Valorization of Selected Biomass-Derived Molecules on Olea europaea Leaves-Biotemplated TiO2-g-C3N4 Photocatalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Photocatalysts
2.2. Characterization of Solids
2.3. Photocatalytic Activity
2.3.1. Hydrogen Production Through Glycerol Photoreforming
2.3.2. Photoacetalization of Cinnamaldehyde with 1,2-Propanediol
3. Results and Discussion
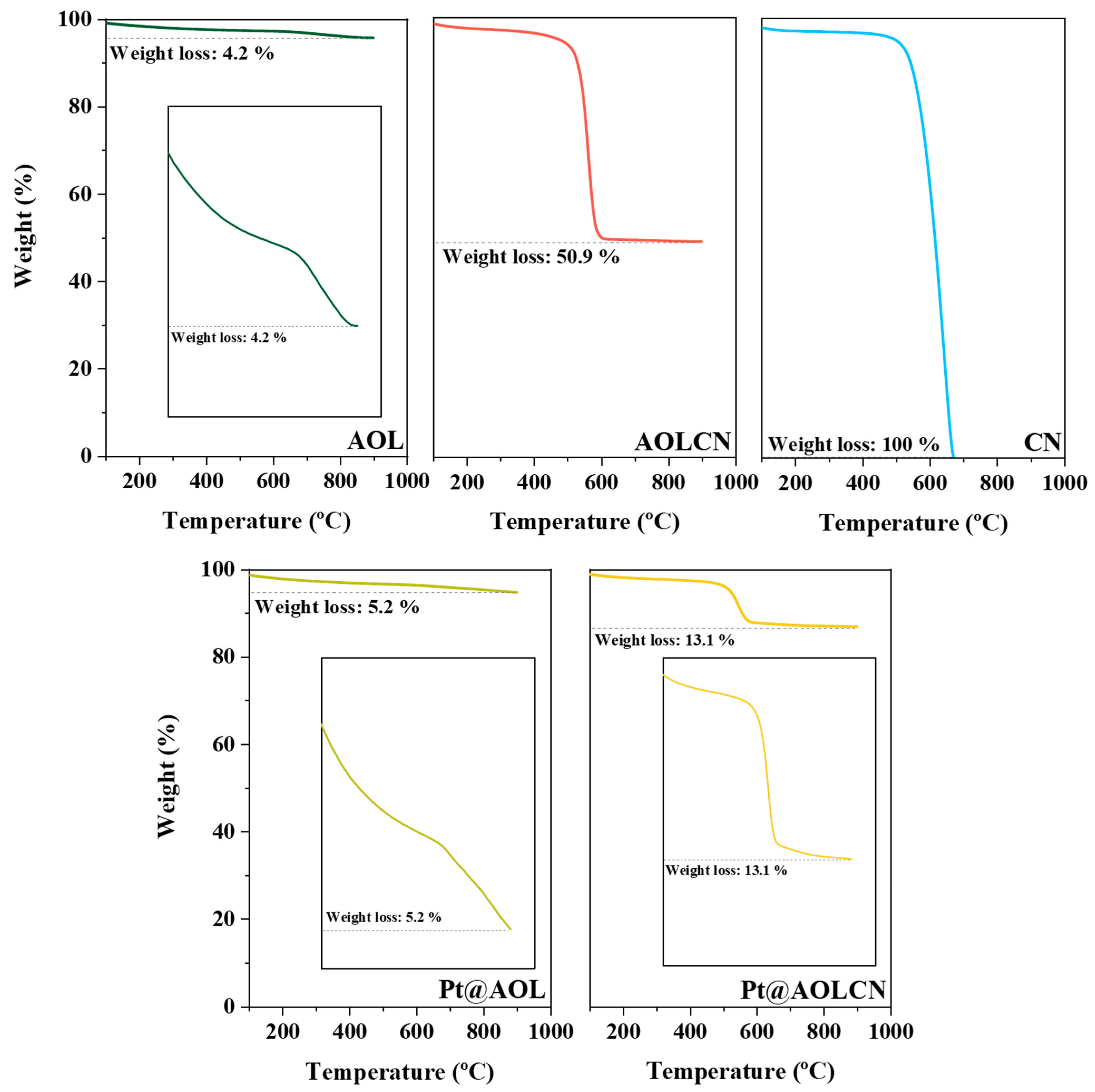
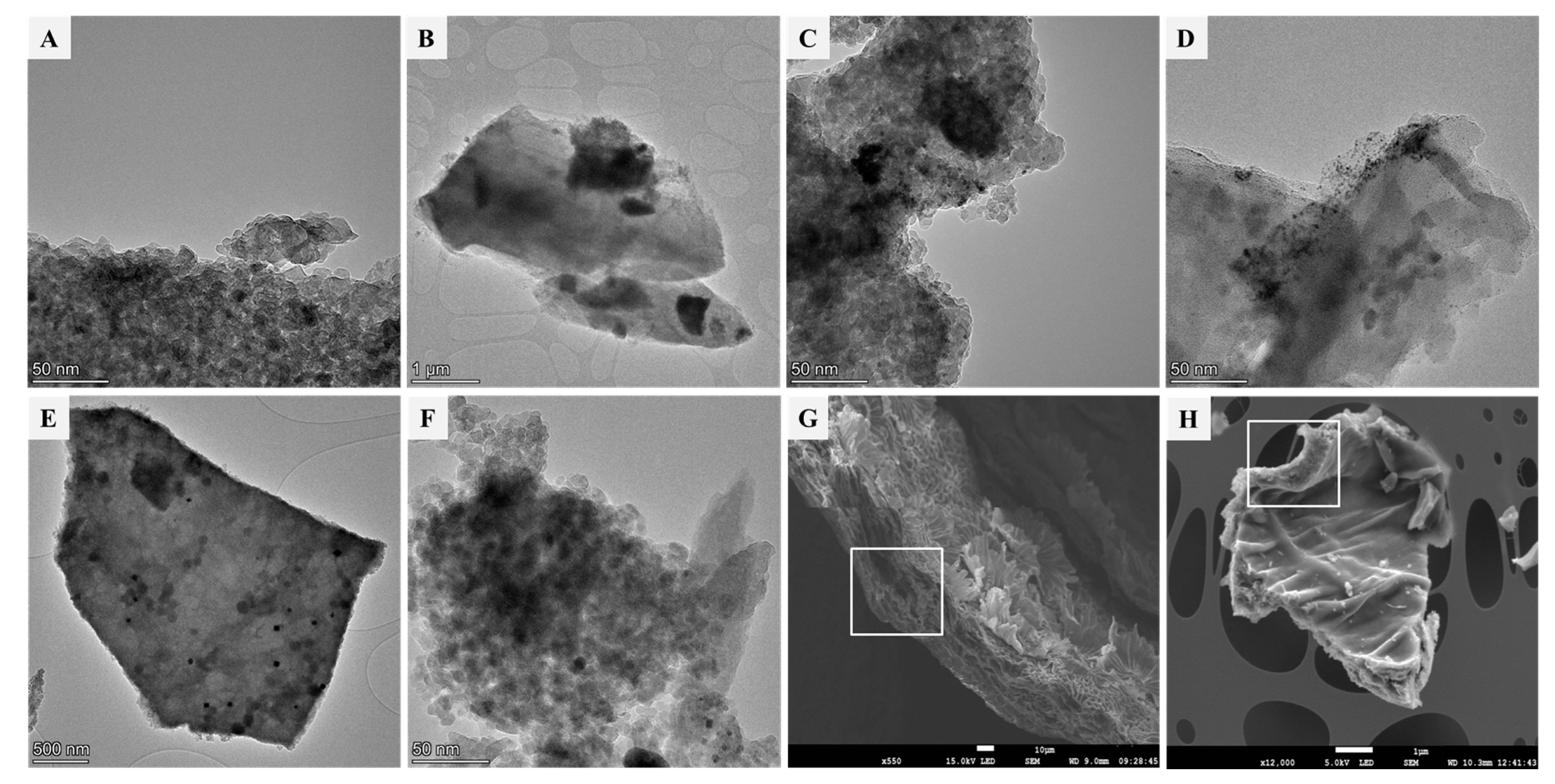
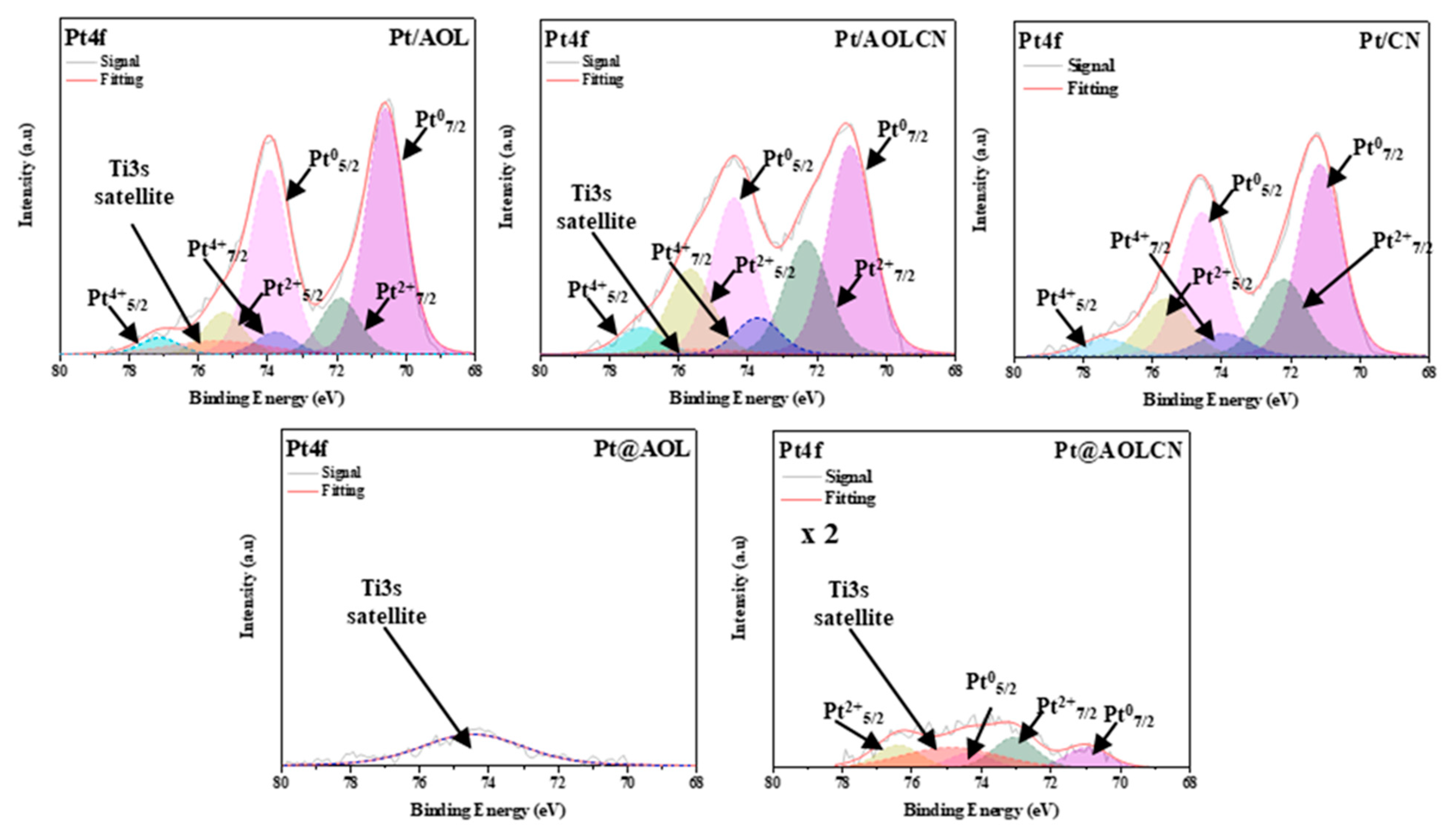
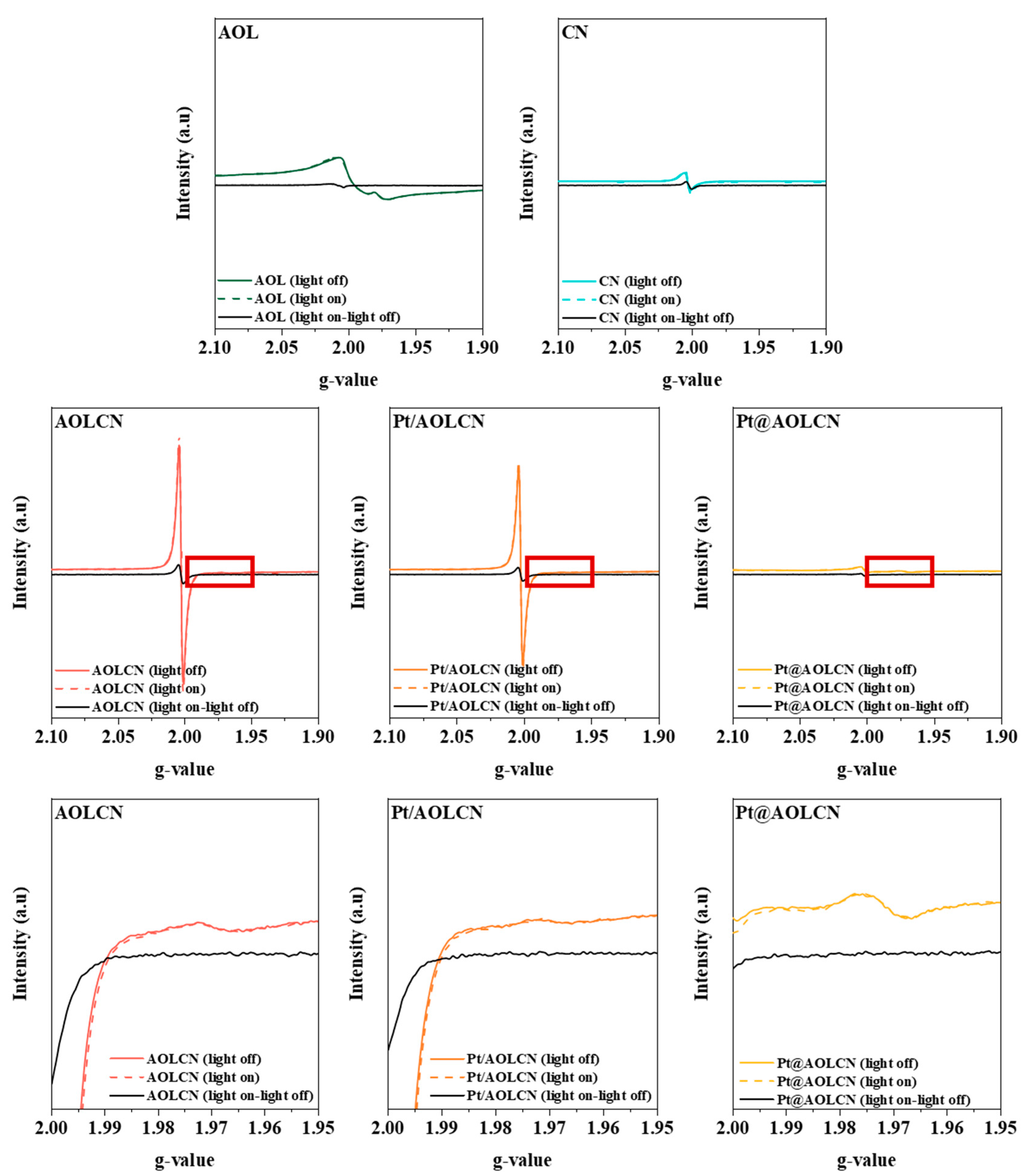
3.1. Characterization Results
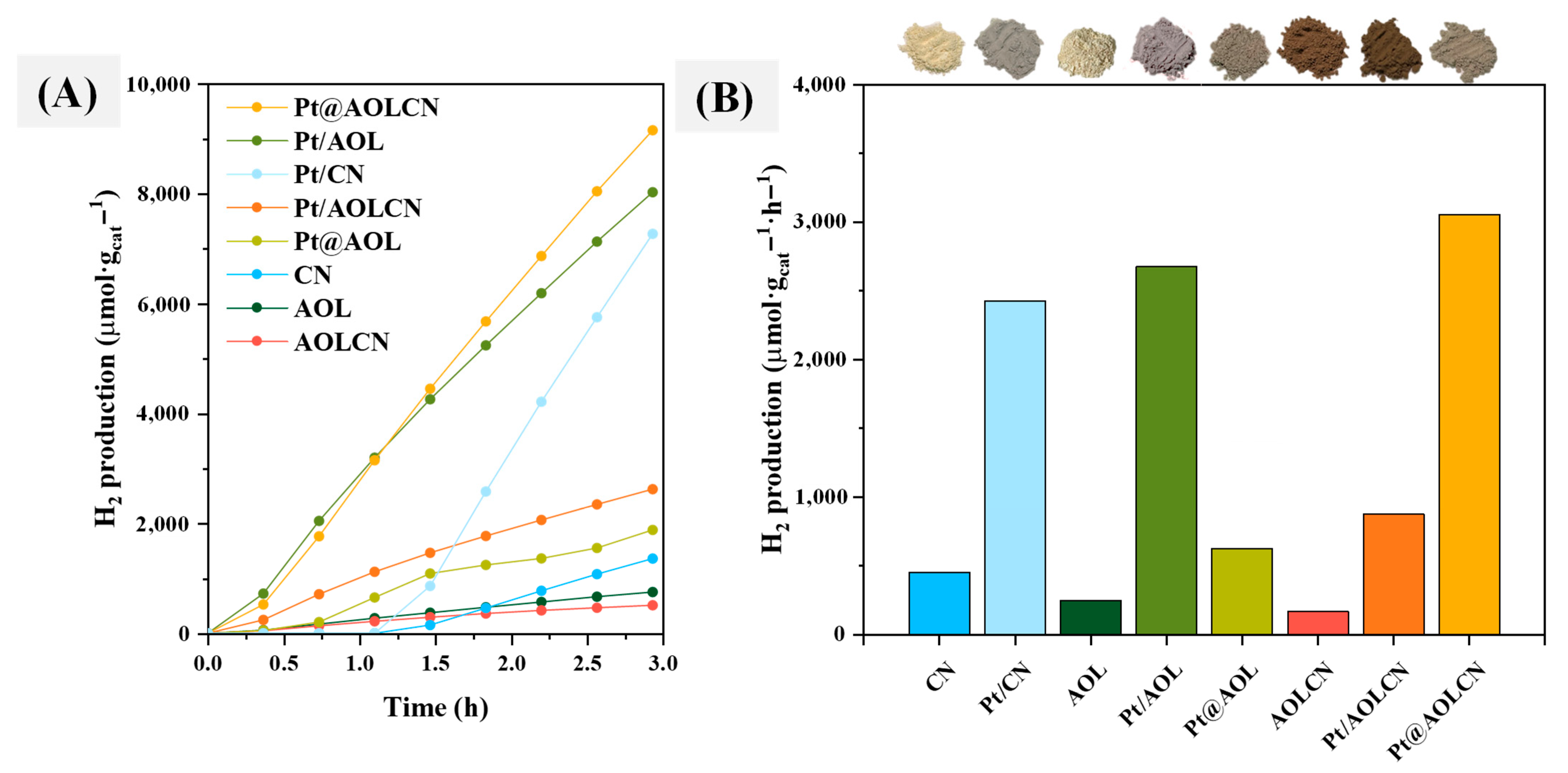
3.2. Photocatalytic Production of Hydrogen
3.3. Photoacetalization of Cinnamaldehyde with 1,2-Propanediol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrera-Beurnio, M.C.; Hidalgo-Carrillo, J.; López-Tenllado, F.J.; Martin-Gómez, J.; Estévez, R.C.; Urbano, F.J.; Marinas, A. Bio-Templating: An Emerging Synthetic Technique for Catalysts. A Review. Catalysts 2021, 11, 1364. [Google Scholar] [CrossRef]
- Roostaei, T.; Rahimpour, M.R. Role of Support Bio-Templating in Ni/Al2O3 Catalysts for Hydrogen Production via Dry Reforming of Methane. Sci. Rep. 2023, 13, 16972. [Google Scholar] [CrossRef] [PubMed]
- Kaplin, I.Y.; Lokteva, E.S.; Golubina, E.V.; Lunin, V.V. Template Synthesis of Porous Ceria-Based Catalysts for Environmental Application. Molecules 2020, 25, 4242. [Google Scholar] [CrossRef]
- Roostaei, T.; Rahimpour, M.R.; Zhao, H.; Eisapour, M.; Chen, Z.; Hu, J. Recent Advances and Progress in Biotemplate Catalysts for Electrochemical Energy Storage and Conversion. Adv. Colloid Interface Sci. 2023, 318, 102958. [Google Scholar] [CrossRef]
- Kim, S.W.; Han, T.H.; Kim, J.; Gwon, H.; Moon, H.S.; Kang, S.W.; Kim, S.O.; Kang, K. Fabrication and Electrochemical Characterization of TiO2 Three- Dimensional Nanonetwork Based on Peptide Assembly. ACS Nano 2009, 3, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.; Plumejeau, S.; Heux, L.; Louvain, N.; Monconduit, L.; Stievano, L.; Boury, B. Conversion of Nanocellulose Aerogel into TiO2 and TiO2@C Nano-Thorns by Direct Anhydrous Mineralization with TiCl4. Evaluation of Electrochemical Properties in Li Batteries. ACS Appl. Mater. Interfaces 2015, 7, 14590–14592. [Google Scholar] [CrossRef]
- Magnabosco, G.; Papiano, I.; Aizenberg, M.; Aizenberg, J.; Falini, G. Beyond Biotemplating: Multiscale Porous Inorganic Materials with High Catalytic Efficiency. Chem. Commun. 2020, 56, 3389–3392. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Mohd Hir, Z.A.; Rosmi, M.S.; Mutalib, M.A.; Ismail, A.F.; Tanemura, M. Regenerated Cellulose Membrane as Bio-Template for in-Situ Growth of Visible-Light Driven C-Modified Mesoporous Titania. Carbohydr. Polym. 2016, 146, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Lu, Y.S.; Prasad Bastakoti, B.; Li, Y.; Pramanik, M.; Shahriar Hossain, M.; Yanmaz, E.; Kuo, S.W. Mesoporous TiO2 Thin Film Formed From a Bioinspired Supramolecular Assembly. ChemistrySelect 2016, 1, 4295–4299. [Google Scholar] [CrossRef]
- Gutbrod, K.; Greil, P.; Zollfrank, C. Carbon Auto-Doping Improves Photocatalytic Properties of Biotemplated Ceramics. Appl. Catal. B 2011, 103, 240–245. [Google Scholar] [CrossRef]
- Hashemizadeh, I.; Golovko, V.B.; Choi, J.; Tsang, D.C.W.; Yip, A.C.K. Photocatalytic Reduction of CO2 to Hydrocarbons Using Bio-Templated Porous TiO2 Architectures under UV and Visible Light. Chem. Eng. J. 2018, 347, 64–73. [Google Scholar] [CrossRef]
- Kumaravel, V.; Imam, M.D.; Badreldin, A.; Chava, R.K.; Do, J.Y.; Kang, M.; Abdel-Wahab, A. Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef]
- García-López, E.I.; Palmisano, L.; Marcì, G. Overview on Photoreforming of Biomass Aqueous Solutions to Generate H2 in the Presence of G-C3N4-Based Materials. ChemEngineering 2023, 7, 11. [Google Scholar] [CrossRef]
- Nair, R.V.; Gummaluri, V.S.; Matham, M.V.; Vijayan, C. A Review on Optical Bandgap Engineering in TiO2 Nanostructures via Doping and Intrinsic Vacancy Modulation towards Visible Light Applications. J. Phys. D Appl. Phys. 2022, 55, 313003. [Google Scholar] [CrossRef]
- Ismael, M. A Review and Recent Advances in Solar-to-Hydrogen Energy Conversion Based on Photocatalytic Water Splitting over Doped-TiO2 Nanoparticles. Solar. Energy. 2020, 211, 522–546. [Google Scholar] [CrossRef]
- Herrera-Beurnio, M.C.; López-Tenllado, F.J.; Hidalgo-Carrillo, J.; Martín-Gómez, J.; Estévez, R.; Castillo-Rodríguez, M.; de Miguel, G.; Urbano, F.J.; Marinas, A. Controlled Photodeposition of Pt onto TiO2-g-C3N4 Systems for Photocatalytic Hydrogen Production. Catal. Today 2023, 413–415, 113967. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Zhang, Y.; Huang, J. Bioinspired Hierarchical Nanotubular Titania Immobilized with Platinum Nanoparticles for Photocatalytic Hydrogen Production. Chem. Eur. J. 2015, 21, 7345–7349. [Google Scholar] [CrossRef]
- Wang, C.; Mouchet, S.R.; Deparis, O.; Li, J.; Paineau, E.; Dragoe, D.; Remita, H.; Ghazzal, M.N. TiO2 Films with Macroscopic Chiral Nematic-Like Structure Stabilized by Copper Promoting Light-Harvesting Capability for Hydrogen Generation. Small 2024, 20, 2402211. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Paineau, E.; Laachachi, A.; Colbeau-Justin, C.; Remita, H.; Ghazzal, M.N. A Sol-Gel Biotemplating Route with Cellulose Nanocrystals to Design a Photocatalyst for Improving Hydrogen Generation. J. Mater. Chem. A 2020, 8, 10779–10786. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, S.; Yang, J.; Wang, H.; Yu, H.; Chen, H.; Zhao, Y.; Yuan, X.; Chu, W.; Li, H. Near-Infrared Light Responsive TiO2 for Efficient Solar Energy Utilization. Adv. Funct. Mater. 2022, 32, 2108977. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, L.; Yang, J.; Zhou, S.; Yuan, X.; Liang, J.; Wang, H.; Wang, H.; Bu, Y.; Li, H. Synthesis and Modification of Ultrathin G-C3N4 for Photocatalytic Energy and Environmental Applications. Renew. Sustain. Energy Rev. 2023, 173, 113110. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Jiang, L.; Yu, H.; Zhao, Y.; Chen, H.; Yuan, X.; Liang, J.; Li, H.; Wu, Z. Defective Polymeric Carbon Nitride: Fabrications, Photocatalytic Applications and Perspectives. Chem. Eng. J. 2022, 427, 130991. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A Metal-Free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, L.; Ye, X.; Yang, Y.; Ren, W.; Ge, J.; Zhang, S.; Zheng, X.; Chen, S. Dual-Defect Modified 2D/2D TiO2/g-C3N4 Heterojunction for Photocatalytic H2 Production. Cryst. Growth Des. 2024, 24, 7605–7616. [Google Scholar] [CrossRef]
- Oseghe, E.O.; Akpotu, S.O.; Mombeshora, E.T.; Oladipo, A.O.; Ombaka, L.M.; Maria, B.B.; Idris, A.O.; Mamba, G.; Ndlwana, L.; Ayanda, O.S.; et al. Multi-Dimensional Applications of Graphitic Carbon Nitride Nanomaterials—A Review. J. Mol. Liq. 2021, 344, 117820. [Google Scholar] [CrossRef]
- Acharya, R.; Parida, K. A Review on TiO2/g-C3N4 Visible-Light-Responsive Photocatalysts for Sustainable Energy Generation and Environmental Remediation. J. Environ. Chem. Eng. 2020, 8, 103896. [Google Scholar] [CrossRef]
- Guo, X.; Duan, J.; Li, C.; Zhang, Z.; Wang, W. Fabrication of G-C3N4/TiO2 Photocatalysts with a Special Bilayer Structure for Visible Light Photocatalytic Application. Colloids. Surf. A. Physicochem. Eng. Asp. 2020, 599, 124931. [Google Scholar] [CrossRef]
- Abdullah Khan, M.; Teixeira, I.F.; Li, M.M.J.; Koito, Y.; Tsang, S.C.E. Graphitic Carbon Nitride Catalysed Photoacetalization of Aldehydes/Ketones under Ambient Conditions. Chem. Commun. 2016, 52, 2772–2775. [Google Scholar] [CrossRef]
- Ruiz, V.R.; Velty, A.; Santos, L.L.; Leyva-Pérez, A.; Sabater, M.J.; Iborra, S.; Corma, A. Gold Catalysts and Solid Catalysts for Biomass Transformations: Valorization of Glycerol and Glycerol-Water Mixtures through Formation of Cyclic Acetals. J. Catal. 2010, 271, 351–357. [Google Scholar] [CrossRef]
- Corrêa, I.; Faria, R.P.V.; Rodrigues, A.E. Continuous Valorization of Glycerol into Solketal: Recent Advances on Catalysts, Processes, and Industrial Perspectives. Sustain. Chem. 2021, 2, 286–324. [Google Scholar] [CrossRef]
- Hidalgo-Carrillo, J.; Estévez-Toledano, R.C.; López-Tenllado, F.J.; Bautista, F.M.; Urbano, F.J.; Marinas, A. Fourth Generation Synthesis of Solketal by Glycerol Acetalization with Acetone: A Solar-Light Photocatalytic Approach. J. Taiwan Inst. Chem. Eng. 2021, 125, 297–303. [Google Scholar] [CrossRef]
- Abdullah Khan, M.; Hussain, A.; Teixeira, I.F.; Al-Humaidi, J.Y.; Hafeez, M.; Liaqat, F. Tandem Photooxidation/Acetalization of Alcohols to Acetals/Ketals with Sulfur-Doped Carbon Nitride. Asian J. Org. Chem. 2023, 12, e202300388. [Google Scholar] [CrossRef]
- Zhao, H.; Zheng, L.; Li, X.; Chen, P.; Hou, Z. Hydrogenolysis of Glycerol to 1,2-Propanediol over Cu-Based Catalysts: A Short Review. Catal. Today 2020, 355, 84–95. [Google Scholar] [CrossRef]
- Woelfel, K.; Hartman, T.G. Mass Spectrometry of the Acetal Derivatives of Selected Generally Recognized as Safe Listed Aldehydes with Ethanol, 1,2-Propylene Glycol and Glycerol. In Flavor Analysis; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1998; Volume 705, pp. 193–210. [Google Scholar] [CrossRef]
- Hidalgo-Carrillo, J.; Martín-Gómez, J.; Herrera-Beurnio, M.C.; Estévez, R.C.; Urbano, F.J.; Marinas, A. Olive Leaves as Biotemplates for Enhanced Solar-Light Harvesting by a Titania-Based Solid. Nanomaterials 2020, 10, 1057. [Google Scholar] [CrossRef]
- Stadnichenko, A.; Svintsitskiy, D.; Kibis, L.; Fedorova, E.; Stonkus, O.; Slavinskaya, E.; Lapin, I.; Fakhrutdinova, E.; Svetlichnyi, V.; Romanenko, A.; et al. Influence of Titania Synthesized by Pulsed Laser Ablation on the State of Platinum during Ammonia Oxidation. Appl. Sci. 2020, 10, 4699. [Google Scholar] [CrossRef]
- Balaji, S.; Guda, R.; Mandal, B.K.; Kasula, M.; Ubba, E.; Khan, F.R.N. Green Synthesis of Nano-Titania (TiO2 NPs) Utilizing Aqueous Eucalyptus Globulus Leaf Extract: Applications in the Synthesis of 4H-Pyran Derivatives. Res. Chem. Intermed. 2021, 47, 3919–3931. [Google Scholar] [CrossRef]
- Kite, S.V.; Sathe, D.J.; Kadam, A.N.; Chavan, S.S.; Garadkar, K.M. Highly Efficient Photodegradation of 4-Nitrophenol over the Nano-TiO2 Obtained from Chemical Bath Deposition Technique. Res. Chem. Intermed. 2020, 46, 1255–1282. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Bagheri, S.; Abd Hamid, S.B. Biotemplated Synthesis of Anatase Titanium Dioxide Nanoparticles via Lignocellulosic Waste Material. Biomed. Res. Int. 2014, 2014, 205636. [Google Scholar] [CrossRef]
- Leelavathi, H.; Abirami, N.; Muralidharan, R.; Kavitha, H.P.; Tamizharasan, S.; Sankeetha, S.; Arulmozhi, R. Sunlight-Assisted Degradation of Textile Pollutants and Phytotoxicity Evaluation Using Mesoporous ZnO/g-C3N4 Catalyst. RSC Adv. 2021, 11, 26800–26812. [Google Scholar] [CrossRef]
- Verma, R.; Gangwar, J.; Srivastava, A.K. Multiphase TiO2 Nanostructures: A Review of Efficient Synthesis, Growth Mechanism, Probing Capabilities, and Applications in Bio-Safety and Health. RSC Adv. 2017, 7, 44199–44224. [Google Scholar] [CrossRef]
- Wetchakun, N.; Phanichphant, S. Effect of Temperature on the Degree of Anatase-Rutile Transformation in Titanium Dioxide Nanoparticles Synthesized by the Modified Sol-Gel Method. Curr. Appl. Phys. 2008, 8, 343–346. [Google Scholar] [CrossRef]
- Phromma, S.; Wutikhun, T.; Kasamechonchung, P.; Eksangsri, T.; Sapcharoenkun, C. Effect of Calcination Temperature on Photocatalytic Activity of Synthesized TiO2 Nanoparticles via Wet Ball Milling Sol-Gel Method. Appl. Sci. 2020, 10, 993. [Google Scholar] [CrossRef]
- Ismael, M.; Wu, Y.; Taffa, D.H.; Bottke, P.; Wark, M. Graphitic Carbon Nitride Synthesized by Simple Pyrolysis: Role of Precursor in Photocatalytic Hydrogen Production. New J. Chem. 2019, 43, 6909–6920. [Google Scholar] [CrossRef]
- Taudul, B.; Tielens, F.; Calatayud, M. On the Origin of Raman Activity in Anatase TiO2 (Nano)Materials: An Ab Initio Investigation of Surface and Size Effects. Nanomaterials 2023, 13, 1856. [Google Scholar] [CrossRef]
- Luo, Y.; Kong, D.; Luo, J.; Chen, S.; Zhang, D.; Qiu, K.; Qi, X.; Zhang, H.; Li, C.M.; Yu, T. Hierarchical TiO2 Nanobelts@MnO2 Ultrathin Nanoflakes Core-Shell Array Electrode Materials for Supercapacitors. RSC Adv. 2013, 3, 14413–14422. [Google Scholar] [CrossRef]
- He, K.; Wang, Q. Activation of Pt Nanoclusters on TiO2 via Tuning the Metallic Sites to Promote Low-Temperature CO Oxidation. Catalysts 2021, 11, 1280. [Google Scholar] [CrossRef]
- Apopei, P.; Catrinescu, C.; Teodosiu, C.; Royer, S. Mixed-Phase TiO2 Photocatalysts: Crystalline Phase Isolation and Reconstruction, Characterization and Photocatalytic Activity in the Oxidation of 4-Chlorophenol from Aqueous Effluents. Appl. Catal. B 2014, 160–161, 374–382. [Google Scholar] [CrossRef]
- Houǎková, V.; Štengl, V.; Bakardjieva, S.; Murafa, N.; Tyrpekl, V. Photocatalytic Properties of Ru-Doped Titania Prepared by Homogeneous Hydrolysis. Cent. Eur. J. Chem. Open Chem. 2009, 7, 259–266. [Google Scholar] [CrossRef]
- Allen, N.S.; Mahdjoub, N.; Vishnyakov, V.; Kelly, P.J.; Kriek, R.J. The Effect of Crystalline Phase (Anatase, Brookite and Rutile) and Size on the Photocatalytic Activity of Calcined Polymorphic Titanium Dioxide (TiO2). Polym. Degrad. Stab. 2018, 150, 31–36. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Eder, D.; Motta, M.; Windle, A.H. Iron-Doped Pt-TiO2 Nanotubes for Photo-Catalytic Water Splitting. Nanotechnology 2009, 20, 055602. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Koirala, A.R.; Akhtar, U.S.; Song, M.K.; Yoon, K.B. First Synthesis of Highly Crystalline, Hexagonally Ordered, Uniformly Mesoporous TiO2-B and Its Optical and Photocatalytic Properties. Chem. Mater. 2015, 27, 6550–6557. [Google Scholar] [CrossRef]
- Wang, L.; Yang, X.; Wang, Q.; Zeng, Y.; Ding, L.; Jiang, W. Effects of Ionic Strength and Temperature on the Aggregation and Deposition of Multi-Walled Carbon Nanotubes. J. Environ. Sci. 2017, 51, 248–255. [Google Scholar] [CrossRef]
- Dolgov, A.; Lopaev, D.; Lee, C.J.; Zoethout, E.; Medvedev, V.; Yakushev, O.; Bijkerk, F. Characterization of Carbon Contamination under Ion and Hot Atom Bombardment in a Tin-Plasma Extreme Ultraviolet Light Source. Appl. Surf. Sci. 2015, 353, 708–713. [Google Scholar] [CrossRef]
- Abdullah, S.A.; Sahdan, M.Z.; Nayan, N.; Embong, Z.; Hak, C.R.C.; Adriyanto, F. Neutron Beam Interaction with Rutile TiO2 Single Crystal (1 1 1): Raman and XPS Study on Ti3+-Oxygen Vacancy Formation. Mater. Lett. 2020, 263, 127143. [Google Scholar] [CrossRef]
- Mazur, M.; Kaczmarek, D.; Prociow, E.; Domaradzki, J.; Wojcieszak, D.; Bocheński, J. Investigation of Structural, Optical and Electrical Properties of (Ti, Nb)Ox Thin Films Deposited by High Energy Reactive Magnetron Sputtering. Mater. Sci. Poland. 2014, 32, 457–464. [Google Scholar] [CrossRef]
- Fang, J.; Fan, H.; Li, M.; Long, C. Nitrogen Self-Doped Graphitic Carbon Nitride as Efficient Visible Light Photocatalyst for Hydrogen Evolution. J. Mater. Chem. A. Mater. 2015, 3, 13819–13826. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Hu, S.; Huang, Q.; Wei, C.; Zhang, W.; Kang, L.; Huang, Z.; Hao, A. Fluorescent Probes for “off-on” Sensitive and Selective Detection of Mercury Ions and l-Cysteine Based on Graphitic Carbon Nitride Nanosheets. J. Mater. Chem. C. Mater. 2015, 3, 2093–2100. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, H.; Zhao, L.; Fang, W.; He, X.; Li, W.; Tian, P. In Suit Inducing Electron-Donating and Electron-Withdrawing Groups in Carbon Nitride by One-Step NH4Cl-Assisted Route: A Strategy for High Solar Hydrogen Production Efficiency. Environ. Int. 2019, 126, 289–297. [Google Scholar] [CrossRef]
- Kurenkova, A.Y.; Saraev, A.A.; Mishchenko, D.D.; Gerasimov, E.Y.; Kozlova, E.A. Influence of Pt Oxidation State on the Activity and Selectivity of G-C3N4-Based Photocatalysts in H2 Evolution Reaction. Appl. Sci. 2023, 13, 11739. [Google Scholar] [CrossRef]
- Zhou, D.; Luo, H.; Zhang, F.; Wu, J.; Yang, J.; Wang, H. Efficient Photocatalytic Degradation of the Persistent PET Fiber-Based Microplastics over Pt Nanoparticles Decorated N-Doped TiO2 Nanoflowers. Adv. Fiber Mater. 2022, 4, 1094–1107. [Google Scholar] [CrossRef]
- Xu, X.; Huang, H.; Zhang, Y.; Xu, Z.; Cao, X. Biochar as Both Electron Donor and Electron Shuttle for the Reduction Transformation of Cr(VI) during Its Sorption. Environ. Pollut. 2019, 244, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Green, U.; Shenberger, Y.; Aizenshtat, Z.; Cohen, H.; Ruthstein, S. Exploring the Radical Nature of a Carbon Surface by Electron Paramagnetic Resonance and a Calibrated Gas Flow. J. Vis. Exp. 2014, 86, 51548. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, X.; Wang, C.; Xu, J.; Du, X.; Li, L. Ti3+ Defect Mediated G-C3N4 /TiO2 Z-Scheme System for Enhanced Photocatalytic Redox Performance. Appl. Surf. Sci. 2018, 448, 288–296. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Antonopoulou, M.; Papavasiliou, J.; Deligiannakis, Y.; Konstantinou, I. Photocatalytic Performance of Pt-TiO2, Pt-N-TiO2 and Pt-N/F-TiO2 towards Simultaneous Cr(VI) Reduction/Benzoic Acid Oxidation: Insights into Photogenerated Charge Carrier Dynamics and Catalyst Properties. J. Photochem. Photobiol. A Chem. 2017, 349, 25–35. [Google Scholar] [CrossRef]
- Herrera-Beurnio, M.C.; López-Tenllado, F.J.; Hidalgo-Carrillo, J.; Martín-Gómez, J.; Estévez, R.; Urbano, F.J.; Marinas, A. Glycerol Photoreforming for Photocatalytic Hydrogen Production on Binary and Ternary Pt-g-C3N4-TiO2 Systems: A Comparative Study. Catal. Today 2024, 430, 114548. [Google Scholar] [CrossRef]
- Ahmed, L.M.; Ivanova, I.; Hussein, F.H.; Bahnemann, D.W. Role of Platinum Deposited on TiO2 in Photocatalytic Methanol Oxidation and Dehydrogenation Reactions. Int. J. Photoenergy 2014, 2014, 503516. [Google Scholar] [CrossRef]


| Catalyst | XRD | ICP-MS | UV-Vis | XPS | |
|---|---|---|---|---|---|
| Anatase Crystallite Size (nm) | Pt Content (%) | Bandgap (eV) | Pt4f (eV, At. %) | Surface Pt wt.% | |
| CN | - | a | 2.82 | a | a |
| AOL | 8.8 | a | 3.06 | a | a |
| AOLCN | 7.8 | a | 2.60 | a | a |
| Pt/CN | - | 0.39 | 2.81 | 71.2, 67.4 (Pt0) | 0.66 |
| 72.3, 22.8 (Pt2+) | |||||
| 73.8, 9.8 (Pt4+) | |||||
| Pt/AOL | 8.5 | 0.47 | 3.06 | 70.6, 75.7 (Pt0) | 0.73 |
| 71.9, 17.3 (Pt2+) | |||||
| 73.8, 7.0 (Pt4+) | |||||
| Pt/AOLCN | 7.6 | 0.45 | 2.60 | 71.0, 57.9 (Pt0) | 0.63 |
| 72.3, 31.8 (Pt2+) | |||||
| 73.7, 10.3 (Pt4+) | |||||
| Pt@AOL | 8.5 | 0.45 | 3.01 | b | b |
| Pt@AOLCN | 9.3 | 0.53 | 3.02 (AOL) | 70.9, 39.9 (Pt0) | 0.40 |
| 2.76 (Heterojunction) | 74.3, 60.1 (Pt2+) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Beurnio, M.C.; López-Tenllado, F.J.; Ariza-Pérez, A.; Hidalgo-Carrillo, J.; Estevez, R.; Martín-Gómez, J.; Urbano, F.J.; Marinas, A. Valorization of Selected Biomass-Derived Molecules on Olea europaea Leaves-Biotemplated TiO2-g-C3N4 Photocatalysts. Biomimetics 2024, 9, 726. https://doi.org/10.3390/biomimetics9120726
Herrera-Beurnio MC, López-Tenllado FJ, Ariza-Pérez A, Hidalgo-Carrillo J, Estevez R, Martín-Gómez J, Urbano FJ, Marinas A. Valorization of Selected Biomass-Derived Molecules on Olea europaea Leaves-Biotemplated TiO2-g-C3N4 Photocatalysts. Biomimetics. 2024; 9(12):726. https://doi.org/10.3390/biomimetics9120726
Chicago/Turabian StyleHerrera-Beurnio, M. Carmen, Francisco J. López-Tenllado, Alejandro Ariza-Pérez, Jesús Hidalgo-Carrillo, Rafael Estevez, Juan Martín-Gómez, Francisco J. Urbano, and Alberto Marinas. 2024. "Valorization of Selected Biomass-Derived Molecules on Olea europaea Leaves-Biotemplated TiO2-g-C3N4 Photocatalysts" Biomimetics 9, no. 12: 726. https://doi.org/10.3390/biomimetics9120726
APA StyleHerrera-Beurnio, M. C., López-Tenllado, F. J., Ariza-Pérez, A., Hidalgo-Carrillo, J., Estevez, R., Martín-Gómez, J., Urbano, F. J., & Marinas, A. (2024). Valorization of Selected Biomass-Derived Molecules on Olea europaea Leaves-Biotemplated TiO2-g-C3N4 Photocatalysts. Biomimetics, 9(12), 726. https://doi.org/10.3390/biomimetics9120726











