001, 11, 129–135. [Google Scholar] [CrossRef]
- Liu, C.C.; Maciel, G.E. The Fumed Silica Surface: A Study by NMR. J. Am. Chem. Soc. 1996, 118, 5103–5119. [Google Scholar] [CrossRef]
- Smith, D.M.; Scherer, G.W.; Anderson, J.M. Shrinkage during Drying of Silica Gel. J. Non-Cryst. Solids 1995, 188, 191–206. [Google Scholar] [CrossRef]
- Feuston1, B.P.; Garofalini, S.H. Oligomerization in Silica Sols. J. Phys. Chem. 1990, 94, 5351–5356. [Google Scholar] [CrossRef]
- Saito, K.; Ikushima, A.J. Reduction of Light-Scattering Loss in Silica Glass by the Structural Relaxation of “‘Frozen-in’” Density Fluctuations. Appl. Phys. Lett. 1997, 70, 3504–3506. [Google Scholar] [CrossRef]
- Merget, R.; Bauer, T.; Küpper, H.; Philippou, S.; Bauer, H.; Breitstadt, R.; Bruening, T. Health Hazards Due to the Inhalation of Amorphous Silica. Arch. Toxicol. 2002, 75, 625–634. [Google Scholar] [CrossRef]
- David, L. Griscom And Fission-Reactor Radiation Effects on the Visible-Range Transparency of Aluminum-Jacketed, All-Silica Optical Fibers. J. Appl. Phys. 1996, 80, 2142–2155. [Google Scholar]
- Kikuchi, Y.; Sudo, H.; Kuzuu, N. Thermal Expansion of Vitreous Silica: Correspondence between Dilatation Curve and Phase Transitions in Crystalline Silica. J. Appl. Phys. 1997, 82, 4121–4123. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhattacharyya, S.K.; Kumar, R.; Mishra, G.; Sharma, U.; Singh, G.; Ahalawat, S. Sol-Gel Processing of Silica Nanoparticles and Their Applications. Adv. Colloid. Interface Sci. 2014, 214, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Koilketsu, N.; Awazu, K.; Wape, H.; Ypmne, B. Llminescence Centers in SiO2 and SiO2: Geop Vad Rods Si-under Reducing or Oxidizing Conditions. J. Non Cryst. Solids 1987, 95, 679–684. [Google Scholar] [CrossRef]
- Kimura, R.; Chatani, S.; Inui, M.; Motozuka, S.; Liu, Z.; Tagaya, M. Control of Biological Surface States on Chlorine-Doped Amorphous Silica Particles and Their Effective Absorptive Ability for Antibody Protein. Langmuir 2024, 40, 8939–8949. [Google Scholar] [CrossRef]
- Kuzuu, N.; Horikoshi, H.; Nishimura, T.; Kokubo, Y. Effects of Heat Treatment on Absorption Bands in OH-Free and OH-Contalning Fused Quartz. J. Appl. Phys. 2003, 93, 9062–9071. [Google Scholar] [CrossRef]
- Brockner, R. Properties and structure of vitreous silica. I. J. Non-Cryst. Solids 1970, 5, 123–175. [Google Scholar] [CrossRef]
- Sacks, M.D.; Sheu, R.-S. Rheological properties of silica sol-gel materials. J. Non-Cryst. Solids 1987, 92, 383–396. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Correa-Duarte, M.A.; Liz-Marzán, L.M. Sol-Gel Processing of Silica-Coated Gold Nanoparticles. Langmuir 2001, 17, 6375–6379. [Google Scholar] [CrossRef]
- Ciriminna, R.; Sciortino, M.; Alonzo, G.; De Schrijver, A.; Pagliaro, M. From Molecules to Systems: Sol-Gel Microencapsulation in Silica-Based Materials. Chem. Rev. 2011, 111, 765–789. [Google Scholar] [CrossRef]
- Horender, S.; Lipowsky, J.; Sommerfeld, M.; Schwerin, M.; Badeke, K.U. Deposition of SiO2 Nanoparticles Produced in a Turbulent H2/O2 Flame. Aerosol Sci. Technol. 2008, 42, 873–883. [Google Scholar] [CrossRef][Green Version]
- Sekiya, E.H.; Torikai, D.; Gusken, E.; Ogata, D.Y.; Cuevas, R.F.; Suzuki, C.K. Crystalline and Amorphous Phases of GeO 2 in VAD Silica±germania Soot Preform. J. Non-Cryst. Solids 2000, 273, 228–232. [Google Scholar] [CrossRef]
- Butov, O.V.; Golant, K.M.; Tomashuk, A.L.; Van Stralen, M.J.N.; Breuls, A.H.E. Refractive Index Dispersion of Doped Silica for Fiber Optics. Opt. Commun. 2002, 213, 301–308. [Google Scholar] [CrossRef]
- Hammond, C.R.; Norman, S.R. Silica Based Binary Glass Systems-Refractive Index Behaviour and Composition in Optical Fibres. Opt. Quantum Electron. 1977, 9, 399–409. [Google Scholar] [CrossRef]
- Pereira, J.C.G.; Catlow, C.R.A.; Price, G.D. Silica Condensation Reaction: An Ab Initio Study. Chem. Commun. 1998, 13, 1387–1388. [Google Scholar] [CrossRef]
- Xiao, Y.; Lasaga, A.C. Quantum Mechanical Studies of the Kinetics and Mechanisms of Silicate Dissolution: H + ( H30’) Catalysis. Geochim. Cosmochlmica Acta 1994, 58, 5379–5400. [Google Scholar] [CrossRef]
- Feya, O.D.; Wang, Q.; Lepeshkin, S.V.; Baturin, V.S.; Uspenskii, Y.A.; Oganov, A.R. Tetrahedral Honeycomb Surface Reconstructions of Quartz, Cristobalite and Stishovite. Sci. Rep. 2018, 8, 11947. [Google Scholar] [CrossRef]
- Loy, D.A.; Carpenter, J.P.; Alam, T.M.; Shaltout, R.; Dorhout, P.K.; Greaves, J.; Small, J.H.; Shea, K.J. Cyclization Phenomena in the Sol-Gel Polymerization of α,ω- Bis(Triethoxysilyl)Alkanes and Incorporation of the Cyclic Structures into Network Silsesquioxane Polymers. J. Am. Chem. Soc. 1999, 121, 5413–5425. [Google Scholar] [CrossRef]
- Pelmenschikov, A.; Strandh, H.; Pettersson, L.G.M.; Leszczynski, J. Lattice Resistance to Hydrolysis of Si-O-Si Bonds of Silicate Minerals: Ab Initio Calculations of a Single Water Attack onto the (001) and (111) β-Cristobalite Surfaces. J. Phys. Chem. B 2000, 104, 5779–5783. [Google Scholar] [CrossRef]
- Hair, M.L. Hydroxyl groups on silica surface. J. Non-Cryst. Solids 1975, 19, 299–309. [Google Scholar] [CrossRef]
- Cypryk, M.; Apeloig, Y. Mechanism of the Acid-Catalyzed Si-O Bond Cleavage in Siloxanes and Siloxanols. A Theoretical Study. Organometallics 2002, 21, 2165–2175. [Google Scholar] [CrossRef]
- Le Page, Y.; Calvert, L.D.; Gabe, E.J. Parameter variation in low-quartz between 94 and 298KtS. Phys. Chem. Solids 1980, 41, 721–725. [Google Scholar] [CrossRef]
- Lisovskii, I.P.; Litovchenko, V.G.; Lozinskii, V.G.; Steblovskii, G.I. IR Spectroscopic Investigation of SiO2 Film Structure. Thin Solid. Films 1992, 213, 164–169. [Google Scholar] [CrossRef]
- Janes, N.; Oldfield, E. Prediction of Silicon-29 Nuclear Magnetic Resonance Chemical Shifts Using a Group Electronegativity Approach: Applications to Silicate and Aluminosilicate Structures. J. Am. Chem. Soc. 1985, 107, 6769–6775. [Google Scholar] [CrossRef]
- Sharma, S.K.; Mammone, J.F.; Nicol, M.F. Raman Investigation of Ring Configurations in Vitreous Silica. Nature 1981, 292, 140–141. [Google Scholar] [CrossRef]
- Walrafen, G.E.; Hokmabadi, M.S.; Holmes, N.C.; Nellis, W.J.; Henning, S. Raman Spectrum and Structure of Silica Aerogel. J. Chem. Phys. 1985, 82, 2472–2476. [Google Scholar] [CrossRef]
- Pasquarello, A.; Car, R. Identification of Raman Defect Lines as Signatures of Ring Structures in Vitreous Silica. Phys. Rev. Lett. 1998, 80, 5145–5147. [Google Scholar] [CrossRef]
- Wallace, S.; West, J.K.; Hench, L.L. Interactions of Water with Trisiloxane Rings. I. Experimental Analysis. J. Non-Cryst. Solids 1993, 152, 101–108. [Google Scholar] [CrossRef]
- Vigil, G.; Xu, Z.; Steinberg, S.; Israelachvili, J. Interactions of Silica Surfaces. J. Colloid. Interface Sci. 1994, 165, 367–385. [Google Scholar] [CrossRef]
- Morrow, B.A.; Mcfarlan, A.J. Surface Vibrational Modes of Silanol Groups on Silica. J. Phys. Chem. 1992, 96, 1395–1400. [Google Scholar] [CrossRef]
- Warring, S.L.; Beattie, D.A.; McQuillan, A.J. Surficial Siloxane-to-Silanol Interconversion during Room-Temperature Hydration/Dehydration of Amorphous Silica Films Observed by ATR-IR and TIR-Raman Spectroscopy. Langmuir 2016, 32, 1568–1576. [Google Scholar] [CrossRef]
- Zhuravlev, L.T. The Surface Chemistry of Amorphous Silica. Zhuravlev Model. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 1–38. [Google Scholar] [CrossRef]
- Dalstein, L.; Potapova, E.; Tyrode, E. The Elusive Silica/Water Interface: Isolated Silanols under Water as Revealed by Vibrational Sum Frequency Spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 10343–10349. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, I.; Mamic, J.; Arlt, W. Adsorption of Drugs on Silica Aerogels. Langmuir 2003, 19, 8521–8525. [Google Scholar] [CrossRef]
- Kosmulski, M. Adsorption of Trivalent Cations on Silica. J. Colloid. Interface Sci. 1997, 195, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Krysztafkiewicz, A.; Binkowski, S.; Jesionowski, T. Adsorption of Dyes on a Silica Surface. Appl. Surf. Sci. 2002, 199, 31–39. [Google Scholar] [CrossRef]
- Hair, M.L.; Hertl, W. Adsorption on Hydroxylated Silica Surfaces. J. Phys. Chem. 1969, 73, 4269–4276. [Google Scholar]
- De Keizer, A.; Van Der Ent, E.M.; Koopal, L.K. Surface and Volume Charge Densities of Monodisperse Porous Silicas. Colloids Surf. A Physicochem. Eng. Asp. 1998, 142, 303–313. [Google Scholar] [CrossRef]
- Kobayashi, M.; Juillerat, F.; Galletto, P.; Bowen, P.; Borkovec, M. Aggregation and Charging of Colloidal Silica Particles: Effect of Particle Size. Langmuir 2005, 21, 5761–5769. [Google Scholar] [CrossRef] [PubMed]
- Antonio Alves Júnior, J.; Baptista Baldo, J. The Behavior of Zeta Potential of Silica Suspensions. New J. Glass Ceram. 2014, 04, 29–37. [Google Scholar] [CrossRef]
- Franks, G.V. Zeta Potentials and Yield Stresses of Silica Suspensions in Concentrated Monovalent Electrolytes: Isoelectric Point Shift and Additional Attraction. J. Colloid. Interface Sci. 2002, 249, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Arcos, D.; Vallet-Regí, M. Sol-Gel Silica-Based Biomaterials and Bone Tissue Regeneration. Acta Biomater. 2010, 6, 2874–2888. [Google Scholar] [CrossRef]
- Jones, J.R. New Trends in Bioactive Scaffolds: The Importance of Nanostructure. J. Eur. Ceram. Soc. 2009, 29, 1275–1281. [Google Scholar] [CrossRef]
- Zhang, K.; Yan, H.; Bell, D.C.; Stein, A.; Francis, L.F. Effects of Materials Parameters on Mineralization and Degradation of Sol-Gel Bioactive Glasses with 3D-Ordered Macroporous Structures. J. Biomed. Mater. Res. A 2003, 66, 860–869. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Wilson, J.; Pigott, G.H.; Schoen, F.J.; Hench, L.L. Toxicology and Biocompatibility of Bioglasses. J. Biomed. Mater. Res. 1981, 15, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Ogino, M.; Ohuchi, F.; Hench, L.L. Compositional Dependence of the Formation of Calcium Phosphate Films on Bioglass. J. Biomed. Mater. Res. 1980, 14, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Xynos, I.D.; Hukkanen, M.V.J.; Batten, J.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Bioglass ®45S5 Stimulates Osteoblast Turnover and Enhances Bone Formation In Vitro: Implications and Applications for Bone Tissue Engineering. Calcif. Tissue Int. 2000, 67, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Clark, A.E.; Hench, L.L. An Investigation of Bioactive Glass Powders by Sol-Gel Processing. J. Appl. Biomater. 1991, 2, 231–239. [Google Scholar] [CrossRef]
- Peters, R.; Kramer, E.; Oomen, A.G.; Herrera Rivera, Z.E.; Oegema, G.; Tromp, P.C.; Fokkink, R.; Rietveld, A.; Marvin, H.J.P.; Weigel, S.; et al. Presence of Nano-Sized Silica during In Vitro Digestion of Foods Containing Silica as a Food Additive. ACS Nano 2012, 6, 2441–2451. [Google Scholar] [CrossRef]
- Mojsiewicz-Pie, N.; Nkowska, K.; Łukasiak, J. Analytical Fractionation of Silicon Compounds in Foodstuffs. Food Control 2003, 14, 153–162. [Google Scholar] [CrossRef]
- Napierska, D.; Thomassen, L.C.J.; Lison, D.; Martens, J.A.; Hoet, P.H. The Nanosilica Hazard: Another Variable Entity. Part. Fibre Toxicol. 2010, 7, 39. [Google Scholar] [CrossRef]
- Winkler, H.C.; Suter, M.; Naegeli, H. Critical Review of the Safety Assessment of Nano-Structured Silica Additives in Food. J. Nanobiotechnol. 2016, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shafiq, M.; Sun, B.; Wu, J.; Wang, W.; EL-Newehy, M.; EL-Hamshary, H.; Morsi, Y.; Ali, O.; Khan, A.U.R.; et al. Composite Superelastic Aerogel Scaffolds Containing Flexible SiO2 Nanofibers Promote Bone Regeneration. Adv. Healthc. Mater. 2022, 11, 2200499. [Google Scholar] [CrossRef]
- Desimone, M.F.; Hélary, C.; Rietveld, I.B.; Bataille, I.; Mosser, G.; Giraud-Guille, M.M.; Livage, J.; Coradin, T. Silica-Collagen Bionanocomposites as Three-Dimensional Scaffolds for Fibroblast Immobilization. Acta Biomater. 2010, 6, 3998–4004. [Google Scholar] [CrossRef]
- Hwang, C.; Min, Y.J.; Seong, Y.J.; Kim, D.E.; Kim, H.E.; Jeong, S.H. Enhanced Biolubrication on Biomedical Devices Using Hyaluronic Acid-Silica Nanohybrid Hydrogels. Colloids Surf. B Biointerfaces 2019, 184, 110503. [Google Scholar] [CrossRef]
- Tsai, C.-P.; Chen, C.-Y.; Hung, Y.; Chang, F.-H.; Mou, C.-Y. Monoclonal Antibody-Functionalized Mesoporous Silica Nanoparticles (MSN) for Selective Targeting Breast Cancer Cells. J. Mater. Chem. 2009, 19, 5737–5743. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Xu, H.; Shan, H.; Lu, J.R. Dynamic Adsorption of Monoclonal Antibody Layers on Hydrophilic Silica Surface: A Combined Study by Spectroscopic Ellipsometry and AFM. J. Colloid. Interface Sci. 2008, 323, 18–25. [Google Scholar] [CrossRef]
- Kontermann, R.E.; Brinkmann, U. Bispecific Antibodies. Drug Discov. Today 2015, 20, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody Therapy of Cancer. Nat. Rev. Cancer 2012, 12, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Mylvaganam, S.E.; Paterson, Y.; Getzoff, E.D. Structural Basis for the Binding of an Anti-Cytochrome c Antibody to Its Antigen: Crystal Structures of FabE8-Cytochrome c Complex to 1.8 A Ê Resolution and FabE8 to 2.26 A Ê Resolution. J. Mol. Biol. 1998, 281, 301–322. [Google Scholar] [CrossRef]
- Javkhlantugs, N.; Bayar, H.; Ganzorig, C.; Ueda, K. Computational Study on the Interactions and Orientation of Monoclonal Human Immunoglobulin G on a Polystyrene Surface. Int. J. Nanomed. 2013, 8, 2487–2496. [Google Scholar] [CrossRef]
- Harris, L.J.; Skaletsky, E.; Mcpherson, A. Crystallographic Structure of an Intact IgG1 Monoclonal Antibody. J. Mol. Biol. 1998, 275, 861–872. [Google Scholar] [CrossRef]
- Sheriff, S.; Silverton, E.W.; Padlan, E.A.; Cohen, G.H.; Smith-Gillt, S.J.; Finzelt, B.C.; Davies, D.R. Three-Dimensional Structure of an Antibody-Antigen Complex (Immunoglobulins/Epitope/X-ray Crystallography/Complementarity/Lysozyme). Proc. Natl. Acad. Sci. USA 1987, 84, 8075–8079. [Google Scholar] [CrossRef]
- Sundberg, E.J.; Mariuzza, R.A. Molecular Recognition in Antibody-Antigen Complexes. Adv. Protein Chem. 2003, 61, 119–157. [Google Scholar]
- Davies, D.R.; Padlan, E.A.; Sheriff, S. Antibody-antigen complexes. Annu. Rev. Biochem. 1990, 59, 439–473. [Google Scholar] [CrossRef]
- Iyonaga, K.; Takeya, M.; Yamamoto, T.; Ando, M.; Takahashi, K. A Novel Monoclonal Antibody, RM-4, Specifically Recognizes Rat Macrophages and Dendritic Cells in Formalin-Fixed, Paraffin- Embedded Tissues. Histochem. J. 1997, 29, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Kraal, G.; Rep, M.; Janse, M. Macrophages in T and B Cell Compartments and Other Tissue Macrophages Recognized by Monoclonal Antibody MOMA-2. Scand. J. Immunol. 1987, 26, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, S.; Gordon, S. The Use and Limitation of Monoclonal Antibodies Against Mononuclear Phagocytes. Immunobiology 1982, 161, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Das, A.A.; Krishna, R.; Suresh Kumar, M.; Mathur, P.P. Structural Epitope Database (SEDB): A Web-Based Database for the Epitope, and Its Intermolecular Interaction along with the Tertiary Structure Information. J. Proteomics Bioinform. 2012, 5, 84–89. [Google Scholar] [CrossRef]
- Sanchez-Trincado, J.L.; Gomez-Perosanz, M.; Reche, P.A. Reche Fundamentals and Methods for T- and B-Cell Epitope Prediction. J. Immunol. Res. 2017, 2017, 2680160. [Google Scholar] [CrossRef]
- van de Bovenkamp, F.S.; Hafkenscheid, L.; Rispens, T.; Rombouts, Y. The Emerging Importance of IgG Fab Glycosylation in Immunity. J. Immunol. 2016, 196, 1435–1441. [Google Scholar] [CrossRef]
- Amit, A.G.; Mariuzza, R.A.; Phillips, S.E.V.; Poljak, R.J. Three-Dimensional Structure of an Antigen-Antibody Complex at 2.8 Å Resolution. Science 1986, 233, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Selby, C. Interference in Immunoassay. Ann. Clin. Biochem. 1999, 36, 704–721. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Michel, B.; Delamarche, E. Micromosaic Immunoassays. Anal. Chem. 2001, 73, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Umeki, K.; Yasuda, A.; Umekita, K.; Megumi, R.; Nomura, H.; Kawaguchi, T.; Matsuda, M.; Takajo, I.; Shimojima, M.; Okayama, A. Detection of Anti-SFTSV Nuclear Protein Antibody in the Acute Phase Sera of Patients Using Double-Antigen ELISA and Immunochromatography. J. Virol. Methods 2020, 285, 113942. [Google Scholar] [CrossRef] [PubMed]
- Niloofa, R.; Fernando, N.; De Silva, N.L.; Karunanayake, L.; Wickramasinghe, H.; Dikmadugoda, N.; Premawansa, G.; Wickramasinghe, R.; De Silva, H.J.; Premawansa, S.; et al. Diagnosis of Leptospirosis: Comparison between Microscopic Agglutination Test, IgM-ELISA and IgM Rapid Immunochromatography Test. PLoS ONE 2015, 10, e129236. [Google Scholar] [CrossRef] [PubMed]
- Khamrin, P.; Nguyen, T.A.; Phan, T.G.; Satou, K.; Masuoka, Y.; Okitsu, S.; Maneekarn, N.; Nishio, O.; Ushijima, H. Evaluation of Immunochromatography and Commercial Enzyme-Linked Immunosorbent Assay for Rapid Detection of Norovirus Antigen in Stool Samples. J. Virol. Methods 2008, 147, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.-H.; Park, J.-W.; Lee, T.G.; Lee, H.; Paek, S.-H. Biophysical Characterization of the Molecular Orientation of an Antibody-Immobilized Layer Using Secondary Ion Mass Spectrometry. Analyst 2011, 136, 1412–1419. [Google Scholar] [CrossRef]
- Welch, N.G.; Madiona, R.M.T.; Scoble, J.A.; Muir, B.W.; Pigram, P.J. ToF-SIMS and Principal Component Analysis Investigation of Denatured, Surface-Adsorbed Antibodies. Langmuir 2016, 32, 10824–10834. [Google Scholar] [CrossRef] [PubMed]
- Awsiuk, K.; Stetsyshyn, Y.; Raczkowska, J.; Lishchynskyi, O.; Dąbczyński, P.; Kostruba, A.; Ohar, H.; Shymborska, Y.; Nastyshyn, S.; Budkowski, A. Temperature-Controlled Orientation of Proteins on Temperature-Responsive Grafted Polymer Brushes: Poly(Butyl Methacrylate) vs. Poly(Butyl Acrylate): Morphology, Wetting, and Protein Adsorption. Biomacromolecules 2019, 20, 2185–2197. [Google Scholar] [CrossRef]
- Hirano, A.; Wada, M.; Kitamura, M.; Kasahara, S.; Kato, K. Interactions between Amino Acids and Zirconia Modified with Ethylenediaminetetra(Methylenephosphonic Acid): Mechanistic Insights into the Selective Binding of Antibodies. Langmuir 2021, 37, 1605–1612. [Google Scholar] [CrossRef]
- Jönsson, U.; Lundström, I.; Rönnberg, I. Immunoglobulin G and Secretory Fibronectin Adsorption to Silica The Influence of Conformational Changes on the Surface. J. Colloid. Interface Sci. 1987, 117, 127–138. [Google Scholar]
- Tosaka, R.; Yamamoto, H.; Ohdomari, I.; Watanabe, T. Adsorption Mechanism of Ribosomal Protein L2 onto a Silica Surface: A Molecular Dynamics Simulation Study. Langmuir 2010, 26, 9950–9955. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Elwing, H.; Karlsson, A.; Nimeri, G.; Dahlgren, C. Surface-Related Triggering of the Neutrophil Respiratory Burst. Characterization of the Response Induced by IgG Adsorbed to Hydrophilic and Hydrophobic Glass Surfaces. Clin. Exp. Immunol. 2003, 109, 204–210. [Google Scholar] [CrossRef]
- Frauenfelder, H.; Fenimore, P.W.; Mcmahon, B.H. Hydration, Slaving and Protein Function. Biophys. Chem. 2002, 98, 35–48. [Google Scholar] [CrossRef]
- Tsuruta, T. On the Role of Water Molecules in the Interface between Biological Systems and Polymers. J. Biomater. Sci. Polym. Ed. 2010, 21, 1831–1848. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Mochizuki, A. Effect of Water Structure on Blood Compatibility— Thermal Analysis of Water in Poly(Meth)Acrylate. J. Biomed. Mater. Res. A 2004, 68A, 684–695. [Google Scholar] [CrossRef]
- Tanaka, M.; Mochizuki, A.; Ishii, N.; Motomura, T.; Hatakeyama, T. Study of Blood Compatibility with Poly(2-Methoxyethyl Acrylate). Relationship between Water Structure and Platelet Compatibility in Poly(2-Methoxyethylacrylate-Co-2-Hydroxyethylmethacrylate). Biomacromolecules 2002, 3, 36–41. [Google Scholar] [CrossRef]
- Tanaka, M.; Motomura, T.; Ishii, N.; Shimura, K.; Onishi, M.; Mochizuki, A.; Hatakeyama, T. Cold Crystallization of Water in Hydrated Poly(2-Methoxyethyl Acrylate) (PMEA). Polym. Int. 2000, 49, 1709–1713. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, K.; Kitakami, E.; Kobayashi, S.; Hoshiba, T.; Fukushima, K. Design of Biocompatible and Biodegradable Polymers Based on Intermediate Water Concept. Polym. J. 2015, 47, 114–121. [Google Scholar] [CrossRef]
- Morita, S.; Tanaka, M.; Ozaki, Y. Time-Resolved In Situ ATR-IR Observations of the Process of Sorption of Water into a Poly(2-Methoxyethyl Acrylate) Film. Langmuir 2007, 23, 3750–3761. [Google Scholar] [CrossRef]
- Sugimoto, K.; Zhou, Y.; Galindo, T.G.P.; Kimura, R.; Tagaya, M. Investigation of Surface Layers on Biological and Synthetic Hydroxyapatites Based on Bone Mineralization Process. Biomimetics 2023, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Tagaya, M. In Situ QCM-D Study of Nano-Bio Interfaces with Enhanced Biocompatibility. Polym. J. 2015, 47, 599–608. [Google Scholar] [CrossRef]
- Sugimoto, K.; Mikami, K.; Kimura, R.; Tagaya, M. Synthesis of Tetraethoxysilane-Reacted Hydroxyapatite Nanoparticles and Their Stabilization in Phosphate-Buffered Saline. Langmuir 2023, 39, 9431–9438. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Chai, Y.; Tagaya, M. PEG Functionalization Effect of Silicate-Containing Hydroxyapatite Particles on Effective Collagen Fibrillation with Hydration Layer State Change. Phys. Chem. Chem. Phys. 2022, 24, 6788–6802. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Kawagoe, D.; Tagaya, M. Nanospacial Effect of Citric Acid-Coordinated Hydroxyapatite Nanoparticle Films on Protein Adsorption and Cell Adhesion States. J. Mater. Chem. B 2022, 10, 9599–9606. [Google Scholar] [CrossRef] [PubMed]
- Peñaflor Galindo, T.G.; Tagaya, M. Interfacial Effect of Hydration Structures of Hydroxyapatite Nanoparticle Films on Protein Adsorption and Cell Adhesion States. ACS Appl. Bio Mater. 2019, 2, 5559–5567. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.; Collins, B.; Goehl, T.; Dickson, I.R.; Glimcher, M.J. The Carbonate Environment in Bone Mineral: A Resolution-Enhanced Fourier Transform Infrared Spectroscopy Study. Calcif. Tissue Int. 1989, 45, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Combes, C.; Rey, C.; Mounic, S. Identification and Evaluation of HPO4 Ions in Biomimetic Poorly Crystalline Apatite and Bone Mineral. Key Eng. Mater. 2001, 192–195, 143–146. [Google Scholar]
- Jarlbring, M.; Sandström, D.E.; Antzutkin, O.N.; Forsling, W. Characterization of Active Phosphorus Surface Sites at Synthetic Carbonate-Free Fluorapatite Using Single-Pulse 1H, 31P, and 31P CP MAS NMR. Langmuir 2006, 22, 4787–4792. [Google Scholar] [CrossRef]
- Aue, W.P.; Roufosse, A.H.; Glimcher, M.J.; Griffin, R.G. Solid-State Phosphorus-31 Nuclear Magnetic Resonance Studies of Synthetic Solid Phases of Calcium Phosphate: Potential Models of Bone Mineral. Biochemistry 1984, 23, 6110–6114. [Google Scholar] [CrossRef]
- Roufosse, A.H.; Aue, W.P.; Roberts, J.E.; Glimcher, M.J.; Griffin, R.G. Investigation of the Mineral Phases of Bone by Solid-State Phosphorus-31 Magic Angle Sample Spinning Nuclear Magnetic Resonance. Biochemistry 1984, 23, 6115–6120. [Google Scholar] [CrossRef] [PubMed]
- Delgado-López, J.M.; Iafisco, M.; Rodríguez, I.; Tampieri, A.; Prat, M.; Gómez-Morales, J. Crystallization of Bioinspired Citrate-Functionalized Nanoapatite with Tailored Carbonate Content. Acta Biomater. 2012, 8, 3491–3499. [Google Scholar] [CrossRef]
- Bolis, V.; Busco, C.; Martra, G.; Bertinetti, L.; Sakhno, Y.; Ugliengo, P.; Chiatti, F.; Corno, M.; Roveri, N. Coordination Chemistry of Ca Sites at the Surface of Nanosized Hydroxyapatite: Interaction with H2O and CO. Philos. Trans. R. Soc. A 2012, 370, 1313–1336. [Google Scholar] [CrossRef]
- Lin, T.-J.; Heinz, H. Accurate Force Field Parameters and PH Resolved Surface Models for Hydroxyapatite to Understand Structure, Mechanics, Hydration, and Biological Interfaces. J. Phys. Chem. C 2016, 120, 4975–4992. [Google Scholar] [CrossRef]
- Mann, S. Molecular Recognition in Biomineralization. Nature 1988, 332, 119–124. [Google Scholar] [CrossRef]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic Systems for Hydroxyapatite Mineralization Inspired by Bone and Enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef]
- Boyan, B.D.; Schwartz, Z.; Swain, L.D.; Khare, A. Role of Lipids in Calcification of Cartilage. Anat. Rec. 1989, 224, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Loveridge, N. Bone: More than a Stick. J. Anim. Sci. 1999, 77, 190–196. [Google Scholar] [CrossRef]
- Gómez-Morales, J.; Iafisco, M.; Delgado-López, J.M.; Sarda, S.; Drouet, C. Progress on the Preparation of Nanocrystalline Apatites and Surface Characterization: Overview of Fundamental and Applied Aspects. Prog. Cryst. Growth Charact. Mater. 2013, 59, 1–46. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; González-Calbet, J.M. Calcium Phosphates as Substitution of Bone Tissues. Progress Solid. State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Porter, A.E.; Patel, N.; Skepper, J.N.; Best, S.M.; Bonfield, W. Comparison of in Vivo Dissolution Processes in Hydroxyapatite and Silicon-Substituted Hydroxyapatite Bioceramics. Biomaterials 2003, 24, 4609–4620. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.F.; Li, G.; Wang, J.F.; Zheng, Q.J.; Wang, Y. Development, Characterization, and Validation of Porous Carbonated Hydroxyapatite Bone Cement. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90 B, 886–893. [Google Scholar] [CrossRef]
- Qiu, Z.Y.; Noh, I.S.; Zhang, S.M. Silicate-Doped Hydroxyapatite and Its Promotive Effect on Bone Mineralization. Front. Mater. Sci. 2013, 7, 40–50. [Google Scholar] [CrossRef]
- Kadu, K.; Kowshik, M.; Roy Ramanan, S. Does the Nanoparticle Morphology Influence Interaction with Protein: A Case Study with Hydroxyapatite Nanoparticles. Mater. Today Commun. 2021, 26, 102172. [Google Scholar] [CrossRef]
- Noda, D.; Saito, N.; Yamada, I.; Tagaya, M. Investigation of Adsorptive Orientation State Change of Anionic Porphyrin with the Hydrolysis Reaction of α-Tricalcium Phosphate. CrystEngComm 2022, 24, 4008–4012. [Google Scholar] [CrossRef]
- Iafisco, M.; Sabatino, P.; Lesci, I.G.; Prat, M.; Rimondini, L.; Roveri, N. Conformational Modifications of Serum Albumins Adsorbed on Different Kinds of Biomimetic Hydroxyapatite Nanocrystals. Colloids Surf. B Biointerfaces 2010, 81, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, Q.; Wu, T.; Pan, H. Understanding Adsorption-Desorption Dynamics of BMP-2 on Hydroxyapatite (001) Surface. Biophys. J. 2007, 93, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Iafisco, M.; Varoni, E.; Di Foggia, M.; Pietronave, S.; Fini, M.; Roveri, N.; Rimondini, L.; Prat, M. Conjugation of Hydroxyapatite Nanocrystals with Human Immunoglobulin G for Nanomedical Applications. Colloids Surf. B Biointerfaces 2012, 90, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, C.; Pettersson, U.; Johansson, K.; Philpson, L. Relationship of MRNA from Productively Infected Cells to the Complementary Strands of Adenovirus Type 2 DNA. J. Virol. 1974, 13, 370–377. [Google Scholar] [CrossRef]
- Kawasaki, T.; Takahashi, S.; Ideda, K. Hydroxyapatite High-Performance Liquid Chromatography: Column Performance for Proteins. Eur. J. Biochem. 1985, 152, 361–371. [Google Scholar] [CrossRef]
- Sang Cho, J.; Um, S.H.; Su Yoo, D.; Chung, Y.C.; Hye Chung, S.; Lee, J.C.; Rhee, S.H. Enhanced Osteoconductivity of Sodium-Substituted Hydroxyapatite by System Instability. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1046–1062. [Google Scholar] [CrossRef]
- Kim, H.S.; Bugli, G.; Djéga-Mariadassou, G. Preparation and Characterization of Niobium Carbide and Carbonitride. J. Solid. State Chem. 1999, 142, 100–107. [Google Scholar] [CrossRef]
- Kim, H.M.; Miyaji, F.; Kokubo, T.; Nakamura, T. Effect of Heat Treatment on Apatite-Forming Ability of Ti Metal Induced by Alkali Treatment. J. Mater. Sci. Mater. Med. 1997, 8, 341–347. [Google Scholar] [CrossRef]
- Kokubo, T.; Miyaji, F.; Kim, H.-M.; Nakamura, T. Spontaneous Formation of Bonelike Apatite Layer on Chemically Treated Titanium Metals. J. Am. Ceram. Soc. 1996, 79, 1127–1129. [Google Scholar] [CrossRef]
- Kim, H.-M.; Miyaji, F.; Kokubo, T.; Nakamura, T. Preparation of Bioactive Ti and Its Alloys via Simple Chemical Surface Treatment. J. Biomed. Mater. Res. 1996, 32, 409–417. [Google Scholar] [CrossRef]
- Cho, J.S.; Yoo, D.S.; Chung, Y.C.; Rhee, S.H. Enhanced Bioactivity and Osteoconductivity of Hydroxyapatite through Chloride Substitution. J. Biomed. Mater. Res. A 2014, 102, 455–469. [Google Scholar] [CrossRef]
- Saito, K.; Ikushima, A.J. Structural Relaxation Enhanced by Cl Ions in Silica Glass. Appl. Phys. Lett. 1998, 73, 1209–1211. [Google Scholar] [CrossRef]
- Sanada, K.; Shiota, T.; Inada, K. Studies of the Chemical Kinetics on the Vapor Phase Axial Deposition Method. J. Non Cryst. Solids 1995, 188, 275–284. [Google Scholar] [CrossRef]
- Shinji, I.; Masashi, O. Method of Making Synthetic Silica Glass. U.S. Patent No. 6,116,055, 12 September 2000. [Google Scholar]
- Awazu, K.; Kawazoe, H.; Muta, K.; Ibuki, T.; Tabayashi, K.; Shobatake, K. Characterization of Silica Glasses Sintered under Cl2 Ambients. J. Appl. Phys. 1991, 69, 1849–1852. [Google Scholar] [CrossRef]
- Hepburn, R.W.; Tomozawa, M. Diffusion of Water in Silica Glasses Containing Different Amounts of Chlorine. J. Non-Cryst. Solids 2001, 281, 162–170. [Google Scholar] [CrossRef]
- Davis, K.M.; Tomozawa, M. An Infrared Spectroscopic Study of Water-Related Species in Silica Glasses. J. Non-Cryst. Solids 1996, 201, 177–198. [Google Scholar] [CrossRef]
- Awazu, K.; Harada, K.; Kawazoe, H.; Muta, K. Structural Imperfections in Silica Glasses with an Optical Absorption Peak at 3.8 EV. J. Appl. Phys. 1992, 72, 4696–4699. [Google Scholar] [CrossRef]
- Griscom, D.L.; Friebele, E.J. Fundamental Radiation-Induced Defect Centers in Synthetic Fused Silicas: Atomic Chlorine, Delocalized E’ Centers, and a Triplet State. Phys. Rev. B 1986, 34, 7524–7533. [Google Scholar] [CrossRef] [PubMed]
- Bogart, K.H.A.; Donnelly, V.M. Composition of Trench Sidewalls and Bottoms for SiO2-Masked Si(100) Etched in Cl2 Plasmas. J. Appl. Phys. 2000, 87, 8351–8360. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, R.; Noda, D.; Liu, Z.; Shi, W.; Akutsu, R.; Tagaya, M. Biological Surface Layer Formation on Bioceramic Particles for Protein Adsorption. Biomimetics 2024, 9, 347. https://doi.org/10.3390/biomimetics9060347
Kimura R, Noda D, Liu Z, Shi W, Akutsu R, Tagaya M. Biological Surface Layer Formation on Bioceramic Particles for Protein Adsorption. Biomimetics. 2024; 9(6):347. https://doi.org/10.3390/biomimetics9060347
Chicago/Turabian StyleKimura, Reo, Daichi Noda, Zizhen Liu, Wanyu Shi, Ryota Akutsu, and Motohiro Tagaya. 2024. "Biological Surface Layer Formation on Bioceramic Particles for Protein Adsorption" Biomimetics 9, no. 6: 347. https://doi.org/10.3390/biomimetics9060347
APA StyleKimura, R., Noda, D., Liu, Z., Shi, W., Akutsu, R., & Tagaya, M. (2024). Biological Surface Layer Formation on Bioceramic Particles for Protein Adsorption. Biomimetics, 9(6), 347. https://doi.org/10.3390/biomimetics9060347





