One-Step Fabrication Process of Silica–Titania Superhydrophobic UV-Blocking Thin Coatings onto Polymeric Films
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Preparation of Titanium and SiO2-Titanium Dispersions for Superhydrophobic Coatings
2.2.2. Facile Fabrication of SiO2-TiO2 Thin Coatings Using a Mayer Rod on Corona-Treated Polypropylene Films
2.3. Characterization Methods
2.3.1. Coated Polypropylene Films
Environmental Scanning Electron Microscope (E-SEM)
Attenuated Total Reflectance (ATR)
Atomic Force Microscope (AFM)
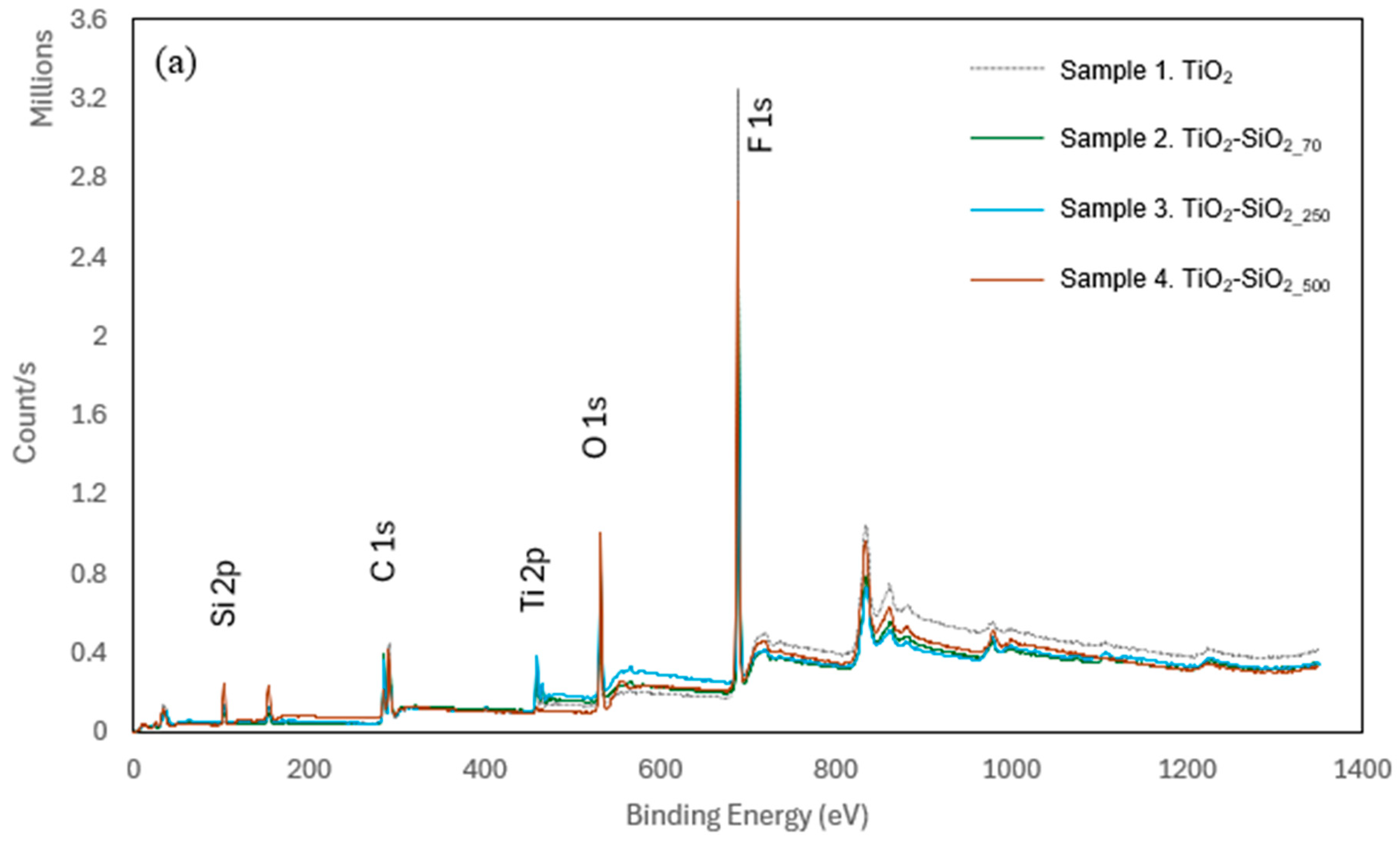
X-Ray Photoelectron Spectroscopy (XPS)
2.3.2. Surface Wettability
Water Contact Angle (WCA) and Rolling Angle (WRA)
Self-Cleaning Ability
Mechanical and Chemical Durability Tests
3. Results and Discussion
3.1. Surface Morphology and Chemical Composition of TiO2 and TiO2-SiO2 Composite Coatings on Polypropylene Films
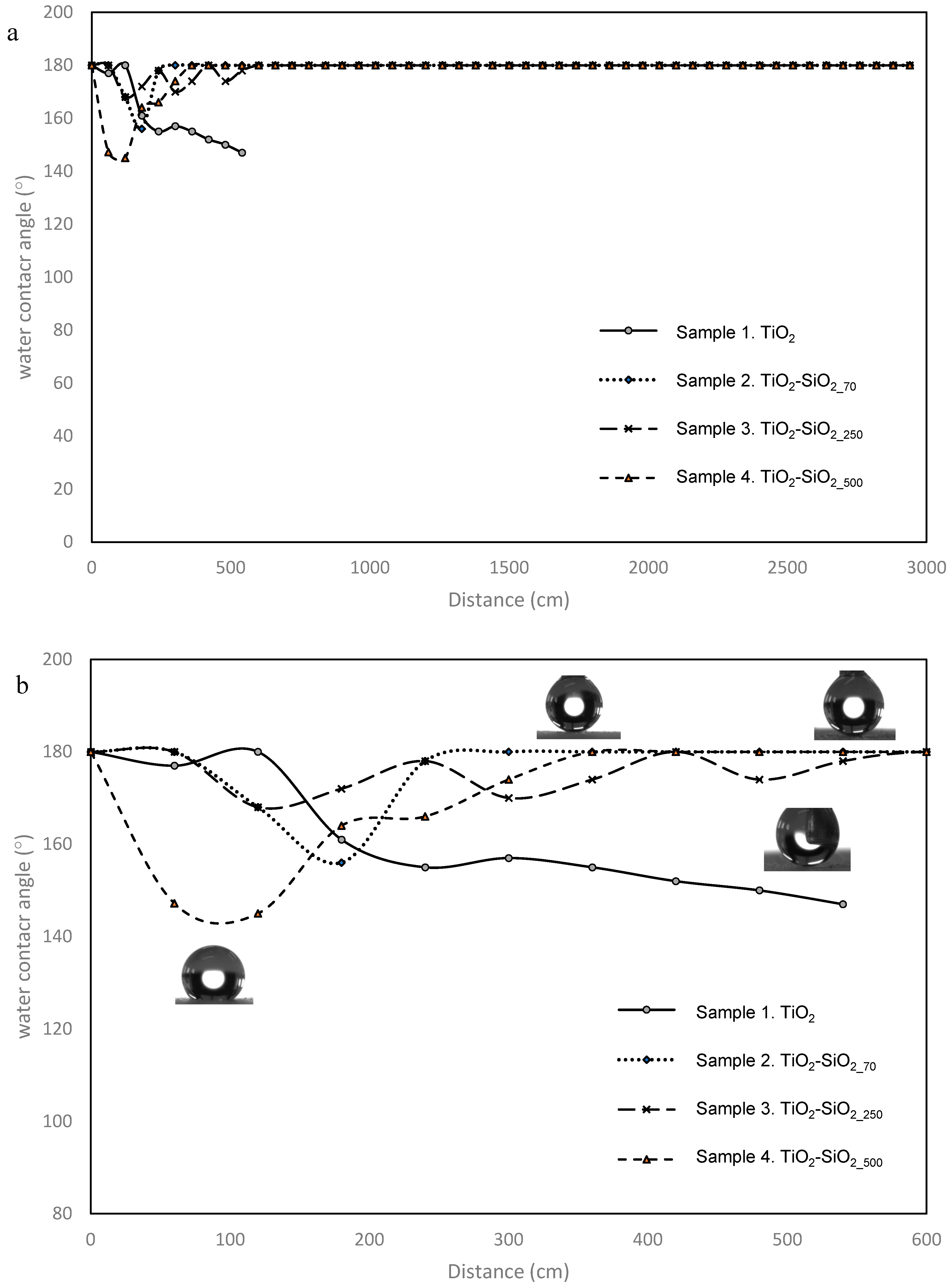
3.2. Wettability Properties
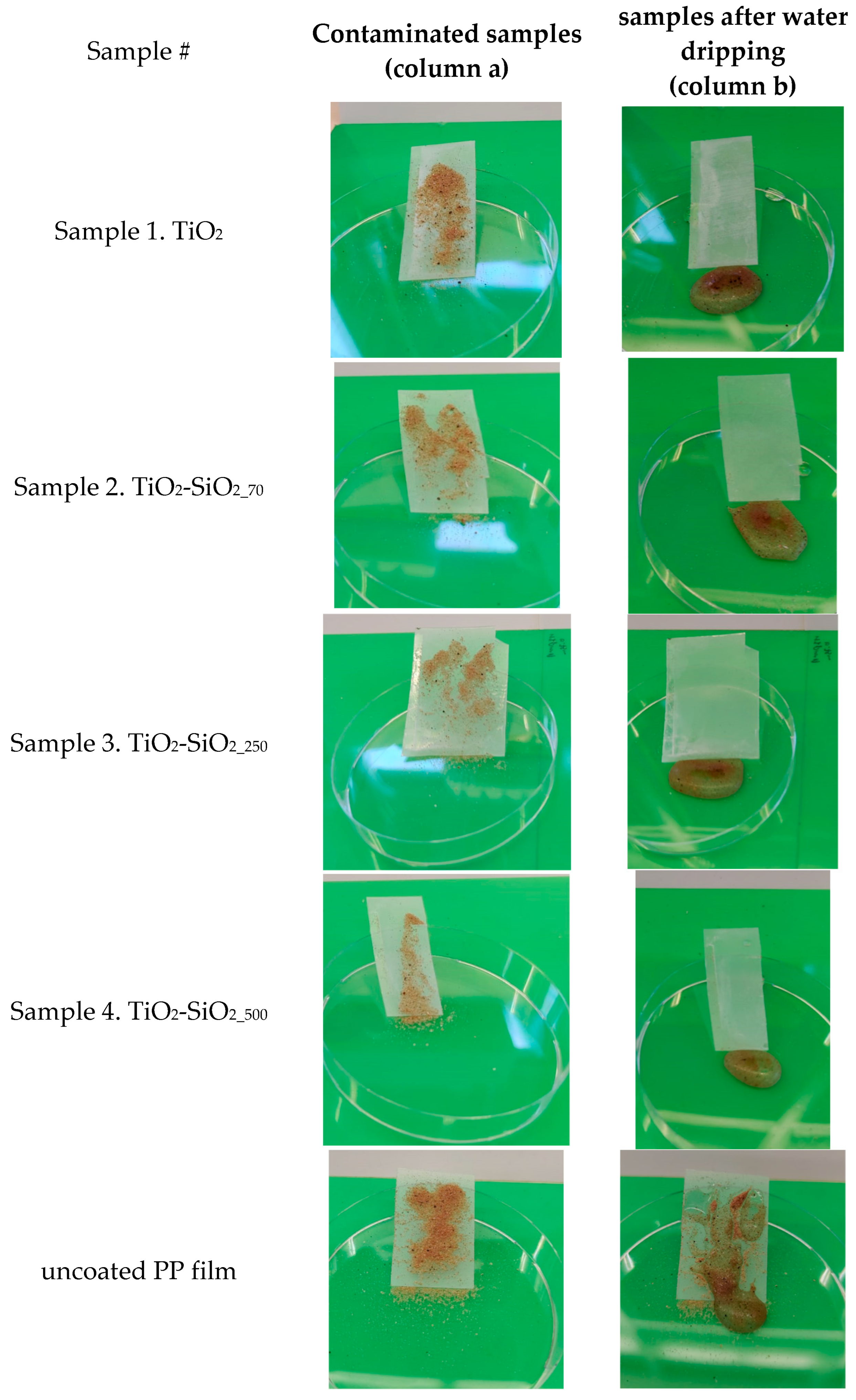
3.3. Self-Cleaning Ability
3.4. Mechanical Properties
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, X.J.; Jiang, L. Design and creation of superwetting/antiwetting surfaces. Adv. Mater. 2006, 18, 3063–3078. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Tran, H.N.; Plamondon, C.O.; Tuduri, L.; Vo, D.V.N.; Nanda, S.; Mishra, A.; Chao, H.-P.; Bajpai, A.K. Recent progress in the preparation, properties and applications of superhydrophobic nano-based coatings and surfaces: A review. Prog. Org. Coat. 2019, 132, 235–256. [Google Scholar] [CrossRef]
- Antinate Shilpa, S.; Kavitha Sri, A.; Jeen Robert, R.B.; Subbulakshmi, M.S.; Hikku, G.S.O. A review focused on the superhydrophobic fabrics with functional properties. J. Appl. Polym. Sci. 2023, 140, e53664. [Google Scholar] [CrossRef]
- Li, B.; Liang, W.; Zhang, J.; Wei, J.; Mao, M.; Zhang, J. Preparation of pressure-resistant and mechanically durable superhydrophobic coatings via non-solvent induced phase separation for anti-icing. Small 2024, 490, 2406490. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, L. Bio-inspired self-cleaning surfaces. Annu. Rev. Mater. Res. 2012, 42, 231–263. [Google Scholar] [CrossRef]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progress in superhydrophobic surface development. Soft Matter 2008, 4, 224–240. [Google Scholar] [CrossRef]
- Kausar, A. Nanomaterials for design and fabrication of superhydrophobic polymer coating. In Superhydrophobic Polymer Coatings; Elsevier: Amsterdam, The Netherlands, 2019; pp. 77–90. [Google Scholar]
- Protsenko, V.S.; Danilov, F.I. Current trends in electrodeposition of electrocatalytic coatings. Methods Electrocatal. Adv. Mater. Allied Appl. 2020, 263–299. [Google Scholar]
- Branzoi, V.; Branzoi, F.; Pilan, L. Characterization of electrodeposited polymeric and composite modified electrodes on cobalt based alloy. Mater. Chem. Phys. 2009, 118, 197–202. [Google Scholar] [CrossRef]
- Wang, J.; Li, A.; Chen, H.; Chen, D. Synthesis of biomimetic superhydrophobic surface through electrochemical deposition on porous alumina. J. Bionic Eng. 2011, 8, 122–128. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, D.; Cao, J.; Zhao, F.; Feng, G.; Xu, C.; Deng, Y.; Xiang, X.D. Development of high-throughput wet-chemical synthesis techniques for material research. Mater. Genome Eng. Adv. 2023, 1, e5. [Google Scholar] [CrossRef]
- Kanovsky, N.; Iline-Vul, T.; Margel, S. In-situ design of hierarchical durable silica-based coatings on polypropylene films with superhydrophilic, superhydrophobic and self-cleaning properties. Results Surf. Interfaces 2023, 10, 100101. [Google Scholar] [CrossRef]
- Contreras, C.B.; Figueroa, F.N.; Weibel, D.E.; Strumia, M.C. Superhydrophobic polypropylene surfaces prepared with TiO2 nanoparticles functionalized by dendritic polymers. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2019–2029. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, H.; Zhang, L.; Sun, Y.; Xu, L.; Sun, Z.; Gu, W.; Chen, Z.; Yang, H. Novel one-step, in situ thermal polymerization fabrication of robust superhydrophobic mesh for efficient oil/water separation. Ind. Eng. Chem. Res. 2017, 56, 11817–11826. [Google Scholar] [CrossRef]
- Ma, M.; Mao, Y.; Gupta, M.; Gleason, K.K.; Rutledge, G.C. Superhydrophobic fabrics produced by electrospinning and chemical vapor deposition. Macromolecules 2005, 38, 9742–9748. [Google Scholar] [CrossRef]
- Kang, M.; Jung, R.; Kim, H.S.; Jin, H.J. Preparation of superhydrophobic polystyrene membranes by electrospinning. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313, 411–414. [Google Scholar] [CrossRef]
- Vanithakumari, S.C.; Athulya, V.; George, R.P.; Philip, J. Fabrication of superhydrophobic and self-cleaning PVA-silica fiber coating on 304L SS surfaces by electrospinning. J. Appl. Polym. Sci. 2021, 138, 50118. [Google Scholar] [CrossRef]
- Cakir, M.; Kartal, I.; Yildiz, Z. The preparation of UV-cured superhydrophobic cotton fabric surfaces by electrospinning method. Text. Res. J. 2014, 84, 1528–1538. [Google Scholar] [CrossRef]
- Mahadik, S.A.; Vhatkara, R.S.; Mahadik, D.B.; Kavale, M.S.; Wagh, P.B.; Gupta, S.; Gurav, J. Superhydrophobic silica coating by dip coating method. Appl. Surf. Sci. 2013, 277, 67–72. [Google Scholar] [CrossRef]
- Gurav, A.B.; Xu, Q.; Latthe, S.S.; Vhatkar, R.S.; Liu, S.; Yoon, H.; Yoon, S.S. Superhydrophobic coatings prepared from methyl-modified silica particles using simple dip-coating method. Ceram. Int. 2015, 41, 3017–3023. [Google Scholar] [CrossRef]
- Bhagat, S.D.; Kim, Y.H.; Ahn, Y.S. Room temperature synthesis of water repellent silica coatings by the dip coat technique. Appl. Surf. Sci. 2006, 253, 2217–2221. [Google Scholar] [CrossRef]
- Gao, L.; He, J. A facile dip-coating approach based on three silica sols to fabrication of broadband antireflective superhydrophobic coatings. J. Colloid Interface Sci. 2013, 400, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, G.; Tong, Q.; Yang, W.; Hao, W. Fluorine-free superhydrophobic coatings from polydimethylsiloxane for sustainable chemical engineering: Preparation methods and applications. Chem. Eng. J. 2021, 426, 130829. [Google Scholar] [CrossRef]
- Caldona, E.B.; Sibaen, J.W.; Tactay, C.B.; Mendiola, S.L.D.; Abance, C.B.; Añes, M.P.; Serrano, F.D.D.; De Guzman, M.M.S. Preparation of spray-coated surfaces from green-formulated superhydrophobic coatings. SN Appl. Sci. 2019, 1, 1657. [Google Scholar] [CrossRef]
- Yang, H.; Dong, Y.; Li, X.; Gao, Y.; He, W.; Liu, Y.; Mu, X.; Zhao, Y. Anti-corrosion superhydrophobic micro-GF/micro-TiB2/nano-SiO2 based coating with braid strengthening structure fabricated by a single-step spray deposition. J. Alloys Compd. 2024, 1008, 176725. [Google Scholar] [CrossRef]
- Ye, H.; Zhu, L.; Li, W.; Liu, H.; Chen, H. Simple spray deposition of a water-based superhydrophobic coating with high stability for flexible applications. J. Mater. Chem. A 2017, 5, 9882–9890. [Google Scholar] [CrossRef]
- Li, Y.; Men, X.; Zhu, X.; Ge, B.; Chu, F.; Zhang, Z. One-step spraying to fabricate nonfluorinated superhydrophobic coatings with high transparency. J. Mater. Sci. 2016, 51, 2411–2419. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, Y.; Li, Y.; Jiang, Y.; Wang, S.; Xiang, J.; Zhang, J.; Cheng, P.; Tang, N. Stable construction of superhydrophobic surface on polypropylene membrane via atomic layer deposition for high salt solution desalination. J. Membr. Sci. 2022, 647, 120289. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, H.; Sun, J.; Shen, J. Layer-by-layer deposition of poly (diallyldimethylammonium chloride) and sodium silicate multilayers on silica-sphere-coated substrate—Facile method to prepare a superhydrophobic surface. Chem. Mater. 2007, 19, 948–953. [Google Scholar] [CrossRef]
- Shao, C.; Jiang, M.; Zhang, J.; Zhang, Q.; Han, L.; Wu, Y. Construction of a superhydrophobic wood surface coating by layer-by-layer assembly: Self-adhesive properties of polydopamine. Appl. Surf. Sci. 2023, 609, 155259. [Google Scholar] [CrossRef]
- Lu, X.; Hu, Y. Layer-by-layer deposition of TiO2 nanoparticles in the wood surface and its superhydrophobic performance. BioResources 2016, 11, 4605–4620. [Google Scholar] [CrossRef]
- Xiong, M.; Ren, Z.; Liu, W. Fabrication of UV-resistant and superhydrophobic surface on cotton fabric by functionalized polyethyleneimine/SiO2 via layer-by-layer assembly and dip-coating. Cellulose 2019, 26, 8951–8962. [Google Scholar] [CrossRef]
- Kothary, P.; Dou, X.; Fang, Y.; Gu, Z.; Leo, S.Y.; Jiang, P. Superhydrophobic hierarchical arrays fabricated by a scalable colloidal lithography approach. J. Colloid Interface Sci. 2017, 487, 484–492. [Google Scholar] [CrossRef]
- Gürsoy, M.; Testici, H.; Çıtak, E.; Kaya, M.; Türk Daği, H.; Öztürk, B.; Karaman, M. Biomimetic surfaces prepared by soft lithography and vapour deposition for hydrophobic and antibacterial performance. Mater. Technol. 2022, 37, 745–752. [Google Scholar] [CrossRef]
- Wang, F.; Li, S.; Wang, L. Fabrication of artificial super-hydrophobic lotus-leaf-like bamboo surfaces through soft lithography. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 389–395. [Google Scholar] [CrossRef]
- Li, Q.; Yan, Y.; Yu, M.; Song, B.; Shi, S.; Gong, Y. Synthesis of polymeric fluorinated sol–gel precursor for fabrication of superhydrophobic coating. Appl. Surf. Sci. 2016, 367, 101–108. [Google Scholar] [CrossRef]
- Aljumaily, M.M.; Alsaadi, M.A.; Das, R.; Hamid, S.B.A.; Hashim, N.A.; AlOmar, M.K.; Alayan, H.M.; Novikov, M.; Alsalhy, Q.F.; Hashim, M.A. Optimization of the synthesis of superhydrophobic carbon nanomaterials by chemical vapor deposition. Sci. Rep. 2018, 8, 2778. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sun, M.; Shi, X.; Shao, J.; Jin, M.; Liu, W.; Zhang, R.; Huang, S.; Ye, Y. Chemical vapor deposition of transparent superhydrophobic anti-icing coatings with tailored polymer nanoarray architecture. Chem. Eng. J. 2023, 454, 139981. [Google Scholar] [CrossRef]
- Kalmoni, J.J.; Heale, F.L.; Blackman, C.S.; Parkin, I.P.; Carmalt, C.J. A single-step route to robust and fluorine-free superhydrophobic coatings via aerosol-assisted chemical vapor deposition. Langmuir 2023, 39, 7731–7740. [Google Scholar] [CrossRef]
- Tombesi, A.; Li, S.; Sathasivam, S.; Page, K.; Heale, F.L.; Pettinari, C.; Carmalt, C.J.; Parkin, I.P. Aerosol-assisted chemical vapour deposition of transparent superhydrophobic film by using mixed functional alkoxysilanes. Sci. Rep. 2019, 9, 7549. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Tian, Y.; Liu, Y.; Fu, K.; Chen, Q.; Zhang, B.; Zhang, H.; Zhang, Q. Facile synthesis of superhydrophobic coating with icing delay ability by the self-assembly of PVDF clusters. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128562. [Google Scholar] [CrossRef]
- Tang, X.; Huang, W.; Xie, Y.; Xiao, Z.; Wang, H.; Liang, D.; Li, J.; Wang, Y. Superhydrophobic hierarchical structures from self-assembly of cellulose-based nanoparticles. ACS Sustain. Chem. Eng. 2021, 9, 14101–14111. [Google Scholar] [CrossRef]
- Zhou, C.; Ma, Y.; Rong, H.; Yu, X.; Liu, S.; Deng, L.; Zhang, J.; Li, S.; Dong, A. Cotton fabric with durable flame retardancy, robust superhydrophobicity and reliable UV shielding. Cellulose 2024, 31, 10025–10043. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, M.; Li, H.; Zhang, Z.; Su, X.; Li, Y.; Han, Z.; Lv, X.; He, J.; Liu, Y.; et al. Facile and effective construction of superhydrophobic, multi-functional and durable coatings on steel structure. Compos. Part B Eng. 2024, 287, 111850. [Google Scholar] [CrossRef]
- Gong, X.; He, S. Highly durable superhydrophobic polydimethylsiloxane/silica nanocomposite surfaces with good self-cleaning ability. ACS Omega 2020, 5, 4100–4108. [Google Scholar] [CrossRef]
- Cao, C.; Yi, B.; Zhang, J.; Hou, C.; Wang, Z.; Lu, G.; Huang, X.; Yao, X. Sprayable superhydrophobic coating with high processibility and rapid damage-healing nature. Chem. Eng. J. 2020, 392, 124834. [Google Scholar] [CrossRef]
- Yang, X.; Sun, H.; Zhang, Y. Fabrication of durable superhydrophobic coatings on aluminum via anodization and hydrophobic layer deposition. J. Mater. Sci. Technol. 2022, 118, 45–53. [Google Scholar]
- Barthwal, S.; Uniyal, S.; Barthwal, S. Nature-Inspired Superhydrophobic Coating Materials: Drawing Inspiration from Nature for Enhanced Functionality. Micromachines 2024, 15, 391. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Lu, Y.; Shi, W.; Tian, H. PDMS/PVDF Electrospinning Membranes for Water-in-Oil Emulsion Separation and UV Protection. Biomimetics 2022, 7, 217. [Google Scholar] [CrossRef]
- Ben Rejeb, Z.; Abidli, A.; Zaoui, A.; Fashandi, M.; Selka, A.; Naguib, H.E.; Park, C.B. One-pot synthesis of rationally-designed flexible, robust, and hydrophobic ambient-dried molecularly-bridged silica aerogels with efficient and versatile oil/water separation applications. Adv. Compos. Hybrid Mater. 2024, 7, 188. [Google Scholar] [CrossRef]
- Kanovsky, N.; Cohen, S.; Margel, S. In-situ design, characterization and use of durable superhydrophobic thin coatings applied on polymeric films. Mater. Res. Bull. 2022, 146, 111598. [Google Scholar] [CrossRef]
- Wu, X.; Chu, F.; Orejon, D.; Mouterde, T. Superhydrophobic surfaces: Fundamentals, manufacture, and applications. Appl. Phys. Lett. 2024, 124, 020401. [Google Scholar] [CrossRef]
- Mittal, T.; Tiwari, S.; Tiwari, S.K. Design of mixed metal oxide nanostructured superhydrophilic surfaces with self-cleaning properties. J. Coat. Technol. Res. 2024, 416, 1–12. [Google Scholar] [CrossRef]
- Schrijnemakers, K.; Impens, N.R.E.N.; Vansant, E.F. Deposition of a titania coating on silica by means of the chemical surface coating. Langmuir 1999, 15, 5807–5813. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M.; Britto Joseph, G.; Durairaj, R.B.; Mageshwaran, G.J.J.O.C.T. Superhydrophobic surfaces: A review on fundamentals, applications, and challenges. J. Coat. Technol. Res. 2018, 15, 231–250. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Multiscale Dissipative Mechanisms and Hierarchical Surfaces: Friction, Superhydrophobicity, and Biomimetics; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- S Latthe, S.S.; Sutar, R.S.; Bhosale, A.K.; Nagappan, S.; Ha, C.S.; Sadasivuni, K.K.; Liu, S.; Xing, R. Recent developments in air-trapped superhydrophobic and liquid-infused slippery surfaces for anti-icing application. Prog. Org. Coat. 2019, 137, 105373. [Google Scholar] [CrossRef]
- Li, W.; Zhan, Y.; Yu, S. Applications of superhydrophobic coatings in anti-icing: Theory, mechanisms, impact factors, challenges and perspectives. Prog. Org. Coat. 2021, 152, 106117. [Google Scholar] [CrossRef]
- Bretler, S.; Kanovsky, N.; Iline-Vul, T.; Cohen, S.; Margel, S. In-situ thin coating of silica micro/nano-particles on polymeric films and their anti-fogging application. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125444. [Google Scholar] [CrossRef]
- Ganguly, S.; Kanovsky, N.; Das, P.; Gedanken, A.; Margel, S. Photopolymerized Thin Coating of Polypyrrole/Graphene Nanofiber/Iron Oxide onto Nonpolar Plastic for Flexible Electromagnetic Radiation Shielding, Strain Sensing, and Non-Contact Heating Applications. Adv. Mater. Interfaces 2021, 8, 2101255. [Google Scholar] [CrossRef]
- Kanovsky, N.; Margel, S. Fabrication of transparent silica/PEG smooth thin coatings on polymeric films for antifogging applications. ACS Omega 2022, 7, 20505–20514. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, M.; Kumar, A. Advance biodegradable jute fabric surface with water-repellent properties. J. Coat. Technol. Innov. 2024, 2, 1–6. [Google Scholar]
- Hayne, S.; Margel, S. Thin coating of silica/polystyrene core-shell nano/microparticles with hierarchical morphology onto polymeric films for fabrication of superhydrophobic surfaces. Mater. Today Chem. 2023, 30, 101497. [Google Scholar] [CrossRef]
- Hayne, S.; Margel, S. In situ coatings of polymeric films with core polystyrene, core–shell polystyrene/SiO2, and hollow SiO2 micro/nanoparticles and potential applications. ACS Omega 2023, 8, 11406–11413. [Google Scholar] [CrossRef]
- Jung, Y.C.; Bhushan, B. Contact angle, adhesion and friction properties of micro-and nanopatterned polymers for superhydrophobicity. Nanotechnology 2006, 17, 4970. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; McHale, G.; Newton, M.I.; Perry, C.C. Intrinsically superhydrophobic organosilica sol−gel foams. Langmuir 2003, 19, 5626–5631. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Boccuti, M.R.; Rao, K.M.; Zecchina, A.; Leofanti, G.; Petrini, G. Spectroscopic characterization of silicalite and titanium-silicalite. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1989; Volume 48, pp. 133–144. [Google Scholar]
- Qian, Z.; Zhang, Z.; Song, L.; Liu, H. A novel approach to raspberry-like particles for superhydrophobic materials. J. Mater. Chem. 2009, 19, 1297–1304. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Q.; Lyons, A.M. Durable, optically transparent, superhydrophobic polymer films. Appl. Surf. Sci. 2019, 470, 187–195. [Google Scholar] [CrossRef]
- Chen, D.; Mai, Z.; Liu, X.; Ye, D.; Zhang, H.; Yin, X.; Zhou, Y.; Liu, M.; Xu, W. UV-blocking, superhydrophobic and robust cotton fabrics fabricated using polyvinylsilsesquioxane and nano-TiO2. Cellulose 2018, 25, 3635–3647. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J. Fabrication of durable superhydrophobic coatings via electrodeposition and chemical modification. Surf. Coat. Technol. 2021, 409, 126892. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Huang, L. Durable superhydrophobic coatings based on layer-by-layer assembly of nanoparticles and polymers. Mater. Lett. 2022, 309, 131356. [Google Scholar]
- Wang, F.; Zhang, R.; Liu, J. A durable superhydrophobic coating fabricated via plasma etching and sol-gel process. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621, 126608. [Google Scholar] [CrossRef]
- Zhao, L.; Li, W. Fabrication of durable superhydrophobic surfaces via electrospinning and chemical vapor deposition. J. Appl. Polym. Sci. 2023, 140, e53357. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.; Zhao, X. Enhanced wear resistance and adhesion of superhydrophobic coatings on polypropylene via sol-gel and plasma treatment. Surf. Coat. Technol. 2020, 383, 125265. [Google Scholar]
- Wang, Y.; Liu, H.; Sun, X. Durability of superhydrophobic coatings reinforced with silica nanoparticle networks. Mater. Sci. Eng. B 2021, 274, 115452. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, W.; Lu, X. Durable superhydrophobic coatings based on silica nanoparticles and silane crosslinking. J. Colloid Interface Sci. 2021, 586, 845–854. [Google Scholar] [CrossRef]
- Zhao, L.; Li, W. Fabrication of wear-resistant superhydrophobic coatings through the combination of sol-gel and nanoparticle reinforcement. Mater. Lett. 2022, 313, 131710. [Google Scholar]
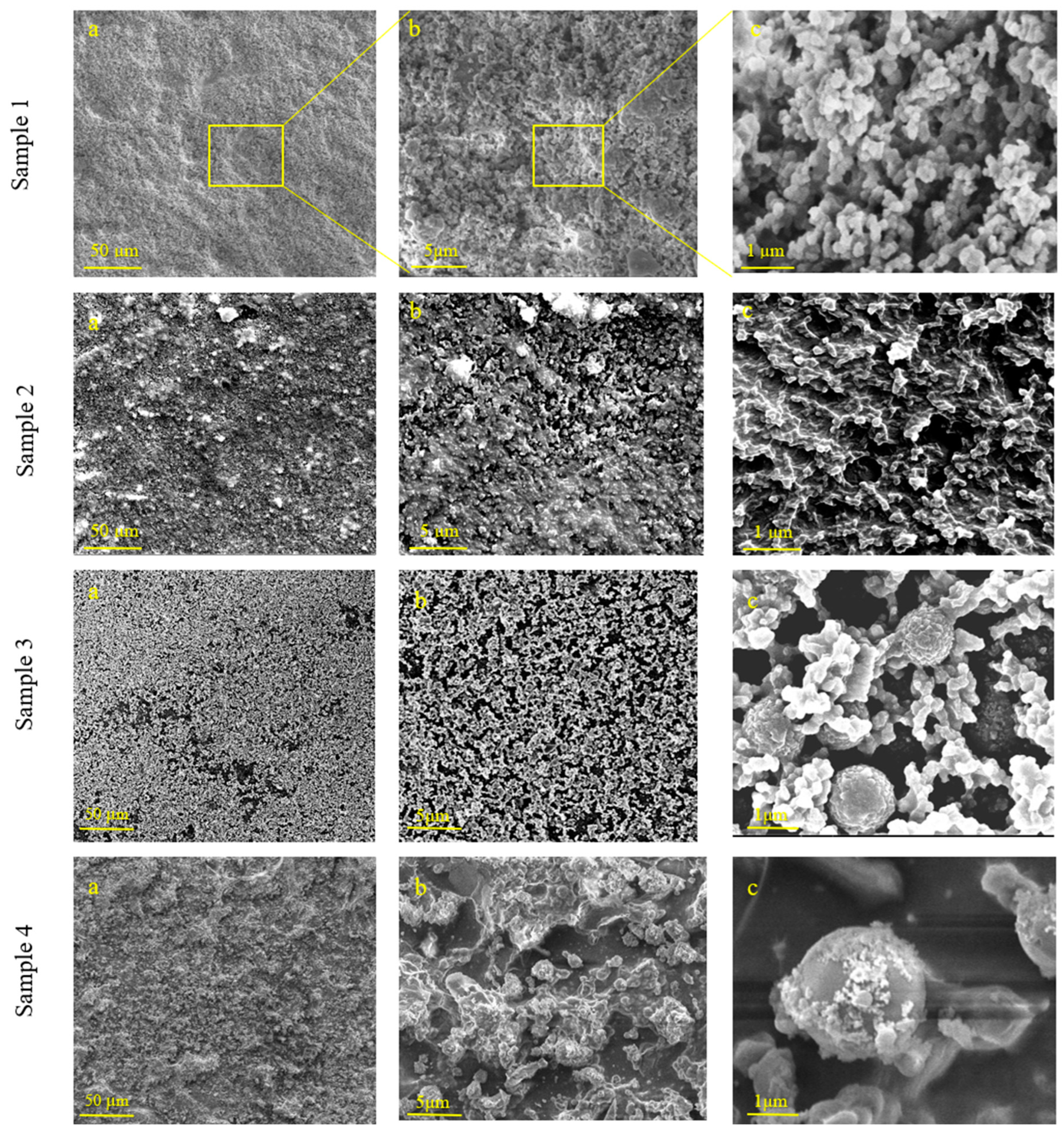
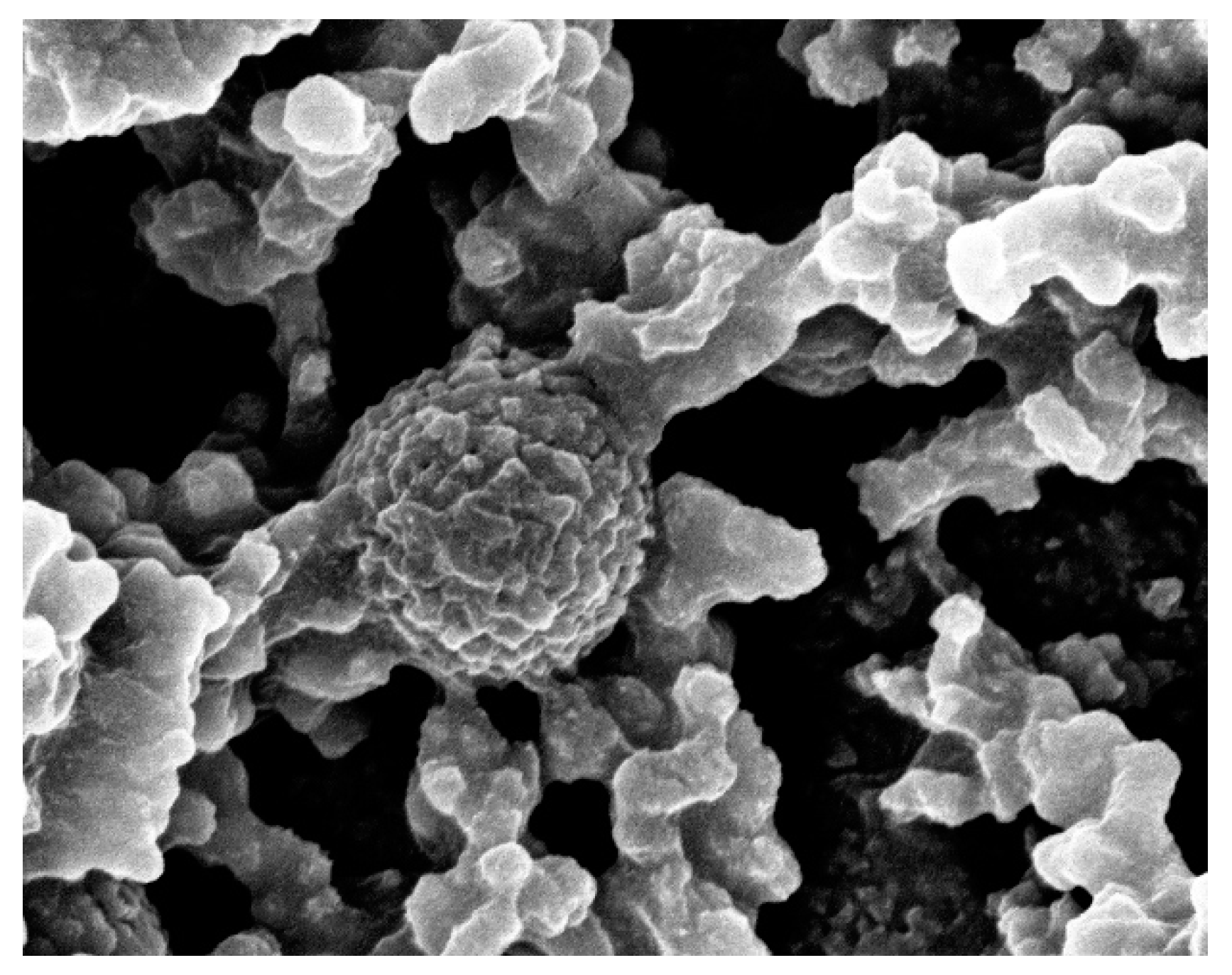




| Sample # | SiO2 Nanoparticle Average Size (nm) | SiO2/Isopropanol Dispersion (mL) | Isopropanol (mL) | FDTES (µL) | TEOS (µL) | TBT (µL) |
|---|---|---|---|---|---|---|
| 1. TiO2 | None | 0.91 | 36.1 | 45.9 | ||
| 2. TiO2-SiO2_70 | 70 | 0.95 | 23 | 7 | 14 | |
| 3. TiO2-SiO2_250 | 250 | 0.95 | 23 | 7 | 14 | |
| 4. TiO2-SiO2_500 | 500 | 0.95 | 23 | 7 | 14 |
| Sample # | Si2p | Ti2p | F1s | O1s | C1s | N1s |
|---|---|---|---|---|---|---|
| 1. TiO2 | 0.22 | 4.68 | 52.38 | 9.58 | 32.49 | 0.55 |
| 2. TiO2-SiO2_70 | 3.61 | 3.89 | 40.7 | 13.65 | 37.41 | 0.74 |
| 3. TiO2-SiO2_250 | 5.2 | 2.99 | 36.51 | 21 | 33.18 | 1.12 |
| 4. TiO2-SiO2_500 | 9.84 | 0.4 | 44.38 | 20.43 | 24.51 | 0.43 |
| Sample # | Ra (nm) | CA (°) |
|---|---|---|
| Corona-treated PP film | 8.7 ± 0.6 | 54.3 ± 1.2 |
| 1. TiO2 | 5.4 ± 2.2 | >180 |
| 2. TiO2-SiO2_70 | 8.9 ± 0.4 | >180 |
| 3. TiO2-SiO2_250 | 22.6 ± 6.3 | >180 |
| 4. TiO2-SiO2_500 | 36.4 ± 5.6 | >180 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayne, S.; Kanovsky, N.; Margel, S. One-Step Fabrication Process of Silica–Titania Superhydrophobic UV-Blocking Thin Coatings onto Polymeric Films. Biomimetics 2024, 9, 756. https://doi.org/10.3390/biomimetics9120756
Hayne S, Kanovsky N, Margel S. One-Step Fabrication Process of Silica–Titania Superhydrophobic UV-Blocking Thin Coatings onto Polymeric Films. Biomimetics. 2024; 9(12):756. https://doi.org/10.3390/biomimetics9120756
Chicago/Turabian StyleHayne, Sharon, Naftali Kanovsky, and Shlomo Margel. 2024. "One-Step Fabrication Process of Silica–Titania Superhydrophobic UV-Blocking Thin Coatings onto Polymeric Films" Biomimetics 9, no. 12: 756. https://doi.org/10.3390/biomimetics9120756
APA StyleHayne, S., Kanovsky, N., & Margel, S. (2024). One-Step Fabrication Process of Silica–Titania Superhydrophobic UV-Blocking Thin Coatings onto Polymeric Films. Biomimetics, 9(12), 756. https://doi.org/10.3390/biomimetics9120756





