The Role of Vasculature and Angiogenic Strategies in Bone Regeneration
Abstract
1. Introduction
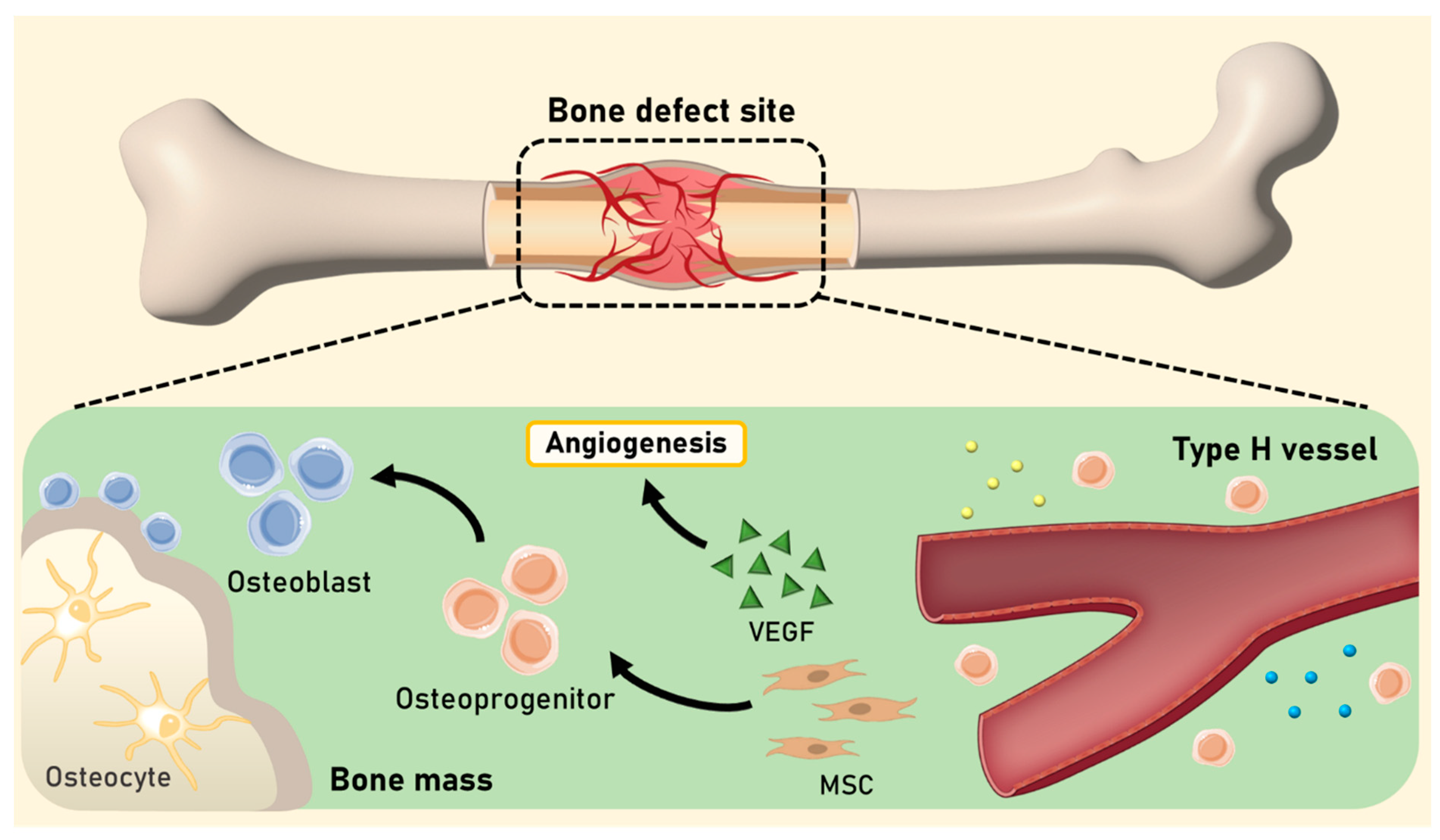
2. Angiogenesis in Bone Regeneration
3. Conventional Strategies for Vascularized Bone Tissue Engineering
4. Angiogenic Strategies for Bone Regeneration
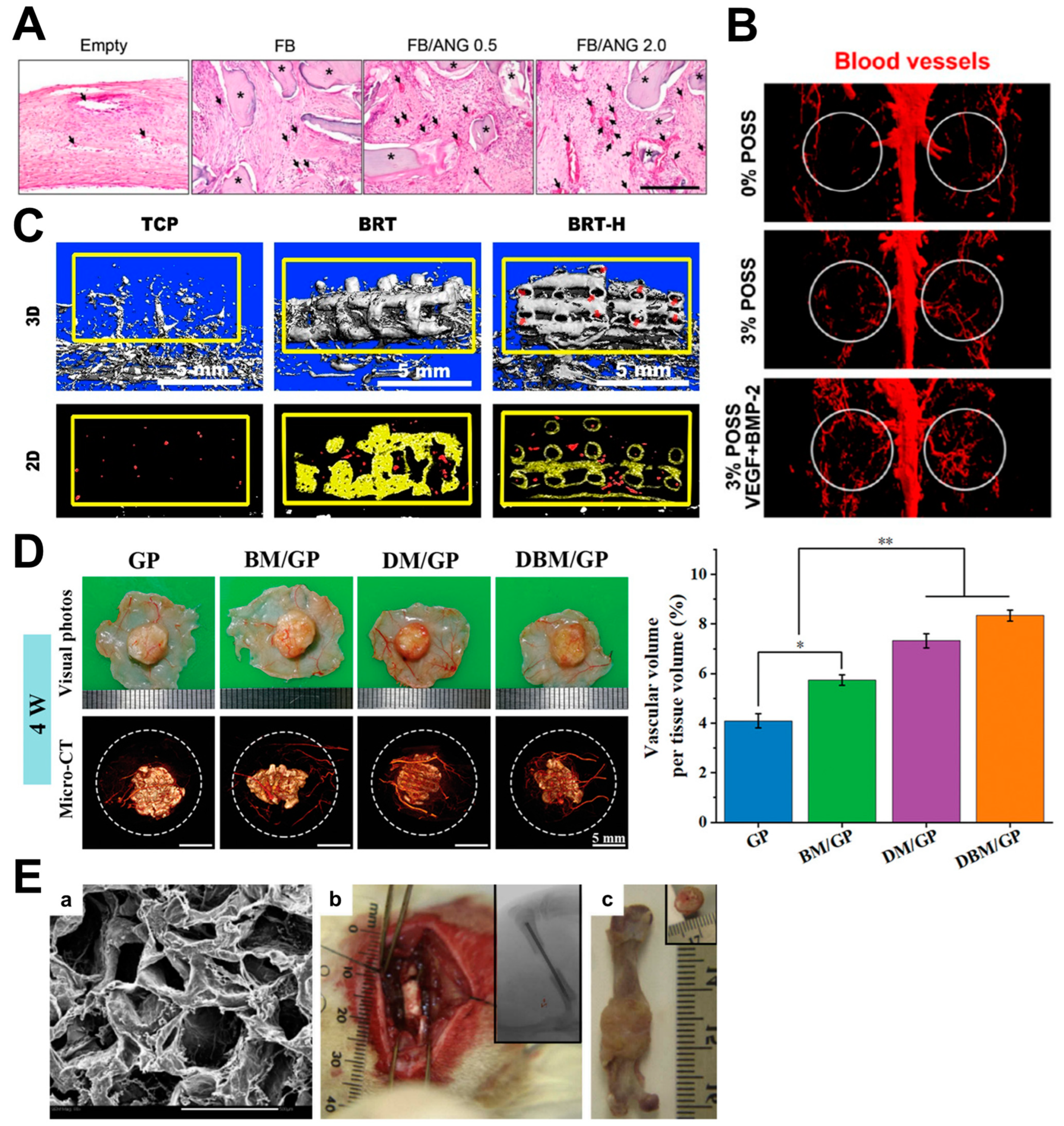
4.1. Angiogenic Factor Delivery
4.2. Cell Delivery
4.3. Gene Delivery
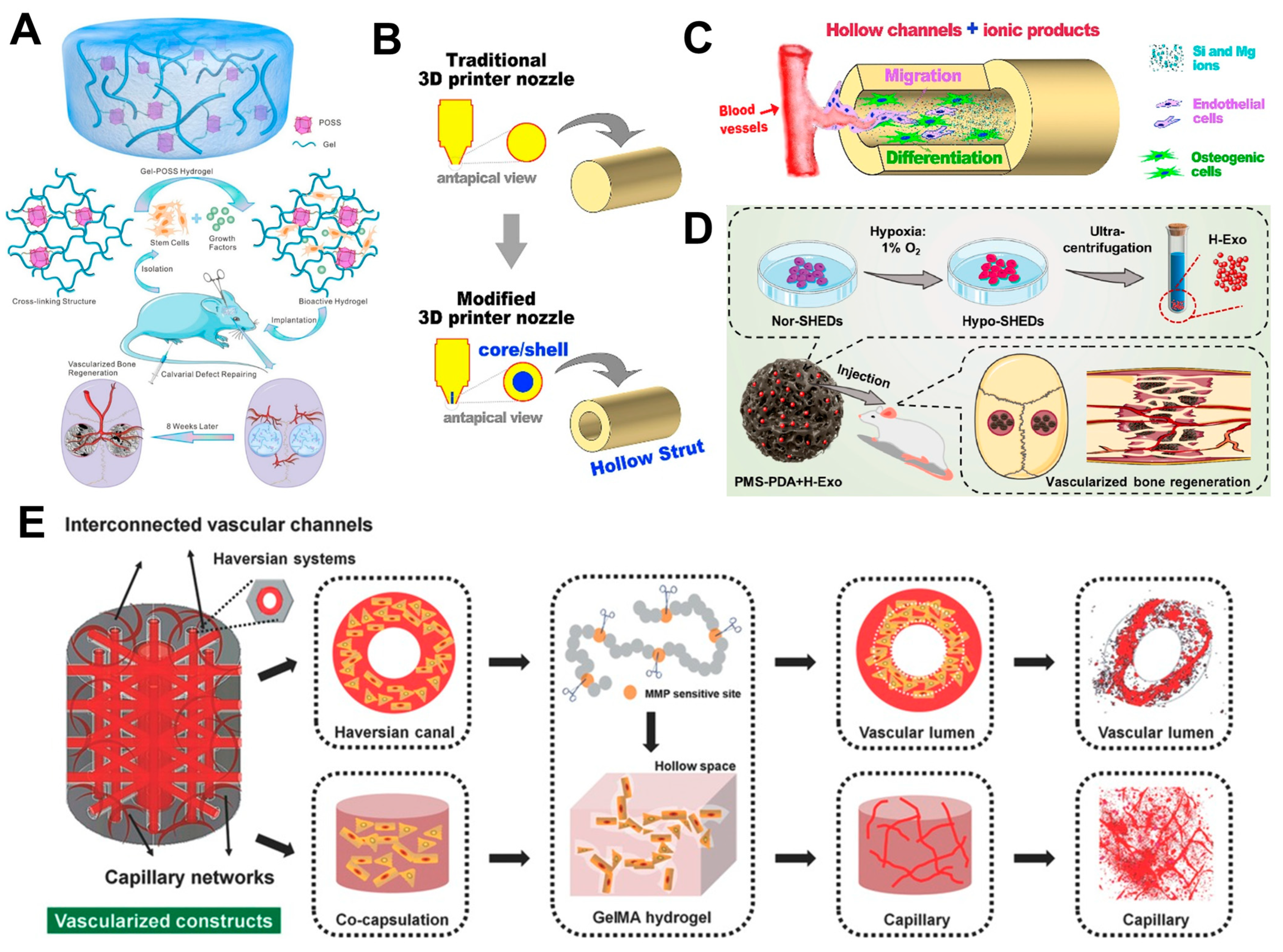
4.4. Perfusable 3D Vascular Network
4.5. Hydrogels Inducing Angiogenesis
4.6. Extracellular Vesicle (EV) Delivery
4.7. Three-Dimensional-Bioprinted Models
4.8. Other Synthetic Models
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, R.; Guo, Q.; Xiao, Y.; Guo, Q.; Huang, Y.; Li, C.; Luo, X. Endocrine role of bone in the regulation of energy metabolism. Bone Res. 2021, 9, 25. [Google Scholar] [CrossRef]
- Heini, P.F.; Berlemann, U.; Kaufmann, M.; Lippuner, K.; Fankhauser, C.; van Landuyt, P. Augmentation of mechanical properties in osteoporotic vertebral bones—A biomechanical investigation of vertebroplasty efficacy with different bone cements. Eur. Spine J. 2001, 10, 164–171. [Google Scholar] [CrossRef]
- Kenkre, J.; Bassett, J. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef]
- Kanczler, J.; Oreffo, R. Osteogenesis and angiogenesis: The potential for engineering bone. Eur. Cell Mater. 2008, 15, 100–114. [Google Scholar] [CrossRef]
- Arvidson, K.; Abdallah, B.; Applegate, L.; Baldini, N.; Cenni, E.; Gomez-Barrena, E.; Granchi, D.; Kassem, M.; Konttinen, Y.; Mustafa, K. Bone regeneration and stem cells. J. Cell. Mol. Med. 2011, 15, 718–746. [Google Scholar] [CrossRef]
- Weinans, H.; Huiskes, R.; Van Rietbergen, B.; Sumner, D.R.; Turner, T.M.; Galante, J.O. Adaptive bone remodeling around bonded noncemented total hip arthroplasty: A comparison between animal experiments and computer simulation. J. Orthop. Res. 1993, 11, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; See, C.W.; Li, X.; Zhu, D. Orthopedic implants and devices for bone fractures and defects: Past, present and perspective. Eng. Regen. 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Wüster, J.; Hesse, B.; Rothweiler, R.; Bortel, E.; Gross, C.; Bakhtiyari, S.; King, A.; Boller, E.; Gerber, J.; Rendenbach, C.; et al. Comparison of the 3D-microstructure of human alveolar and fibula bone in microvascular autologous bone transplantation: A synchrotron radiation μ-CT study. Front. Bioeng. Biotechnol. 2023, 11, 1169385. [Google Scholar] [CrossRef] [PubMed]
- Ebina, H.; Hatakeyama, J.; Onodera, M.; Honma, T.; Kamakura, S.; Shimauchi, H.; Sasano, Y. Micro-CT analysis of alveolar bone healing using a rat experimental model of critical-size defects. Oral Dis. 2009, 15, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M.; Mooney, M.P.; Gosain, A.K.; Campbell, P.G.; Losee, J.E.; Huard, J. Testing the “critical-size” in calvarial bone defects: Revisiting the concept of a critical-sized defect (CSD). Plast. Reconstr. Surg. 2010, 125, 1685. [Google Scholar] [CrossRef] [PubMed]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef]
- Schmitz, J.P.; Hollinger, J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. (1976–2007) 1986, 205, 299–308. [Google Scholar] [CrossRef]
- Masquelet, A.C.; Kishi, T.; Benko, P.E. Very long-term results of post-traumatic bone defect reconstruction by the induced membrane technique. Orthop. Traumatol. Surg. Res. 2019, 105, 159–166. [Google Scholar] [CrossRef]
- Lasanianos, N.G.; Kanakaris, N.K.; Giannoudis, P.V. Current management of long bone large segmental defects. Orthop. Trauma 2010, 24, 149–163. [Google Scholar] [CrossRef]
- Laubach, M.; Suresh, S.; Herath, B.; Wille, M.-L.; Delbrück, H.; Alabdulrahman, H.; Hutmacher, D.W.; Hildebrand, F. Clinical translation of a patient-specific scaffold-guided bone regeneration concept in four cases with large long bone defects. J. Orthop. Transl. 2022, 34, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Pape, H.C.; Evans, A.; Kobbe, P. Autologous bone graft: Properties and techniques. J. Orthop. Trauma 2010, 24, S36–S40. [Google Scholar] [CrossRef]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous bone grafts in oral implantology—Is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int. J. Implant Dent. 2017, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, M.J.; Sayres, S.C.; Saleem, O.; Levine, D.; Roberts, M.; Deland, J.T.; Ellis, S. Morbidity and complications following percutaneous calcaneal autograft bone harvest. Foot Ankle Int. 2014, 35, 30–37. [Google Scholar] [CrossRef]
- Schwartz, C.E.; Martha, J.F.; Kowalski, P.; Wang, D.A.; Bode, R.; Li, L.; Kim, D.H. Prospective evaluation of chronic pain associated with posterior autologous iliac crest bone graft harvest and its effect on postoperative outcome. Health Qual. Life Outcomes 2009, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, P.; Barsa, P.; Buchvald, P.; Svobodnik, A.; Vanickova, E. Autologous versus allogenic bone grafts in instrumented anterior cervical discectomy and fusion: A prospective study with respect to bone union pattern. Eur. Spine J. 2004, 13, 510–515. [Google Scholar] [CrossRef]
- Eppley, B.L.; Pietrzak, W.S.; Blanton, M.W. Allograft and alloplastic bone substitutes: A review of science and technology for the craniomaxillofacial surgeon. J. Craniofac. Surg. 2005, 16, 981–989. [Google Scholar] [CrossRef]
- Bauer, T.W.; Muschler, G.F. Bone graft materials: An overview of the basic science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, allograft, and bone graft substitutes: Clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.E.; Guzman, M.E.; Lopes, G.; Hurley, J. Immune checkpoint inhibitors and the risk of allograft rejection: A comprehensive analysis on an emerging issue. Oncologist 2019, 24, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R.J.; Post, D.R.; Mannon, R.B.; Sebastian, A.; Wright, H.I.; Sigle, G.; Burdick, J.; Elmagd, K.A.; Zeevi, A.; Lopez-Cepero, M. Assessing relative risks of infection and rejection: A meta-analysis using an immune function assay. Transplantation 2006, 82, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Lafage-Proust, M.-H.; Roche, B.; Langer, M.; Cleret, D.; Bossche, A.V.; Olivier, T.; Vico, L. Assessment of bone vascularization and its role in bone remodeling. BoneKEy Rep. 2015, 4, 662. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.; Wallkamm, B.; Hämmerle, C.H.; Gogolewski, S.; Lang, N.P. The significance of angiogenesis in guided bone regeneration. A case report of a rabbit experiment. Clin. Oral. Implant. Res. 1997, 8, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Bouland, C.; Philippart, P.; Dequanter, D.; Corrillon, F.; Loeb, I.; Bron, D.; Lagneaux, L.; Meuleman, N. Cross-talk between mesenchymal stromal cells (MSCs) and endothelial progenitor cells (EPCs) in bone regeneration. Front. Cell Dev. Biol. 2021, 9, 674084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, C.; Wu, Y.; Ye, D.; Wang, S.; Zou, D.; Zhang, X.; Kaplan, D.L.; Jiang, X. VEGF and BMP-2 promote bone regeneration by facilitating bone marrow stem cell homing and differentiation. Eur. Cell Mater. 2014, 27, 1–11; discussion 11–12. [Google Scholar] [CrossRef]
- Potier, E.; Ferreira, E.; Andriamanalijaona, R.; Pujol, J.-P.; Oudina, K.; Logeart-Avramoglou, D.; Petite, H. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone 2007, 40, 1078–1087. [Google Scholar] [CrossRef]
- Mistry, A.S.; Mikos, A.G. Tissue engineering strategies for bone regeneration. In Regenerative Medicine II: Clinical and Preclinical Applications; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–22. [Google Scholar]
- Adachi, T.; Osako, Y.; Tanaka, M.; Hojo, M.; Hollister, S.J. Framework for optimal design of porous scaffold microstructure by computational simulation of bone regeneration. Biomaterials 2006, 27, 3964–3972. [Google Scholar] [CrossRef]
- Kaigler, D.; Pagni, G.; Park, C.H.; Braun, T.M.; Holman, L.A.; Yi, E.; Tarle, S.A.; Bartel, R.L.; Giannobile, W.V. Stem cell therapy for craniofacial bone regeneration: A randomized, controlled feasibility trial. Cell Transplant. 2013, 22, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, R.; Ruehle, M.A.; Klosterhoff, B.S.; Lin, A.S.; Hettiaratchi, M.H.; Willett, N.J.; Bertassoni, L.E.; García, A.J.; Guldberg, R.E. Triple growth factor delivery promotes functional bone regeneration following composite musculoskeletal trauma. Acta Biomater. 2021, 127, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, W.; Sakaguchi, K.; Kawai, T.; Wakayama, Y.; Osugi, M.; Hibi, H. A defined mix of cytokines mimics conditioned medium from cultures of bone marrow-derived mesenchymal stem cells and elicits bone regeneration. Cell Prolif. 2017, 50, e12333. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Jin, C.; Ma, L.; Feng, X.; Deng, X.; Wu, S.; Liu, X.; Yang, C. Accelerated bone regeneration by gold-nanoparticle-loaded mesoporous silica through stimulating immunomodulation. ACS Appl. Mater. Interfaces 2019, 11, 41758–41769. [Google Scholar] [CrossRef] [PubMed]
- Kent Leach, J.; Kaigler, D.; Wang, Z.; Krebsbach, P.H.; Mooney, D.J. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials 2006, 27, 3249–3255. [Google Scholar] [CrossRef]
- Zhou, J.; See, C.W.; Sreenivasamurthy, S.; Zhu, D. Customized Additive Manufacturing in Bone Scaffolds—The Gateway to Precise Bone Defect Treatment. Research 2023, 6, 0239. [Google Scholar] [CrossRef] [PubMed]
- Raisz, L.G. Physiology and pathophysiology of bone remodeling. Clin. Chem. 1999, 45, 1353–1358. [Google Scholar]
- Duda, G.N.; Geissler, S.; Checa, S.; Tsitsilonis, S.; Petersen, A.; Schmidt-Bleek, K. The decisive early phase of bone regeneration. Nat. Rev. Rheumatol. 2023, 19, 78–95. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Feng, Q.; Xie, E.; Meng, Q.; Shu, W.; Wu, J.; Bian, L.; Han, F.; Li, B. Building Osteogenic Microenvironments with a Double-Network Composite Hydrogel for Bone Repair. Research 2023, 6, 0021. [Google Scholar] [CrossRef]
- Kurkalli, B.G.S.; Gurevitch, O.; Sosnik, A.; Cohn, D.; Slavin, S. Repair of bone defect using bone marrow cells and demineralized bone matrix supplemented with polymeric materials. Curr. Stem Cell Res. Ther. 2010, 5, 49–56. [Google Scholar] [CrossRef]
- Schmidt-Bleek, K.; Schell, H.; Lienau, J.; Schulz, N.; Hoff, P.; Pfaff, M.; Schmidt, G.; Martin, C.; Perka, C.; Buttgereit, F. Initial immune reaction and angiogenesis in bone healing. J. Tissue Eng. Regen. Med. 2014, 8, 120–130. [Google Scholar] [CrossRef]
- Shiu, H.T.; Leung, P.C.; Ko, C.H. The roles of cellular and molecular components of a hematoma at early stage of bone healing. J. Tissue Eng. Regen. Med. 2018, 12, e1911–e1925. [Google Scholar] [CrossRef]
- Oryan, A.; Monazzah, S.; Bigham-Sadegh, A. Bone injury and fracture healing biology. Biomed. Environ. Sci. 2015, 28, 57–71. [Google Scholar]
- Wang, L.; Fan, H.; Zhang, Z.-Y.; Lou, A.-J.; Pei, G.-X.; Jiang, S.; Mu, T.-W.; Qin, J.-J.; Chen, S.-Y.; Jin, D. Osteogenesis and angiogenesis of tissue-engineered bone constructed by prevascularized β-tricalcium phosphate scaffold and mesenchymal stem cells. Biomaterials 2010, 31, 9452–9461. [Google Scholar] [CrossRef]
- Kolar, P.; Gaber, T.; Perka, C.; Duda, G.N.; Buttgereit, F. Human early fracture hematoma is characterized by inflammation and hypoxia. Clin. Orthop. Relat. Res. 2011, 469, 3118–3126. [Google Scholar] [CrossRef] [PubMed]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenesis in bone regeneration. Injury 2011, 42, 556–561. [Google Scholar] [CrossRef]
- Schott, N.G.; Friend, N.E.; Stegemann, J.P. Coupling osteogenesis and vasculogenesis in engineered orthopedic tissues. Tissue Eng. Part B Rev. 2021, 27, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Pearson, H.B.; Mason, D.E.; Kegelman, C.D.; Zhao, L.; Dawahare, J.H.; Kacena, M.A.; Boerckel, J.D. Effects of bone morphogenetic protein-2 on neovascularization during large bone defect regeneration. Tissue Eng. Part A 2019, 25, 1623–1634. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, F.; Zhang, P.; Wang, H.; Qu, Z.; Jia, P.; Yao, Z.; Shen, G.; Li, G.; Zhao, G. Human type H vessels are a sensitive biomarker of bone mass. Cell Death Dis. 2017, 8, e2760. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, S.; Li, Y.; Crane, J.L. Type H blood vessels in bone modeling and remodeling. Theranostics 2020, 10, 426–436. [Google Scholar] [CrossRef]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Zelzer, E.; Mamluk, R.; Ferrara, N.; Johnson, R.S.; Schipani, E.; Olsen, B.R. VEGFA is necessary for chondrocyte survival during bone development. Development 2004, 131, 2161–2171. [Google Scholar] [CrossRef]
- Xie, H.; Cui, Z.; Wang, L.; Xia, Z.; Hu, Y.; Xian, L.; Li, C.; Xie, L.; Crane, J.; Wan, M.; et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat. Med. 2014, 20, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Percival, C.J.; Richtsmeier, J.T. Angiogenesis and intramembranous osteogenesis. Dev. Dyn. 2013, 242, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef]
- Saleh, F.A.; Whyte, M.; Genever, P.G. Effects of endothelial cells on human mesenchymal stem cell activity in a three-dimensional in vitro model. Eur. Cell Mater. 2011, 22, 242–257; discussion 257. [Google Scholar] [CrossRef] [PubMed]
- Pennings, I.; van Dijk, L.A.; van Huuksloot, J.; Fledderus, J.O.; Schepers, K.; Braat, A.K.; Hsiao, E.C.; Barruet, E.; Morales, B.M.; Verhaar, M.C.; et al. Effect of donor variation on osteogenesis and vasculogenesis in hydrogel cocultures. J. Tissue Eng. Regen. Med. 2019, 13, 433–445. [Google Scholar] [CrossRef]
- Younger, E.M.; Chapman, M.W. Morbidity at Bone Graft Donor Sites. J. Orthop. Trauma 1989, 3, 192–195. [Google Scholar] [CrossRef]
- William Jr, G.; Einhorn, T.A.; Koval, K.; McKee, M.; Smith, W.; Sanders, R.; Watson, T. Bone grafts and bone graft substitutes in orthopaedic trauma surgery: A critical analysis. JBJS 2007, 89, 649–658. [Google Scholar]
- Yang, K.; Ren, Y. Nickel-free austenitic stainless steels for medical applications. Sci. Technol. Adv. Mater. 2010, 11, 014105. [Google Scholar] [CrossRef]
- Yasuoka, S.; Usukura, Y.; Fuse, M.; Okada, H.; Hayakawa, T.; Kato, T. Stainless and titanium fibers as non-degradable three-dimensional scaffolds for bone reconstruction. J. Hard Tissue Biol. 2014, 23, 407–414. [Google Scholar] [CrossRef][Green Version]
- Zhang, Q.-H.; Cossey, A.; Tong, J. Stress shielding in periprosthetic bone following a total knee replacement: Effects of implant material, design and alignment. Med. Eng. Phys. 2016, 38, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Noyama, Y.; Miura, T.; Ishimoto, T.; Itaya, T.; Niinomi, M.; Nakano, T. Bone loss and reduced bone quality of the human femur after total hip arthroplasty under stress-shielding effects by titanium-based implant. Mater. Trans. 2012, 53, 565–570. [Google Scholar] [CrossRef]
- Mi, Z.; Shuib, S.; Hassan, A.; Shorki, A.; Ibrahim, M. Problem of stress shielding and improvement to the hip Implat designs: A review. J. Med. Sci. 2007, 7, 460–467. [Google Scholar]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R. Applications of metals for bone regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef] [PubMed]
- Blázquez-Carmona, P.; Mora-Macías, J.; Martínez-Vázquez, F.J.; Morgaz, J.; Domínguez, J.; Reina-Romo, E. Mechanics Predicts Effective Critical-Size Bone Regeneration Using 3D-Printed Bioceramic Scaffolds. Tissue Eng. Regen. Med. 2023, 20, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.L.; Zheng, G.B.; Park, J.; Kim, H.D.; Baek, H.R.; Lee, H.K.; Lee, K.; Han, H.N.; Lee, C.K.; Hwang, N.S. In vitro and in vivo evaluation of whitlockite biocompatibility: Comparative study with hydroxyapatite and β-tricalcium phosphate. Adv. Healthc. Mater. 2016, 5, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Szcześ, A.; Hołysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef]
- Heimann, R.B. Structure, properties, and biomedical performance of osteoconductive bioceramic coatings. Surf. Coat. Technol. 2013, 233, 27–38. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef]
- Thompson, I.D.; Hcnch, L.L. Mechanical properties of bioactive glasses, glass-ceramics and composites. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 127–136. [Google Scholar] [CrossRef]
- Mala, R.; Celsia, A.R. Bioceramics in orthopaedics: A review. In Fundamental Biomaterials: Ceramics; Woodhead Publishing: Sawston, UK, 2018; pp. 195–221. [Google Scholar]
- Lin, T.-H.; Wang, H.-C.; Cheng, W.-H.; Hsu, H.-C.; Yeh, M.-L. Osteochondral tissue regeneration using a tyramine-modified bilayered PLGA scaffold combined with articular chondrocytes in a porcine model. Int. J. Mol. Sci. 2019, 20, 326. [Google Scholar] [CrossRef]
- Wang, C.; Xu, D.; Li, S.; Yi, C.; Zhang, X.; He, Y.; Yu, D. Effect of pore size on the physicochemical properties and osteogenesis of Ti6Al4V porous scaffolds with bionic structure. ACS Omega 2020, 5, 28684–28692. [Google Scholar] [CrossRef] [PubMed]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Wagoner Johnson, A.J. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar]
- Makihara, T.; Sakane, M.; Noguchi, H.; Tsukanishi, T.; Suetsugu, Y.; Yamazaki, M. Formation of osteon-like structures in unidirectional porous hydroxyapatite substitute. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2665–2672. [Google Scholar]
- Quade, M.; Münch, P.; Lode, A.; Duin, S.; Vater, C.; Gabrielyan, A.; Rösen-Wolff, A.; Gelinsky, M. The secretome of hypoxia conditioned hMSC loaded in a central depot induces chemotaxis and angiogenesis in a biomimetic mineralized collagen bone replacement material. Adv. Healthc. Mater. 2020, 9, 1901426. [Google Scholar] [CrossRef]
- Song, P.; Hu, C.; Pei, X.; Sun, J.; Sun, H.; Wu, L.; Jiang, Q.; Fan, H.; Yang, B.; Zhou, C. Dual modulation of crystallinity and macro-/microstructures of 3D printed porous titanium implants to enhance stability and osseointegration. J. Mater. Chem. B 2019, 7, 2865–2877. [Google Scholar] [CrossRef]
- Klompmaker, J.; Jansen, H.W.; Veth, R.H.; Nielsen, H.K.; de Groot, J.H.; Pennings, A.J. Porous implants for knee joint meniscus reconstruction: A preliminary study on the role of pore sizes in ingrowth and differentiation of fibrocartilage. Clin. Mater. 1993, 14, 1–11. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Klenke, F.M.; Liu, Y.; Yuan, H.; Hunziker, E.B.; Siebenrock, K.A.; Hofstetter, W. Impact of pore size on the vascularization and osseointegration of ceramic bone substitutes in vivo. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008, 85, 777–786. [Google Scholar] [CrossRef]
- Patel, Z.S.; Young, S.; Tabata, Y.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008, 43, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kim, J.S.; Yang, S.S.; Kim, H.W.; Lim, H.J.; Lee, J. Angiogenin-loaded fibrin/bone powder composite scaffold for vascularized bone regeneration. Biomater. Res. 2015, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, Y.; Zhang, W.; Li, J. Polyhedral Oligomeric Silsesquioxane-Incorporated Gelatin Hydrogel Promotes Angiogenesis during Vascularized Bone Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 22410–22425. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Park, J.; Amirthalingam, S.; Jayakumar, R.; Hwang, N.S. Bioinspired inorganic nanoparticles and vascular factor microenvironment directed neo-bone formation. Biomater. Sci. 2020, 8, 2627–2637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Feng, C.; Yang, G.; Li, G.; Ding, X.; Wang, S.; Dou, Y.; Zhang, Z.; Chang, J.; Wu, C.; et al. 3D-printed scaffolds with synergistic effect of hollow-pipe structure and bioactive ions for vascularized bone regeneration. Biomaterials 2017, 135, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Ma, X.; Li, S.; Li, T.; Li, Z.; Qian, Y.; Shafiq, M.; Wang, J.; Zhou, X.; He, C. Bone Microenvironment-Mimetic Scaffolds with Hierarchical Microstructure for Enhanced Vascularization and Bone Regeneration. Adv. Funct. Mater. 2022, 32, 2200011. [Google Scholar] [CrossRef]
- Fitzpatrick, V.; Martín-Moldes, Z.; Deck, A.; Torres-Sanchez, R.; Valat, A.; Cairns, D.; Li, C.; Kaplan, D.L. Functionalized 3D-printed silk-hydroxyapatite scaffolds for enhanced bone regeneration with innervation and vascularization. Biomaterials 2021, 276, 120995. [Google Scholar] [CrossRef] [PubMed]
- Kaigler, D.; Krebsbach, P.H.; West, E.R.; Horger, K.; Huang, Y.-C.; Mooney, D.J. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2005, 19, 1–26. [Google Scholar] [CrossRef]
- Yu, H.; VandeVord, P.J.; Mao, L.; Matthew, H.W.; Wooley, P.H.; Yang, S.-Y. Improved tissue-engineered bone regeneration by endothelial cell mediated vascularization. Biomaterials 2009, 30, 508–517. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, H.; Fang, T.; Li, X.; Dai, W.; Uemura, T.; Dong, J. The repair of large segmental bone defects in the rabbit with vascularized tissue engineered bone. Biomaterials 2010, 31, 1171–1179. [Google Scholar] [CrossRef]
- Curtin, C.M.; Tierney, E.G.; McSorley, K.; Cryan, S.A.; Duffy, G.P.; O’Brien, F.J. Combinatorial gene therapy accelerates bone regeneration: Non-viral dual delivery of VEGF and BMP2 in a collagen-nanohydroxyapatite scaffold. Adv. Healthc. Mater. 2015, 4, 223–227. [Google Scholar] [CrossRef]
- Geiger, F.; Bertram, H.; Berger, I.; Lorenz, H.; Wall, O.; Eckhardt, C.; Simank, H.G.; Richter, W. Vascular endothelial growth factor gene-activated matrix (VEGF165-GAM) enhances osteogenesis and angiogenesis in large segmental bone defects. J. Bone Miner. Res. 2005, 20, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liu, X.; Liu, S.; Jiang, Y.; Zhang, X.; Zhang, C.; Zeng, B. Experimental repair of segmental bone defects in rabbits by angiopoietin-1 gene transfected MSCs seeded on porous β-TCP scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1229–1236. [Google Scholar] [CrossRef]
- Feng, H.; Li, Z.; Xie, W.; Wan, Q.; Guo, Y.; Chen, J.; Wang, J.; Pei, X. Delivery of therapeutic miRNAs using nanoscale zeolitic imidazolate framework for accelerating vascularized bone regeneration. Chem. Eng. J. 2022, 430, 132867. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, W.; Holmes, B.; Zhang, L.G. Biologically Inspired Smart Release System Based on 3D Bioprinted Perfused Scaffold for Vascularized Tissue Regeneration. Adv. Sci. 2016, 3, 1600058. [Google Scholar] [CrossRef]
- Hann, S.Y.; Cui, H.; Esworthy, T.; Zhou, X.; Lee, S.-j.; Plesniak, M.W.; Zhang, L.G. Dual 3D printing for vascularized bone tissue regeneration. Acta Biomater. 2021, 123, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh-Narbat, M.; Rouwkema, J.; Annabi, N.; Cheng, H.; Ghaderi, M.; Cha, B.-H.; Aparnathi, M.; Khalilpour, A.; Byambaa, B.; Jabbari, E.; et al. Engineering Photocrosslinkable Bicomponent Hydrogel Constructs for Creating 3D Vascularized Bone. Adv. Healthc. Mater. 2017, 6, 1601122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, R.; Ruan, H.; Cai, Z.; Zhuang, Y.; Han, Z.; Zhang, M.; Cui, W.; Cai, M. Biological signal integrated microfluidic hydrogel microspheres for promoting bone regeneration. Chem. Eng. J. 2022, 436, 135176. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, C.; He, L.; Zhou, J.; Chen, T.; Ouyang, L.; Guo, X.; Qu, Y.; Luo, Z.; Duan, D. Stratified-structural hydrogel incorporated with magnesium-ion-modified black phosphorus nanosheets for promoting neuro-vascularized bone regeneration. Bioact. Mater. 2022, 16, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, Y.; Li, W.; Zhao, Z.; Xia, X.; Liu, J.; Deng, Y.; Wu, Y.; Pan, X.; He, F.; et al. A Self-Adaptive Biomimetic Periosteum Employing Nitric Oxide Release for Augmenting Angiogenesis in Bone Defect Regeneration. Adv. Healthc. Mater. 2023, e2302153. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wang, X.; Zhang, W.; Sun, L.; Han, X.; Tong, X.; Yu, L.; Ding, J.; Yu, L.; Liu, Y. Versatile Hypoxic Extracellular Vesicles Laden in an Injectable and Bioactive Hydrogel for Accelerated Bone Regeneration. Adv. Funct. Mater. 2023, 33, 2211664. [Google Scholar] [CrossRef]
- Gao, Y.; Yuan, Z.; Yuan, X.; Wan, Z.; Yu, Y.; Zhan, Q.; Zhao, Y.; Han, J.; Huang, J.; Xiong, C.; et al. Bioinspired porous microspheres for sustained hypoxic exosomes release and vascularized bone regeneration. Bioact. Mater. 2022, 14, 377–388. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, W.; Nowicki, M.; Zhou, X.; Khademhosseini, A.; Zhang, L.G. Hierarchical Fabrication of Engineered Vascularized Bone Biphasic Constructs via Dual 3D Bioprinting: Integrating Regional Bioactive Factors into Architectural Design. Adv. Healthc. Mater. 2016, 5, 2174–2181. [Google Scholar] [CrossRef]
- Han, X.; Sun, M.; Chen, B.; Saiding, Q.; Zhang, J.; Song, H.; Deng, L.; Wang, P.; Gong, W.; Cui, W. Lotus seedpod-inspired internal vascularized 3D printed scaffold for bone tissue repair. Bioact. Mater. 2021, 6, 1639–1652. [Google Scholar] [CrossRef]
- Leucht, A.; Volz, A.C.; Rogal, J.; Borchers, K.; Kluger, P.J. Advanced gelatin-based vascularization bioinks for extrusion-based bioprinting of vascularized bone equivalents. Sci. Rep. 2020, 10, 5330. [Google Scholar] [CrossRef]
- Jusoh, N.; Oh, S.; Kim, S.; Kim, J.; Jeon, N.L. Microfluidic vascularized bone tissue model with hydroxyapatite-incorporated extracellular matrix. Lab Chip 2015, 15, 3984–3988. [Google Scholar] [CrossRef]
- Yang, G.; Liu, H.; Cui, Y.; Li, J.; Zhou, X.; Wang, N.; Wu, F.; Li, Y.; Liu, Y.; Jiang, X.; et al. Bioinspired membrane provides periosteum-mimetic microenvironment for accelerating vascularized bone regeneration. Biomaterials 2021, 268, 120561. [Google Scholar] [CrossRef]
- Yun, H.-M.; Ahn, S.-J.; Park, K.-R.; Kim, M.-J.; Kim, J.-J.; Jin, G.-Z.; Kim, H.-W.; Kim, E.-C. Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials 2016, 85, 88–98. [Google Scholar] [CrossRef]
- Rady, A.A.M.; Hamdy, S.M.; Abdel-Hamid, M.A.; Hegazy, M.G.A.; Fathy, S.A.; Mostafa, A.A. The role of VEGF and BMP-2 in stimulation of bone healing with using hybrid bio-composite scaffolds coated implants in animal model. Bull. Natl. Res. Cent. 2020, 44, 131. [Google Scholar] [CrossRef]
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional relationship between osteogenesis and angiogenesis in tissue regeneration. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, S.J.; Jo, H.H.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. Fabrication and characterization of 3D-printed bone-like β-tricalcium phosphate/polycaprolactone scaffolds for dental tissue engineering. J. Ind. Eng. Chem. 2017, 46, 175–181. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, J.Y.; Yang, G.H.; Seo, J.-H.; Ryu, H.-S.; Kim, G. 3D-printed PCL/bioglass (BGS-7) composite scaffolds with high toughness and cell-responses for bone tissue regeneration. J. Ind. Eng. Chem. 2019, 79, 163–171. [Google Scholar] [CrossRef]
- Bajuri, M.Y.; Selvanathan, N.; Dzeidee Schaff, F.N.; Abdul Suki, M.H.; Ng, A.M.H. Tissue-Engineered Hydroxyapatite Bone Scaffold Impregnated with Osteoprogenitor Cells Promotes Bone Regeneration in Sheep Model. Tissue Eng. Regen. Med. 2021, 18, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Park, J.S.; Shin, Y.M.; Lee, D.H.; Yoon, J.-K.; Kim, D.-H.; Ko, U.H.; Kim, Y.; Bae, S.H.; Sung, H.-J. Implantable Vascularized Liver Chip for Cross-Validation of Disease Treatment with Animal Model. Adv. Funct. Mater. 2019, 29, 1900075. [Google Scholar] [CrossRef]
- Thurston, G.; Suri, C.; Smith, K.; McClain, J.; Sato, T.N.; Yancopoulos, G.D.; McDonald, D.M. Leakage-Resistant Blood Vessels in Mice Transgenically Overexpressing Angiopoietin-1. Science 1999, 286, 2511–2514. [Google Scholar] [CrossRef]
- Lee, J.B.; Kim, D.H.; Yoon, J.K.; Park, D.B.; Kim, H.S.; Shin, Y.M.; Baek, W.; Kang, M.L.; Kim, H.J.; Sung, H.J. Microchannel network hydrogel induced ischemic blood perfusion connection. Nat. Commun. 2020, 11, 615. [Google Scholar] [CrossRef]
| Strategy | Main Factors | Findings and Observations | Ref. |
|---|---|---|---|
| Angiogenic factor delivery | VEGF, BMP-2 | Vessel formation ↑/Bone formation, bone bridging ↑ | [85] |
| VEGF | Bone bridging, bone volume fraction, bone mineral density ↑ | [37] | |
| Angiogenin | EC proliferation, adhesion ↑/Vessel formation ↑/Bone formation ↑ | [86] | |
| VEGF, BMP-2 | Vessel number ↑/Bone formation ↑ | [87] | |
| VEGF | Osteogenic differentiation ↑/Vessel formation ↑/Bone formation ↑ | [88] | |
| Bioactive ions | EC migration ↑/Vessel formation ↑/Stem cell, growth factor delivery ↑/Bone formation ↑ | [89] | |
| Dimethyloxalylglycine | Angiogenic, Osteogenic marker expression ↑/ALP activity ↑/Vessel formation ↑/Bone formation ↑ | [90] | |
| BMP-2, VEGF, NGF | Osteogenic differentiation ↑/HUVEC migration ↑ | [91] | |
| Cell delivery | ECs, BMSCs | Osteogenic marker expression ↑/Vessel formation ↑ | [92] |
| ECs, osteoblasts | Vessel formation ↑/Bone formation ↑ | [93] | |
| MSCs, MSC-derived ECs | Mechanical properties ↑/Capillary network ↑ | [94] | |
| Gene delivery | VEGF, BMP-2 gene | Calcium deposition, mineralization ↑/Host cell recruitment, transfection ↑/Vessel formation ↑ | [95] |
| VEGF gene | Bone gap ↓/Vessel formation ↑ Osteoid formation ↑ | [96] | |
| Ang1 gene | Vessel growth ↑/Faster bone union/Bone formation ↑ | [97] | |
| miR-21, miR-5106 | Vessel formation ↑/Bone mineral density, trabecular number, thickness ↑/Angiogenic ability ↑ | [98] | |
| Perfusable 3D vascular network | Perfused microstructure | Osteogenic differentiation ↑/Vascular like network ↑ | [99] |
| Perfusable vascular channels | Osteogenic differentiation ↑/Vessel formation ↑ | [100] | |
| Angiogenesis-inducing hydrogel | osteogenic and angiogenic niche-incorporated hydrogel | EC, MSC self-assembly/Bone formation ↑ | [101] |
| Biological signal peptide | Bone formation ↑/Vascularization ↑ | [102] | |
| Bilayer hydrogel | EC proliferation, adhesion ↑/Bone mineral density, bone score values, bone formation ↑/Angiogenesis ↑ | [103] | |
| NO-releasing biomimetic periosteum | Angiogenesis ↑/Bone volume fraction, bone mineral density ↑ | [104] | |
| EV delivery | Bone marrow-derived hypoxic EVs | Vascularization ↑/Osteoblast proliferation, migration and differentiation ↑/Bone formation ↑ | [105] |
| SHED-derived hypoxic exosomes | Vascularization ↑/Bone formation ↑ | [106] | |
| 3D-bioprinting | HUVECs, MSCs, GelMA hydrogel | Cell adhesion, proliferation ↑/VEGF, collagen I ↑/Osteogenic, angiogenic differentiation ↑/Capillary network ↑ | [107] |
| DFO liposome-containing scaffold | Osteogenic marker expression ↑/Bone growth ↑/Early-stage internal vascularization ↑/Vascular network maturation ↑ | [108] | |
| HDMECs, ASCs, gelatin-based bioink | Capillary-mimicking structure formation ↑/Bone-specific protein ↑/Vascularization ↑ | [109] | |
| Other synthetic methods | HA-incorporated microfluidic model | Vessel sprout length, velocity, number, and lumen diameter ↑/ECs–stromal cells paracrine communication/Functional endothelial marker expression ↑ | [110] |
| Biomimetic membrane with HA nanoparticle | Cell adhesion, alignment, differentiation ↑/Vessel formation ↑/Ossification ↑ | [111] | |
| Static magnetic field, magnetic scaffold | Osteoblast function ↑/VEGF expression ↑/Capillary tube formation ↑/Bone formation ↑ | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.-J.; Yoon, J.-K. The Role of Vasculature and Angiogenic Strategies in Bone Regeneration. Biomimetics 2024, 9, 75. https://doi.org/10.3390/biomimetics9020075
Jang H-J, Yoon J-K. The Role of Vasculature and Angiogenic Strategies in Bone Regeneration. Biomimetics. 2024; 9(2):75. https://doi.org/10.3390/biomimetics9020075
Chicago/Turabian StyleJang, Hye-Jeong, and Jeong-Kee Yoon. 2024. "The Role of Vasculature and Angiogenic Strategies in Bone Regeneration" Biomimetics 9, no. 2: 75. https://doi.org/10.3390/biomimetics9020075
APA StyleJang, H.-J., & Yoon, J.-K. (2024). The Role of Vasculature and Angiogenic Strategies in Bone Regeneration. Biomimetics, 9(2), 75. https://doi.org/10.3390/biomimetics9020075





