Effects of Coaxial Nozzle’s Inner Nozzle Diameter on Filament Strength and Gelation in Extrusion-Based 3D Printing with In Situ Ionic Crosslinking
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.1.1. Preparation of Bioink (Sodium Alginate Solution)
2.1.2. Preparation of Crosslinking Solution (Calcium Chloride Solution)
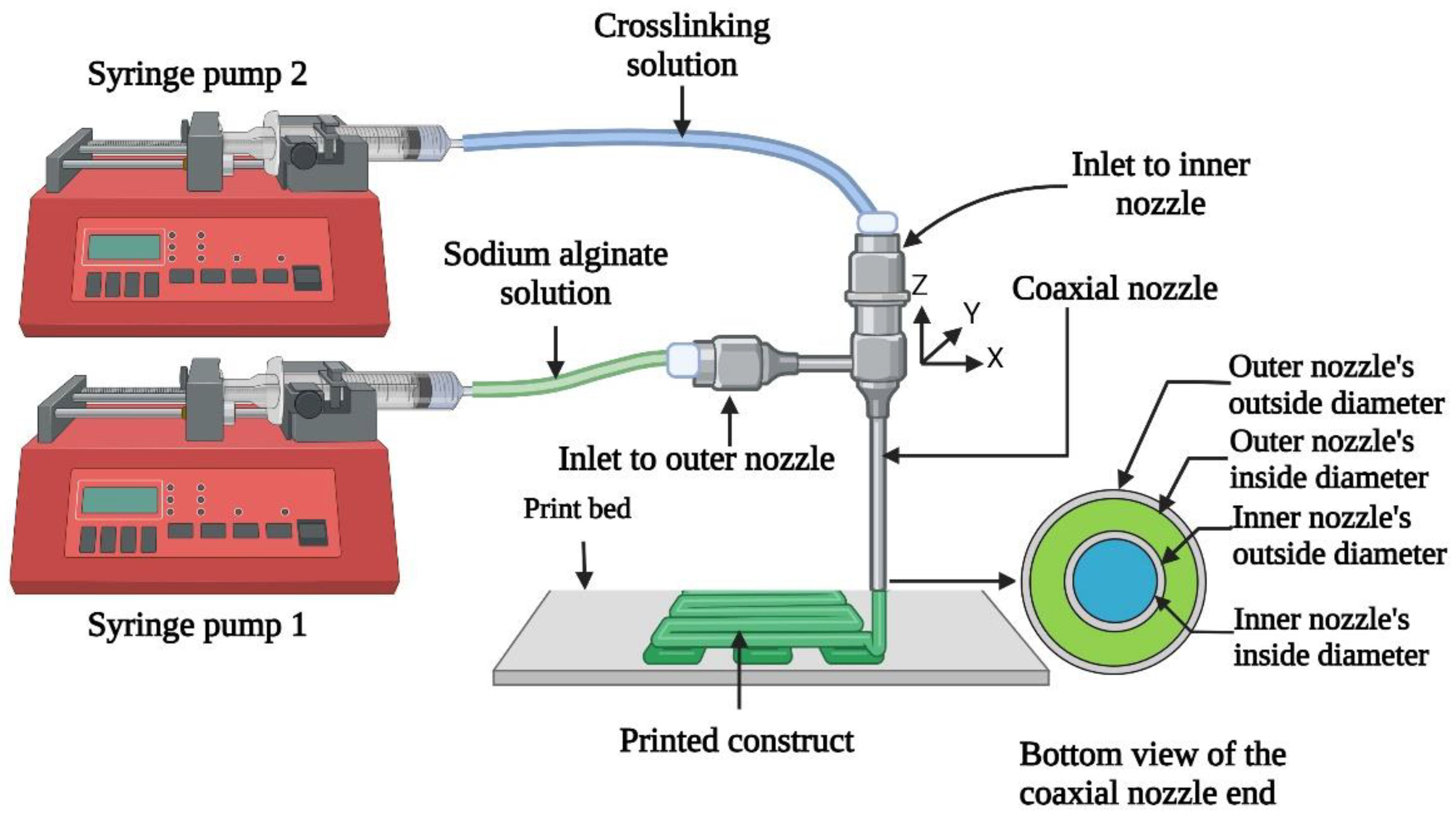
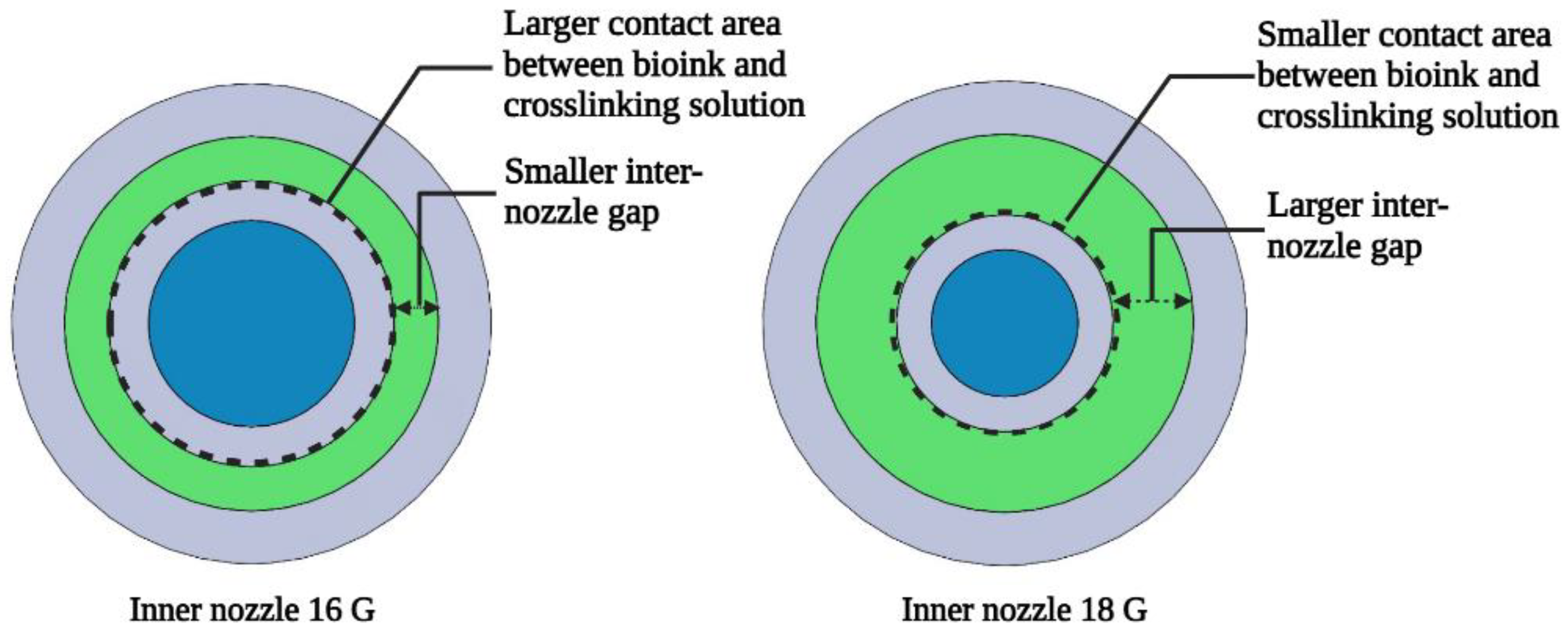
2.2. 3D Printing
2.3. Measurement of Filament Strength
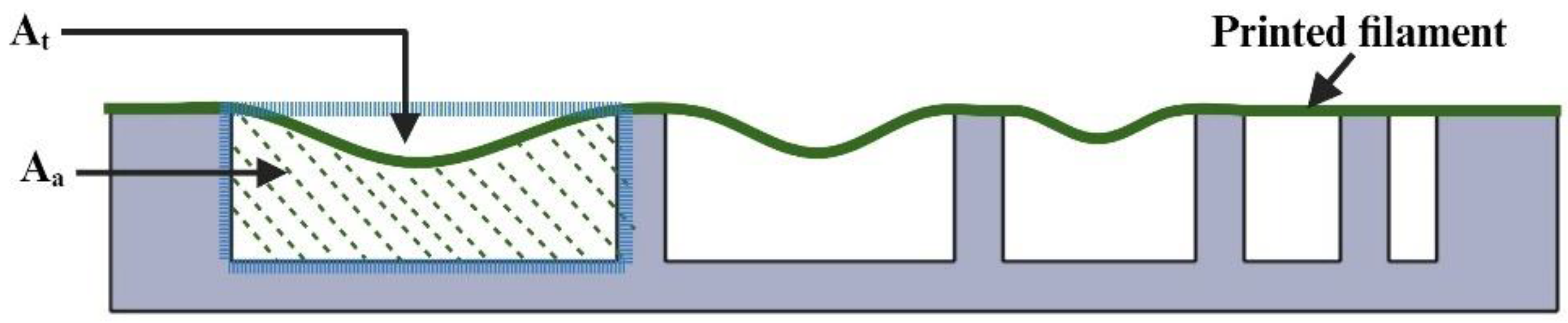
2.4. Measurement of Filament Gelation
2.5. Statistical Analysis
3. Results and Discussions
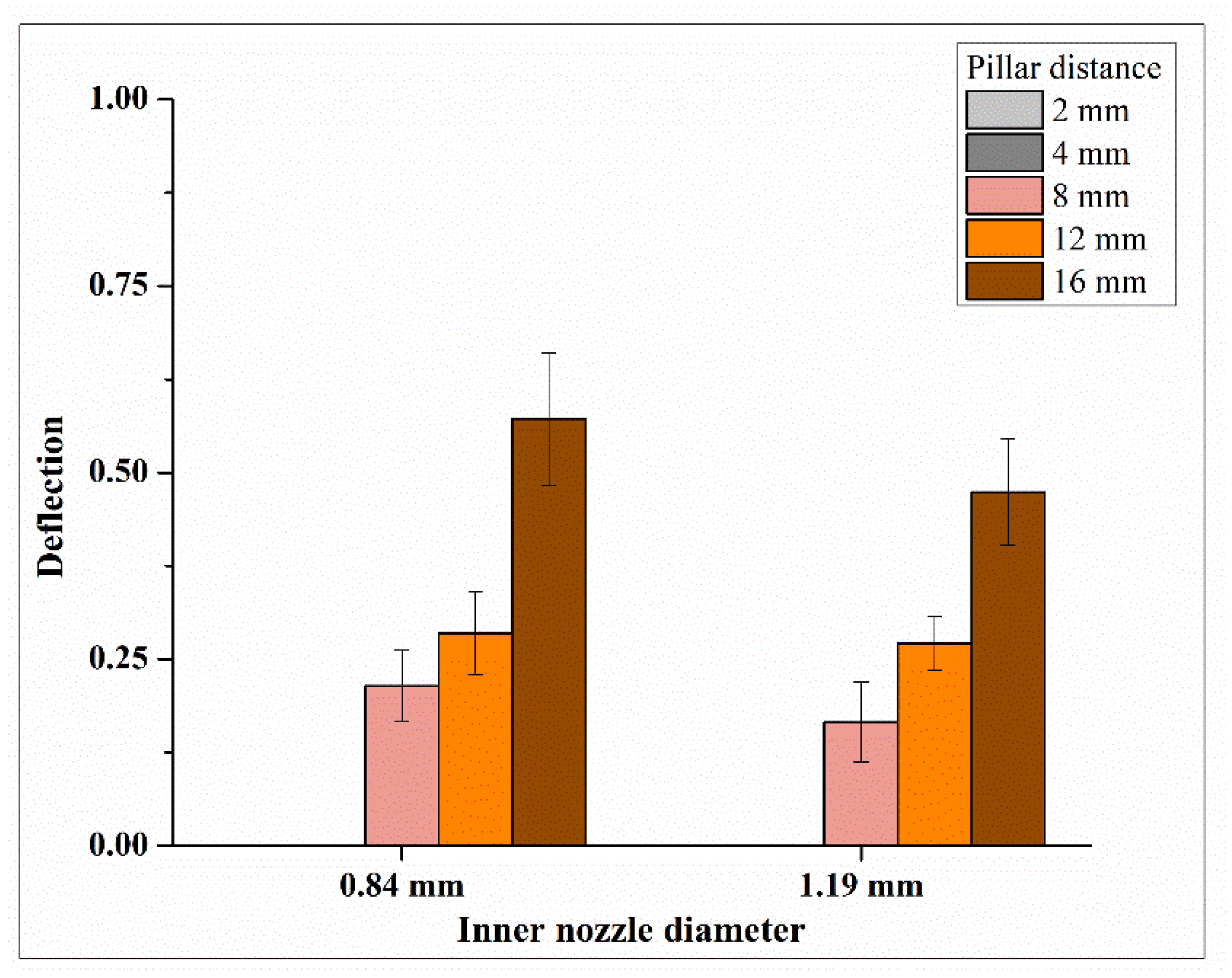
3.1. Effects on Filament Strength
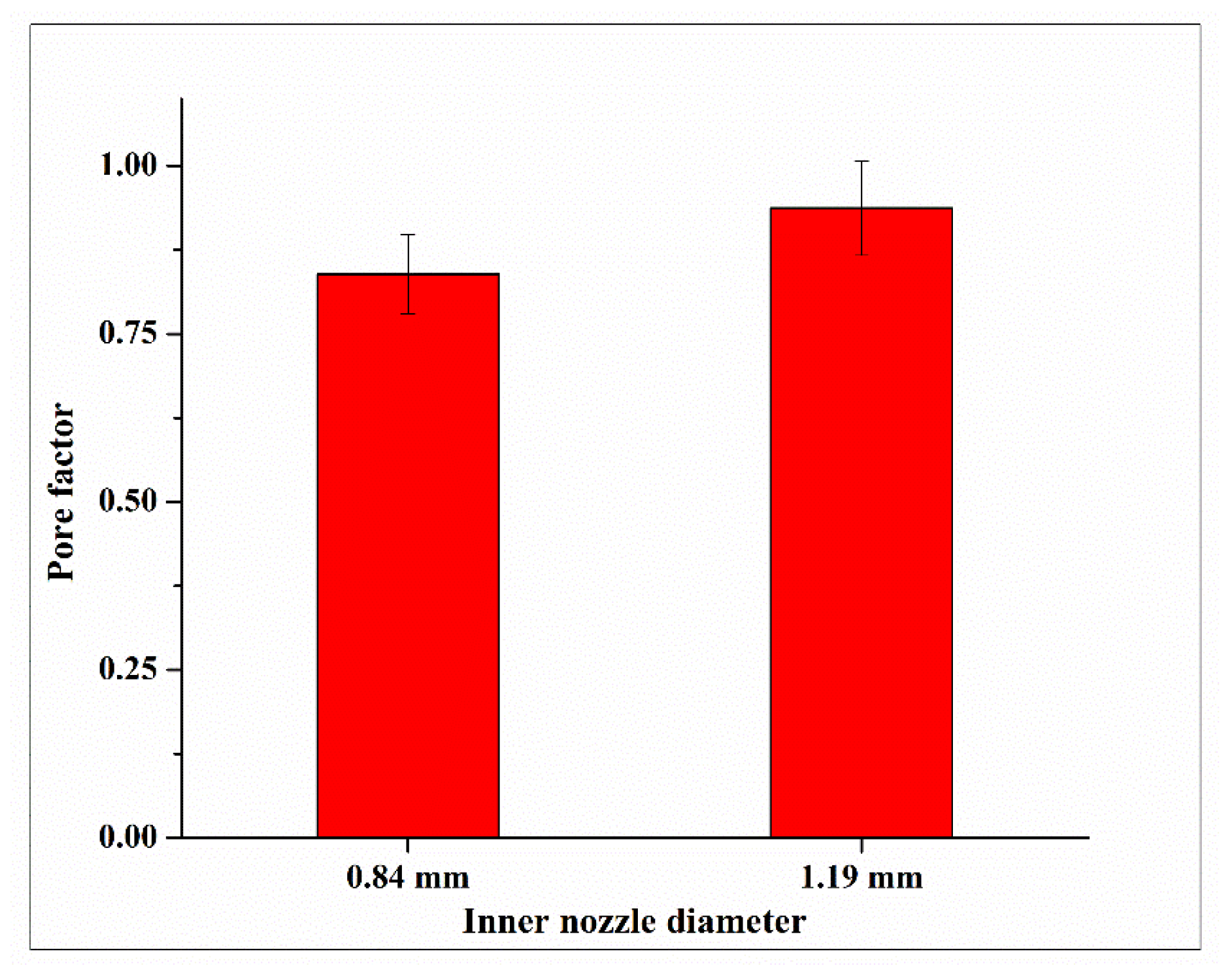
3.2. Effects on Gelation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozbolat, I.T. 3D Bioprinting: Fundamentals, Principles and Applications; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar] [CrossRef]
- Wang, Y.; Pereira, R.F.; Peach, C.; Huang, B.; Vyas, C.; Bartolo, P. Robotic in situ bioprinting for cartilage tissue engineering. Int. J. Extrem. Manuf. 2023, 5, 032004. [Google Scholar] [CrossRef]
- Xie, Z.; Gao, M.; Lobo, A.O.; Webster, T.J. 3D bioprinting in tissue engineering for medical applications: The classic and the hybrid. Polymers 2020, 12, 1717. [Google Scholar] [CrossRef] [PubMed]
- Zimmerling, A.; Dahlan, N.A.; Zhou, Y.; Chen, X. Recent frontiers in biofabrication for respiratory tissue engineering. Bioprinting 2024, 40, e00342. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Liu, P.; Li, Q.; Zhang, R.; Yang, H.; Zhou, H. Simultaneous multi-material embedded printing for 3D heterogeneous structures. Int. J. Extrem. Manuf. 2023, 5, 035001. [Google Scholar] [CrossRef]
- Rahman, T.T.; Arman, M.S.; Perez, V.; Xu, B.; Li, J. Analysis of the operating conditions of pulse electric field–assisted EHD for sodium alginate printing using design of experiment approach. Int. J. Adv. Manuf. Technol. 2021, 115, 2037–2047. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Yan, W.-C.; Lu, W.F.; Wang, C.-H.; Fuh, J.Y.H. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef]
- Zhou, D.; Dou, B.; Kroh, F.; Wang, C.; Ouyang, L. Biofabrication strategies with single-cell resolution: A review. Int. J. Extrem. Manuf. 2023, 5, 042005. [Google Scholar] [CrossRef]
- Wang, X.; Jia, J.; Niu, M.; Li, W.; Zhao, Y. Living Chinese herbal scaffolds from microfluidic bioprinting for wound healing. Research 2023, 6, 0138. [Google Scholar] [CrossRef]
- Thakare, K.; Jerpseth, L.; Pei, Z.; Elwany, A.; Quek, F.; Qin, H. Bioprinting of organ-on-chip systems: A literature review from a manufacturing perspective. J. Manuf. Mater. Process. 2021, 5, 91. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Tang, R.; Feng, W.; Gao, J.; Wu, B.; Deng, Z.; Liu, J.; Li, L. Liquid metal neuro-electrical interface. Soft Sci. 2024, 4, 23. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, X.; Li, Y.; Cui, J.; Shi, Q.; Huang, H.-W.; Huang, Q.; Wang, H. Integrated Cross-Scale Manipulation and Modulable Encapsulation of Cell-Laden Hydrogel for Constructing Tissue-Mimicking Microstructures. Research 2024, 7, 0414. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Munguia-Lopez, J.G.; Flores-Torres, S.; Kort-Mascort, J.; Kinsella, J.M. Extrusion bioprinting of soft materials: An emerging technique for biological model fabrication. Appl. Phys. Rev. 2019, 6, 011310. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef]
- Ramesh, S.; Harrysson, O.L.; Rao, P.K.; Tamayol, A.; Cormier, D.R.; Zhang, Y.; Rivero, I.V. Extrusion bioprinting: Recent progress, challenges, and future opportunities. Bioprinting 2021, 21, e00116. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Rahman, T.T.; Wood, N.; Rahman, A.M.; Pei, Z.; Qin, H. Applying In Situ Ionic Crosslinking in Bioprinting Using Algae Cells. J. Manuf. Sci. Eng. 2024, 146, 034501. [Google Scholar] [CrossRef]
- Gillispie, G.; Prim, P.; Copus, J.; Fisher, J.; Mikos, A.G.; Yoo, J.J.; Atala, A.; Lee, S.J. Assessment methodologies for extrusion-based bioink printability. Biofabrication 2020, 12, 022003. [Google Scholar] [CrossRef]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Mohan, T.S.; Datta, P.; Nesaei, S.; Ozbolat, V.; Ozbolat, I.T. 3D coaxial bioprinting: Process mechanisms, bioinks and applications. Prog. Biomed. Eng. 2022, 4, 022003. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; He, Y.; Fu, J.Z.; Liu, A.; Ma, L. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials 2015, 61, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Albalawi, H.I.; Khan, Z.N.; Rawas, R.H.; Valle-Pérez, A.U.; Abdelrahman, S.; Hauser, C.A.E. 3D-Printed disposable nozzles for cost-efficient extrusion-based 3D bioprinting. Mater. Sci. Addit. Manuf. 2023, 2, 52. [Google Scholar] [CrossRef]
- Habib, A.; Quigley, C.; Sarah, R.; Hurd, W.; Clark, S. Design and fabrication of in-house nozzle system to extrude multi-hydrogels for 3D bioprinting process. J. Manuf. Sci. Eng. 2024, 146, 021003. [Google Scholar] [CrossRef]
- Li, H.; Li, N.; Zhang, H.; Zhang, Y.; Suo, H.; Wang, L.; Xu, M. Three-dimensional bioprinting of perfusable hierarchical microchannels with alginate and silk fibroin double cross-linked network. 3D Print. Addit. Manuf. 2020, 7, 78–84. [Google Scholar] [CrossRef]
- Perez, V.J. Investigation of Cell Behavior in 3D Printed Lumen Structures for Capillary Regeneration. Master’s Thesis, The University of Texas Rio Grande Valley, Edinburg, TX, USA, 2021. [Google Scholar]
- Rahman, T.T. Bioprinting a 3D Tubular Structure with Vascular Smooth Muscle Cells to Observe the Process Compatibility. Master’s Thesis, The University of Texas Rio Grande Valley, Edinburg, TX, USA, 2021. [Google Scholar]
- Sun, Z.; Di, Z.; Zhang, Y.; Wen, H.; Zhang, S.; Zhang, Z.; Zhang, J.; Yu, Z. Photosynthetic living fibers fabrication from algal-bacterial consortia with controlled spatial distribution. ACS Biomater. Sci. Eng. 2023, 9, 6481–6489. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Yu, Y.; Zhao, Y. In Situ 3D Bioprinting Living Photosynthetic Scaffolds for Autotrophic Wound Healing. Research 2022, 2022, 9794745. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Chen, H.; Ozbolat, I.T. Characterization of printable cellular micro-fluidic channels for tissue engineering. Biofabrication 2013, 5, 025004. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Akkouch, A.; Dababneh, A.; Dolati, F.; Ozbolat, I.T. In Vitro Study of Directly Bioprinted Perfusable Vasculature Conduits. Biomater. Sci. 2015, 3, 134–143. [Google Scholar] [CrossRef]
- Ahmadi Soufivand, A.; Faber, J.; Hinrichsen, J.; Budday, S. Multilayer 3D bioprinting and complex mechanical properties of alginate-gelatin mesostructures. Sci. Rep. 2023, 13, 11253. [Google Scholar] [CrossRef]
- Habib, A.; Sathish, V.; Mallik, S.; Khoda, B. 3D Printability of Alginate-Carboxymethyl Cellulose Hydrogel. Materials 2018, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Nasehi, R.; Aveic, S.; Fischer, H. Wall shear stress during impingement at the building platform can exceed nozzle wall shear stress in microvalve-based bioprinting. Int. J. Bioprint. 2023, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.T.; Rahman, A.M.; Pei, Z.; Thakare, K.; Qin, H.; Khan, A. 3D Printing of Microalgae-Enriched Cookie Dough: Determining Feasible Regions of Process Parameters for Continuous Extrusion; OAE Publishing Inc.: Alhambra, CA, USA, 2023. [Google Scholar] [CrossRef]
- Ribeiro, A.; Blokzijl, M.M.; Levato, R.; Visser, C.W.; Castilho, M.; Hennink, W.E.; Vermonden, T.; Malda, J. Assessing bioink shape fidelity to aid material development in 3D bioprinting. Biofabrication 2017, 10, 014102. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef]
- Ostadfar, A. Biofluid Mechanics: Principles and Applications; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays; Markossian, S., Grossman, A., Arkin, M., Eds.; Eli Lilly & Company: Indianapolis, IN, USA; National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Rahman, T.T.; Wood, N.; Akib, Y.M.; Qin, H.; Pei, Z. Experimental Study on Compatibility of Human Bronchial Epithelial Cells in Collagen–Alginate Bioink for 3D Printing. Bioengineering 2024, 11, 862. [Google Scholar] [CrossRef]





| Parameter | Response Variable | Reference |
|---|---|---|
| Bioink concentration and flow rate, crosslinking solution concentration and flow rate, inner nozzle diameter, and printing speed | Diameter and shape of printed filaments, filament fusion, ultimate strength and failure strain of printed constructs | [23] |
| Bioink concentration and flow rate, and crosslinking solution concentration and flow rate | Dimensions of printed filaments | [28] |
| Crosslinking solution flow rate and inner nozzle diameter | Inner diameter of printed filaments | [29] |
| Bioink concentration and flow rate, and crosslinking solution concentration and flow rate | Dimensions of printed filaments | [31] |
| Bioink concentration | Shrinkage, swelling, and dimension of printed filaments | [32] |
| Inner Nozzle Size (Gauge) | Inside Diameter of the Inner Nozzle (mm) | Outside Diameter of the Inner Nozzle (mm) | Inter-Nozzle Gap of the Coaxial Nozzle System (mm) |
|---|---|---|---|
| 14 G | 1.60 | 2.11 | 0.025 |
| 15 G | 1.37 | 1.83 | 0.165 |
| 16 G | 1.19 | 1.65 | 0.255 |
| 18 G | 0.84 | 1.24 | 0.46 |
| 20 G | 0.584 | 0.889 | 0.6355 |
| Parameter | Unit | Value |
|---|---|---|
| Flow rate of the syringe pump for sodium alginate solution | µL/min | 600 |
| Flow rate of the syringe pump for crosslinking solution | µL/min | 600 |
| Coaxial nozzle’s outer nozzle diameter | mm | 2.16 |
| Printing speed | mm/s | 8 |
| Layer thickness | mm | 1.6 |
| Source of Variance | Degree of Freedom | Adj Sum of Squares | F-Value | p-Value |
|---|---|---|---|---|
| Inner nozzle diameter | 2 | 0.014 | 2.256 | 0.208 |
| Error | 6 | 0.026 | ||
| Total | 8 | 0.040 |
| Source of Variance | Degree of Freedom | Adj Sum of Squares | F-Value | p-Value |
|---|---|---|---|---|
| Inner nozzle diameter | 2 | 0.014 | 3.453 | 0.137 |
| Error | 6 | 0.017 | ||
| Total | 8 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, T.T.; Rahman, A.M.; Pei, Z.; Wood, N.; Qin, H. Effects of Coaxial Nozzle’s Inner Nozzle Diameter on Filament Strength and Gelation in Extrusion-Based 3D Printing with In Situ Ionic Crosslinking. Biomimetics 2024, 9, 589. https://doi.org/10.3390/biomimetics9100589
Rahman TT, Rahman AM, Pei Z, Wood N, Qin H. Effects of Coaxial Nozzle’s Inner Nozzle Diameter on Filament Strength and Gelation in Extrusion-Based 3D Printing with In Situ Ionic Crosslinking. Biomimetics. 2024; 9(10):589. https://doi.org/10.3390/biomimetics9100589
Chicago/Turabian StyleRahman, Taieba Tuba, Al Mazedur Rahman, Zhijian Pei, Nathan Wood, and Hongmin Qin. 2024. "Effects of Coaxial Nozzle’s Inner Nozzle Diameter on Filament Strength and Gelation in Extrusion-Based 3D Printing with In Situ Ionic Crosslinking" Biomimetics 9, no. 10: 589. https://doi.org/10.3390/biomimetics9100589
APA StyleRahman, T. T., Rahman, A. M., Pei, Z., Wood, N., & Qin, H. (2024). Effects of Coaxial Nozzle’s Inner Nozzle Diameter on Filament Strength and Gelation in Extrusion-Based 3D Printing with In Situ Ionic Crosslinking. Biomimetics, 9(10), 589. https://doi.org/10.3390/biomimetics9100589








