Modeling of the Effect of Subperiosteal Hydrostatic Pressure Conductivity between Joints on Decreasing Contact Loads on Cartilage and of the Effect of Myofascial Relief in Treating Trigger Points: The Floating Skeleton Theory
Abstract
1. Introduction
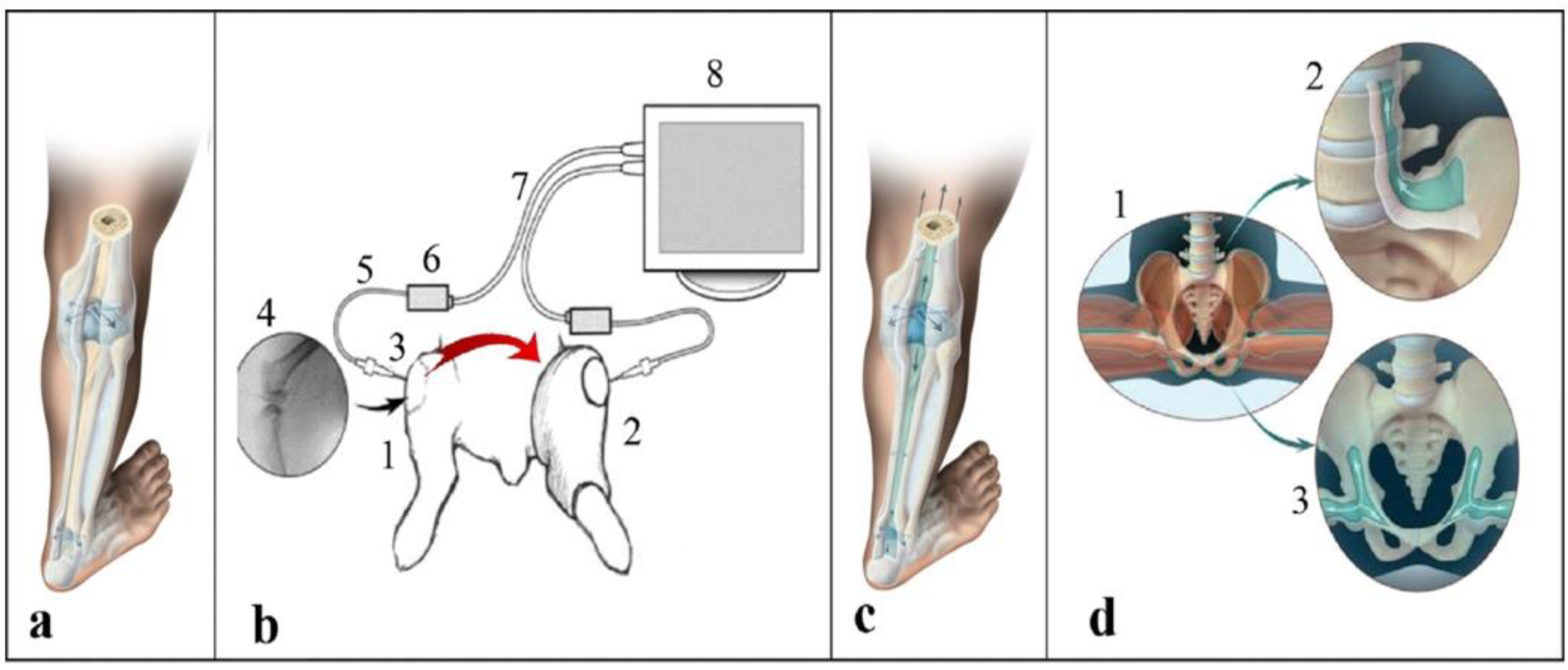
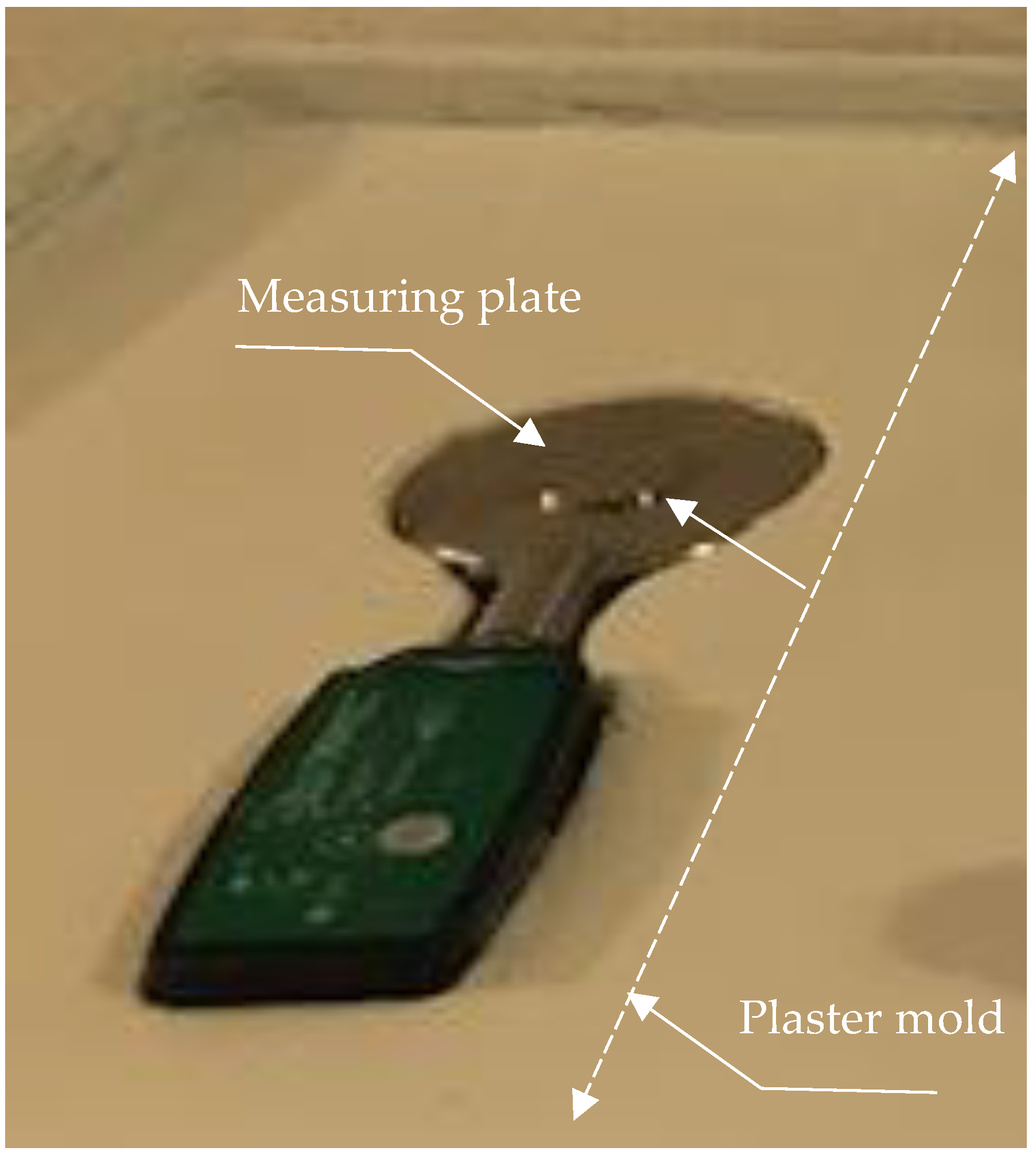
2. Methods
Hydromechanical Model
3. Data Analysis
4. Results
5. Discussion
Trigger Points: Additional Consideration
6. Conclusions
- The experiments presented in this paper modeled:
- (a)
- Loading between two surfaces without a layer of fluid between them (experiment 1) and with a layer of fluid between them (experiment 2).
- A documented reduction in loading in experiment 2 may help to provide a better understanding of the effect of recently discovered pressure sharing between the joint capsules (Floating Skeleton system) protecting contacting cartilages.
- Future studies may suggest that methods focused on maintaining/restoring the normal functioning of the Floating Skeleton system can be harnessed by more efficient rehabilitative strategies.
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, C.R.; Won Choi, K.; Negrut, D.; Thelen, D.G. Efficient computation of cartilage contact pressures within dynamic simulations of movement. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2018, 6, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Thambyah, A.; Goh, J.C.H.; De, S.D. Contact stresses in the knee joint in deep flexion. Med. Eng. Phys. 2005, 27, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Morrell, K.C.; Hodge, W.A.; Krebs, D.E.; Mann, R.W. Corroboration of in vivo cartilage pressures with implications for synovial joint tribology and osteoarthritis causation. Proc. Natl. Acad. Sci. USA 2005, 102, 14819–14824. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.W.; Sorvig, K. Sustainable Landscape Construction: A Guide to Green Building Outdoors; Island Press: Washington, DC, USA, 2008. [Google Scholar]
- Mak, A.; Lai, W.M.; Mow, V.C. Biphasic indentation of articular cartilage—I. Theoretical analysis. J. Biomech. 1987, 20, 703–714. [Google Scholar] [CrossRef]
- Sun, H.B. Mechanical loading, cartilage degradation, and arthritis. Ann. N. Y. Acad. Sci. 2010, 1211, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.T.; Cao, Y.P.; Zhou, X.H.; Han, D. Interstitial Fluid Behavior and Diseases. Adv. Sci. 2022, 9, e2100617. [Google Scholar] [CrossRef] [PubMed]
- Ateshian, G.A. The role of interstitial fluid pressurization in articular cartilage lubrication. J. Biomech. 2009, 42, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Pitkin, M.; Cassidy, C.; Muppavarapu, R.; Pitkin, E. Subperiosteal Transmission of Intra-Articular Pressure Between Articulated and Stationary Joints. Nat. Sci. Rep. 2015, 5, 8103. [Google Scholar] [CrossRef] [PubMed]
- Gray, H.; Goss, C.M. Anatomy of the Human Body; Lea & Febiger: Philadelphia, PA, USA, 1966. [Google Scholar]
- Levick, J.R. An investigation into the validity of subatmospheric pressure recordings from synovial fluid and their dependence on joint angle. J. Physiol. 1979, 289, 55–67. [Google Scholar] [CrossRef]
- Jayson, M. Intra-articular Pressure. Clin. Rheum. Dis. 1981, 7, 149–166. [Google Scholar] [CrossRef]
- Prange, H.D. Laplace’s law and the alveolus: A misconception of anatomy and a misapplication of physics. Adv. Physiol. Educ. 2003, 27, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Tarasevicius, S.; Kesteris, U.; Gelmanas, A.; Smailys, A.; Wingstrand, H. Intracapsular pressure and elasticity of the hip joint capsule in osteoarthritis. J. Arthroplast. 2007, 22, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Pitkin, M. Biomechanics for Life. In Introduction to Sanomechanics; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Verdonck, P.; Dumont, K. Biofluid mechanics and the circulatory system. Technol. Health Care 2011, 19, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Pitkin, M. New Training System Based on the Discovery of Subperiosteal Transmission of Pressures Between Joint Capsules. Mil. Med. 2021, 186, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Greene, W.B.; Netter, F.H. Netter’s Orthopaedics; Saunders Elsevier: Philadelphia, PA, USA, 2006. [Google Scholar]
- Dwek, J.R. The periosteum: What is it, where is it, and what mimics it in its absence? Skelet. Radiol. 2010, 39, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Popowics, T.E.; Zhu, Z.; Herring, S.W. Mechanical properties of the periosteum in the pig, Sus scrofa. Arch. Oral Biol. 2002, 47, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.D.; Levick, J.R. The density and distribution of capillaries around a synovial cavity. Exp. Physiol. 1983, 68, 629. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.D.; Carter, D.R. The mechanobiological effects of periosteal surface loads. Biomech. Model. Mechanobiol. 2008, 7, 227–242. [Google Scholar] [CrossRef]
- Chartier, S.R.; Mitchell, S.A.T.; Majuta, L.A.; Mantyh, P.W. The Changing Sensory and Sympathetic Innervation of the Young, Adult and Aging Mouse Femur. Neuroscience 2018, 387, 178–190. [Google Scholar] [CrossRef] [PubMed]
- McBride, S.H.; Evans, S.F.; Knothe Tate, M.L. Anisotropic mechanical properties of ovine femoral periosteum and the effects of cryopreservation. J. Biomech. 2011, 44, 1954–1959. [Google Scholar] [CrossRef]
- Reid, H.; Wood, S. Achilles Tendinopathy: Advice and Management; Oxford University Hospitals NHS Foundation Trust: Oxford, UK, 2015. [Google Scholar]
- Benjamin, M.; Kumai, T.; Milz, S.; Boszczyk, B.M.; Boszczyk, A.A.; Ralphs, J.R. The skeletal attachment of tendons—Tendon “entheses”. Comp Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Dorfl, J. Migration of tendinous insertions. I. Cause and mechanism. J. Anat. 1980, 131, 179–195. [Google Scholar] [PubMed]
- Lu, H.H.; Thomopoulos, S. Functional attachment of soft tissues to bone: Development, healing, and tissue engineering. Annu. Rev. Biomed. Eng. 2013, 15, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Pitkin, M. Sanomechanical criterion of correctness of exercising based on a floating skeleton concept. J. Nov. Physiother. 2016, 6, 97. [Google Scholar]
- Zugel, M.; Maganaris, C.N.; Wilke, J.; Jurkat-Rott, K.; Klingler, W.; Wearing, S.C.; Findley, T.; Barbe, M.F.; Steinacker, J.M.; Vleeming, A.; et al. Fascial tissue research in sports medicine: From molecules to tissue adaptation, injury and diagnostics: Consensus statement. Br. J. Sports Med. 2018, 52, 1497. [Google Scholar] [CrossRef] [PubMed]
- Stecco, A.; Giordani, F.; Fede, C.; Pirri, C.; De Caro, R.; Stecco, C. From Muscle to the Myofascial Unit: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 4527. [Google Scholar] [CrossRef] [PubMed]
- Gerwin, R.D. A New Unified Theory of Trigger Point Formation: Failure of Pre-and Post-Synaptic Feedback Control Mechanisms. Int. J. Mol. Sci. 2023, 24, 8142. [Google Scholar] [CrossRef] [PubMed]
- Travell, J.; Rinzler, S.H. The myofascial genesis of pain. Postgrad. Med. 1952, 11, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Hua, N.K.; Van der Does, E. The occurrence and inter-rater reliability of myofascial trigger points in the quadratus lumborum and gluteus medius: A prospective study in non-specific low back pain patients and controls in general practice. Pain 1994, 58, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Bogduk, N. The anatomy and pathophysiology of neck pain. Phys. Med. Rehabil. Clin. 2003, 14, 455–472. [Google Scholar] [CrossRef]
- Schroeder, B.; Sanfilippo, J.S.; Hertweck, S.P. Musculoskeletal pelvic pain in a pediatric and adolescent gynecology practice. J. Pediatr. Adolesc. Gynecol. 2000, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, S.; Shah, J.P.; Gebreab, T.; Yen, R.H.; Gilliams, E.; Danoff, J.; Gerber, L.H. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Arch. Phys. Med. Rehabil. 2009, 90, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Bron, C.; Dommerholt, J.D. Etiology of myofascial trigger points. Curr. Pain Headache Rep. 2012, 16, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Vahdatinia, R.; Humbert, S.; Stecco, A. Myofascial injection using fascial layer-specific hydromanipulation technique (FLuSH) and the delineation of multifactorial myofascial pain. Medicina 2020, 56, 717. [Google Scholar] [CrossRef]
- Jacobs, J. Myofascial Release Method. U.S. Patent 9649244B1, 16 May 2017. [Google Scholar]
- Liu, C.; Wang, Y.; Yu, W.; Xiang, J.; Ding, G.; Liu, W. Comparative effectiveness of non-invasive therapeutic interventions for myofascial pain syndrome: A network meta-analysis of randomized controlled trials. Int. J. Surg. 2023, 110, 1099–1112. [Google Scholar] [CrossRef]


| Experimental Run | Condition 1 (with Obstruction) | Condition 2 (without Obstruction) | Difference |
|---|---|---|---|
| 1 | 0.1 | 0.7 | |
| 2 | 0.1 | 0.7 | |
| 3 | 0.1 | 0.7 | |
| 4 | 0.1 | 0.7 | |
| 5 | 0.1 | 0.7 | |
| 6 | 0.1 | 0.7 | |
| 7 | 0.1 | 0.6 | |
| 8 | 0.1 | 0.7 | |
| 9 | 0.1 | 0.7 | |
| 10 | 0.1 | 0.7 | |
| Average | 0.1 | 0.969 | 0.031 |
| SD | 0.0 | 0.0032 | 0.0032 |
| t-statistics | 9.8 | ||
| p-value | <0.000001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitkin, M.R. Modeling of the Effect of Subperiosteal Hydrostatic Pressure Conductivity between Joints on Decreasing Contact Loads on Cartilage and of the Effect of Myofascial Relief in Treating Trigger Points: The Floating Skeleton Theory. Biomimetics 2024, 9, 222. https://doi.org/10.3390/biomimetics9040222
Pitkin MR. Modeling of the Effect of Subperiosteal Hydrostatic Pressure Conductivity between Joints on Decreasing Contact Loads on Cartilage and of the Effect of Myofascial Relief in Treating Trigger Points: The Floating Skeleton Theory. Biomimetics. 2024; 9(4):222. https://doi.org/10.3390/biomimetics9040222
Chicago/Turabian StylePitkin, Mark R. 2024. "Modeling of the Effect of Subperiosteal Hydrostatic Pressure Conductivity between Joints on Decreasing Contact Loads on Cartilage and of the Effect of Myofascial Relief in Treating Trigger Points: The Floating Skeleton Theory" Biomimetics 9, no. 4: 222. https://doi.org/10.3390/biomimetics9040222
APA StylePitkin, M. R. (2024). Modeling of the Effect of Subperiosteal Hydrostatic Pressure Conductivity between Joints on Decreasing Contact Loads on Cartilage and of the Effect of Myofascial Relief in Treating Trigger Points: The Floating Skeleton Theory. Biomimetics, 9(4), 222. https://doi.org/10.3390/biomimetics9040222







