Warm Air Delivery in Adhesive Application: Effect on Bonding Performance and Morphological Outcomes
Abstract
1. Introduction
2. Materials and Methods
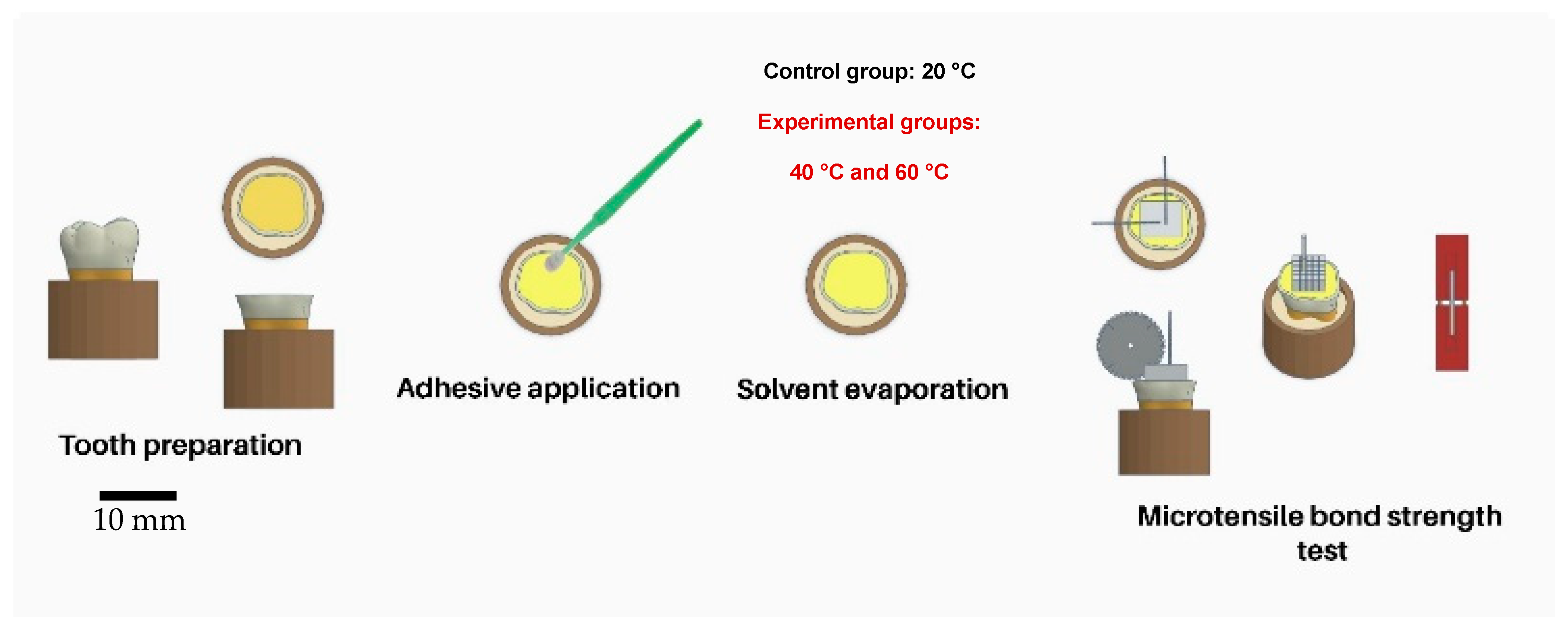
2.1. Tooth Selection
2.2. Dentin Specimen Preparation
2.3. Bonding Procedures
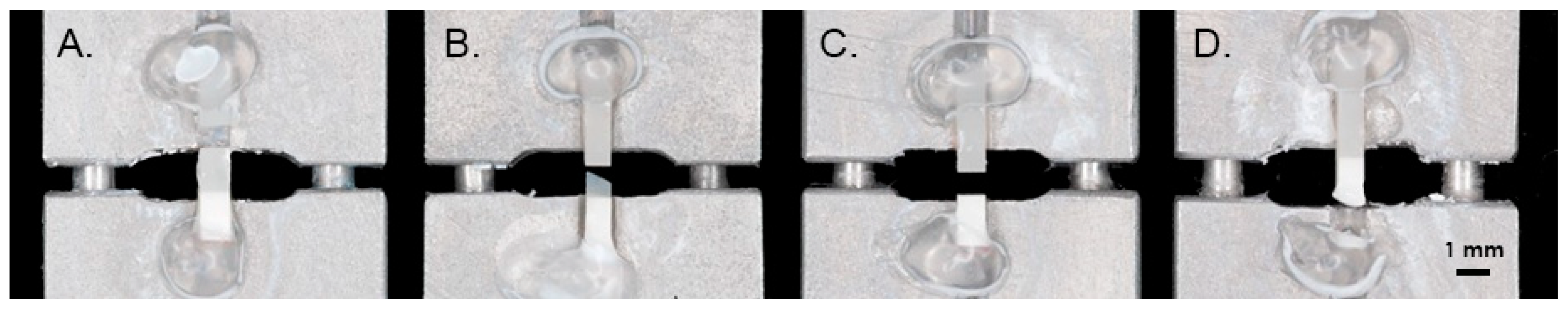
2.4. Micro-Tensile Bond Strength Testing
2.5. Failure Analysis
2.6. Scanning Electron Microscopy of Resin–Dentin-Bonded Interface
2.7. Solvent Evaporation Rate
2.8. Statistical Analysis
3. Results
3.1. Micro-Tensile Bond Strength Testing
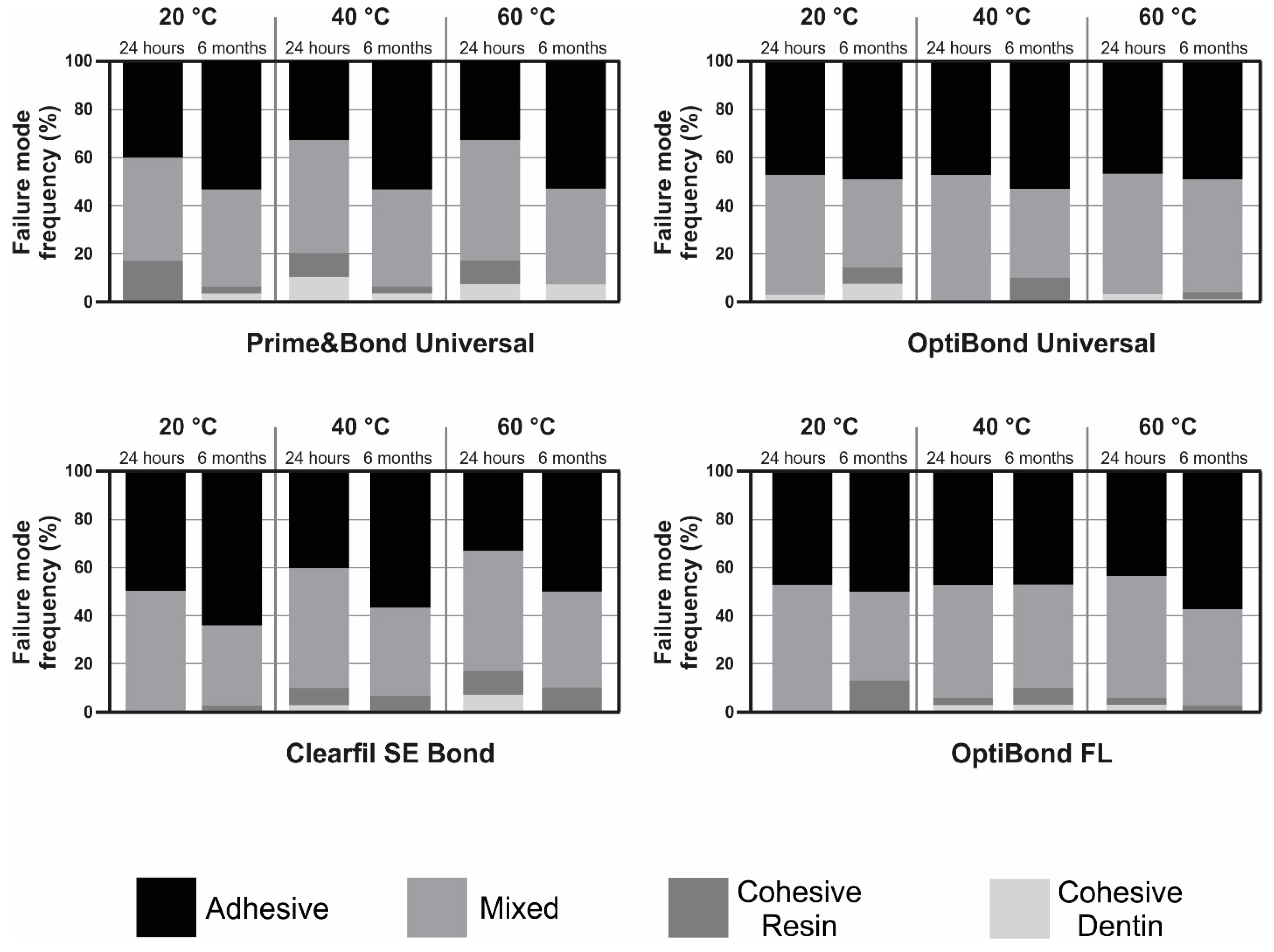
3.2. Failure Mode Analysis
3.3. Scanning Electron Microscopy of Resin–Dentin-Bonded Interface
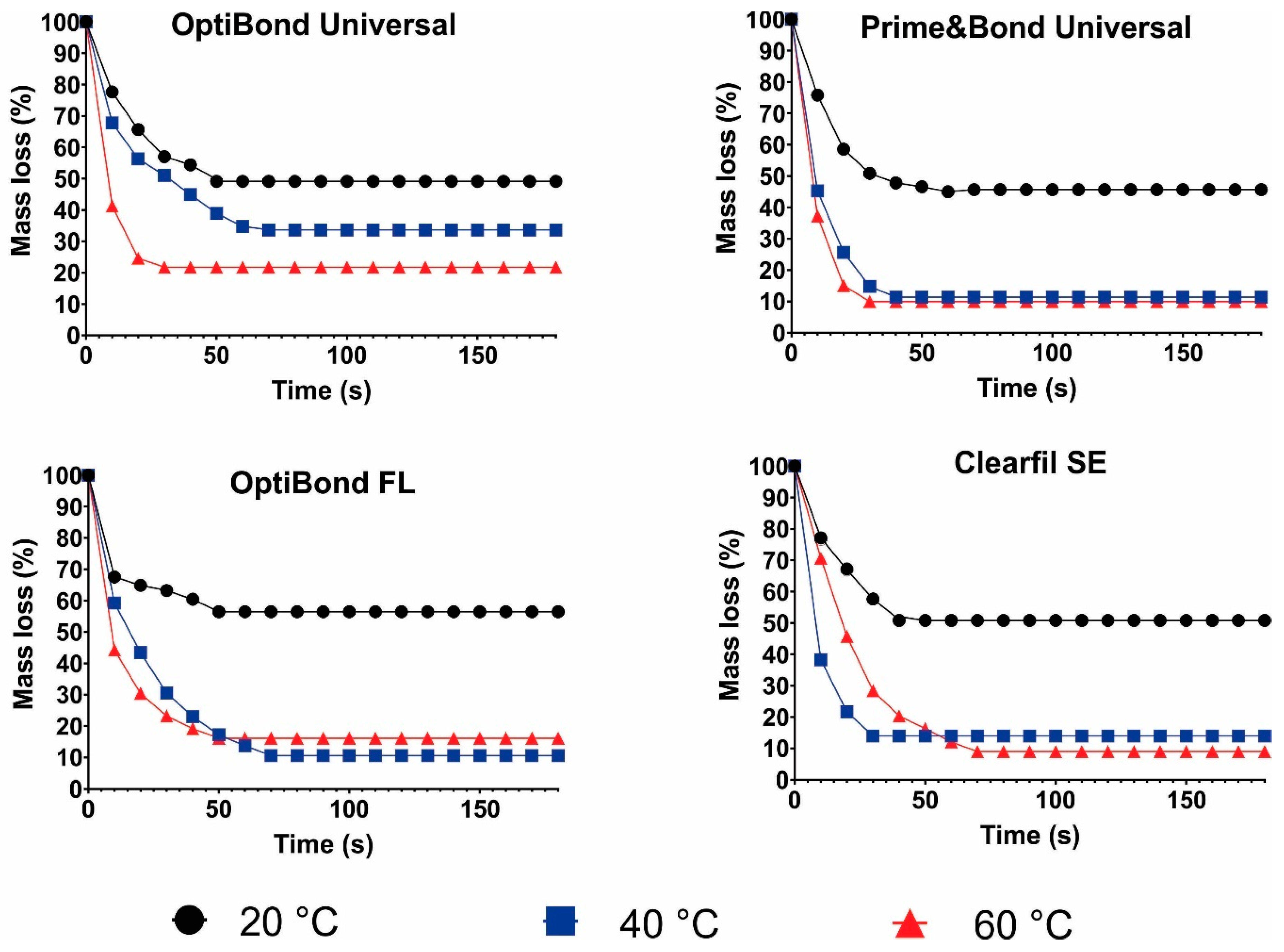
3.4. Solvent Evaporation Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Yan, X.; Li, K.; Zheng, S.; Sano, H.; Zhan, D.; Fu, J. Effect of air-blowing temperature and water storage time on the bond strength of five universal adhesive systems to dentin. Dent. Mater. J. 2021, 40, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.; de Almeida Neves, A.; Mine, A.; Coutinho, E.; Van Landuyt, K.; De Munck, J.; Van Meerbeek, B. Current Aspects on Bonding Effectiveness and Stability in Adhesive Dentistry. Aust. Dent. J. 2011, 56, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, A.D.; Luque-Martinez, I.; Muñoz, M.A.; Szesz, A.L.; Cuadros-Sánchez, J.; Reis, A. A Comprehensive Laboratory Screening of Three-Step Etch-and-Rinse Adhesives. Oper. Dent. 2014, 39, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Alex, G. Universal Adhesives: The Next Evolution in Adhesive Dentistry? Compend. Contin. Educ. Dent. 2015, 36, 15–26. [Google Scholar] [PubMed]
- Bedran-Russo, A.; Leme-Kraus, A.A.; Vidal, C.M.P.; Teixeira, E.C. An Overview of Dental Adhesive Systems and the Dynamic Tooth–Adhesive Interface. Dent. Clin. N. Am. 2017, 61, 713–731. [Google Scholar] [CrossRef] [PubMed]
- Kambaram, M.; Yiu, C.K.Y.; Matinlinna, J.P. An overview of solvents in resin-dentin bonding. Int. J. Adhes. Adhes. 2015, 57, 22–33. [Google Scholar] [CrossRef]
- Fontes, S.T.; Fernández, M.R.; Ogliari, F.A.; de Carvalho, R.V.; de Moraes, R.R.; Pinto, M.B.; Piva, E. Tetrahydrofuran as solvent in dental adhesives: Cytotoxicity and dentin bond stability. Clin. Oral Investig. 2013, 17, 237–242. [Google Scholar] [CrossRef]
- Tjäderhane, L.; Mehtälä, P.; Scaffa, P.; Vidal, C.; Pääkkönen, V.; Breschi, L.; Hebling, J.; Tay, F.R.; Nascimento, F.D.; Pashley, D.H.; et al. The effect of dimethyl sulfoxide (DMSO) on dentin bonding and nanoleakage of etch-and-rinse adhesives. Dent. Mater. 2013, 29, 1055–1062. [Google Scholar] [CrossRef]
- Klein-Junior, C.A.; Zander-Grande, C.; Amaral, R.; Stanislawczuk, R.; Garcia, E.J.; Baumhardt-Neto, R.; Meier, M.M.; Loguercio, A.D.; Reis, A. Evaporating Solvents with a Warm Air-Stream: Effects on Adhesive Layer Properties and Resin–Dentin Bond Strengths. J. Dent. 2008, 36, 618–625. [Google Scholar] [CrossRef]
- Bourgi, R.; Hardan, L.; Rivera-Gonzaga, A.; Cuevas-Suárez, C.E. Effect of Warm-Air Stream for Solvent Evaporation on Bond Strength of Adhesive Systems: A Systematic Review and Meta-Analysis of in Vitro Studies. Int. J. Adhes. Adhes. 2021, 105, 102794. [Google Scholar] [CrossRef]
- Bail, M.; Malacarne-Zanon, J.; Silva, S.; Anauate-Netto, A.; Nascimento, F.; Amore, R.; Lewgoy, H.; Pashley, D.H.; Carrilho, M. Effect of Air-Drying on the Solvent Evaporation, Degree of Conversion and Water Sorption/Solubility of Dental Adhesive Models. J. Mater. Sci. Mater. Med. 2012, 23, 629–638. [Google Scholar] [CrossRef]
- Taguchi, K.; Hosaka, K.; Ikeda, M.; Kishikawa, R.; Foxton, R.; Nakajima, M.; Tagami, J. The Effect of Warm Air-Blowing on the Microtensile Bond Strength of One-Step Self-Etch Adhesives to Root Canal Dentin. J. Prosthodont. Res. 2018, 62, 330–336. [Google Scholar] [CrossRef]
- Bourgi, R.; Hardan, L.; Cuevas-Suárez, C.E.; Scavello, F.; Mancino, D.; Kharouf, N.; Haikel, Y. The Use of Warm Air for Solvent Evaporation in Adhesive Dentistry: A Meta-Analysis of In Vitro Studies. J. Funct. Biomater. 2023, 14, 285. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Shimizu, Y.; Shiratsuchi, K.; Tsujimoto, A.; Takamizawa, T.; Ando, S.; Miyazaki, M. Effect of Warm Air-Drying on Dentin Bond Strength of Single-Step Self-Etch Adhesives. Dent. Mater. J. 2012, 31, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Santana, F.R.; Pereira, J.C.; Pereira, C.A.; Fernandes Neto, A.J.; Soares, C.J. Influence of Method and Period of Storage on the Microtensile Bond Strength of Indirect Composite Resin Restorations to Dentine. Braz. Oral Res. 2008, 22, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, K.; Hosaka, K.; Tichy, A.; Taguchi, K.; Ikeda, M.; Thanatvarakorn, O.; Prasansuttiporn, T.; Nakajima, M.; Tagami, J. Air-Blowing Strategies for Improving the Microtensile Bond Strength of One-Step Self-Etch Adhesives to Root Canal Dentin. Dent. Mater. J. 2020, 39, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.; Breschi, L.; Özcan, M.; Pfefferkorn, F.; Ferrari, M.; Van Meerbeek, B. Academy of Dental Materials Guidance on in Vitro Testing of Dental Composite Bonding Effectiveness to Dentin/Enamel Using Micro-Tensile Bond Strength (μTBS) Approach. Dent. Mater. 2017, 33, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.C.; Almeida, J.C.; Osorio, R.; Carvalho, R.M.; Toledano, M. Influence of Drying Time and Temperature on Bond Strength of Contemporary Adhesives to Dentine. J. Dent. 2009, 37, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Hanabusa, M.; Mine, A.; Kuboki, T.; Momoi, Y.; Van Ende, A.; Van Meerbeek, B.; De Munck, J. Bonding effectiveness of a new «multi-mode» adhesive to enamel and dentine. J. Dent. 2012, 40, 475–484. [Google Scholar] [CrossRef]
- Tsujimoto, A.; Fischer, N.G.; Barkmeier, W.W.; Latta, M.A. Bond durability of two-step HEMA-free universal adhesive. J. Funct. Biomater. 2022, 13, 134. [Google Scholar] [CrossRef]
- Pashley, D.H.; Agee, K.; Nakajima, M.; Tay, F.; Carvalho, R.; Terada, R.; Harmon, F.; Lee, W.; Rueggeberg, F. Solvent-induced Dimensional Changes in EDTA-demineralized Dentin Matrix. J. Biomed. Mater. Res. 2001, 56, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Saeed, N.A.; Tichy, A.; Kuno, Y.; Hosaka, K.; Tagami, J.; Nakajima, M. Effect of Surface Moisture on Bur-Cut Dentin on Bonding of HEMA-Free and HEMA-Containing Universal Adhesives with or without Methacrylamide Monomer. J. Adhes. Dent. 2021, 23, 327–334. [Google Scholar] [PubMed]
- Paken, G.; Çömlekoğlu, M.E.; Sonugelen, M. Detection of the hybrid layer biodegradation initiation factor with a scanning electron microscope. Microsc. Res. Tech. 2021, 84, 2166–2175. [Google Scholar] [CrossRef] [PubMed]
- Breschi, L.; Maravic, T.; Cunha, S.R.; Comba, A.; Cadenaro, M.; Tjäderhane, L.; Pashley, D.H.; Tay, F.R.; Mazzoni, A. Dentin bonding systems: From dentin collagen structure to bond preservation and clinical applications. Dent. Mater. 2018, 34, 78–96. [Google Scholar] [CrossRef] [PubMed]
- Bourgi, R.; Kharouf, N.; Cuevas-Suárez, C.E.; Lukomska-Szymańska, M.; Devoto, W.; Kassis, C.; Hasbini, O.; Mancino, D.; Haikel, Y.; Hardan, L. Effect of Modified Triple-Layer Application on the Bond Strength of Different Dental Adhesive Systems to Dentin. J. Funct. Biomater. 2023, 14, 522. [Google Scholar] [CrossRef] [PubMed]
- Hardan, L.; Bourgi, R.; Kharouf, N.; Mancino, D.; Zarow, M.; Jakubowicz, N.; Haikel, Y.; Cuevas-Suárez, C.E. Bond Strength of Universal Adhesives to Dentin: A Systematic Review and Meta-Analysis. Polymers 2021, 13, 814. [Google Scholar] [CrossRef] [PubMed]
- Al-Salamony, H.; Naguibb, E.A.; Hamzac, H.S.; Younisd, S.H. The Effect of Warm Air Solvent Evaporation on Microtensile Bond Strength of Two Different Self-Etch Adhesives to Dentin (In Vitro Study). SYLWAN 2020, 164, 479–497. [Google Scholar]
- Van Meerbeek, B.; Van Landuyt, K.; De Munck, J.; Hashimoto, M.; Peumans, M.; Lambrechts, P.; Yoshida, Y.; Inoue, S.; Suzuki, K. Technique-sensitivity of contemporary adhesives. Dent. Mater. J. 2005, 24, 1–13. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Snauwaert, J.; Peumans, M.; De Munck, J.; Lambrechts, P.; Van Meerbeek, B. The role of HEMA in one-step self-etch adhesives. Dent. Mater. 2008, 24, 1412–1419. [Google Scholar] [CrossRef]
- Frassetto, A.; Breschi, L.; Turco, G.; Marchesi, G.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Cadenaro, M. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability—A literature review. Dent. Mater. 2016, 32, 41–53. [Google Scholar] [CrossRef]
- Spencer, P.; Wang, Y.; Walker, M.P.; Wieliczka, D.M.; Swafford, J.R. Interfacial chemistry of the dentin/adhesive bond. J. Dent. Res. 2000, 79, 1458–1463. [Google Scholar] [CrossRef]
- Choi, A.N.; Lee, J.H.; Son, S.A.; Jung, K.H.; Kwon, Y.H.; Park, J.K. Effect of Dentin Wetness on the Bond Strength of Universal Adhesives. Materials 2017, 10, 1224. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.; Wang, Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J. Biomed. Mater. Res. 2002, 62, 447–456. [Google Scholar] [CrossRef]
- Nagura, Y.; Tsujimoto, A.; Fischer, N.G.; Baruth, A.G.; Barkmeier, W.W.; Takamizawa, T.; Latta, M.A.; Miyazaki, M. Effect of Reduced Universal Adhesive Application Time on Enamel Bond Fatigue and Surface Morphology. Oper. Dent. 2019, 44, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, A.; Shimatani, Y.; Nojiri, K.; Barkmeier, W.W.; Markham, M.D.; Takamizawa, T.; Latta, M.A.; Miyazaki, M. Influence of surface wetness on bonding effectiveness of universal adhesives in etch-and-rinse mode. Eur. J. Oral Sci. 2019, 127, 162–169. [Google Scholar] [CrossRef]
- Han, F.; Dai, S.; Yang, J.; Shen, J.; Liao, M.; Xie, H.; Chen, C. Glycerol Phosphate Dimethacrylate: An Alternative Functional Phosphate Ester Monomer to 10-Methacryloyloxydecyl Dihydrogen Phosphate for Enamel Bonding. ACS Omega 2020, 5, 24826–24837. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi, M.R.; Tambare, O.H.; More, A.P. Performance of various fillers in adhesives applications: A review. Polym. Bull. 2022, 79, 10491–10553. [Google Scholar] [CrossRef]
- Jacobsen, T.; Söderholm, K. Effect of primer solvent, primer agitation, and dentin dryness on shear bond strength to dentin. Am. J. Dent. 1998, 11, 225–228. [Google Scholar]
- Nihi, F.M.; Fabre, H.S.C.; Garcia, G.; Fernandes, K.B.P.; Ferreira, F.B.D.A.; Wang, L. In vitro assessment of solvent evaporation from commercial adhesive systems compared to experimental systems. Braz. Dent. J. 2009, 20, 396–402. [Google Scholar] [CrossRef]
- De Munck, J.; Van Landuyt, K.; Peumans, M.; Poitevin, A.; Lambrechts, P.; Braem, M.; Van Meerbeek, B. A critical review of the durability of adhesion to tooth tissue: Methods and results. J. Dent. Res. 2005, 84, 118–132. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.J.; De Munck, J.; Van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef]
- Peumans, M.; De Munck, J.; Van Landuyt, K.; Van Meerbeek, B. Thirteen-year randomized controlled clinical trial of a twostep self-etch adhesive in non-carious cervical lesions. Dent. Mater. 2015, 31, 308–314. [Google Scholar] [CrossRef]
- Cuevas-Suárez, C.E.; Ramos, T.S.; Rodrigues, S.B.; Collares, F.M.; Zanchi, C.H.; Lund, R.G.; da Silva, A.F.; Piva, E. Impact of Shelf-Life Simulation on Bonding Performance of Universal Adhesive Systems. Dent. Mater. 2019, 35, e204–e219. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.B.; Petzhold, C.L.; Gamba, D.; Leitune, V.C.B.; Collares, F.M. Acrylamides and methacrylamides as alternative monomers for dental adhesives. Dent. Mater. 2018, 34, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Peumans, M.; De Munck, J.; Van Landuyt, K.L.; Poitevin, A.; Lambrechts, P.; Van Meerbeek, B. Eight-year clinical evaluation of a 2-step self-etch adhesive with and without selective enamel etching. Dent. Mater. 2010, 26, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, A.D.; Bittencourt, D.D.; Baratieri, L.N.; Reis, A. A 36-month evaluation of self-etch and etch- and-rinse adhesives in noncarious cervical lesions. J. Am. Dent. Assoc. 2007, 138, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Teshima, I. Degradation of 10-Methacryloyloxydecyl Dihydrogen Phosphate. J. Dent. Res. 2010, 89, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Yoshihara, K.; Van Landuyt, K.; Yoshida, Y.; Peumans, M. From Buonocore’s Pioneering Acid-Etch Technique to Self-Adhering Restoratives. A Status Perspective of Rapidly Advancing Dental Adhesive Technology. J. Adhes. Dent. 2020, 22, 7–34. [Google Scholar] [PubMed]
- Luque-Martinez, I.V.; Perdigão, J.; Muñoz, M.A.; Sezinando, A.; Reis, A.; Loguercio, A.D. Effects of solvent evaporation time on immediate adhesive properties of universal adhesives to dentin. Dent. Mater. 2014, 30, 1126–1135. [Google Scholar] [CrossRef]
- Cho, B.; Dickens, S.H. Effects of the acetone content of single solution dentin bonding agents on the adhesive layer thickness and the microtensile bond strength. Dent. Mater. 2004, 20, 107–115. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Yoshihara, K.; Yao, C.; Okazaki, Y.; Van Landuyt, K.; Peumans, M.; Van Meerbeek, B. Multiparameter evaluation of acrylamide HEMA alternative monomers in 2-step adhesives. Dent. Mater. 2021, 37, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Fukegawa, D.; Hayakawa, S.; Yoshida, Y.; Suzuki, K.; Osaka, A.; Van Meerbeek, B. Chemical interaction of phosphoric acid ester with hydroxyapatite. J. Dent. Res. 2006, 85, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Valsan, D.; Bhaskaran, S.; Mathew, J.; Hari, K.; Joy, J. Comparative Evaluation of the Bonding Efficacy of Multimode Adhesive, Two-Step Self-Etch Adhesive, and a Total-Etch System to Pulpal Floor Dentin—An In vitro Study. Contemp. Clin. Dent. 2023, 14, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the Art Etch-and-Rinse Adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef]
- Moura, S.K.; Lemos, L.V.; Myszkovisk, S.; Provenzano, M.G.; Balducci, I.; Myaki, S.I. Bonding durability of dental sealants to deciduous and permanent teeth. Braz. J. Oral Sci. 2014, 13, 198–202. [Google Scholar] [CrossRef]
- El Zohairy, A.; De Gee, A.; De Jager, N.; Van Ruijven, L.; Feilzer, A. The Influence of Specimen Attachment and Dimension on Microtensile Strength. J. Dent. Res. 2004, 83, 420–424. [Google Scholar] [CrossRef]
- Takahashi, M.; Nakajima, M.; Hosaka, K.; Ikeda, M.; Foxton, R.M.; Tagami, J. Long-term evaluation of water sorption and ultimate tensile strength of HEMA-containing/-free one-step self-etch adhesives. J. Dent. 2011, 39, 506–512. [Google Scholar] [CrossRef]
- Sano, H.; Chowdhury, A.F.M.A.; Saikaew, P.; Matsumoto, M.; Hoshika, S.; Yamauti, M. The microtensile bond strength test: Its historical background and application to bond testing. Jpn. Dent. Sci. Rev. 2020, 56, 24–31. [Google Scholar] [CrossRef]
- Armstrong, S.; Geraldeli, S.; Maia, R.; Raposo, L.H.A.; Soares, C.J.; Yamagawa, J. Adhesion to tooth structure: A critical review of “micro” bond strength test methods. Dent. Mater. 2010, 26, 50–62. [Google Scholar] [CrossRef]
- Reis, A.; Klein-Junior, C.A.; de Souza, F.C.; Stanislawczuk, R.; Loguercio, A.D. The Use of Warm Air Stream for Solvent Evaporation: Effects on the Durability of Resin-Dentin Bonds. Oper. Dent. 2010, 35, 29–36. [Google Scholar] [CrossRef]
- Kharouf, N.; Ashi, T.; Eid, A.; Maguina, L.; Zghal, J.; Sekayan, N.; Bourgi, R.; Hardan, L.; Sauro, S.; Haikel, Y. Does Adhesive Layer Thickness and Tag Length Influence Short/Long-Term Bond Strength of Universal Adhesive Systems? An in-Vitro Study. Appl. Sci. 2021, 11, 2635. [Google Scholar] [CrossRef]
- Sinhoreti, M.A.C.; Soares, E.F.; Abuna, G.F.; Correr, L.; Roulet, J.-F.; Geraldeli, S. Microtensile Bond Strength of Adhesive Systems in Different Dentin Regions on a Class II Cavity Configuration. Braz. Dent. J. 2017, 28, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Michelsen, V.B.; Moe, G.; Skålevik, R.; Jensen, E.; Lygre, H. Quantification of organic eluates from polymerized resin-based dental restorative materials by use of GC/MS. J. Chromatogr. B. 2007, 850, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Beloica, M.; Goracci, C.; Carvalho, C.-A.; Radovic, I.; Margvelashvili, M.; Vulicevic, Z.-R.; Ferrari, M. Microtensile vs microshear bond strength of all-in-one adhesives to unground enamel. J. Adhes. Dent. 2010, 12, 427–433. [Google Scholar]
- Sahadi, B.O.; Sebold, M.; André, C.B.; Nima, G.; Dos Santos, A.; Nascimento, F.D.; dos Santos Tersariol, I.L.; Giannini, M. Effect of experimental dentin etchants on dentin bond strength, metalloproteinase inhibition, and antibiofilm activity. Dent. Mater. 2024. [Google Scholar] [CrossRef]

| Adhesive and Manufacturer | Classification of the Material | Main Composition * | Adhesive Application Protocol |
|---|---|---|---|
| PBU (Dentsply DeTrey GmbH, Konstanz, Germany) | Mild Universal pH = 2.5 | 10-MDP, PENTA, isopropanol, water, photoinitiator, bi- and multifunctional acrylate | Using a microbrush applicator, dispense one drop of PBU to all dentinal surfaces. Avoid pooling. Keep PBU slightly agitated for 20 s. Evaporate solvent with air delivered from the device (cold or warm) for at least 10 s. Light-irradiate for 20 s. |
| OBU (Kerr Co, Orange, CA, USA) | Universal pH = 2.5–3.0 | Acetone, HEMA, GDMA, ethanol, GPDM | Using a microbrush applicator, a generous amount of OBU adhesive is applied to the dentinal surfaces. Scrub the surface with a brushing motion for 20 s. Dry the adhesive with air delivered from the device (cold or warm) for at least 10 s. Light-irradiate for 20 s. |
| OBFL (Kerr Co, Orange, CA, USA) | Three-step etch-and-rinse pH primer: 1.9; pH bonding: 6.9 | Etchant: 37.5% H3PO4 Primer: HEMA, GPDM, MMEP, water, ethanol, CQ, and BHT Adhesive: Bis-GMA, HEMA, GDMA, CQ, and filler (fumed SiO2, barium aluminoborosilicat, Na2SiF6), coupling factor A174 | Using a microbrush applicator, apply OBFL primer over dentin surfaces with a light scrubbing motion for 20 s. Dry the primer with air delivered from the device (cold or warm) for at least 10 s. At this point, the dentin surface should have a slightly shiny appearance. Using a new microbrush applicator, apply OBFL adhesive to the prepared dentin surfaces with a light scrubbing motion for 20 s, creating a thin coating. Gently air-dry for approximately 5 s from the device (cold air). Light-irradiate for 20 s. |
| CSE (Kuraray Noritake Dental Inc., Tokyo, Japan) | Two-step self-etch pH primer = 1.76 pH bond = 2 | Primer: 10-MDP, HEMA, hydrophilic dimethacrylate, CQ, DEPT, water Bond: MDP, HEMA, Bis-GMA, hydrophobic dimethacrylate, CQ, DEPT, silanized colloidal silica | Using a microbrush applicator, apply primer for 20 s. Dry with air delivered from the device (cold or warm) for at least 10 s. Using a new microbrush applicator, apply bond. Apply air flow gently from the device (cold air). Light-irradiate for 20 s. |
| Temperature for Solvent Evaporation | Prime&Bond Universal | OptiBond Universal | OptiBond FL | Clearfil SE |
|---|---|---|---|---|
| 20 °C | A 13.3 (3.4) a | A 20.9 (3.1) a | A 20.5 (5.5) a | A 20.5 (5.5) a |
| 40 °C | A 22.1 (9.9) ab | A 26.8 (7.6) a | A 29.3 (4.5) a | A 26.2 (8.2) a |
| 60 °C | A 24.6 (4.1) b | A 24.2 (3.8) a | A 29.9 (6.3) a | A 28.6 (10.4) a |
| Temperature for Solvent Evaporation | Prime&Bond Universal | OptiBond Universal | OptiBond FL | Clearfil SE |
|---|---|---|---|---|
| 20 °C | A 12.69 (6.6) a | A 16.08 (7.3) a | A 16.0 (7.4) a | A 12.7 (5.0) a |
| 40 °C | A 19.24 (6.1) a | A 24.3 (5.99) a | A 22.1 (11.4) a | A 22.5 (6.1) ab |
| 60 °C | A 19.61 (8.6) a | A 21.47 (6.6) a | A 26.2 (6.6) a | A 26.7 (4.5) b |
| Adhesive/Temperature | Aging | |
|---|---|---|
| Prime&Bond Universal | 24 h | 6 months |
| 20 °C | 13.3 (3.4) | 12.69 (6.6) |
| 40 °C | 22.1 (9.9) | 19.24 (6.1) |
| 60 °C | 24.6 (4.1) | 19.61 (8.6) |
| OptiBond Universal | 24 h | 6 months |
| 20 °C | 20.9 (3.1) | 16.08 (7.3) |
| 40 °C | 26.8 (7.6) | 24.3 (5.99) |
| 60 °C | 24.2 (3.8) | 21.47 (6.6) |
| Clearfil SE | 24 h | 6 months |
| 20 °C | 20.5 (5.5) | 12.7 (5.0) |
| 40 °C | 26.2 (8.2) | 22.5 (6.1) |
| 60 °C | 28.6 (10.4) | 26.7 (4.5) |
| OptiBond FL | 24 h | 6 months |
| 20 °C | 20.5 (5.5) | 16.0 (7.4) |
| 40 °C | 29.3 (4.5) | 22.1 (11.4) |
| 60 °C | 29.9 (6.3) | 26.2 (6.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourgi, R.; Kharouf, N.; Cuevas-Suárez, C.E.; Lukomska-Szymanska, M.; Kharma, K.; Moussa, F.H.; Metlej, M.; Haikel, Y.; Hardan, L. Warm Air Delivery in Adhesive Application: Effect on Bonding Performance and Morphological Outcomes. Biomimetics 2024, 9, 194. https://doi.org/10.3390/biomimetics9040194
Bourgi R, Kharouf N, Cuevas-Suárez CE, Lukomska-Szymanska M, Kharma K, Moussa FH, Metlej M, Haikel Y, Hardan L. Warm Air Delivery in Adhesive Application: Effect on Bonding Performance and Morphological Outcomes. Biomimetics. 2024; 9(4):194. https://doi.org/10.3390/biomimetics9040194
Chicago/Turabian StyleBourgi, Rim, Naji Kharouf, Carlos Enrique Cuevas-Suárez, Monika Lukomska-Szymanska, Khalil Kharma, Fabienne Hajj Moussa, Manar Metlej, Youssef Haikel, and Louis Hardan. 2024. "Warm Air Delivery in Adhesive Application: Effect on Bonding Performance and Morphological Outcomes" Biomimetics 9, no. 4: 194. https://doi.org/10.3390/biomimetics9040194
APA StyleBourgi, R., Kharouf, N., Cuevas-Suárez, C. E., Lukomska-Szymanska, M., Kharma, K., Moussa, F. H., Metlej, M., Haikel, Y., & Hardan, L. (2024). Warm Air Delivery in Adhesive Application: Effect on Bonding Performance and Morphological Outcomes. Biomimetics, 9(4), 194. https://doi.org/10.3390/biomimetics9040194












