A Bioinspired Astrocyte-Derived Coating Promotes the In Vitro Proliferation of Human Neural Stem Cells While Maintaining Their Stemness
Abstract
:1. Introduction
2. Materials and Methods
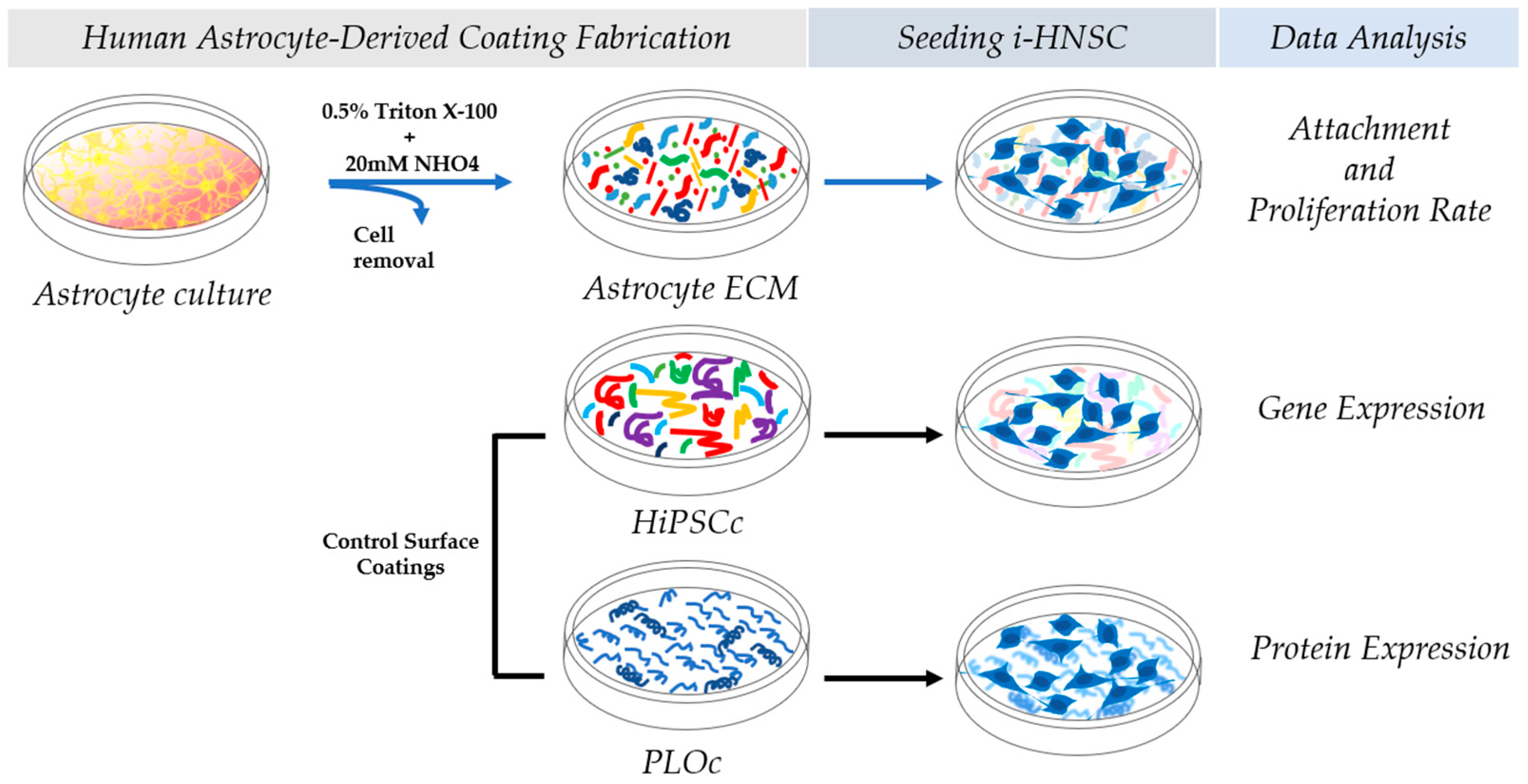
2.1. Human Astrocyte-Derived Coating Fabrication
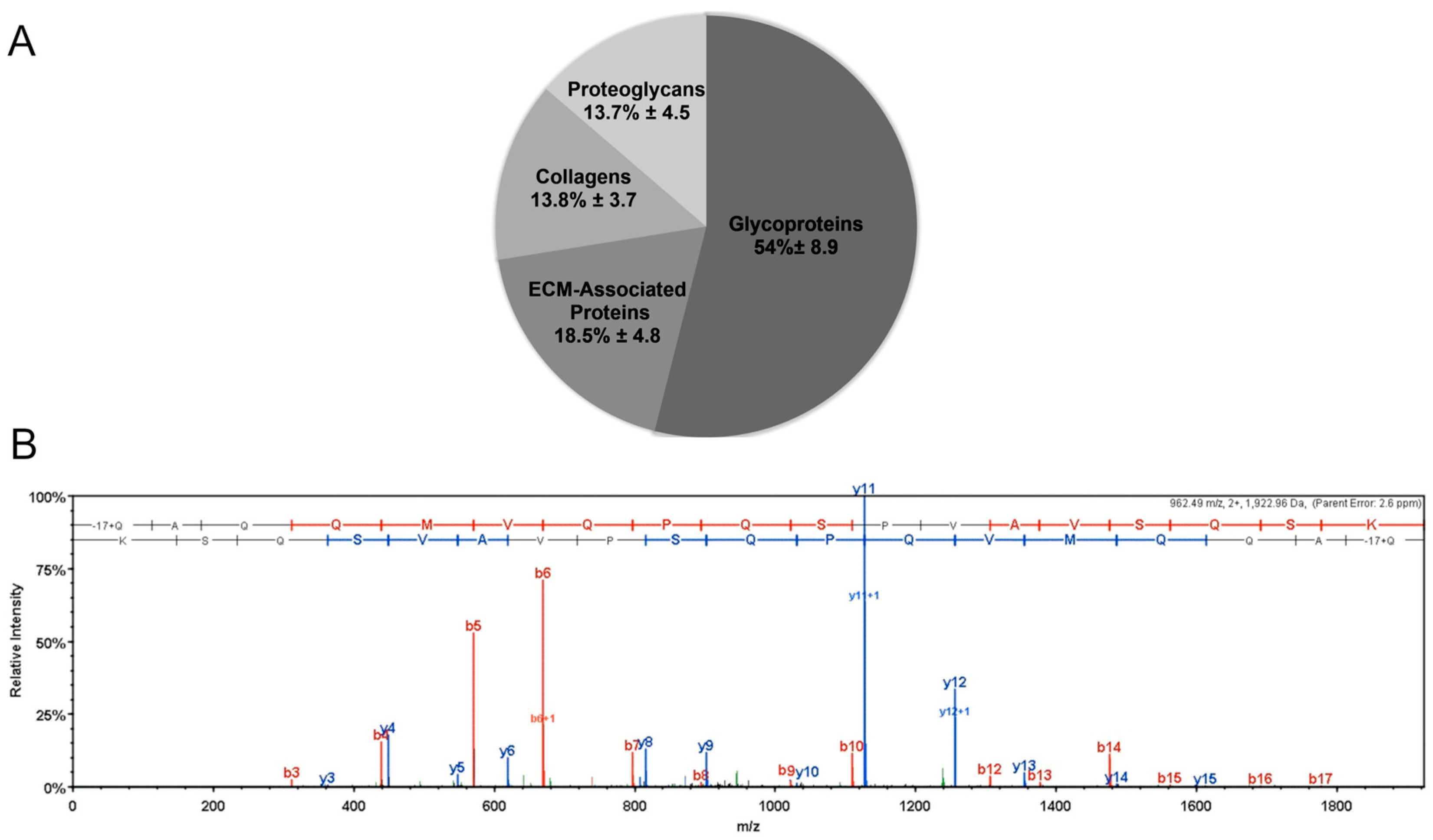
2.2. Mass Spectroscopy
2.3. Control Surface Coatings
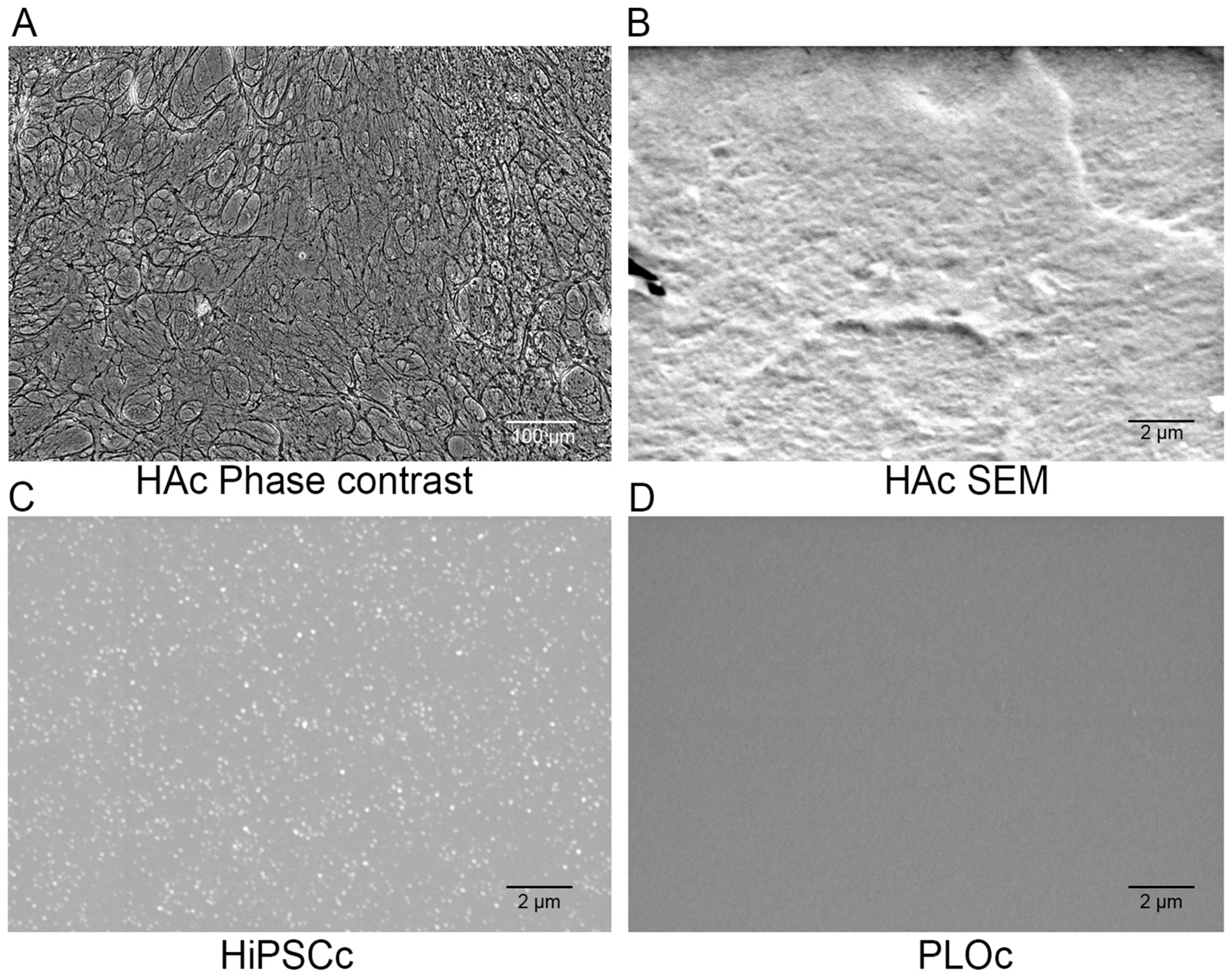
2.4. Microscopic Characterization
2.5. Cell Culture
2.6. Attachment and Proliferation Rate Analysis
2.7. Gene Expression Characterization of Stemness Markers
2.8. Protein Expression Assessments of Stemness Markers
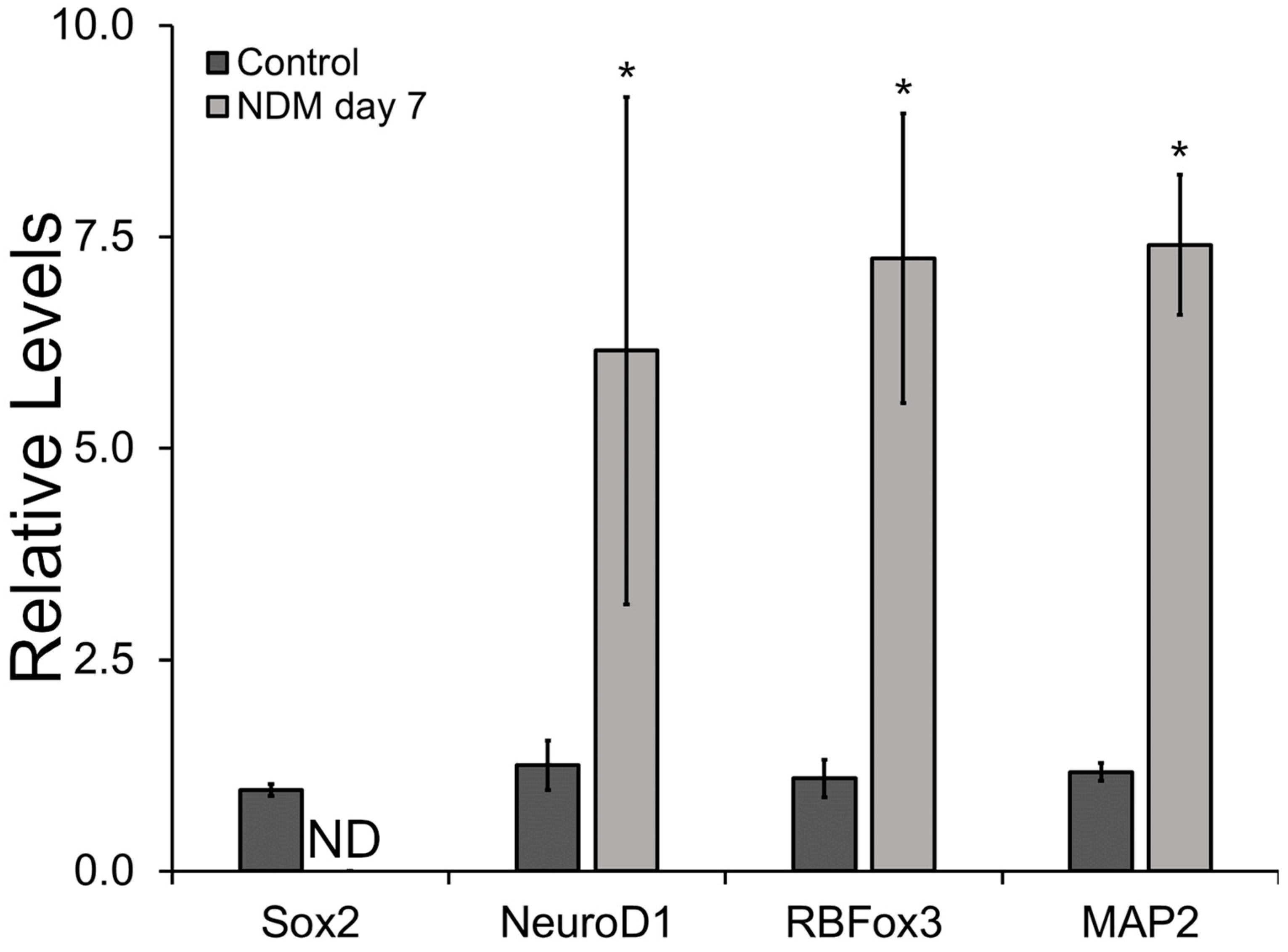
2.9. Gene Expression Characterization of Neural Differentiation Markers
2.10. Statistical Analyses
3. Results
3.1. Mass Spectroscopy
3.2. Microscopic Characterization
3.3. Attachment and Proliferation of i-HNSCs
3.4. Relative Gene and Protein Expression Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s disease: Treatment strategies and their limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, V.A.; Bobotis, B.C.; Correia, F.F.; Lima-Vasconcellos, T.H.; Chiarantin, G.M.; De La Vega, L.; Lombello, C.B.; Willerth, S.M.; Malmonge, S.M.; Paschon, V. The Impact of Biomaterial Surface Properties on Engineering Neural Tissue for Spinal Cord Regeneration. Int. J. Mol. Sci. 2023, 24, 13642. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, J.S.; Song, B.; Herrington, T.M.; Park, T.-Y.; Lee, N.; Ko, S.; Jeon, J.; Cha, Y.; Kim, K.; Li, Q. Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N. Engl. J. Med. 2020, 382, 1926–1932. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Funayama, M.; Tsukita, K.; Hotta, A.; Yasuda, A.; Nori, S.; Kaneko, S.; Nakamura, M.; Takahashi, R.; Okano, H. Focal transplantation of human iPSC-derived glial-rich neural progenitors improves lifespan of ALS mice. Stem Cell Rep. 2014, 3, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Asai, M.; Tsukita, K.; Kutoku, Y.; Ohsawa, Y.; Sunada, Y.; Imamura, K.; Egawa, N.; Yahata, N.; Okita, K. Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness. Cell Stem Cell 2013, 12, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-H.; Shaltouki, A.; Gonzalez, A.E.; da Cruz, A.B.; Burbulla, L.F.; Lawrence, E.S.; Schüle, B.; Krainc, D.; Palmer, T.D.; Wang, X. Functional impairment in miro degradation and mitophagy is a shared feature in familial and sporadic Parkinson’s disease. Cell Stem Cell 2016, 19, 709–724. [Google Scholar] [CrossRef]
- Little, D.; Luft, C.; Mosaku, O.; Lorvellec, M.; Yao, Z.; Paillusson, S.; Kriston-Vizi, J.; Gandhi, S.; Abramov, A.Y.; Ketteler, R. A single cell high content assay detects mitochondrial dysfunction in iPSC-derived neurons with mutations in SNCA. Sci. Rep. 2018, 8, 9033. [Google Scholar] [CrossRef]
- Wang, C.; Ward, M.E.; Chen, R.; Liu, K.; Tracy, T.E.; Chen, X.; Xie, M.; Sohn, P.D.; Ludwig, C.; Meyer-Franke, A. Scalable production of iPSC-derived human neurons to identify tau-lowering compounds by high-content screening. Stem Cell Rep. 2017, 9, 1221–1233. [Google Scholar] [CrossRef]
- Hayashi, Y.; Furue, M.K. Biological effects of culture substrates on human pluripotent stem cells. Stem Cells Int. 2016, 2016, 5380560. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Nogueira, D.E.; Cabral, J.M.; Rodrigues, C.A. Neural progenitor cell-derived extracellular matrix as a new platform for neural differentiation of human induced pluripotent stem cells. Biomater. Biosyst. 2022, 8, 100070. [Google Scholar] [CrossRef]
- Macri-Pellizzeri, L.; Pelacho, B.; Sancho, A.; Iglesias-Garcia, O.; Simón-Yarza, A.M.; Soriano-Navarro, M.; González-Granero, S.; García-Verdugo, J.M.; De-Juan-Pardo, E.M.; Prosper, F. Substrate stiffness and composition specifically direct differentiation of induced pluripotent stem cells. Tissue Eng. Part A 2015, 21, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Conover, J.C.; Notti, R.Q. The neural stem cell niche. Cell Tissue Res. 2008, 331, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Llorente, V.; Velarde, P.; Desco, M.; Gómez-Gaviro, M.V. Current understanding of the neural stem cell niches. Cells 2022, 11, 3002. [Google Scholar] [CrossRef] [PubMed]
- Regalado-Santiago, C.; Juárez-Aguilar, E.; Olivares-Hernández, J.D.; Tamariz, E. Mimicking neural stem cell niche by biocompatible substrates. Stem Cells Int. 2016, 2016, 1513285. [Google Scholar] [CrossRef]
- Gonzalez, H.; Narasipura, S.D.; Shull, T.; Shetty, A.; Teppen, T.L.; Naqib, A.; Al-Harthi, L. An Efficient and Cost-Effective Approach to Generate Functional Human Inducible Pluripotent Stem Cell-Derived Astrocytes. Cells 2023, 12, 2357. [Google Scholar] [CrossRef]
- Julia, T.; Wang, M.; Pimenova, A.A.; Bowles, K.R.; Hartley, B.J.; Lacin, E.; Machlovi, S.I.; Abdelaal, R.; Karch, C.M.; Phatnani, H. An efficient platform for astrocyte differentiation from human induced pluripotent stem cells. Stem Cell Rep. 2017, 9, 600–614. [Google Scholar] [CrossRef]
- Kaukonen, R.; Jacquemet, G.; Hamidi, H.; Ivaska, J. Cell-derived matrices for studying cell proliferation and directional migration in a complex 3D microenvironment. Nat. Protoc. 2017, 12, 2376–2390. [Google Scholar] [CrossRef]
- Harris, G.M.; Raitman, I.; Schwarzbauer, J.E. Cell-derived decellularized extracellular matrices. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 143, pp. 97–114. [Google Scholar]
- Mao, Y.; Block, T.; Singh-Varma, A.; Sheldrake, A.; Leeth, R.; Griffey, S.; Kohn, J. Extracellular matrix derived from chondrocytes promotes rapid expansion of human primary chondrocytes in vitro with reduced dedifferentiation. Acta Biomater. 2019, 85, 75–83. [Google Scholar] [CrossRef]
- Marinkovic, M.; Tran, O.N.; Block, T.J.; Rakian, R.; Gonzalez, A.O.; Dean, D.D.; Yeh, C.-K.; Chen, X.-D. Native extracellular matrix, synthesized ex vivo by bone marrow or adipose stromal cells, faithfully directs mesenchymal stem cell differentiation. Matrix Biol. Plus 2020, 8, 100044. [Google Scholar] [CrossRef]
- Block, T.; Creech, J.; da Rocha, A.M.; Marinkovic, M.; Ponce-Balbuena, D.; Jiménez-Vázquez, E.N.; Griffey, S.; Herron, T.J. Human perinatal stem cell derived extracellular matrix enables rapid maturation of hiPSC-CM structural and functional phenotypes. Sci. Rep. 2020, 10, 19071. [Google Scholar] [CrossRef]
- Cho, A.-N.; Jin, Y.; Kim, S.; Kumar, S.; Shin, H.; Kang, H.-C.; Cho, S.-W. Aligned brain extracellular matrix promotes differentiation and myelination of human-induced pluripotent stem cell-derived oligodendrocytes. ACS Appl. Mater. Interfaces 2019, 11, 15344–15353. [Google Scholar] [CrossRef] [PubMed]
- Marinkovic, M.; Block, T.J.; Rakian, R.; Li, Q.; Wang, E.; Reilly, M.A.; Dean, D.D.; Chen, X.-D. One size does not fit all: Developing a cell-specific niche for in vitro study of cell behavior. Matrix Biol. 2016, 52, 426–441. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.-A.; Cistulli, P.A.; Cook, K.M. A Cell Culture Model that Mimics Physiological Tissue Oxygenation Using Oxygen-permeable Membranes. Bio-Protocol 2019, 9, e3371. [Google Scholar] [CrossRef] [PubMed]
- Clevenger, A.J.; Jimenez-Vergara, A.C.; Tsai, E.H.; de Barros Righes, G.; Díaz-Lasprilla, A.M.; Ramírez-Caballero, G.E.; Munoz-Pinto, D.J. Growth Factor Binding Peptides in Poly (Ethylene Glycol) Diacrylate (PEGDA)-Based Hydrogels for an Improved Healing Response of Human Dermal Fibroblasts. Gels 2022, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Comella-Bolla, A.; Orlandi, J.G.; Miguez, A.; Straccia, M.; García-Bravo, M.; Bombau, G.; Galofré, M.; Sanders, P.; Carrere, J.; Segovia, J.C. Human pluripotent stem cell-derived neurons are functionally mature in vitro and integrate into the mouse striatum following transplantation. Mol. Neurobiol. 2020, 57, 2766–2798. [Google Scholar] [CrossRef]
- Seki, T. Expression patterns of immature neuronal markers PSA-NCAM, CRMP-4 and NeuroD in the hippocampus of young adult and aged rodents. J. Neurosci. Res. 2002, 70, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Baltus, G.A.; Kowalski, M.P.; Zhai, H.; Tutter, A.V.; Quinn, D.; Wall, D.; Kadam, S. Acetylation of sox2 induces its nuclear export in embryonic stem cells. Stem Cells 2009, 27, 2175–2184. [Google Scholar] [CrossRef]
- Schaefer, T.; Lengerke, C. SOX2 protein biochemistry in stemness, reprogramming, and cancer: The PI3K/AKT/SOX2 axis and beyond. Oncogene 2020, 39, 278–292. [Google Scholar] [CrossRef]
- Negredo, P.N.; Yeo, R.W.; Brunet, A. Aging and rejuvenation of neural stem cells and their niches. Cell Stem Cell 2020, 27, 202–223. [Google Scholar] [CrossRef]
- White III, C.W.; Fan, X.; Maynard, J.C.; Wheatley, E.G.; Bieri, G.; Couthouis, J.; Burlingame, A.L.; Villeda, S.A. Age-related loss of neural stem cell O-GlcNAc promotes a glial fate switch through STAT3 activation. Proc. Natl. Acad. Sci. USA 2020, 117, 22214–22224. [Google Scholar] [CrossRef]
- Carpentier, P.A.; Palmer, T.D. Immune influence on adult neural stem cell regulation and function. Neuron 2009, 64, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhou, L.; Wagner, A.M.; Marchetto, M.C.; Muotri, A.R.; Gage, F.H.; Chen, G. Astroglial cells regulate the developmental timeline of human neurons differentiated from induced pluripotent stem cells. Stem Cell Res. 2013, 11, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Oberheim, N.A.; Goldman, S.A.; Nedergaard, M. Heterogeneity of astrocytic form and function. In Astrocytes: Methods and Protocols; Humana Press: La Jolla, CA, USA, 2012; Volume 814, pp. 23–45. [Google Scholar] [CrossRef]
- Liu, D.; Pavathuparambil Abdul Manaph, N.; Al-Hawwas, M.; Bobrovskaya, L.; Xiong, L.-L.; Zhou, X.-F. Coating materials for neural stem/progenitor cell culture and differentiation. Stem Cells Dev. 2020, 29, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Clément, J.-P.; Al-Alwan, L.; Glasgow, S.D.; Stolow, A.; Ding, Y.; Quevedo Melo, T.; Khayachi, A.; Liu, Y.; Hellmund, M.; Haag, R. Dendritic polyglycerol amine: An enhanced substrate to support long-term neural cell culture. ASN Neuro 2022, 14, 17590914211073276. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Tan, L.; Wu, P.; Yin, Y.; Liu, X.; Meng, H.; Cui, G.; Wu, N.; Lin, J.; Hu, R. Poly-L-ornithine promotes preferred differentiation of neural stem/progenitor cells via ERK signalling pathway. Sci. Rep. 2015, 5, 15535. [Google Scholar] [CrossRef] [PubMed]
- Milky, B.; Zabolocki, M.; Al-Bataineh, S.A.; van den Hurk, M.; Greenberg, Z.; Turner, L.; Mazzachi, P.; Williams, A.; Illeperuma, I.; Adams, R. Long-term adherence of human brain cells in vitro is enhanced by charged amine-based plasma polymer coatings. Stem Cell Rep. 2022, 17, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Wiese, S.; Karus, M.; Faissner, A. Astrocytes as a source for extracellular matrix molecules and cytokines. Front. Pharmacol. 2012, 3, 120. [Google Scholar] [CrossRef]
- Hirano, S.; Yonezawa, T.; Hasegawa, H.; Hattori, S.; Greenhill, N.S.; Davis, P.F.; Sage, E.H.; Ninomiya, Y. Astrocytes express type VIII collagen during the repair process of brain cold injury. Biochem. Biophys. Res. Commun. 2004, 317, 437–443. [Google Scholar] [CrossRef]
- Pintér, P.; Alpár, A. The role of extracellular matrix in human neurodegenerative diseases. Int. J. Mol. Sci. 2022, 23, 11085. [Google Scholar] [CrossRef]
- Diniz, L.P.; Matias, I.; Siqueira, M.; Stipursky, J.; Gomes, F.C.A. Astrocytes and the TGF-β1 pathway in the healthy and diseased brain: A double-edged sword. Mol. Neurobiol. 2019, 56, 4653–4679. [Google Scholar] [CrossRef]
- Welser-Alves, J.V.; Milner, R. Microglia are the major source of TNF-α and TGF-β1 in postnatal glial cultures; regulation by cytokines, lipopolysaccharide, and vitronectin. Neurochem. Int. 2013, 63, 47–53. [Google Scholar] [CrossRef]
- Chen, H.T.; Lee, M.J.; Chen, C.H.; Chuang, S.C.; Chang, L.F.; Ho, M.L.; Hung, S.H.; Fu, Y.C.; Wang, Y.H.; Wang, H.I. Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J. Cell Mol. Med. 2012, 16, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Haragopal, H.; Yu, D.; Zeng, X.; Kim, S.-W.; Han, I.-B.; Ropper, A.E.; Anderson, J.E.; Teng, Y.D. Stemness enhancement of human neural stem cells following bone marrow MSC coculture. Cell Transplant. 2015, 24, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cui, W. Sox2, a key factor in the regulation of pluripotency and neural differentiation. World J. Stem Cells 2014, 6, 305. [Google Scholar] [CrossRef] [PubMed]
- Pagin, M.; Pernebrink, M.; Giubbolini, S.; Barone, C.; Sambruni, G.; Zhu, Y.; Chiara, M.; Ottolenghi, S.; Pavesi, G.; Wei, C.-L. Sox2 controls neural stem cell self-renewal through a Fos-centered gene regulatory network. Stem Cells 2021, 39, 1107–1119. [Google Scholar] [CrossRef]
- Wilhelmsson, U.; Lebkuechner, I.; Leke, R.; Marasek, P.; Yang, X.; Antfolk, D.; Chen, M.; Mohseni, P.; Lasič, E.; Bobnar, S.T. Nestin regulates neurogenesis in mice through notch signaling from astrocytes to neural stem cells. Cereb. Cortex 2019, 29, 4050–4066. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.; Mrowetz, H.; Kreutzer, C.; Rotheneichner, P.; Zaunmair, P.; Lange, S.; Coras, R.; Couillard-Despres, S.; Rivera, F.J.; Aigner, L. DCX+ neuronal progenitors contribute to new oligodendrocytes during remyelination in the hippocampus. Sci. Rep. 2020, 10, 20095. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Paredes, M.F.; Zhang, Z.; Kang, G.; Pastor-Alonso, O.; Biagiotti, S.; Page, C.E.; Sandoval, K.; Knox, A.; Connolly, A. Positive controls in adults and children support that very few, if any, new neurons are born in the adult human hippocampus. J. Neurosci. 2021, 41, 2554–2565. [Google Scholar] [CrossRef]
- Piccirilli, G.; Gabrielli, L.; Bonasoni, M.P.; Chiereghin, A.; Turello, G.; Borgatti, E.C.; Simonazzi, G.; Felici, S.; Leone, M.; Salfi, N.C.M. Fetal brain damage in human fetuses with congenital cytomegalovirus infection: Histological features and viral tropism. Cell Mol. Neurobiol. 2023, 43, 1385–1399. [Google Scholar] [CrossRef]


| Gene | Sequence | Manufacturer |
|---|---|---|
| β-actin | F: CACCATTGGCAATGAGCGGTTC | Fisher-Eurofins |
| R: AGGTCTTTGCGGATGTCCACGT | Fisher-Eurofins | |
| Sox2 | Proprietary sequence | Qiagen |
| Nestin | F: TCAAGATGTCCCTCAGCCTGGA | Fisher-Eurofins |
| R: AAGCTGAGGGAAGTCTTGGAGC | Fisher-Eurofins | |
| DCX | Proprietary sequence | Qiagen |
| NeuroD1 | Proprietary sequence | Qiagen |
| RBFox3 | Proprietary sequence | Qiagen |
| MAP2 | F: AGGCTGTAGCAGTCCTGAAAGG | Fisher-Eurofins |
| R: CTTCCTCCACTGTGACAGTCTG | Fisher-Eurofins |
| Marker Type | Primary Antibody | Clone | Catalog Number |
|---|---|---|---|
| Stemness | Sox2 | E-4 | sc-365823 |
| Stemness | Nestin | 10c2 | sc-23927 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez-Vergara, A.C.; Avina, J.; Block, T.J.; Sheldrake, A.; Koch, C.; Gonzalez, A.; Steele, J.; Díaz-Lasprilla, A.M.; Munoz-Pinto, D.J. A Bioinspired Astrocyte-Derived Coating Promotes the In Vitro Proliferation of Human Neural Stem Cells While Maintaining Their Stemness. Biomimetics 2023, 8, 589. https://doi.org/10.3390/biomimetics8080589
Jimenez-Vergara AC, Avina J, Block TJ, Sheldrake A, Koch C, Gonzalez A, Steele J, Díaz-Lasprilla AM, Munoz-Pinto DJ. A Bioinspired Astrocyte-Derived Coating Promotes the In Vitro Proliferation of Human Neural Stem Cells While Maintaining Their Stemness. Biomimetics. 2023; 8(8):589. https://doi.org/10.3390/biomimetics8080589
Chicago/Turabian StyleJimenez-Vergara, Andrea C., Jacob Avina, Travis Jackson Block, Anne Sheldrake, Carson Koch, Anna Gonzalez, Jennifer Steele, Ana M. Díaz-Lasprilla, and Dany J. Munoz-Pinto. 2023. "A Bioinspired Astrocyte-Derived Coating Promotes the In Vitro Proliferation of Human Neural Stem Cells While Maintaining Their Stemness" Biomimetics 8, no. 8: 589. https://doi.org/10.3390/biomimetics8080589
APA StyleJimenez-Vergara, A. C., Avina, J., Block, T. J., Sheldrake, A., Koch, C., Gonzalez, A., Steele, J., Díaz-Lasprilla, A. M., & Munoz-Pinto, D. J. (2023). A Bioinspired Astrocyte-Derived Coating Promotes the In Vitro Proliferation of Human Neural Stem Cells While Maintaining Their Stemness. Biomimetics, 8(8), 589. https://doi.org/10.3390/biomimetics8080589





