Abstract
Mycelium-based green composites (MBCs) represent an eco-friendly material innovation with vast potential across diverse applications. This paper provides a thorough review of the factors influencing the production and properties of MBCs, with a particular focus on interdisciplinary collaboration and long-term sustainability goals. It delves into critical aspects such as fungal species selection, substrate type selection, substrate preparation, optimal conditions, dehydrating methods, post-processing techniques, mold design, sterilization processes, cost comparison, key recommendations, and other necessary factors. Regarding fungal species selection, the paper highlights the significance of considering factors like mycelium species, decay type, hyphal network systems, growth rate, and bonding properties in ensuring the safety and suitability of MBCs fabrication. Substrate type selection is discussed, emphasizing the importance of chemical characteristics such as cellulose, hemicellulose, lignin content, pH, organic carbon, total nitrogen, and the C: N ratio in determining mycelium growth and MBC properties. Substrate preparation methods, optimal growth conditions, and post-processing techniques are thoroughly examined, along with their impacts on MBCs quality and performance. Moreover, the paper discusses the importance of designing molds and implementing effective sterilization processes to ensure clean environments for mycelium growth. It also evaluates the costs associated with MBCs production compared to traditional materials, highlighting potential cost savings and economic advantages. Additionally, the paper provides key recommendations and precautions for improving MBC properties, including addressing fungal strain degeneration, encouraging research collaboration, establishing biosecurity protocols, ensuring regulatory compliance, optimizing storage conditions, implementing waste management practices, conducting life cycle assessments, and suggesting parameters for desirable MBC properties. Overall, this review offers valuable insights into the complex interplay of factors influencing MBCs production and provides guidance for optimizing processes to achieve sustainable, high-quality composites for diverse applications.
1. Introduction
In recent years, the increasing environmental concerns associated with traditional synthetic materials and the need to transition to sustainable practices have made the exploration of innovative materials a primary focus in contemporary research [1]. Among these materials, MBCs stand out as technologically advanced and highly environmentally friendly, being produced through a 100% biotechnological process. This presents a promising avenue to revolutionize industries and contribute significantly to long-term environmental sustainability goals [2,3]. MBCs represent a type of green material offering a unique blend of biological suitability, versatility, and impressive physical and mechanical properties [3,4]. This material is typically derived from mycelium, the vegetative part of fungi, and serves as a natural binder for organic substrates, forming robust and versatile composite materials that exhibit remarkable characteristics with advantages in several MBC fields [5]. This paper aims to comprehensively review and delve into the primary factors influencing the production of MBCs and their resulting properties, focusing on their applicability towards achieving long-term sustainability targets.
Currently, the increasing demand and expanding applications of MBC materials drive researchers to explore innovative solutions beyond conventional options [3]. Understanding the intricacies of MBCs’ production is paramount to harnessing their full potential and ensuring their seamless integration into sustainable practices [6]. Moreover, studying factors related to additional production processes, improving properties, addressing limitations, and delving into basic microbiological knowledge related to mushroom mycelium growth is essential for unlocking the MBC material’s complete capabilities [7,8]. This paper will comprehensively delve into key aspects and provide a detailed overview of MBCs production. Through a thorough analysis of these factors, our goal is to offer valuable insights into optimizing MBCs production for both efficiency and sustainability in the future. Furthermore, a comprehensive analysis of resulting material properties will offer potential applications in diverse industries, ranging from construction, packaging, furniture, and agriculture to fashion design and household items [5,9,10].
Through a critical analysis of current research and development, this paper seeks to enrich the ongoing discourse on sustainable materials by integrating knowledge from biological science (microbiology), chemistry, physics, materials science, engineering, environmental science, architecture, business management, and multidisciplinary. The insights derived from this review will not only enhance our understanding of MBCs but also inform future research directions, paving the way for the widespread adoption of MBCs in mainstream industries. As we navigate the complexities of achieving long-term sustainability targets, MBCs stand as a beacon of innovation, offering a tangible and scalable solution to lead in a more environmentally conscious era of material production and advancements in the realm of eco-friendly composites [11].
2. Basic Details about Mycelium-Based Green Composites
Green composites, a particular type of biocomposites, are created from lignocellulosic materials and a biopolymer matrix. They have emerged as essential alternatives to nondegradable materials due to the growing demand for eco-friendly options [12]. These materials are versatile and find application across various industries such as agriculture, automotive, aviation, construction, containers, flame retardancy, household items, medical applications, and packaging, aligning with the principles of environmental sustainability [12,13]. Green composites incorporate various lignocellulosic materials like plant leaves, peels, stalks, husks, hulls, straws, and sawdust, blended with a biopolymer matrix that binds the substrate particles together during manufacturing, rendering them highly eco-friendly [14,15]. However, ongoing research on green composite materials is increasingly exploring novel biopolymer matrices to produce distinctive, high-quality materials with unique characteristics [12,16,17]. Fungal mycelium, a natural polymer, has emerged as a promising candidate for creating green composites, also known as MBCs, offering distinct features and characteristics [7,9,18,19,20].
MBCs are an eco-friendly, innovative class of biodegradable materials with significant potential across numerous applications [21,22]. This material is produced by cultivating the vegetative portion of fungal mycelia on various lignocellulosic substrates, often sourced from agricultural and forestry wastes. The mycelium acts as a natural binder, forming a network that binds substrate particles together through a fully biotechnological process. This results in a cohesive and robust structure (Figure 1) [3,19,23]. MBCs require low energy consumption during growth, and zero byproducts, which allows them to be produced at a low cost [19,24]. While MBCs offer versatility in applications such as packaging, fashion designs, and household items, along with agriculture materials due to their biodegradability, they may need more strength and durability compared to conventional materials [25]. Addressing and improving these issues is crucial to fully unlocking the potential of MBCs and enabling their use across various industries [3].
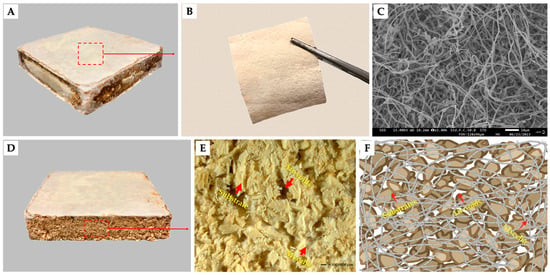
Figure 1.
Illustrates the primary structural components of MBCs, showcasing both their surface and cross-section ((A) depicts the MBCs sample; (B) shows the surface of MBCs removed from the samples; (C) offers scanning electron microscopic images of the MBCs surface; (D,E) display the cross-section area of MBCs; (F) presents a model of the cross-section area of MBCs).
Key components in MBCs production encompass factors are related to both the species of fungal mycelium (matrix phase) and the selection of substrate types (dispersed phase) [26]. Fungal mycelium, typically sourced from mushroom genera like Pleurotus spp., Ganoderma spp., Trametes spp., Pycnoporus spp., Lentinus spp., and Polyporus spp., functions as the primary structural component, providing cohesion and strength to the composite [9,23,27,28]. Simultaneously, the substrate, often composed of agro-industrial wastes such as sawdust, wood chips, husks, peels, straw, cotton, flax, hemp, spent mushrooms, sugarcane bagasse, or other lignocellulosic materials, significantly influences the MBCs properties [9,19,27,28,29,30]. Moreover, other factors, including substrate preparation, optimal growth conditions, post-processing techniques, mold design, sterilization processes, guidelines for improving properties, and other related considerations, play crucial roles in determining the quality of MBCs [3,9,19,20,23,31]. These factors collectively contribute to the versatility and sustainability of MBCs, making them promising alternatives in various industries.
A current preliminary search in [32] (https://www.scopus.com/search/form.uri?display=basic#basic; accessed on 2 March 2024) conducted using the keywords “mycelium & based & composites” over the past five years (2020–2024) revealed over 180 studies on MBCs, predominantly within the fields of engineering, materials science, and environmental science. Moreover, there are studies across more than ten related fields, indicating a growing interest in this material (Figure 2). In the future, the seamless cooperation and integration across these different fields like microbiology, chemistry, physics, materials science, engineering, environmental science, architecture, and business management, will be crucial for advancing MBCs production. For example, in microbiology, scientists investigate different fungal species to select and optimize the growing conditions for mycelium [2,33,34,35,36,37]. Chemistry helps understand the chemical composition and mechanisms in mycelium growth [19,30,38,39,40]. Physics examines MBCs’ mechanical properties, while materials science focuses on improving them [3,41,42,43]. Engineering plans and controls production processes for efficiency [44] and environmental science assesses their impact and sustainability [45]. Architecture explores and designs MBCs’ applications [46,47,48,49], and business management develops models and strategies [50]. Ultimately, multidisciplinary collaboration is paramount for driving innovation in MBCs development [49,51].
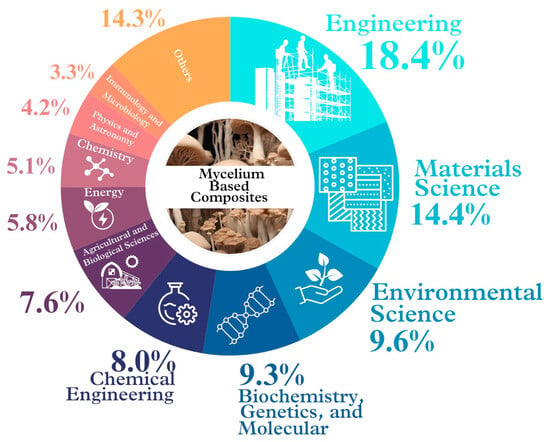
Figure 2.
Current studies on MBC materials conducted throughout the previous five years (2020–2024) [32] in a variety of fields around the world (modified from Sydor et al. [7], Li et al. [10]).
3. Fungal Species Selection
The selection of fungal species is a critical factor in the production of MBCs and significantly influences the properties and applications of the final product [11,24]. Currently, many research studies on MBCs have reported numerous fungal species with the potential to produce high-quality MBCs, each exhibiting varying characteristics. This section delves into the significance of considering fungal species and provides guidelines for their selection in MBCs production.
Firstly, the fungal mycelium species is crucial for considering and ensuring the safety of the final MBC products, while its decay type indicates its performance in producing different enzymes and degrading various lignocellulosic materials [23,28]. Some fungal species may produce toxins or allergens that could transfer to the products during growth [52]. Additionally, the decay type influences its ability to colonize different environmental contexts [53]. By carefully selecting the fungal species (edible and medical fungi should be considered first) and considering its decay type (such as Pleurotus spp., Ganoderma spp., Trametes spp., Pycnoporus spp., Lentinus spp., and Polyporus spp.), producers can mitigate potential safety risks (Table 1) [20,23]. This involves choosing nontoxic, nonallergenic fungal species and ensuring optimal decay types to promote healthy mycelial growth and minimize contamination risks. Wood-decay fungi are generally classified based on the decay type of mushroom, including brown rot, soft rot, and white rot, each with unique enzymatic activities and environmental preferences [10]. Brown-rot and soft-rot fungi primarily degrade cellulose and may induce minor changes to lignin, whereas white-rot fungi focus on breaking down lignin [28,54]. Overall, these considerations are crucial for ensuring the safety and suitability of the MBCs fabrication for various applications.

Table 1.
Fungal classification and mycelium growth characteristics have been reported in previous studies on the production of MBCs.
Secondly, concerning the hyphal network systems, which directly impact MBCs quality, particularly its mechanical properties (flexural, impact, and tensile strength), different fungal species exhibit unique structural traits in their mycelium [19,36]. Some species may yield denser and sturdier mycelial networks, while others present a more delicate structure [59]. These structural attributes directly influence the strength and overall performance of the MBCs. Previous studies have indicated that, in general, monomitic species tend to display lower strength values compared to dimitic and trimitic hyphal species [19,33,36]. This observation is linked to the three main types of hyphal network systems, where monomitic species predominantly feature generative hyphae, dimitic species form both generative as well as skeletal or ligative hyphae, and trimitic species consist of all three types of hyphae (Figure 3) [19]. The binding hyphae, characterized by their thickness, density, and hardness, contribute significantly to the stiffness of the MBCs [24,60]. These findings are consistent with Appels et al. [33], who reported that MBCs produced from T. multicolor (trimitic hyphal system) exhibited higher flexural and tensile strength (0.22 and 0.04 MPa, respectively) than those from P. ostreatus, which has a monomitic hyphal system (0.06 and 0.01 MPa, respectively), when grown on rapeseed straw. Furthermore, these findings are supported by Aiduang et al. [22,36], who highlighted that MBCs produced from trimitic hyphal species like G. fornicatum, G. williamsianum, and L. sajor-caju displayed superior mechanical properties compared to S. commune (monomitic hyphal system), particularly in terms of compressive strength, flexural strength, impact strength, and tensile strength across various substrates used in production. Additionally, hydrophobic hyphae with thick walls and high density can improve the physical properties by reducing water absorption when covering material surfaces [36,60].
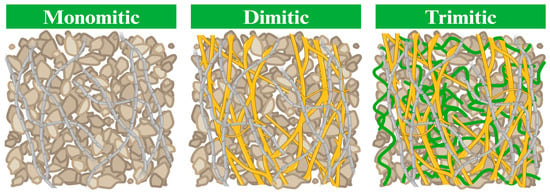
Figure 3.
The different types of hyphal network systems (modified from Porter and Naleway [58]). Gray lines represent generative hyphae, yellow lines represent skeletal hyphae, green lines represent ligative hyphae, and brown particles represent the substrate. Created by BioRender.com (https://www.biorender.com/; access date: 19 March 2024 [61]).
Thirdly, the growth rate of fungal species is a crucial factor influencing the production timeline of MBCs [23,40]. Faster-growing species may expedite the manufacturing process, ensuring efficient colonization and high density on composite surfaces [20]. However, the optimal growth must align with the intended production schedule and efficiency goals [51]. Fourthly, bonding properties vary among fungal species, with some producing stronger natural binders that enhance substrate particle binding, contributing to improved material integrity in the MBCs [62]. Moreover, differences in the number of secretomes, acting like a form of adhesive, including relative concentrations of polysaccharides, lipids, proteins, and chitin among fungal species, result in varied morphologies and mechanical properties of the materials. The generation of elevated quantities of these secretomes could enhance the mechanical properties and other characteristics of MBCs [24,41,63]. Importantly, compatibility with substrates is a critical consideration, as different fungal species thrive on specific substrates. The compatibility between the selected fungal species and the chosen substrate is crucial for successful cultivation and mycelium colonization, ultimately influencing the overall quality and performance of the MBCs [20]. Therefore, the careful selection of fungal species is paramount for optimizing the production process and tailoring the properties of MBCs to meet specific functional and sustainability requirements. It involves thoughtful consideration of product safety, hyphal network systems, growth characteristics, and bonding properties, ensuring that the chosen fungal species aligns with the desired outcomes for the final MBC products.
4. Substrate Type Selection
Substrate type selection is a critical aspect that influences the mycelial growth and quality of MBC products [24]. Several essential chemical factors must be considered, including cellulose, hemicellulose, lignin content, pH, organic carbon, total nitrogen, and the C: N ratio. These factors play crucial roles in determining the growth and performance of the mycelium, as well as the properties of the MBCs [9,20,36,39,47]. Table 2 summarizes the chemical composition of substrates used in previous research on MBCs production, serving as a valuable reference for substrate selection and production process guidelines.
Cellulose, a primary component of plant cell walls, acts as a major carbon source for mycelium growth. Substrates with higher cellulose content can provide ample nutrients, fostering greater growth and potentially yielding stronger composites. Similarly, hemicellulose, another plant cell wall component, can serve as a carbon source, supporting mycelium growth and contributing to mechanical properties [64,65]. Conversely, lignin, a complex organic polymer providing structural support to plant cell walls, can pose challenges for mycelium digestion due to its complexity. Substrates with lower lignin content are generally more conducive to mycelium growth [66]. Moreover, the pH of the substrate significantly affects mycelium growth, with most species thriving in slightly acidic conditions (pH 5–8). Adjusting the substrate pH to match the optimal range for the specific mycelium species is crucial [24].

Table 2.
The chemical composition of the substrates utilized in previous studies for producing MBCs.
Table 2.
The chemical composition of the substrates utilized in previous studies for producing MBCs.
| Substrate Types | Cellulose (%) | Hemicellulose (%) | Lignin (%) | pH | Organic Carbon (%) | Total Nitrogen (%) | C: N Ratio |
|---|---|---|---|---|---|---|---|
| Bamboo | 45.7–46.5 | 18.8–26.6 | 21.1–25.7 | 5.4–6.76 | 34.4 | 1.03 | 33.4:1 |
| Cardboard pulp | 56.4–60 | 13.8–15 | 10–12 | 7.1 | 29.5 | 1.18 | 25:1 |
| Coconut coir | 36–44.2 | 0.3–22.1 | 32.8–45 | 5.7 | 29.38 | 0.44 | 66:1 |
| Coconut shell powder | 24.2 | 38.56 | 29.35 | 5.2–5.7 | 52.6 | 2 | 26.3:1 |
| Spent coffee grounds | 9–33.1 | 30–39 | 24–29 | 5.4 | 48 | 2.29 | 21:1 |
| Corn cobs | 33.7–45 | 31.9–39 | 6.1–15.9 | 7.8–9 | 47.54 | 1.32 | 36:1 |
| Corn husk | 45–55 | 27–39 | 7.5–20 | 5.4–9 | 45.98 | 0.97 | 47:1 |
| Corn stalk | 33.8–35 | 21.1–25 | 19.9–35 | 5.9–8.2 | 47.1 | 0.81 | 58.1:1 |
| Corn stover | 35–44 | 24–30 | 7–21.8 | 5.5–5.7 | 41.4 | 0.8 | 51.8:1 |
| Cotton stalk | 35–67 | 16–25 | 13–35 | 4.74 | 41.5 | 1.81 | 22.9:1 |
| Cotton waste | 83–96 | 1–2 | 0.3–1 | 5.74 | 48.8 | 1.16 | 42.1:1 |
| Flax | 63–88 | 5–12 | 3–5 | 6.8–6.9 | 49.3 | 0.59 | 84:1 |
| Fruit peels | 11.2–43.6 | 5.4–33.3 | 2.2–64 | 3.5–5.9 | 36–43.2 | 0.42–2.23 | 19–84.7:1 |
| Hardwood | 20–55 | 25–50 | 20–25 | 5.1–8.8 | 46.3–50 | 0.2–0.83 | 60–231:1 |
| Hemp fibers | 53.2–85 | 5.7–16 | 3.3–13 | 5 | 45.6 | 0.3 | 152:1 |
| Hemp hurds | 43.3–85.1 | 37.2–39.5 | 20.7–23.1 | 5 | 46.4 | 0.27 | 171.9:1 |
| Jute fibers | 61–73 | 13.6–23 | 12–26 | 7 | 41.1 | 0.97 | 42.4:1 |
| Lavender straw | 18–38 | 28 | 25 | – | 48.1 | 1.3 | 37:1 |
| Miscanthus biomass | 38–40 | 18–24 | 24–25 | – | 51.52 | 0.18 | 286.2:1 |
| Nutshell | 18.6–37 | 18.7–49.3 | 21–49.8 | 5.4–7.1 | 38.5–48.6 | 0.42–0.72 | 61–116:1 |
| Oat husk | 34.9–38.7 | 35.3–37 | 5.6–10.1 | – | 34.6 | 0.71 | 48.7:1 |
| Peach palm sheath | 34.2 | 21.3 | 19.5 | 5.8–6 | 40.87 | 1.14 | 35.8:1 |
| Rapeseed cake | 43.8–46.5 | 15.6–17.5 | 8.7–15.8 | 5.9 | 46.1 | 0.71 | 65:1 |
| Rapeseed straw | 37.5–53 | 18.1–31.4 | 9.6–21.3 | – | 44.4 | 1.29 | 34.4:1 |
| Rice husks | 25–40 | 16–31.6 | 26–31 | 6.8 | 39.8 | 0.55 | 79.6:1 |
| Rice straw | 28–38.1 | 22–31.1 | 12–26.4 | 5.9–7.2 | 35.7 | 0.7 | 51:1 |
| Rose flowers | 29.13 | 14.57 | 18.57 | 5.2 | 13.9 | 0.82 | 16.8:1 |
| Sawdust | 15–44 | 35–60 | 15–30 | 3.5–8.8 | 27.1–57.6 | 0.1–0.5 | 115–271:1 |
| Softwood | 27–50 | 20–40 | 25–31 | 5.1–8.8 | 41.3–55.1 | 0.2–1.3 | 42–206:1 |
| Spent mushroom substrates | 36.19 | 22.24 | 11.99 | 7.5 | 32.61–49.82 | 0.85–1.72 | 29–38.4:1 |
| Sugarcane bagasse | 37–48 | 19–25 | 19–42 | 8.75 | 45 | 0.3 | 150:1 |
| Textile waste | 97–98 | 1–2 | <1 | – | 15.9–24.3 | 0.15–0.24 | 101–106:1 |
| Vegetable peels | 17–63 | 11.4–32 | 3–36 | 3.9–6.1 | 34.5–41 | 0.65–1.9 | 21.6–53:1 |
| Wheat bran | 11.65–13.15 | 49.7 | 5.3 | 6.3–6.8 | 36.92 | 1.98 | 18.6:1 |
| Wheat straw | 30–38 | 25.2–32 | 13–32 | 7.5–17 | 41.7 | 0.4 | 104:1 |
Note = “–”is not reported; “<”is less than [39,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123].
Organic carbon content serves as an essential energy and building block source for mycelium growth. Higher organic carbon levels support robust mycelium growth [19,124]. Similarly, total nitrogen content is vital for numerous metabolic activities, especially the synthesis of proteins and enzymes, as well as for cellular processes, with higher nitrogen content promoting better mycelium growth [125]. Maintaining a balanced carbon-to-nitrogen (C: N) ratio (around 19:1 to 80:1) is crucial for optimal mycelium growth [126]. Substrates with higher C: N ratios may require additional nitrogen supplementation. Overall, substrate selection profoundly influences MBCs production, impacting growth, quality, and final product properties. By considering these essential factors, producers can optimize substrate formulations for robust mycelium growth and high-quality composites.
5. Substrate Preparation
Substrate preparation stands as a pivotal phase in the production of MBCs, employing mycelium, the vegetative part of fungi, to bind organic substrates into a strong and eco-friendly material. This substrate serves both as the growth media for mycelium and the source of essential nutrients pivotal for its development [20]. The combination of optimal pH level, moisture content, and nutritional components, coupled with an effective sterilization process, emerges as crucial factors influencing the successful growth of mycelium and the ultimate quality of the MBCs [5,23,24,41].
Primarily, mycelium growth is directly linked to the pH level of the substrate. Typically, a slightly acidic to neutral pH range is deemed favorable for most fungi, including mycelium. Within this range, an environment is fostered where mycelium thrives, efficiently decomposing organic materials [24]. Additionally, sufficient moisture is essential for mycelium growth, facilitating nutrient absorption, and the expansion of the mycelial network. Thus, the moisture content of the substrate should be carefully controlled. It is typically maintained between 50% and 70%, but the specific requirements may vary depending on the fungal species and the type of substrate being used [127].
Mycelium growth also necessitates a diverse array of nutrients, with commonly used substrates such as agricultural residues (e.g., sawdust, rice straw, wheat straw, sugarcane bagasse, corn cobs, wood chips) providing a mix of essential carbon and nitrogen sources [128]. However, some substrates may require supplementation with additional nutrients like nitrogen, phosphorus, and trace elements to enhance mycelial growth and overall biomass production [129]. Nutrient supplements can be derived from sources such as rice bran, wheat bran, corn gluten meal, soybean meal, potato dextrose, cassava peel, olive byproducts, food waste compost, tea waste, or other lignocellulosic materials [130,131].
Crucially, substrate preparation involves sterilization to eliminate competing microorganisms and prevent contamination. Various techniques, including treatment with chemical or microbial agents and subjecting the substrate to high temperatures, are frequently employed in this step [23,132]. Notably, several studies have reported that sterilization at high temperatures (around 121 °C or 250 °F) and pressure (15 PSI or 1.05 kg/cm2) for a specific duration (typically, 15 to 60 min) enhances the efficiency of the sterilization process, ensuring a clean environment for mycelium colonization [23,133,134]. However, the time required can vary depending on the type and amount of substrate used in production, each with a different density. Substrates with lower densities may take less time to sterilize because heat is more easily accessible [133,135]. Moreover, the performance of sterilizer machines can vary based on region, brand, or model, leading to adjustments in determining variables related to pressure, temperature, and time levels to achieve maximum disinfection efficiency. As a result, alternative sterilization techniques that consider the relationship between these factors have been proposed, as illustrated in Figure 4.
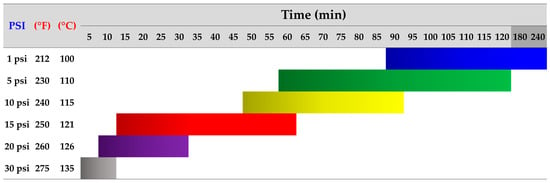
Figure 4.
Recommendations for utilizing high-heat sterilization methods to prepare substrates for mycelium cultivation, adapted from Mycobio [133] and Salish Sea Mushrooms [135], supplemented by additional references, including Sydor et al. [23], Bellettini et al. [129], and Freak of Natural [136]. Sterilization efficiency is indicated by color: blue for 1 PSI at 100 °C for 90–240 min, green for 5 PSI at 110 °C for 60–120 min, yellow for 10 PSI at 115 °C for 50–90 min, red for 15 PSI at 121 °C for 15–60 min, purple for 20 PSI at 126 °C for 10–30 min, and gray for 30 PSI at 135 °C for 5–10 min.
Thus, attaining the optimal pH level, moisture content, and nutritional composition in the substrate is paramount for successful MBCs production. Simultaneously, an effective sterilization process mitigates contamination, fostering optimal mushroom mycelium growth. Careful control of these factors promotes efficient mycelial growth, enhances substrate colonization, and contributes to the development of durable and high-quality MBC products.
6. Factors Controlling Production for MBCs
Creating optimal conditions is pivotal for the successful production of MBCs [137]. The recommended conditions encompass various factors, including temperature, humidity, aeration, gas exchange, incubation time, and light exposure.
- Temperature: Maintain an ideal temperature range for mycelium growth, typically between 20 °C to 30 °C (68 °F to 86 °F). Specific strains may have slight variations in their preferred temperature range [138].
- Relative humidity: Maintaining appropriate humidity levels is essential in MBCs production. Different fungal groups have varied optimal humidity requirements. White rot fungi, commonly used in MBCs fabrication, grow best in humidity levels ranging from 70% to 80%. In contrast, brown rot fungi prefer humidity levels exceeding 95%. Soft rot fungi and other species show the highest growth rates when relative humidity ranges from 60% to 75%. Ensuring adequate moisture content prevents drying and promotes healthy mycelial development [28].
- Aeration and gas exchange: Provide sufficient aeration throughout the substrate for healthy mycelium growth. Oxygen is crucial for mycelial metabolism, making proper gas exchange vital. Incorporate ventilation features to facilitate air exchange, preventing anaerobic conditions and ensuring uniform colonization [139].
- Incubation time: The duration of the incubation period varies depending on factors such as the strain of mycelium, substrate composition, and production goals. Typically ranging from several days to a few weeks or even longer (5–42 days), the duration is determined by the desired level of colonization, the purposeful application, and the volume of the inoculated substrate [24].
- Light exposure: During the colonization phase, mycelium thrives in darkness. Limit exposure to light during this stage [11].
These optimal conditions serve as general guidelines, with potential adjustments needed based on specific mycelium strain requirements and substrate characteristics. Regular monitoring and adjustments to environmental parameters contribute to a successful and efficient MBCs production process.
7. Dehydrating Methods and Post-Processing Techniques
7.1. Dehydrating
Ensuring proper drying is crucial for attaining the desired strength, durability, and dimensional stability of MBCs. Among the most prevalent methods for the dehydration and densification of MBCs post-incubation are hot-pressing and oven drying, often resulting in notable enhancements of their properties [23,60,140].
In the oven drying method, MBCs undergo accelerated drying by being placed in an oven with controlled temperature and airflow. The advantages of this method include faster drying and improved control over drying conditions, with temperatures generally ranging between 40 and 125 °C (104–257 °F) for 2 to 72 h [24,141]. Various authors have explored diverse drying techniques, such as oven baking at temperatures of 40 °C for 72 h, 50–60 °C (122–140 °F) for 2 to 48 h, 70 °C (158 °F) for 5 to 10 h, 80–82 °C (176–179.6 °F) for 12 to 24 h, 100 °C (212 °F) for 2 to 4 h, and at a high temperature of 125 °C for 2 h [24,33,41,51,141,142,143,144,145,146]. However, the selection of the appropriate temperature within each range should be considered based on the size of the workpiece [141]. Additionally, air drying involves exposing MBCs to ambient air to gradually reduce moisture content. This method is typically conducted at room temperature, ranging from 20–25 °C (68–77 °F). Its advantages lie in its simplicity, energy efficiency, and suitability for smaller-scale productions [23,147]. Choosing the optimal drying method and temperature range hinges on factors such as production scale, mycelium strain, composite composition, and intended application [141]. Striking a delicate balance between effective moisture removal and preserving the structural integrity of the MBCs is paramount to the success of the overall drying process [26,51].
7.2. Pressing
Post-processing techniques play a crucial role in MBCs production, influencing material properties, density, and overall performance [5,19]. Preliminary pressing ensures an even distribution of force for consistent MBC properties [48]. The choice of post-processing technique depends on intended applications, such as construction, furniture, packaging, or architectural components [5,148]. Typically, cold pressing, heat pressing, and nonpressing are common methods, each contributing distinct characteristics [9,33].
- Cold pressing: The first option involves applying pressure to MBC samples at or near room temperature [51]. This method is often used to shape and consolidate the material into specific forms without the application of heat. The effect on material properties maintains a softer texture and allows the mycelium to continue growing, resulting in greater flexibility compared to heat pressing. Furthermore, it preserves some of the inherent characteristics of the mycelium, resulting in the obtained MBCs with a more natural feel. This is suitable for applications where a softer and more porous structure is desired, such as producing acoustic panels or packaging materials [149].
- Heat pressing: The second option involves applying pressure to the MBCs at elevated temperatures. This method aims to increase the material’s density, strength, and durability. The effect on material properties involves enhancing material density and structural integrity, resulting in a more rigid and durable product. Importantly, this can contribute to a smoother surface finish, making it suitable for applications where a polished appearance is desirable, along with dimensional stability. This is particularly applicable to materials used in semi-construction (building) and furniture that require strength-bearing properties [24,33,150]. However, the heat may induce some level of color change in the MBCs, potentially transitioning to a gradient from grey to brown [140]. Previous studies have reported various heat pressing conditions for MBCs production, such as temperatures of 250 °C for 20 min [142], pressures less than 30 kN at 150 °C for 20 min [33], pressures of 3.5–4.0 MPa at 160 °C for 6 min [151], and pressures of 20 MPa at 120 °C for 20 min [152]. These conditions offer different advantages for the final MBCs.
- Nonpressing: the third option refers to processes without external pressure, achieving shaping and consolidation through methods like hand pressure, mold casting, or 3D printing. This preserves the delicate structure of the mycelium more effectively than pressing methods, allowing intricate and detailed designs [4,153]. It may result in a lighter and more porous material compared to pressing methods, suitable for applications not requiring strength, like certain types of packaging materials, insulation materials, or materials used in agricultural applications [33,149].
However, choosing the appropriate post-processing technique for MBCs production depends on desired material properties and intended applications. Whether using cold pressing, heat pressing, or nonpressing methods, careful consideration of factors such as texture, rigidity, overall aesthetics, and targets is essential for producing high-quality and functional MBC products.
8. Designing Molds and Sterilization Processes
Designing molds and implementing effective sterilization techniques are crucial aspects of MBCs production [153]. The mold design plays a pivotal role in determining the final shape and dimensions of the MBCs, while sterilization ensures a clean and uncontaminated environment for mycelium growth. An overview of these two aspects directly influences the characteristics and properties of the obtained MBCs [24].
In general, selecting materials compatible with mycelium growth, such as acrylics, polypropylene, silicone, or other materials that withstand the sterilization process and provide a smooth surface for mycelium colonization, is essential [22,154]. Opting for materials that are easily disinfected, resistant to disinfectant solutions (70–75% ethanol, sodium hypochlorite, hydrogen peroxide, etc.), and capable of withstanding high temperatures can streamline production, save time, and reduce costs [155]. This section introduces synthetic materials like high-density polyethylene, polypropylene, and acrylic plastic [22,33].
Moreover, consideration of the complexity and level of detail required for the final product is essential. Molds can be designed for simple geometric shapes or intricate patterns based on the intended application [156]. Knowing the exact shrinkage percentage of MBCs, depending on key production factors like the species of mushroom mycelium and substrate used, is another crucial part of mold design [3]. Additionally, incorporating ventilation features into the mold design for proper air exchange during mycelium growth is important to prevent anaerobic conditions and ensure uniform colonization [157]. It is also crucial to consider whether the mold is reusable or not, as this directly impacts the cost of producing MBCs. Reusable molds may be preferred for larger-scale production to minimize material waste. Furthermore, designing molds that allow for customization and scalability is essential, enabling the production of a variety of shapes and sizes [153]. This flexibility might be particularly valuable for diverse applications in industries such as packaging, building and construction, or furniture.
Various sterilization techniques are applied to clean molds before their use in MBCs production, and the method chosen depends on the material used in mold design. Firstly, autoclaving is a common and effective sterilization method. Mold and any equipment in contact with MBCs can undergo autoclaving, exposing them to high-pressure steam at elevated temperatures to effectively eliminate contaminants. It is crucial, however, that molds and equipment are crafted from materials resistant to high temperatures, such as polypropylene plastic, glass, stainless steel, and aluminum [158]. Secondly, ultraviolet (UV-C) sterilization employs light to sterilize surfaces and air. UV-C light disrupts the DNA of microorganisms, preventing their growth. This method serves as another effective option for sterilizing molds, tools, working surfaces, and the air within clean rooms [159]. Thirdly, chemical sterilization involves soaking the mold in a solution containing agents like 70–75% ethanol, sodium hypochlorite, and hydrogen peroxide. This method is applicable for materials sensitive to heat. However, it is imperative to ensure that the residues of the sterilizing agent do not negatively impact mycelium growth. Consequently, after chemical sterilization, thorough washing with autoclaved water is recommended [3,22,141].
In the overall context, both mold design and sterilization techniques are indispensable for the success of MBCs production. The synergy of a well-designed mold and effective sterilization practices ensures the production of high-quality, uncontaminated MBC products for diverse applications. This integrated approach not only ensures product quality but also contributes to reducing production steps, time, and costs.
9. Other Related Factors
Several factors influence the production process and material properties of MBCs. Two other crucial aspects are the inoculum type and microbial contamination control [2,20,160]. Understanding and carefully managing these additional factors contributes to the optimization of the MBCs production process and ensures the attainment of desired material properties.
In general, various types of mycelium inoculum, including sawdust spawn, solid or grain inoculum, liquid inoculum, and sticks inoculum, present distinct advantages and disadvantages [161]. Sawdust spawn is commonly preferred due to its ease of preparation, low cost, and minimal equipment investment. Solid or grain inoculum offers advantages such as uniform growth, easy handling, and enhanced stability. However, drawbacks include lower colonization speed and limited surface interaction. On the other hand, liquid inoculum showcases rapid colonization, versatile distribution, and scalability, but it is susceptible to contamination and has a shorter shelf life. Sticks inoculum brings advantages like easy handling, controlled placement, and reduced contamination risk, while facing limitations in surface interaction and slower colonization [161,162,163]. However, factors like size, density, and quality can also impact the colonization rate and overall growth of mycelium [7]. The choice among these types depends on specific production goals and substrate characteristics. Thoughtful consideration is essential for the successful cultivation of mycelium.
Beyond mold sterilization, ensuring microbial contamination control throughout the MBCs manufacturing processes is essential to prevent unwanted contamination. Effective sterilization protocols play a pivotal role in creating a clean and uncontaminated environment. The integration of aseptic techniques throughout production minimizes the risk of introducing contaminants [153]. This includes maintaining a sterile working environment and employing sterile tools and equipment. Moreover, controlling environmental factors such as temperature, humidity, and air quality is crucial for preventing microbial contamination [164].
10. Guidelines for Improving Properties
Enhancing properties in MBCs requires meticulous optimization and balance of various factors throughout the production process to improve the material’s strength, durability, and overall performance [3,23]. Key considerations, such as substrate selection and formulation, fungal species selection, controlled growth conditions, optimized nutrient content, mold design, and pressing techniques, as well as other factors related to inoculum type and microbial contamination control, are essential aspects to consider in the production processes [23,24,33,140,153]. Moreover, there is a growing interest in exploring additional approaches to further improve the properties of MBCs.
An intriguing approach to improving MBCs properties is the application of surface treatments. Applying coatings or finishes, such as resins, natural oils, shellac, carnauba wax, or beeswax, can enhance water resistance, fire resistance, and the overall visual appeal of the MBC materials [24]. Additionally, the incorporation of composite reinforcements, such as natural fibers, hemp, or other biodegradable materials, plays a pivotal role in enhancing mechanical properties like tensile strength and toughness [24,60]. Recent advancements in MBCs studies have revealed innovative methods for property enhancement. For instance, Teeraphantuvat et al. [3] demonstrated that adding paper waste to substrates can enhance properties such as density, compressive, flexural, and impact strengths, along with reducing water absorption. Furthermore, studies have indicated that using carbonated sand, silica, and montmorillonite clay as a natural reinforcement can improve density, compression strength, and fire resistance [5,60,165,166,167]. Following guidelines, such as combining substrates in more than two or three phases and incorporating other materials into the substrate, offers further avenues for property improvement [3].
Quality control measures, adherence to specified standards, ongoing research, and development to stay informed about advancements in MBCs technology, and processing techniques contribute significantly to the development of commercial products with long-term sustainability [3,19,20]. Producers, by carefully considering and optimizing these factors, can continually refine their MBCs production processes, resulting in materials with enhanced properties suitable for a wide range of applications. Regular experimentation, combined with a commitment to sustainable practices, ensures the continuous improvement of MBCs [168].
11. Comparison of Costs
Table 3 presents a cost model comparing the production of MBCs with conventional composite materials. The cost of producing MBCs compared with traditional materials varies across different fields, influenced by factors such as raw material availability, production processes, labor costs, and market conditions [20,169]. Although MBCs hold the potential for cost-effectiveness due to their utilization of agricultural or industrial waste materials, energy-efficient production processes, and reduced environmental impact, providing an exact cost comparison and determining the percentage of cost savings compared to traditional materials is challenging [20].

Table 3.
Comparison of MBCs manufacturing costs with traditional materials in each field.
Several studies and industry reports indicate that MBCs can provide cost savings exceeding 65% compared to paper-based materials, and over 90% when compared to fabric composites, gypsum-based materials, polymer materials, and wood–PHA composites [20,171,172,173]. However, the extent of these savings depends on factors such as the specific application, scale of production, and regional considerations [137]. Additionally, MBCs production costs were similar to those of cement-based materials [170]. These cost advantages are significant, particularly concerning materials source, production processes, and waste management. With ongoing technological advancements in MBCs production leading to economies of scale and enhanced production efficiency, the cost disparity between MBCs and traditional materials is expected to diminish further. This trend positions MBCs as an increasingly competitive and cost-effective alternative across various industries.
12. Additional Key Recommendations and Precautions
- Fungal strain degeneration: Frequent strain degradation is a significant concern in fungal product production, particularly affecting economically important varieties like edible and medicinal mushrooms, resulting in substantial production losses. Although tip mycelium subculture is commonly used for vitality maintenance, successive subculturing can lead to strain degradation, affecting mycelium growth efficiency over time [174]. Continuous subculturing without reintroducing genetic diversity can cause a decline in vitality [175,176]. For example, Yin et al. [177] observed strain degeneration from the third generation, displaying incomplete growth by the fourth. Similarly, Kim et al. [178] reported symptoms of degraded strains during continuous subculturing, such as slowed vegetative mycelial growth and less-tight mycelial pads. Inducing mushroom mycelium to produce fruiting bodies completes the fungal life cycle, generating complex mycelium structures and spores with the original genetic information (Zhao et al. [174]). Directly isolating mycelium and spores from fruiting bodies (first generation) is a more effective approach, aiding in maintaining genetic diversity and initiating fresh cultures with lower risks of mutations or degradations in strain characteristics (Figure 5) (Sakamoto et al. [179]). This part supports the efficient growth of fungal mycelium, effective bonding with substrate particles, and dense surface coverage, resulting in great composite properties.
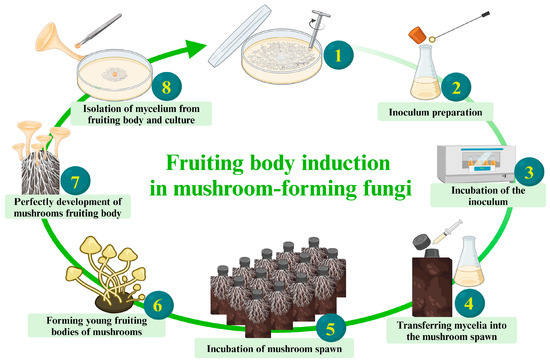 Figure 5. A step-by-step breakdown of mushroom growth: Demonstrating how to induce mushroom mycelium to produce fruiting bodies, thus completing the fungal life cycle and maintaining the viability of the strains. Created by BioRender.com (https://www.biorender.com/; access date: 19 March 2024 [61]).
Figure 5. A step-by-step breakdown of mushroom growth: Demonstrating how to induce mushroom mycelium to produce fruiting bodies, thus completing the fungal life cycle and maintaining the viability of the strains. Created by BioRender.com (https://www.biorender.com/; access date: 19 March 2024 [61]). - Research collaboration: Encourage collaboration with research institutions, industry partners, and experts in biotechnology science, physics, materials science, architecture and design, engineering, environmental science, chemistry, and multidisciplinary. This collaborative approach ensures staying updated on the latest developments, sharing knowledge, and fostering continuous improvements in production processes [7,22]
- Biosecurity protocols: Establish biosecurity protocols to prevent contamination during the production process. Strict hygiene measures, including personnel training, cleanroom practices, and equipment sterilization, are essential to ensure the purity of the MBC products [180].
- Regulatory compliance: Stay informed about and adhere to relevant regulatory guidelines and standards for MBCs production. Compliance ensures the products meet safety, environmental, and quality standards. Moreover, a comprehensive evaluation of properties encompassing physical, mechanical, chemical, and biological aspects should align with internationally accepted standards like the International Organization for Standardization (ISO), American Society for Testing and Materials (ASTM), European Standards (EN), and other relevant benchmarks [24].
- Storage conditions: To ensure optimal conditions for the dry storage of raw materials and MBC samples, it is recommended to maintain a relative humidity level below 60% (preferably between 30% and 50%). This measure effectively prevents unwanted fungal growth and enhances the long-term stability of the samples [181]. Maintaining a low relative humidity is essential for preserving the integrity and properties of MBCs. Adhering to proper storage practices plays a key role in sustaining the qualities and characteristics of MBCs, ensuring their longevity and overall quality.
- Waste management: Developing and creating an effective waste management plan for byproducts and unused materials ensures a clean workspace and successful contamination prevention. Responsible disposal or recycling practices contribute to the sustainability of the overall production process [182].
- Life cycle assessment (LCA): Performing life cycle assessments is integral to understanding and evaluating the environmental footprint of MBCs. This comprehensive analysis empowers stakeholders to make well-informed decisions concerning the sustainability and eco-friendliness of the product [183].
- Suggested parameters: The diverse properties of MBCs are influenced by many production parameters. Table 4 provides suggestions for desirable properties of MBCs across various aspects based on findings from previous studies employing different parameters and techniques. These suggestions serve as both examples and guidelines for producing efficient and high-quality MBCs materials, helping the selection process of appropriate parameters, and reducing the time required.
 Table 4. Recommended parameters for producing high-quality MBCs from previous studies, compared to other materials.
Table 4. Recommended parameters for producing high-quality MBCs from previous studies, compared to other materials.
For example, utilizing fungal mycelium of G. lucidum and Chinese albizia sawdust with a heat pressing technique in MBCs production yields materials with higher density than traditional materials like synthetic foams, wood-based composites, and paper-based materials. Similarly, these MBCs also exhibit compression and tensile strength comparable to those traditional materials [22,36,60,152]. For high dimensional stability, employing fungal mycelium from L. sajor-caju and bamboo sawdust through cold pressing might be a good alternative, resulting in samples with shrinkage percentages comparable to foams, wood-based, and paper-based products [9,22,36]. Moreover, using T. versicolor and chopped hemp with cold pressing leads to MBCs with low water absorption rates, which might be beneficial for the construction fields [9,22,26,36,60]. Meanwhile, utilizing fungal mycelium from G. carnosum and oak shavings without pressing creates MBCs with lower swelling compared to wood composites, but akin to paper-based materials [3,22,184]. Regarding thermal properties like conductivity and degradation, fungal mycelium from G. resinaceum and Miscanthus fibers without pressing, as well as T. versicolor and wheat grain without pressing, result in MBCs with properties similar to synthetic foams, wood-based composites, and paper-based materials [9,22,36,60,185,186,187]. Furthermore, using fungal mycelium from P. ostreatus and rubber sawdust with heat pressing, and P. ostreatus and wastepaper-based substrates without pressing, produces MBCs with flexural strength and sound absorption frequencies similar to foams, wood-based, and paper-based materials [3,9,22,36,60,188,189,190]. Notably, combining fungal mycelium of L. sajor-caju with corn husk mixed with paper waste using cold pressing in MBC production can produce MBCs with higher impact strength levels than many foam materials, but still within the range of wood-based and paper-based products [3,22,36]. However, these guidelines may be subject to change and adjustment based on resource availability in each area for obtaining MBCs with favorable properties.
These additional recommendations and precautions will contribute to generating and enhancing the overall properties, characteristics, and sustainability of MBCs production by addressing factors such as basic principles, environmental control, quality assurance, and regulatory considerations. Importantly, this also provides guidelines for selecting appropriate production factors in various fields to obtain MBCs with high quality, desired properties, and unique characteristics that are similar to other traditional materials.
13. Conclusions
This paper outlines factors influencing MBCs production and properties for long-term sustainability. It covers essential aspects including MBC basics, fungal species and substrate selection, preparation, optimal conditions, dehydration, post-processing, mold design, sterilization, and other related factors. Fungal species choice is critical for safety, growth rate, and substrate compatibility. Substrate type selection considers cellulose, hemicellulose, lignin content, pH, organic carbon, total nitrogen, and C: N ratio, impacting MBC properties. These, however, might vary depending on the types of biological resources available in each area. Substrate preparation involves pH and moisture control, while sterilization eliminates contaminants. Optimal conditions like temperature, humidity, and aeration are crucial for mycelium growth. Dehydrating and post-processing methods influence MBC properties. Effective mold design and sterilization ensure contamination-free production. Additionally, different inoculum types have unique advantages and disadvantages. Importantly, controlling microbial contamination is essential throughout manufacturing. Enhancing MBC properties involves optimizing substrate combination, growth conditions, and mold design. Surface treatments and composite reinforcements improve their properties. The paper also discusses cost comparison, research collaboration, regulatory compliance, waste management, and suggested parameters for desirable MBC properties, aiming to enhance sustainability and quality. Overall, it provides comprehensive insights into MBCs production, emphasizing interdisciplinary collaboration for sustainable alternatives in various industries. Crucially, we expect that our literature review will greatly benefit researchers entering the field of MBC materials soon.
Author Contributions
Conceptualization, W.A. and S.L.; software, W.A. and P.J.; validation, W.A., K.J., T.L., W.T., T.T. and S.L.; formal analysis, W.A., K.J. and S.L.; data curation, W.A., K.J., T.L. and S.L.; writing—original draft preparation, W.A., K.J., T.L., P.J. and T.T.; writing—review and editing, W.A., W.T., T.W. and S.L.; supervision, S.L.; project administration, W.A.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the CMU Proactive Researcher program, Chiang Mai University (grant number 828/2566), Chiang Mai, Thailand.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This research work was partially supported by Chiang Mai University, Chiang Mai, Thailand.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bradu, P.; Biswas, A.; Nair, C.; Sreevalsakumar, S.; Patil, M.; Kannampuzha, S.; Mukherjee, A.G.; Wanjari, U.R.; Renu, K.; Vellingiri, B.; et al. Recent advances in green technology and Industrial Revolution 4.0 for a sustainable future. Environ. Sci. Pollut. Res. Int. 2023, 30, 124488–124519. [Google Scholar] [CrossRef] [PubMed]
- Balaeș, T.; Radu, B.M.; Tănase, C. Mycelium-composite materials—A promising alternative to plastics? J. Fungi 2023, 9, 210. [Google Scholar] [CrossRef]
- Teeraphantuvat, T.; Jatuwong, K.; Jinanukul, P.; Thamjaree, W.; Lumyong, S.; Aiduang, W. Improving the physical and mechanical properties of mycelium-based green composites using paper waste. Polymers 2024, 16, 262. [Google Scholar] [CrossRef] [PubMed]
- Butu, A.; Rodino, S.; Miu, B.; Butu, M. Mycelium-based materials for the ecodesign of bioeconomy. Digest J. Nanomater. Biostruct. 2020, 15, 1129–1140. [Google Scholar] [CrossRef]
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Atta, O.M.; Yang, G. Synthesis and applications of fungal mycelium-based advanced functional materials. J. Bioresour. Bioprod. 2021, 6, 1–10. [Google Scholar] [CrossRef]
- Al-Qahtani, S.; Koç, M.; Isaifan, R.J. Mycelium-based thermal insulation for domestic cooling footprint reduction: A review. Sustainability 2023, 15, 13217. [Google Scholar] [CrossRef]
- Sydor, M.; Bonenberg, A.; Doczekalska, B.; Cofta, G. Mycelium-based composites in art, architecture, and interior design: A review. Polymers 2021, 14, 145. [Google Scholar] [CrossRef]
- Vašatko, H.; Gosch, L.; Jauk, J.; Stavric, M. Basic research of material properties of mycelium-based composites. Biomimetics 2022, 7, 51. [Google Scholar] [CrossRef]
- Aiduang, W.; Chanthaluck, A.; Kumla, J.; Jatuwong, K.; Srinuanpan, S.; Waroonkun, T.; Oranratmanee, W.; Lumyong, S.; Suwannarach, N. Amazing fungi for eco-friendly composite materials: A comprehensive review. J. Fungi 2022, 8, 842. [Google Scholar] [CrossRef]
- Li, K.; Jia, J.; Wu, N.; Xu, Q. Recent advances in the construction of biocomposites based on fungal mycelia. Front. Bioeng. BioTechnol. 2022, 10, 1067869. [Google Scholar] [CrossRef]
- Barta, D.G.; Simion, I.; Tiuc, A.E.; Vasile, O. Mycelium-based composites as a sustainable solution for waste management and circular economy. Materials 2024, 17, 404. [Google Scholar] [CrossRef] [PubMed]
- Payal, R. Green composites: Versatile uses and applications in life. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2022; pp. 165–193. [Google Scholar]
- La Mantia, F.P.; Morreale, M. Green composites: A brief review. Compos. Appl. Sci. Manuf. 2011, 42, 579–588. [Google Scholar] [CrossRef]
- Jadhav, A.C.; Pandit, P.; Gayatri, T.N.; Chavan, P.P.; Jadhav, N.C. Production of green composites from various sustainable raw materials. In Green Composites Sustainable Raw Materials; Spring: Berlin/Heidelberg, Germany, 2019; pp. 1–24. [Google Scholar] [CrossRef]
- Chen, N.; Pilla, S. A comprehensive review on transforming lignocellulosic materials into biocarbon and its utilization for composites applications. Compos. C Open Access 2022, 7, 100225. [Google Scholar] [CrossRef]
- Andrew, J.J.; Dhakal, H.N. Sustainable biobased composites for advanced applications: Recent trends and future opportunities–A critical review. Compos. C Open Access 2022, 7, 100220. [Google Scholar] [CrossRef]
- Phiri, R.; Rangappa, S.M.; Siengchin, S.; Oladijo, O.P.; Dhakal, H.N. Development of sustainable biopolymer-based composites for lightweight applications from agricultural waste biomass: A review. Adv. Ind. Eng. Polym. Res. 2023, 6, 436–450. [Google Scholar] [CrossRef]
- Pelletier, M.G.; Holt, G.A.; Wanjura, J.D.; Greetham, L.; McIntyre, G.; Bayer, E.; Kaplan-Bie, J. Acoustic evaluation of mycological biopolymer, an all-natural closed cell foam alternative. Ind. Crops Prod. 2019, 139, 111533. [Google Scholar] [CrossRef]
- Yang, L.; Park, D.; Qin, Z. Material function of mycelium-based bio-composite: A review. Front. Mater. 2021, 8, 737377. [Google Scholar] [CrossRef]
- Alemu, D.; Tafesse, M.; Mondal, A.K. Mycelium-based composite: The future sustainable biomaterial. Int. J. Biomater. 2020, 2022, 8401528. [Google Scholar] [CrossRef]
- Lingam, D.; Narayan, S.; Mamun, K.; Charan, D. Engineered mycelium-based composite materials: Comprehensive study of various properties and applications. Constr. Build. Mater. 2023, 391, 131841. [Google Scholar] [CrossRef]
- Aiduang, W.; Jatuwong, K.; Jinanukul, P.; Suwannarach, N.; Kumla, J.; Thamjaree, W.; Teeraphantuvat, T.; Waroonkun, T.; Oranratmanee, R.; Lumyong, S. Sustainable Innovation: Fabrication and characterization of mycelium-based green composites for modern interior materials using agro-industrial wastes and different species of fungi. Polymers 2024, 16, 550. [Google Scholar] [CrossRef]
- Sydor, M.; Cofta, G.; Doczekalska, B.; Bonenberg, A. Fungi in mycelium-based composites: Usage and recommendations. Materials 2022, 15, 6283. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Anaele, J.U.; Oke, T.M.; Kareem, S.A.; Adediran, M.; Ajibuwa, O.A.; Anabaranze, Y.O. Mycelium based composites: A review of their bio-fabrication procedures, material properties and potential for green building and construction applications. Alex. Eng. J. 2023, 83, 234–250. [Google Scholar] [CrossRef]
- Javadian, A.; Le Ferrand, H.; Hebel, D.E.; Saeidi, N. Application of mycelium-bound composite materials in construction industry: A short review. SOJ Mater. Sci. Eng. 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Brancart, J.; Peeters, E.; Laet, L.D. Mechanical, physical and chemical characterisation of myce-lium–based composites with different types of lignocellulosic substrates. PLoS ONE 2019, 7, e0213954. [Google Scholar]
- Sierra, L.A.B.; Mendes-Pereira, T.; García, G.J.Y.; Werkhaizer, C.Q.; de Rezende, J.B.; Rodrigues, T.A.B.; Badotti, F.; Cardoso, E.S.d.C.; da Costa, A.M.; Uetanabaro, A.P.; et al. Current situation and future perspectives for the use of fungi in the biomaterial industry and proposal for a new classification of fungal-derived materials. PeerJ Mater. Sci. 2023, 5, e31. [Google Scholar] [CrossRef]
- Huang, Z.; Wei, Y.; Hadigheh, S.A. Variations in the properties of engineered mycelium-bound composites (mbcs) under different manufacturing conditions. Buildings 2024, 14, 155. [Google Scholar] [CrossRef]
- Wimmers, G.; Klick, J.; Tackaberry, L.; Zwiesigk, C.; Egger, K.; Massicotte, H. Fundamental studies for designing insulation panels from wood shavings and filamentous fungi. BioResources 2019, 14, 5506–5520. [Google Scholar] [CrossRef]
- Schritt, H.; Vidi, S.; Pleissner, D. Spent mushroom substrate and sawdust to produce mycelium-based thermal insulation composites. J. Clean. Prod. 2021, 313, 127910. [Google Scholar] [CrossRef]
- Tsao, Y. Characterization of Mycelium-Based Composites as Foam-like Wall Insulation Material. Ph.D. Dissertation, Eidhoven University of Technology, Eindhoven, The Netherlands, 2020. [Google Scholar]
- Scopus.com. Available online: https://www.scopus.com/search/form.uri?display=basic#basic (accessed on 2 March 2024).
- Appels, F.V.W.; Camere, S.; Montalti, M.; Karana, E.; Jansen, K.M.B.; Dijksterhuis, J.; Krijgsheld, P.; Wosten, H.A.B. Fabrication factors influencing mechanical, moisture and water related properties of mycelium-based composites. Mater. Des. 2019, 161, 64–71. [Google Scholar] [CrossRef]
- Meyer, V.; Basenko, E.Y.; Benz, J.P.; Braus, G.H.; Caddick, M.X.; Csukai, M.; De Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N.; et al. Growing a circular economy with fungal biotechnology: A white paper. Fungal Biol. Biotechnol. 2020, 7, 5. [Google Scholar] [CrossRef]
- Vandelook, S.; Elsacker, E.; Van Wylick, A.; De Laet, L.; Peeters, E. Current state and future prospects of pure mycelium materials. Fungal Biol. Biotechnol. 2021, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Aiduang, W.; Kumla, J.; Srinuanpan, S.; Thamjaree, W.; Lumyong, S.; Suwannarach, N. Mechanical, physical, and chemical properties of mycelium-based composites produced from various lignocellulosic residues and fungal species. J. Fungi 2022, 8, 1125. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.; Salueña Martin, J.; Vandelook, S. Growing sustainable materials from filamentous fungi. Biochemist 2023, 45, 8–13. [Google Scholar] [CrossRef]
- Antinori, M.E.; Ceseracciu, L.; Mancini, G.; Heredia-Guerrero, J.A.; Athanassiou, A. Fine-tuning of physicochemical properties and growth dynamics of mycelium-based materials. ACS Appl. Bio Mater. 2020, 32, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- De Lima, G.G.; Schoenherr, Z.C.P.; Magalhães, W.L.E.; Tavares, L.B.B.; Helm, C.V. Enzymatic activities and analysis of a mycelium-based composite formation using peach palm (Bactris gasipaes) residues on Lentinula edodes. Bioresour. Bioprocess. 2020, 7, 58. [Google Scholar] [CrossRef]
- Nashiruddin, N.I.; Chua, K.S.; Mansor, A.F.A.; Rahman, R.; Lai, J.C.; Wan Azelee, N.I.; El Enshasy, H. Effect of growth factors on the production of mycelium-based biofoam. Clean Technol. Environ. Policy 2022, 24, 351–361. [Google Scholar] [CrossRef]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced materials from fungal mycelium: Fabrication and tuning of physical properties. Sci. Rep. 2017, 7, srep41292. [Google Scholar] [CrossRef]
- Tudryn, G.J.; Smith, L.C.; Freitag, J.; Bucinell, R.; Schadler, L.S. Processing and morphology impacts on mechanical properties of fungal based biopolymer composites. J. Polym. Environ. 2018, 26, 1473–1483. [Google Scholar] [CrossRef]
- Santos, I.S.; Nascimento, B.L.; Marino, R.H.; Sussuchi, E.M.; Matos, M.P.; Griza, S. Influence of drying heat treatments on the mechanical behavior and physico-chemical properties of mycelial biocomposite. Compos. B Eng. 2021, 217, 108870. [Google Scholar] [CrossRef]
- Bähner, F.D.; Prado-Rubio, O.A.; Huusom, J.K. Challenges in optimization and control of biobased process systems: An industrial-academic perspective. Ind. Eng. Chem. Res. 2021, 60, 14985–15003. [Google Scholar] [CrossRef]
- Livne, A.; Wösten, H.A.; Pearlmutter, D.; Gal, E. Fungal mycelium bio-composite acts as a CO2-sink building material with low embodied energy. ACS Sustain Chem Eng. 2022, 10, 12099–12106. [Google Scholar] [CrossRef]
- Ghazvinian, A.; Farrokhsiar, P.; Vieira, F.; Pecchia, J.; Gursoy, B. Mycelium-based bio-composites for architecture: Assessing the effects of cultivation factors on compressive strength. Mater. Res. Innov. 2019, 2, 505–514. [Google Scholar]
- Attias, N.; Danai, O.; Ezov, N.; Tarazi, E.; Grobman, Y.J. Developing novel applications of mycelium based bio-composite materials for design and architecture. In Proceedings of the Building with Biobased Materials: Best Practice And Performance Specification, Zagreb, Croatia, 9–16 September 2017; pp. 1–10. [Google Scholar]
- Abdelhady, O.; Spyridonos, E.; Dahy, H. Bio-modules: Mycelium-based composites forming a modular interlocking system through a computational design towards sustainable architecture. Designs 2023, 7, 20. [Google Scholar] [CrossRef]
- Santulli, C. Mycelium-grown composites as a multidisciplinary way for the development of innovative materials for design and architecture. Cuad. Cent. Estud. Diseño Y Comun. 2023, 190, 165–179. [Google Scholar] [CrossRef]
- Citraresmi, A.D.P.; Haryati, N. The strategy of business model development in mushroom agroindustry. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 924, p. 012057. [Google Scholar]
- Elsacker, E.V. Mycelium Matters-An Interdisciplinary Exploration of the Fabrication and Properties of Mycelium-Based Materials. Ph.D. Thesis, Vrije Universiteit Brussel, Brussel, Belgium, 2021. [Google Scholar]
- Kamali, M.; Sarvtin, M.T. Fungal colonization of wood and wood products inside the buildings of Sari, northern Iran. South Asian J. Exp. Biol. 2016, 6, 101–104. [Google Scholar]
- Martín, J.A.; López, R. Biological Deterioration and Natural Durability of Wood in Europe. Forests 2023, 14, 283. [Google Scholar] [CrossRef]
- Aslanzadeh, S.; Ishola, M.M.; Richards, T.; Taherzadeh, M.J. An overview of existing individual unit operations. Biorefineries 2014, 3–36. [Google Scholar] [CrossRef]
- Jones, M.; Huynh, T.; John, S. Inherent species characteristic influence and growth performance assessment for mycelium composite applications. Adv. Mater. Lett. 2018, 9, 71–80. [Google Scholar] [CrossRef]
- Tacer-Caba, Z.; Varis, J.J.; Lankinen, P.; Mikkonen, K.S. Comparison of novel fungal mycelia strains and sustainable growth substrates to produce humidity-resistant biocomposites. Mater. Des. 2020, 192, 108728. [Google Scholar] [CrossRef]
- Porter, D.L.; Bradshaw, A.J.; Nielsen, R.H.; Newell, P.; Dentinger, B.T.; Naleway, S.E. The melanized layer of Armillaria ostoyae rhizomorphs: Its protective role and functions. J. Mech. Behav. Biomed. Mater. 2022, 125, 104934. [Google Scholar] [CrossRef]
- Porter, D.L.; Naleway, S.E. Hyphal systems and their effect on the mechanical properties of fungal sporocarps. Acta Biomater. 2022, 145, 272–282. [Google Scholar] [CrossRef]
- Heaton, L.; Obara, B.; Grau, V.; Jones, N.; Nakagaki, T.; Boddy, L.; Fricker, M.D. Analysis of fungal networks. Fungal Biol. Rev. 2012, 26, 12–29. [Google Scholar] [CrossRef]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered mycelium composite construction materials from fungal biorefineries: A critical review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- BioRender.com. Available online: https://www.biorender.com/ (accessed on 19 March 2024).
- Sun, W. Fungal mycelia: From innovative materials to promising products: Insights and challenges. Biointerphases 2024, 19, 018502. [Google Scholar] [CrossRef] [PubMed]
- Läkk, H.; Krijgsheld, P.; Montalti, M.; Wösten, H. Fungal based biocomposite for habitat structures on the Moon and Mars. In Proceedings of the International Astronautical Congress (IAC), Bremen, Germany, 1–5 October 2018; International Astronautical Federation, IAF. pp. 1–11. [Google Scholar]
- Xiao, Q.; Ma, F.; Li, Y.; Yu, H.; Li, C.; Zhang, X. Differential proteomic profiles of Pleurotus ostreatus in response to lignocellulosic components provide insights into divergent adaptive mechanisms. Front. Microbiol. 2017, 8, 480. [Google Scholar] [CrossRef] [PubMed]
- Rigobello, A.; Ayres, P. Compressive behaviour of anisotropic mycelium-based composites. Sci. Rep. 2022, 12, 6846. [Google Scholar] [CrossRef] [PubMed]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.; Pyle, L.; Subramanian, S.K. Biogas Technology in the Third World: A Multi-Disciplinary Review; IDRC: Ottawa, ON, Canada, 1978. [Google Scholar]
- Tetreault, J. Coatings for Display and Storage in Museums; Canadian Conservation Institute: Ottawa, ON, Canada, 1999. [Google Scholar]
- Goodale, C.L.; Lajtha, K.; Nadelhoffer, K.J.; Boyer, E.W.; Jaworski, N.A. Forest nitrogen sinks in large eastern US watersheds: Estimates from forest inventory and an ecosystem model. In The Nitrogen Cycle at Regional to Global Scales; Springer: Berlin/Heidelberg, Germany, 2002; pp. 239–266. [Google Scholar]
- Thompson, R.K.; McKinnon, J.J.; Mustafa, A.F.; Maenz, D.D.; Racz, V.J.; Christensen, D.A. Chemical composition, ruminal kinetic parameters, and nutrient digestibility of ammonia treated oat hulls. Can. J. Anim. Sci. 2002, 82, 103–109. [Google Scholar] [CrossRef]
- Lamlom, S.H.; Savidge, R.A. A reassessment of carbon content in wood: Variation within and between 41 North American species. Biomass Bioenergy 2003, 25, 381–388. [Google Scholar] [CrossRef]
- Abdelhamid, M.T.; Horiuchi, T.; Oba, S. Composting of rice straw with oilseed rape cake and poultry manure and its effects on faba bean (Vicia faba L.) growth and soil properties. Bioresour. Technol. 2004, 93, 183–189. [Google Scholar] [CrossRef]
- Roldán-Martín, A.; Esparza-García, F.; Calva-Calva, G.; Rodríguez-Vázquez, R. Effects of mixing low amounts of orange peel (Citrus reticulata) with hydrocarbon-contaminated soil in solid culture to promote remediation. J. Environ. Sci. Health A 2006, 41, 2373–2385. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Halim, M.A.; Islam, M.S.; Biswas, C.K. Analysis the plant nutrients and organic matter in textile sludge in Gazipur, Bangladesh. J. Environ. Sci. Technol. 2009, 2, 63–67. [Google Scholar] [CrossRef]
- Kargarfard, A.; Latibari, A.J. The performance of corn and cotton stalks for medium density fiberboard production. BioResources 2011, 6, 1147–1157. [Google Scholar] [CrossRef]
- Agarry, S.E.; Aremu, M.O. Batch equilibrium and kinetic studies of simultaneous adsorption and biodegradation of phenol by pineapple peels immobilized Pseudomonas aeruginosa NCIB 950. Br. Biotechnol. J. 2012, 2, 26–48. [Google Scholar] [CrossRef]
- Dębska, B.; Drąg, M.; Tobiasova, E. Effect of post-harvest residue of maize, rapeseed, and sunflower on humic acids properties in various soils. Pol. J. Environ. Stud. 2012, 21, 603–613. [Google Scholar]
- Radenkovs, V.; Klava, D. Physically-chemical characterization of industrial wheat bran from Latvia. In Proceedings of the Annual 18th International Scientific Conference Proceedings, Raesfeld, Germany, 16–19 September 2012; Research for Rural Development. pp. 155–159. [Google Scholar]
- Tripetchkul, S.; Pundee, K.; Koonsrisuk, S.; Akeprathumchai, S. Co-composting of coir pith and cow manure: Initial C/N ratio vs physico-chemical changes. Int. J. Recycl. Org. Waste Agric. 2012, 1, 15. [Google Scholar] [CrossRef]
- Wang, A.S.; Hu, P.; Hollister, E.B.; Rothlisberger, K.L.; Somenahally, A.; Provin, T.L.; Hons, F.M.; Gentry, T.J. Impact of Indian mustard (Brassica juncea) and flax (Linum usitatissimum) seed meal applications on soil carbon, nitrogen, and microbial dynamics. Appl. Environ. Soil Sci. 2011, 2012, 351609. [Google Scholar] [CrossRef]
- Isikhuemhen, O.S.; Mikiashvili, N.A.; Senwo, Z.N.; Ohimain, E.I. Biodegradation and sugar release from canola plant biomass by selected white rot fungi. Adv. Biol. Chem. 2014, 4, 395. [Google Scholar] [CrossRef]
- Grover, R.; Goel, A.; Wati, L.; Raj, K. Ethanol production from spent oyster mushroom substrate. Pollut. Res. 2015, 34, 121–124. [Google Scholar]
- Ioelovich, M. Recent findings and the energetic potential of plant biomass as a renewable source of biofuels—A review. BioResources 2015, 10, 1879–1914. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Bou, M.; Sigoillot, J.C.; Faulds, C.B.; Lomascolo, A. Essential oils and distilled straws of lavender and lavandin: A review of current use and potential application in white biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 3375–3385. [Google Scholar] [CrossRef] [PubMed]
- Lesage-Meessen, L.; Bou, M.; Ginies, C.; Chevret, D.; Navarro, D.; Drula, E.; Bonnin, E.; del Río, J.C.; Odinot, E.; Bisotto, A.; et al. Lavender-and lavandin-distilled straws: An untapped feedstock with great potential for the production of high-added value compounds and fungal enzymes. Biotechnol. Biofuels 2018, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, C.D.; Pieris, M. A physico-chemical analysis of coconut shell powder. Procedia Chem. 2015, 16, 222–228. [Google Scholar] [CrossRef]
- Boneberg, B.S.; Machado, G.D.; Santos, D.F.; Gomes, F.; Faria, D.J.; Gomes, L.A.; Santos, F.A. Biorefinery of lignocellulosic biopolymers. Rev. Eletrônica Cient. UERGS 2016, 2, 79–100. [Google Scholar] [CrossRef]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Characterizing fruit and vegetable peels as bioadsorbents. Curr. Sci. 2016, 110, 2114–2123. [Google Scholar] [CrossRef]
- Gervásio, E.S.; Ishikawa, F.H.; da Silva, V.D.; de Melo Júnior, J.C.F. Water levels depletion in substrate in the development of Heliconia psittacorum L.F. CV. Red Opal. Irriga 2017, 22, 44–58. [Google Scholar] [CrossRef]
- Gismatulina, Y.A.; Budaeva, V.V. Chemical composition of five Miscanthus sinensis harvests and nitric-acid cellulose therefrom. Ind Crops Prod. 2017, 109, 227–232. [Google Scholar] [CrossRef]
- Mishra, A.; Taing, K.; Hall, M.W.; Shinogi, Y. Effects of rice husk and rice husk charcoal on soil physicochemical properties, rice growth and yield. Agric. Sci. 2017, 8, 1014–1032. [Google Scholar] [CrossRef]
- Pan-in, S.; Sukasem, N. Methane production potential from anaerobic co-digestions of different animal dungs and sweet corn residuals. Energy Procedia 2017, 138, 943–948. [Google Scholar] [CrossRef]
- Toushik, S.H.; Lee, K.T.; Lee, J.S.; Kim, K.S. Functional applications of lignocellulolytic enzymes in the fruit and vegetable processing industries. J. Food Sci. 2017, 82, 585–593. [Google Scholar] [CrossRef]
- Chowdhury, R.; Ghosh, S.; Debnath, B.; Manna, D. Indian agro-wastes for 2G biorefineries: Strategic decision on conversion processes. Sustain. Energy Technol. Policies A Transform. J. 2018, 1, 353–373. [Google Scholar]
- Rajendran, K.; Drielak, E.; Sudarshan Varma, V.; Muthusamy, S.; Kumar, G. Updates on the pretreatment of lignocellulosic feedstocks for bioenergy production—A review. Biomass Convers. Biorefinery 2018, 8, 471–483. [Google Scholar] [CrossRef]
- Zhang, J.; Ying, Y.; Li, X.; Yao, X. Evaluation of three kinds of nutshell with respect to utilization as culture media. BioResources 2018, 13, 7508–7518. [Google Scholar] [CrossRef]
- Zhou, J.L.; Song, S.; Huang, Z.X.; Yang, L.; Jiao, A.G.; Liu, Y.; Wang, S.X. Cultivation of Pleurotus ostreatus, a potential candidate for biogas residues degradation. BioResources 2018, 13, 5432–5449. [Google Scholar] [CrossRef]
- Ibrahim, M.I.J.; Sapuan, S.M.; Zainudin, E.S.; Yusoff, M.Z.M. Extraction, Chemical composition, and characterization of the potential lignocellulosic biomasses and polymers from corn plant parts. BioResources 2019, 14, 6485–6500. [Google Scholar] [CrossRef]
- Ajayi-Banji, A.A.; Rahman, S.; Sunoj, S.; Igathinathane, C. Impact of corn stover particle size and C/N ratio on reactor performance in solid-state anaerobic co-digestion with dairy manure. J. Air Waste Manag. Assoc. 2020, 70, 436–454. [Google Scholar] [CrossRef]
- Chikri, R.; Elhadiri, N.; Benchanaa, M.; El Maguana, Y. Efficiency of sawdust as low-cost adsorbent for dyes removal. J. Chem. 2020, 2020, 8813420. [Google Scholar] [CrossRef]
- Gummert, M.; Hung, N.V.; Chivenge, P.; Douthwaite, B. Sustainable Rice Straw Management; Springer Nature: Berlin/Heidelberg, Germany, 2020; p. 192. [Google Scholar]
- Kaur, P.; Taggar, M.S.; Kaur, J. Cellulolytic microorganisms: Diversity and role in conversion of rice straw to bioethanol. Cell Chem Technol. 2020, 54, 613–634. [Google Scholar] [CrossRef]
- Carmo, C.O.; Rodrigues, M.D.S.; Silva, F.D.; Irineu, T.G.; Soares, A.C.F. Spent mushroom substrate of Pleurotus ostreatus kummer increases basil biomass and essential oil yield. Rev. Caatinga 2021, 34, 548–558. [Google Scholar] [CrossRef]
- Angelova, G.; Brazkova, M.; Stefanova, P.; Blazheva, D.; Vladev, V.; Petkova, N.; Slavov, A.; Denev, P.; Karashanova, D.; Zaharieva, R.; et al. Waste rose flower and lavender straw biomass—An innovative lignocellulose feedstock for mycelium bio-materials development using newly isolated Ganoderma resinaceum GA1M. J. Fungi 2021, 7, 866. [Google Scholar] [CrossRef] [PubMed]
- Chong, T.Y.; Law, M.C.; Chan, Y.S. The potentials of corn waste lignocellulosic fibre as an improved reinforced bioplastic composites. J. Polym. Environ. 2021, 29, 363–381. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Omran, A.A.B.; Hasan, Z.; Ilyas, R.A.; Sapuan, S.M. Wheat biocomposite extraction, structure, properties and characterization: A review. Polymers 2021, 13, 3624. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Ahmed, N.A.; Ogunkunle, O. Optimization of biogas yield from lignocellulosic materials with different pretreatment methods: A review. Biotechnol. Biofuels 2021, 14, 159. [Google Scholar] [CrossRef]
- Voća, N.; Leto, J.; Karažija, T.; Bilandžija, N.; Peter, A.; Kutnjak, H.; Šurić, J.; Poljak, M. Energy properties and biomass yield of miscanthus x giganteus fertilized by municipal sewage sludge. Molecules 2021, 26, 4371. [Google Scholar] [CrossRef]
- Spacki, K.d.C.; Corrêa, R.C.G.; Uber, T.M.; Barros, L.; Ferreira, I.C.F.R.; Peralta, R.A.; Moreira, R.d.F.P.M.; Helm, C.V.; de Lima, E.A.; Bracht, A.; et al. Full exploitation of peach palm (Bactris gasipaes Kunth): State of the art and perspectives. Plants 2022, 11, 3175. [Google Scholar] [CrossRef]
- Hadrović, S.; Jovanović, F.; Braunović, S.; Mitrović, T.Ć.; Rakonjac, L.; Jandrić, M.; Hadrović, D. Biomass carbon and nitrogen content of softwood broadleaves in southwestern serbia. Hortic. Sci. 2022, 57, 684–685. [Google Scholar] [CrossRef]
- Hajj Obeid, M.; Douzane, O.; Freitas Dutra, L.; Promis, G.; Laidoudi, B.; Bordet, F.; Langlet, T. Physical and mechanical properties of rapeseed straw concrete. Materials 2022, 15, 8611. [Google Scholar] [CrossRef]
- Oyeleke, A.M.; Olajumoke, A.O.; Rofiyat, A.; Oluwatosin, A.J. Effect of pyrolysis temperature on chemical and structural properties of raw agricultural wastes. Food Sci. 2022, 6, 69–85. [Google Scholar]
- Marrot, L.; Candelier, K.; Valette, J.; Lanvin, C.; Horvat, B.; Legan, L.; DeVallance, D.B. Valorization of hemp stalk waste through thermochemical conversion for energy and electrical applications. Waste Biomass Valori. 2022, 13, 2267–2285. [Google Scholar] [CrossRef]
- Shahinur, S.; Sayeed, M.A.; Hasan, M.; Sayem, A.S.M.; Haider, J.; Ura, S. Current development and future perspective on natural jute fibers and their biocomposites. Polymers 2022, 14, 1445. [Google Scholar] [CrossRef] [PubMed]
- Zawadzińska, A.; Salachna, P.; Nowak, J.S.; Kowalczyk, W.; Piechocki, R.; Łopusiewicz, Ł.; Pietrak, A. Compost based on pulp and paper mill sludge, fruit-vegetable waste, mushroom spent substrate and rye straw improves yield and nutritional value of tomato. Agronomy 2021, 12, 13. [Google Scholar] [CrossRef]
- Ajien, A.; Idris, J.; Md Sofwan, N.; Husen, R.; Seli, H. Coconut shell and husk biochar: A review of production and activation technology, economic, financial aspect and application. Waste Manag. Res. 2023, 41, 37–51. [Google Scholar] [CrossRef]
- Balea, A.; Monte, M.C.; Fuente, E.; Sanchez-Salvador, J.L.; Tarrés, Q.; Mutjé, P.; Delgado-Aguilar, M.; Negro, C. Fit-for-use nanofibrillated cellulose from recovered paper. Nanomaterials 2023, 13, 2536. [Google Scholar] [CrossRef]
- Chiarelotto, M.; Melo, D.C.D.; Santos, M.V.A.D. Does the initial C/N ratio interfere with the performance of sewage sludge composting and cotton waste? Environ. Technol. 2023, 45, 2673–2683. [Google Scholar] [CrossRef]
- Chutimanukul, P.; Phatthanamas, W.; Thepsilvisut, O.; Chantarachot, T.; Thongtip, A.; Chutimanukul, P. Commercial scale production of Yamabushitake mushroom (Hericium erinaceus (Bull.) Pers. 1797) using rubber and bamboo sawdust substrates in tropical regions. Sci. Rep. 2023, 13, 13316. [Google Scholar] [CrossRef]
- De Nijs, E.A.; Maas, L.M.E.; Bol, R.; Tietema, A. Assessing the potential of co-composting rose waste as a sustainable waste management strategy: Nutrient availability and disease control. J. Clean. Prod. 2023, 399, 136685. [Google Scholar] [CrossRef]
- Mridha, N.; Ray, D.P.; Singha, A.; Das, A.; Bhowmick, M.; Ghosh, R.K.; Manjunatha, B.S.; Saha, B.; Roy, A.N.; Nayak, L.; et al. Composting of natural fibre wastes for preparation of organic manures and bio-enhancers. Econ. Aff. 2023, 68, 1121–1128. [Google Scholar]
- Palanikumar, K.; Natarajan, E.; Markandan, K.; Ang, C.K.; Franz, G. Targeted pre-treatment of hemp fibers and the effect on mechanical properties of polymer composites. Fibers 2023, 11, 43. [Google Scholar] [CrossRef]
- Chaowana, P.; Hnoocham, W.; Chaiprapat, S.; Yimlamai, P.; Chitbanyong, K.; Wanitpinyo, K.; Chaisan, T.; Paopun, Y.; Pisutpiched, S.; Khantayanuwong, S.; et al. Utilization of hemp stalk as a potential resource for bioenergy. Mater. Sci. Energy Technol. 2024, 7, 19–28. [Google Scholar] [CrossRef]
- Wu, J.; Wang, R.; Liu, X.; Ni, Y.; Sun, H.; Deng, X.; Wan, L.; Liu, F.; Tang, J.; Yu, J.; et al. Calcium dynamics during the growth of Agaricus bisporus: Implications for mushroom development and nutrition. Chem. Biol. Technol. Agric. 2023, 10, 99. [Google Scholar] [CrossRef]
- Upadhyay, R.C.; Verma, R.N.; Singh, S.K.; Yadav, M.C. Effect of organic nitrogen supplementation in Pleurotus species. Mushroom Biol. Mushroom Prod. 2002, 105, 225–232. [Google Scholar]
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of mushrooms and their lignocellulolytic enzyme production through the utilization of agro-industrial waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef]
- Webb, E.; Cullen, N. Ultimate Guide to Mushroom Substrates. Available online: https://urban-farm-it.com/blogs/mushroom-cultivation/guide-to-mushroom-substrates (accessed on 22 March 2024).
- Suwannarach, N.; Kumla, J.; Zhao, Y.; Kakumyan, P. Impact of cultivation substrate and microbial community on improving mushroom productivity: A review. Biology 2022, 11, 569. [Google Scholar] [CrossRef]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Júnior, A.M.; Ribani, R.H. Factors affecting mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef]
- Carrasco, J.; Zied, D.C.; Pardo, J.E.; Preston, G.M.; Pardo-Giménez, A. Supplementation in mushroom crops and its impact on yield and quality. AMB Express 2018, 8, 146. [Google Scholar] [CrossRef]
- Soh, E.; Saeidi, N.; Javadian, A.; Hebel, D.E.; Le Ferrand, H. Effect of common foods as supplements for the mycelium growth of Ganoderma lucidum and Pleurotus ostreatus on solid substrates. PLoS ONE 2021, 16, e0260170. [Google Scholar] [CrossRef]
- Colavolpe, M.B.; Mejía, S.J.; Albertó, E. Efficiency of treatments for controlling Trichoderma spp. during spawning in cultivation of lignicolous mushrooms. Braz. J. Microbiol. 2014, 45, 1263–1270. [Google Scholar] [CrossRef]
- Mycobio. Ultimate Guide to Sterilizing Mushroom Substrate. Available online: https://mycobio.co.nz/ultimate-guide-to-sterilising-mushroom-substrate/ (accessed on 23 March 2024).
- Tangjang, L.; Megu, T.; Rina, T. Effect of substrate pre-treatment methods on the fruit body production of Pleurotus sajor-caju (Fr.) Singer. J. Bioresour. 2022, 9, 53–56. [Google Scholar]
- Salish Sea Mushrooms. Pasteurization and Sterilization. Available online: https://salishmushrooms.com/cultivation/pasteurization-and-sterilization/ (accessed on 23 March 2024).
- Freak of Natural. Growing Mushrooms: Notions of Sterilization. Available online: https://freakofnatural.com/growing-mushrooms-notions-of-sterilization/ (accessed on 23 March 2024).
- Peeters, S.S. Assessing Modifications on Mycelium-Based Composites and the Effects on Fungal Degradation and Material Properties. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2023. [Google Scholar]
- Ahmadjian, V.; Alexopoulos, C.J.; Moore, D. Fungus Organism. Available online: https://www.britannica.com/science/fungus (accessed on 25 March 2024).
- Silvestri, L. TERRAFORMA-Material Investigation on the Possibilities to Combine Natural Growth of Mycelium and Unfired Clay for Novel Sustainable Product Design Applications. 2022. Available online: https://www.politesi.polimi.it/handle/10589/214660 (accessed on 5 March 2024).
- Ghazvinian, A. A sustainable alternative to architectural materials: Mycelium-based bio-composites. In Proceedings of the Divergence in Architectural Research, Atlanta, GA, USA, 5–6 March 2021; Volume 15, pp. 159–167. [Google Scholar]
- Houette, T.; Maurer, C.; Niewiarowski, R.; Gruber, P. Growth and mechanical characterization of mycelium-based composites towards future bioremediation and food production in the material manufacturing cycle. Biomimetics 2022, 7, 103. [Google Scholar] [CrossRef]
- Jiang, L.; Walczyk, D.; McIntyre, G.; Bucinell, R.; Tudryn, G. Manufacturing of biocomposite sandwich structures using mycelium-bound cores and preforms. J. Manuf. Process. 2017, 28, 50–59. [Google Scholar] [CrossRef]
- Islam, M.R.; Tudryn, G.; Bucinell, R.; Schadler, L.; Picu, R.C. Mechanical behavior of mycelium-based particulate composites. J. Mater. Sci. 2018, 53, 16371–16382. [Google Scholar] [CrossRef]
- Karana, E.; Blauwhoff, D.; Hultink, E.J.; Camere, S. When the material grows: A case study on designing (with) mycelium-based materials. Int. J. Des. 2018, 12, 119–136. [Google Scholar]
- Attias, N.; Danai, O.; Tarazi, E.; Pereman, I.; Grobman, Y.J. Implementing bio-design tools to develop mycelium-based products. Des. J. 2019, 22, 1647–1657. [Google Scholar] [CrossRef]
- Attias, N.; Danai, O.; Abitbol, T.; Tarazi, E.; Ezov, N.; Pereman, I.; Grobman, Y.J. Mycelium bio-composites in industrial design and architecture: Comparative review and experimental analysis. J. Clean. Prod. 2020, 246, 119037. [Google Scholar] [CrossRef]
- Kaiser, M.; Kleber, M.; Berhe, A.A. How air-drying and rewetting modify soil organic matter characteristics: An assessment to improve data interpretation and inference. Soil Biol. Biochem. 2015, 80, 324–340. [Google Scholar] [CrossRef]
- Gan, J.K.; Soh, E.; Saeidi, N.; Javadian, A.; Hebel, D.E.; Le Ferrand, H. Temporal characterization of biocycles of mycelium-bound composites made from bamboo and Pleurotus ostreatus for indoor usage. Sci. Rep. 2022, 12, 19362. [Google Scholar] [CrossRef] [PubMed]
- Blauwhoff, D.; La Bianca, I. From Biomass to Mycelium Composite: An Exploration on Cellulose and Weed Residues. Stichting Toegepast Onderzoek Waterbeheer (STOWA). 2019. Available online: https://edepot.wur.nl/515208 (accessed on 5 March 2024).
- Yang, L.; Qin, Z. Mycelium-based wood composites for light weight and high strength by experiment and machine learning. Cell Rep. Phys. Sci. 2023, 4, 1–16. [Google Scholar] [CrossRef]
- Liu, R.; Long, L.; Sheng, Y.; Xu, J.; Qiu, H.; Li, X.; Wang, Y.; Wu, H. Preparation of a kind of novel sustainable mycelium/cotton stalk composites and effects of pressing temperature on the properties. Ind. Crops Prod. 2019, 141, 111732. [Google Scholar] [CrossRef]
- Chan, X.Y.; Saeidi, N.; Javadian, A.; Hebel, D.E.; Gupta, M. Mechanical properties of dense mycelium-bound composites under accelerated tropical weathering conditions. Sci. Rep. 2021, 11, 22112. [Google Scholar] [CrossRef]
- Mohseni, A.; Vieira, F.R.; Pecchia, J.A.; Gürsoy, B. Three-Dimensional Printing of living mycelium-based composites: Material compositions, workflows, and ways to mitigate contamination. Biomimetics 2023, 8, 257. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Peeters, E. Recent technological innovations in mycelium materials as leather substitutes: A patent review. Front. Bioeng. Biotechnol. 2023, 11, 1204861. [Google Scholar] [CrossRef]
- Petersen, J.; McLaughlin, S. Laboratory Exercises in Microbiology: Discovering the Unseen World through Hands-On Investigation; CUNY Academic Works: New York, NY, USA, 2016. [Google Scholar]
- Bubu, V. Mold Design and Maintenance: Key Elements for Manufacturing Excellence. Available online: https://www.linkedin.com/pulse/mold-design-maintenance-key-elements-manufacturing-excellence-babu (accessed on 25 March 2024).
- Etinosa, O.P. Design and Testing of Mycelium Biocomposite. Ph.D. Dissertation, African University of Science and Technology, Galadimawa, Nigeria, 2019. [Google Scholar]
- Lee, T.; Choi, J. Mycelium-composite panels for atmospheric particulate matter adsorption. Results Mater. 2021, 11, 100208. [Google Scholar] [CrossRef]
- Pereira, A.R.; Braga, D.F.; Vassal, M.; Gomes, I.B.; Simões, M. Ultraviolet C irradiation: A promising approach for the disinfection of public spaces? Sci. Total Environ. 2023, 879, 163007. [Google Scholar] [CrossRef]
- Sertkaya, S.N. Assessment of Mycelium Composite Building Elements Produced via Biodesign. 2022. Available online: https://avesis.deu.edu.tr/dosya?id=e5f3bab1-c260-4413-a78f-121439a9f2ad (accessed on 5 March 2024).
- Zhang, W.R.; Liu, S.R.; Kuang, Y.B.; Zheng, S.Z. Development of a novel spawn (block spawn) of an edible mushroom, Pleurotus ostreatus, in liquid culture and its cultivation evaluation. Mycobiology 2019, 47, 97–104. [Google Scholar] [CrossRef]
- Rosado, F.R.; Kemmelmeier, C.; Gomes Da Costa, S.M. Alternative method of inoculum and spawn production for the cultivation of the edible Brazilian mushroom Pleurotus ostreatoroseus Sing. J. Basic Microbiol. 2002, 42, 37–44. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Hu, D.D.; Ma, X.T.; Li, S.G.; Gu, J.G.; Hu, Q.X. Adopting stick spawn reduced the spawn running time and improved mushroom yield and biological efficiency of Pleurotus eryngii. Sci. Hortic. 2014, 175, 156–159. [Google Scholar] [CrossRef]
- Coté, R.J. Aseptic technique for cell culture. Curr. Protoc. Cell Biol. 1998, 1, 1–3. [Google Scholar] [CrossRef]
- Gou, L.; Li, S.; Yin, J.; Li, T.; Liu, X. Morphological and physico-mechanical properties of mycelium biocomposites with natural reinforcement particles. Constr. Build. Mater. 2021, 304, 124656. [Google Scholar] [CrossRef]
- Elsacker, E.; De Laet, L.; Peeters, E. Functional grading of mycelium materials with inorganic particles: The effect of nanoclay on the biological, chemical and mechanical properties. Biomimetics 2022, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Fan, X.; Wnek, G.; Liao, Y.T.T.; Yu, X. Development and characterization of novelly grown fire-resistant fungal fibers. Sci. Rep. 2022, 12, 10836. [Google Scholar] [CrossRef] [PubMed]
- Bonenberg, A.; Sydor, M.; Cofta, G.; Doczekalska, B.; Grygorowicz-Kosakowska, K. Mycelium-based composite materials: Study of acceptance. Materials 2023, 16, 2164. [Google Scholar] [CrossRef]
- Jiang, L.; Walczyk, D.; McIntyre, G.; Chan, W.K. Cost modeling and optimization of a manufacturing system for mycelium-based biocomposite parts. J. Manuf. Syst. 2016, 41, 8–20. [Google Scholar] [CrossRef]
- Indexbox. Cement Price per kg. Available online: https://www.indexbox.io/search/cement-price-per-kg/ (accessed on 25 March 2024).
- Monteiro, S.N.; de Assis, F.S.; Ferreira, C.L.; Simonassi, N.T.; Weber, R.P.; Oliveira, M.S.; Colorado, H.A.; Pereira, A.C. Fique fabric: A promising reinforcement for polymer composites. Polymers 2018, 10, 246. [Google Scholar] [CrossRef]
- Sai, S. What Is the Total Cost of Production of 1 kg of Cardboard? Available online: https://www.quora.com/What-is-the-total-cost-of-production-of-1-kg-of-cardboard (accessed on 25 March 2024).
- Vandi, L.J.; Chan, C.M.; Werker, A.; Richardson, D.; Laycock, B.; Pratt, S. Wood-PHA composites: Mapping opportunities. Polymers 2018, 10, 751. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, X.; Chen, C.; Cheng, Z.; Wang, W.; Yun, J. Successive mycelial subculturing decreased lignocellulase activity and increased ROS accumulation in Volvariella volvacea. Front. Microbiol. 2022, 13, 997485. [Google Scholar] [CrossRef]
- Sattayaphisut, W. Genetic Instability of Mushrooms as Affected by Continuous Subculturing for Production. Ph.D. Thesis, Suranaree University of Technology, Nakhon Ratchasima, Thailand, 2000. [Google Scholar]
- Chen, X.; Zhang, Z.; Liu, X.; Cui, B.; Miao, W.; Cheng, W.; Zhao, F. Characteristics analysis reveals the progress of Volvariella volvacea mycelium subculture degeneration. Front. Microbiol. 2019, 10, 2045. [Google Scholar] [CrossRef]
- Yin, J.; Xin, X.; Weng, Y.; Gui, Z. Transcriptome-wide analysis reveals the progress of Cordyceps militaris subculture degeneration. PLoS ONE 2017, 12, e0186279. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, K.H.; Im, C.H.; Ali, A.; Lee, C.Y.; Kong, W.S.; Ryu, J.S. Identification of degenerate nuclei and development of a SCAR marker for Flammulina velutipes. PLoS ONE 2014, 9, e107207. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Sato, S.; Takizawa, M.; Goto, F.; Eda, K.; Yamauchi, T. Marker-assisted selection of a novel Lentinula edodes cultivation strain with improved post-harvest quality. Mushroom Sci. Biotechnol. 2022, 30, 121–128. [Google Scholar]
- Corrales, C.; Astrin, J.J. Biodiversity Biobanking—A Handbook on Protocols and Practices. 2023. Available online: https://ab.pensoft.net/book/101876/element/8/182816// (accessed on 10 March 2024).
- Gromicko, B.; Gromicko, N. How to Perform a Mold Inspection. 2009. Available online: https://education.nachi.org/coursemedia/course-50/documents/Course-material.pdf (accessed on 10 March 2024).
- Chiu, S.W.; Law, S.C.; Ching, M.L.; Cheung, K.W.; Chen, M.J. Themes for mushroom exploitation in the 21st century: Sustainability, waste management, and conservation. J. Gen. Appl. Microbiol. 2000, 46, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, L.; Hoberg, F.; Bach, V.; Schmidt, B.; Pfeiffer, S.; Meyer, V.; Finkbeiner, M. Life cycle assessment of fungal-based composite bricks. Sustainability 2021, 13, 11573. [Google Scholar] [CrossRef]
- Charpentier-Alfaro, C.; Benavides-Hernández, J.; Poggerini, M.; Crisci, A.; Mele, G.; Della Rocca, G.; Emiliani, G.; Frascella, A.; Torrigiani, T.; Palanti, S. Wood-decaying fungi: From timber degradation to sustainable insulating biomaterials production. Materials 2023, 16, 3547. [Google Scholar] [CrossRef]
- Dias, P.P.; Jayasinghe, L.B.; Waldmann, D. Investigation of mycelium-miscanthus composites as building insulation material. Results Mater. 2021, 10, 100189. [Google Scholar] [CrossRef]
- Thermtest. Materials Thermal Properties Database. Available online: https://thermtest.com/thermal-resources/materials-database (accessed on 23 March 2024).
- Jones, M.; Bhat, T.; Kandare, E.; Thomas, A.; Joseph, P.; Dekiwadia, C.; Yuen, R.; John, S.; Ma, J.; Wang, C.-H. Thermal degradation and fire properties of fungal mycelium and mycelium-biomass composite materials. Sci. Rep. 2018, 8, 17583. [Google Scholar] [CrossRef]
- Shakir, M.A.; Azahari, B.; Yusup, Y.; Yhaya, M.F.; Salehabadi, A.; Ahmad, M.I. Preparation and characterization of mycelium as a bio-matrix in fabrication of bio-composite. J. Adv. Res. Fluid Mech. Therm. Sci. 2020, 65, 253–263. [Google Scholar]
- Walter, N.; Gürsoy, B. A study on the sound absorption properties of mycelium-based composites cultivated on waste paper-based substrates. Biomimetics 2022, 7, 100. [Google Scholar] [CrossRef]
- Sim, J.S.; Zulkifli, R.; Tahir, M.M.; Elwaleed, A.K. Recycled paper fibres as sound absorbing material. Appl. Mech. Mater. 2014, 663, 459–463. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).