Piezoelectric and Triboelectric Nanogenerators for Enhanced Wound Healing
Abstract
:1. Introduction
2. Wound Healing and Skin Regeneration
2.1. Skin Anatomy and Physiology
2.2. Wounds
2.3. Process of Wound Healing
2.3.1. Hemostasis Phase
2.3.2. Inflammatory Phase
2.3.3. Proliferation Phase
2.3.4. Remodeling Phase
3. Bioelectrical Factors in Skin Tissue
3.1. Bioelectrical Properties in Normal Skin
3.1.1. Epidermal Ion Distribution
3.1.2. Transepithelial Potential (TEP)
3.1.3. Bioimpedance and Barrier Properties of Skin
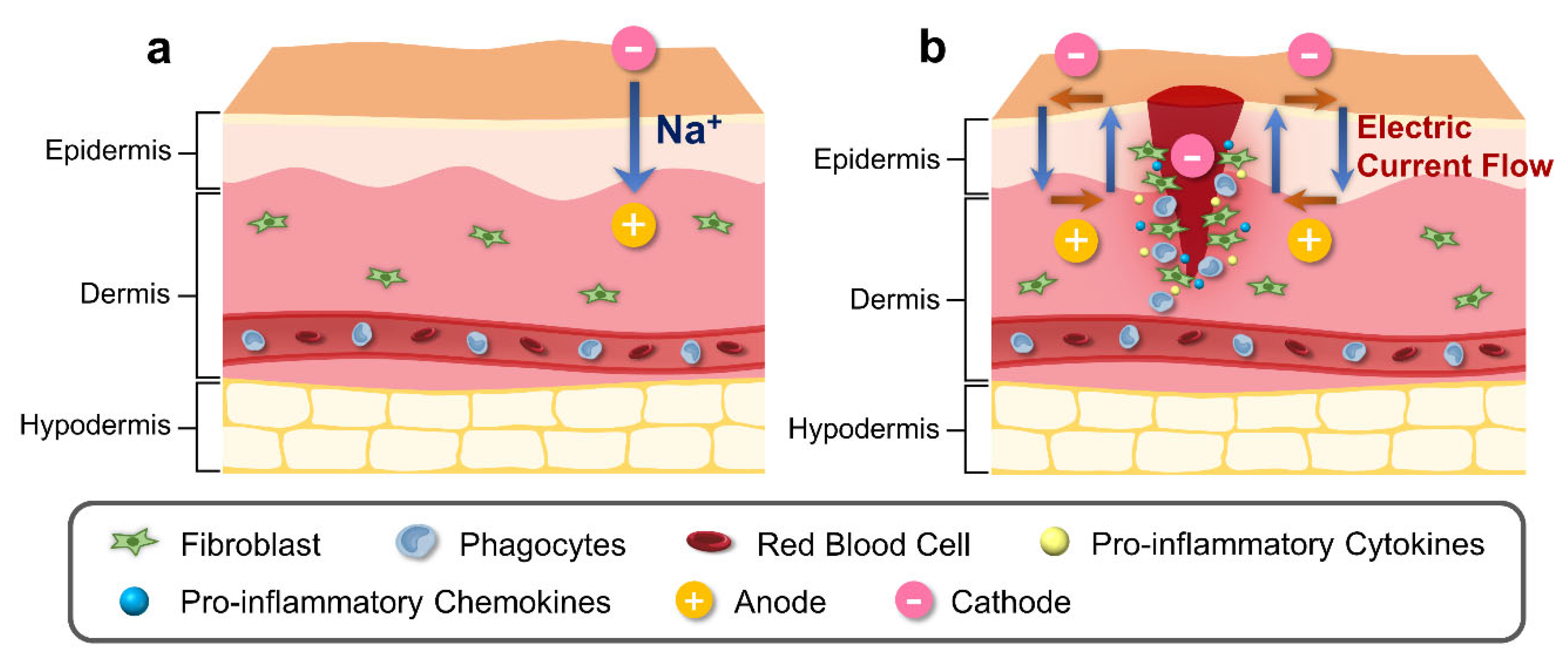
3.2. Bioelectrical Factors during Wound Healing
3.2.1. Alteration in Epidermal Ion Distribution and Electric Potential
3.2.2. Electric Field Profiles in Wound Sites
3.2.3. Effects of Electric Field Alteration on Cell Components
4. Nanogenerators for Tissue Regeneration
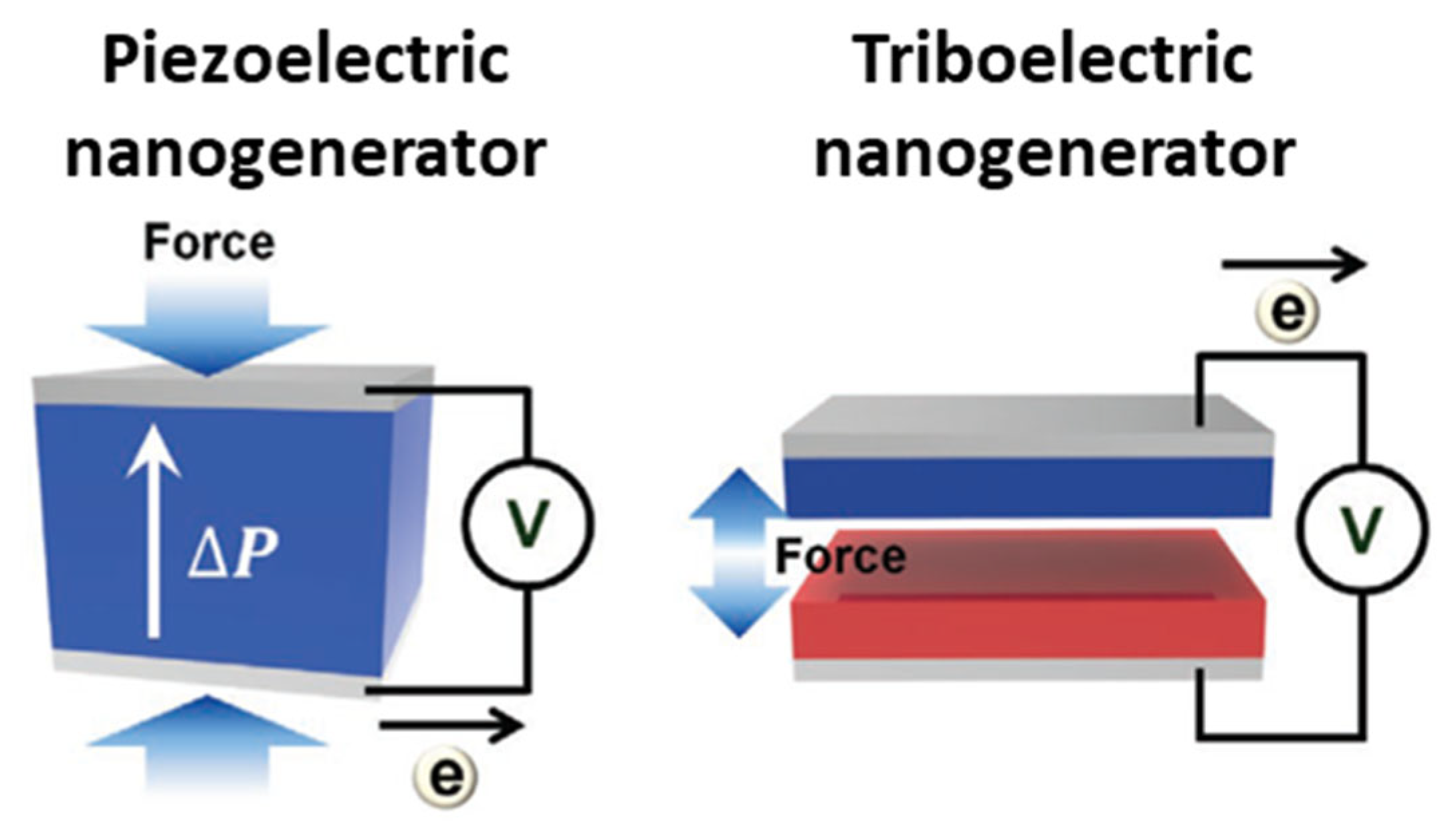
4.1. Types and Principles of Nanogenerators
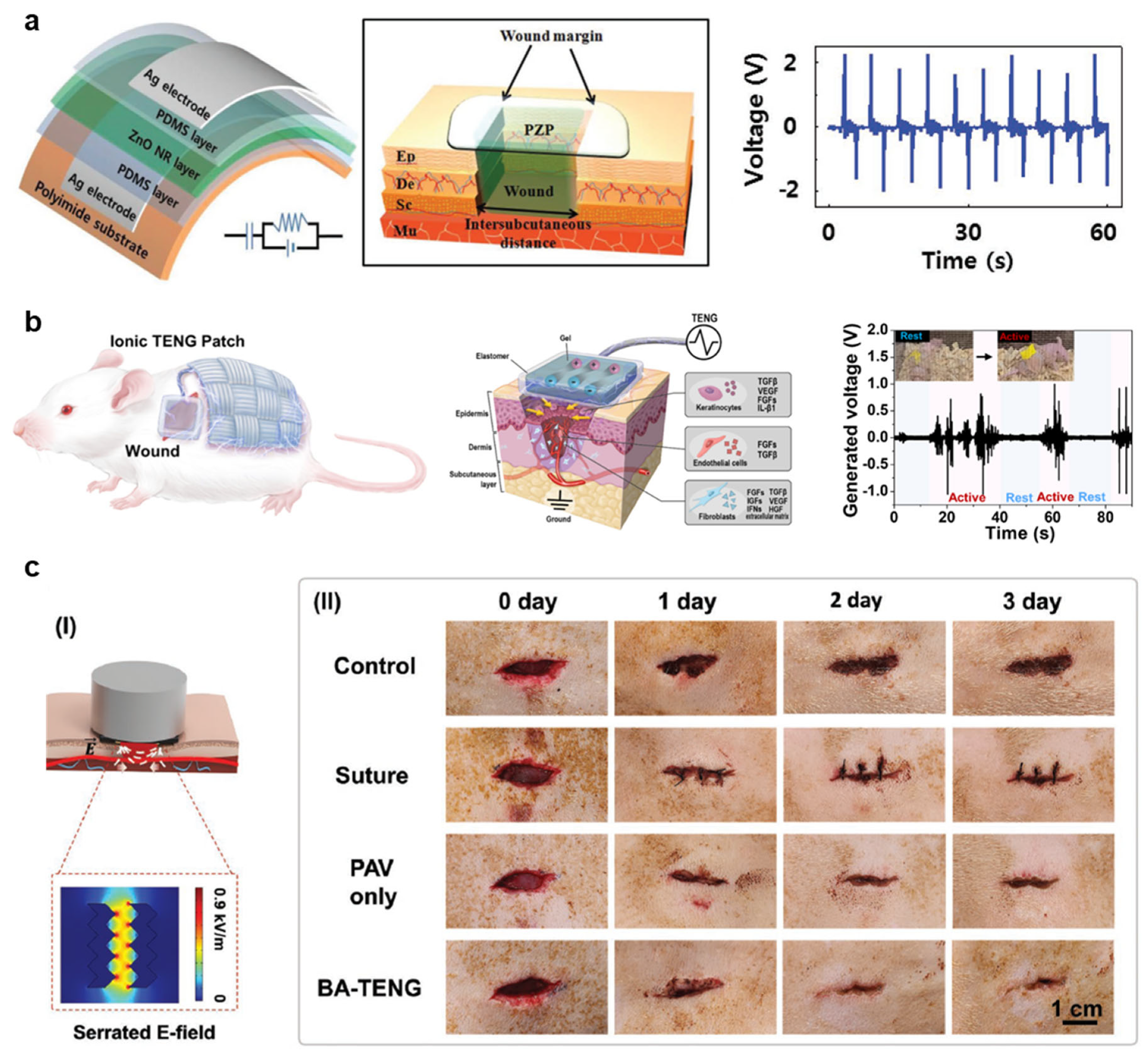
4.2. Applications of Nanogenerators for Enhanced Wound Healing
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Scotton, M.F.; Miot, H.A.; Abbade, L.P. Factors that influence healing of chronic venous leg ulcers: A retrospective cohort. An. Bras Dermatol. 2014, 89, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Brem, H.; Sheehan, P.; Rosenberg, H.J.; Schneider, J.S.; Boulton, A.J.M. Evidence-Based Protocol for Diabetic Foot Ulcers. Plast. Reconstr. Surg. 2006, 117, 193S–209S. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.; Bjarnsholt, T.; James, G.A.; Leaper, D.J.; McBain, A.J.; Malone, M.; Stoodley, P.; Swanson, T.; Tachi, M.; Wolcott, R.D.; et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017, 25, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef]
- Price, P.; Harding, K. Cardiff Wound Impact Schedule: The development of a condition-specific questionnaire to assess health-related quality of life in patients with chronic wounds of the lower limb. Int. Wound J. 2004, 1, 10–17. [Google Scholar] [CrossRef]
- Fahey, T.J.; Sadaty, A.; Jones, W.G.; Barber, A.; Smoller, B.; Shires, G.T. Diabetes impairs the late inflammatory response to wound healing. J. Surg. Res. 1991, 50, 308–313. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- Simman, R. Wound Closure and the Reconstructive Ladder in Plastic Surgery. J. Am. Coll. Certif. Wound Spec. 2009, 1, 6–11. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Vijayan, A.; Sabareeswaran, A.; Kumar, G.S.V. PEG grafted chitosan scaffold for dual growth factor delivery for enhanced wound healing. Sci. Rep. 2019, 9, 19165. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Hui, Q.; Tang, L.; Zheng, L.; Jin, Z.; Yu, B.; Wang, Z.; Lin, P.; Yu, W.; Li, H.; et al. TAT-Mediated Acidic Fibroblast Growth Factor Delivery to the Dermis Improves Wound Healing of Deep Skin Tissue in Rat. PLoS ONE 2015, 10, e0135291. [Google Scholar] [CrossRef] [PubMed]
- Briquez, P.S.; Hubbell, J.A.; Martino, M.M. Extracellular Matrix-Inspired Growth Factor Delivery Systems for Skin Wound Healing. Adv. Wound Care 2015, 4, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, L.; Li, Y.; Zhang, F.; Lin, L.; Niu, S.; Chenet, D.; Zhang, X.; Hao, Y.; Heinz, T.F.; et al. Piezoelectricity of single-atomic-layer MoS2 for energy conversion and piezotronics. Nature 2014, 514, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-Y.; Choi, D.; Jin, M.-J.; Kim, I.; Kim, S.-H.; Choi, J.-Y.; Lee, S.Y.; Kim, J.M.; Kim, S.-W. Mechanically Powered Transparent Flexible Charge-Generating Nanodevices with Piezoelectric ZnO Nanorods. Adv. Mater. 2009, 21, 2185–2189. [Google Scholar] [CrossRef]
- Fan, F.-R.; Tian, Z.-Q.; Lin Wang, Z. Flexible triboelectric generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Hinchet, R.; Yoon, H.-J.; Ryu, H.; Kim, M.-K.; Choi, E.-K.; Kim, D.-S.; Kim, S.-W. Transcutaneous ultrasound energy harvesting using capacitive triboelectric technology. Science 2019, 365, 491–494. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Rajagopalan, P.; Zhang, L.; Zhan, S.; Huang, S.; Li, W.; Zeng, X.; Ye, Q.; Liu, Y.; et al. Toward Controlled Electrical Stimulation for Wound Healing Based on a Precision Layered Skin Model. ACS Appl. Bio. Mater. 2020, 3, 8901–8910. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, Z.; Chen, T.; Lee, C. Low cost exoskeleton manipulator using bidirectional triboelectric sensors enhanced multiple degree of freedom sensory system. Nat. Commun. 2021, 12, 2692. [Google Scholar] [CrossRef]
- Yuan, H.; Lei, T.; Qin, Y.; Yang, R. Flexible electronic skins based on piezoelectric nanogenerators and piezotronics. Nano Energy 2019, 59, 84–90. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Zhou, Z.; Li, J.; Shen, B.; Zhu, T.; Gao, X.; Tao, R.; Guo, X.; Hu, X.; Shi, Y.; et al. Coupling Enhanced Performance of Triboelectric–Piezoelectric Hybrid Nanogenerator Based on Nanoporous Film of Poly(vinylidene fluoride)/BaTiO3 Composite Electrospun Fibers. ACS Mater. Lett. 2022, 4, 847–852. [Google Scholar] [CrossRef]
- Wang, X.; Yang, B.; Liu, J.; Zhu, Y.; Yang, C.; He, Q. A flexible triboelectric-piezoelectric hybrid nanogenerator based on P(VDF-TrFE) nanofibers and PDMS/MWCNT for wearable devices. Sci. Rep. 2016, 6, 36409. [Google Scholar] [CrossRef] [PubMed]
- Kolarsick, P.A.J.; Kolarsick, M.A.; Goodwin, C. Anatomy and Physiology of the Skin. J. Dermatol. Nurses’ Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef]
- Kalinin, A.; Marekov, L.N.; Steinert, P.M. Assembly of the epidermal cornified cell envelope. J. Cell Sci. 2001, 114, 3069–3070. [Google Scholar] [CrossRef]
- Van Den Bossche, K.; Naeyaert, J.M.; Lambert, J. The quest for the mechanism of melanin transfer. Traffic 2006, 7, 769–778. [Google Scholar] [CrossRef]
- Romani, N.; Schuler, G. The immunologic properties of epidermal Langerhans cells as a part of the dendritic cell system. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 265–279. [Google Scholar]
- Maksimovic, S.; Nakatani, M.; Baba, Y.; Nelson, A.M.; Marshall, K.L.; Wellnitz, S.A.; Firozi, P.; Woo, S.-H.; Ranade, S.; Patapoutian, A. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 2014, 509, 617–621. [Google Scholar] [CrossRef]
- Corcuff, P.; Bertrand, C.; Leveque, J. Morphometry of human epidermis in vivo by real-time confocal microscopy. Arch. Dermatol. Res. 1993, 285, 475–481. [Google Scholar] [CrossRef]
- Matsui, T.; Amagai, M. Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 2015, 27, 269–280. [Google Scholar] [CrossRef]
- Briggaman, R.A.; Wheeler, C.E., Jr. The epidermal-dermal junction. J. Investig. Dermatol. 1975, 65, 71–84. [Google Scholar] [CrossRef]
- McGrath, J.; Eady, R.; Pope, F. Anatomy and organization of human skin. Rook’s Textb. Dermatol. 2004, 1, 3.2–3.80. [Google Scholar]
- Klingberg, F.; Hinz, B.; White, E.S. The myofibroblast matrix: Implications for tissue repair and fibrosis. J. Pathol. 2013, 229, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Venus, M.; Waterman, J.; McNab, I. Basic physiology of the skin. Surgery 2010, 28, 469–472. [Google Scholar]
- Cinti, S. The adipose organ: Morphological perspectives of adipose tissues. Proc. Nutr. Soc. 2001, 60, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G. Antimicrobial and antiseptic strategies in wound management. Int. Wound J. 2013, 10, 9–14. [Google Scholar] [CrossRef]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef]
- Mittal, K.; Kaur, R. Platelet storage lesion: An update. Asian J. Transfus. Sci. 2015, 9, 1–3. [Google Scholar]
- Bray, M.A.; Sartain, S.E.; Gollamudi, J.; Rumbaut, R.E. Microvascular thrombosis: Experimental and clinical implications. Transl. Res. 2020, 225, 105–130. [Google Scholar] [CrossRef]
- Li, R.; Liu, K.; Huang, X.; Li, D.; Ding, J.; Liu, B.; Chen, X. Bioactive materials promote wound healing through modulation of cell behaviors. Adv. Sci. 2022, 9, 2105152. [Google Scholar] [CrossRef]
- Pierce, G.F.; Mustoe, T.A.; Altrock, B.W.; Deuel, T.F.; Thomason, A. Role of platelet-derived growth factor in wound healing. J. Cell. Biochem. 1991, 45, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Ridiandries, A.; Tan, J.T.; Bursill, C.A. The role of chemokines in wound healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Duan, Z.; Chen, X.; Li, M. The immune function of dermal fibroblasts in skin defence against pathogens. Exp. Dermatol. 2023, 32, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.-O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 101, e56–e60. [Google Scholar] [CrossRef]
- Singhal, A.; Kumar, S. Neutrophil and remnant clearance in immunity and inflammation. Immunology 2022, 165, 22–43. [Google Scholar] [CrossRef]
- Oschman, J.L.; Chevalier, G.; Brown, R. The effects of grounding (earthing) on inflammation, the immune response, wound healing, and prevention and treatment of chronic inflammatory and autoimmune diseases. J. Inflamm. Res. 2015, 8, 83–96. [Google Scholar] [CrossRef]
- Lucas, S.M.; Rothwell, N.J.; Gibson, R.M. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006, 147, S232–S240. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef]
- Schreier, T.; Degen, E.; Baschong, W. Fibroblast migration and proliferation during in vitro wound healing: A quantitative comparison between various growth factors and a low molecular weight blood dialyzate used in the clinic to normalize impaired wound healing. Res. Exp. Med. 1993, 193, 195–205. [Google Scholar] [CrossRef]
- Luo, S.; Benathan, M.; Raffoul, W.; Panizzon, R.G.; Egloff, D.V. Abnormal balance between proliferation and apoptotic cell death in fibroblasts derived from keloid lesions. Plast. Reconstr. Surg. 2001, 107, 87–96. [Google Scholar] [CrossRef]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 410–412. [Google Scholar]
- Hardy, M.A. The biology of scar formation. Phys. Ther. 1989, 69, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Grazul-Bilska, A.T.; Johnson, M.L.; Bilski, J.J.; Redmer, D.A.; Reynolds, L.P.; Abdullah, A.; Abdullah, K.M. Wound healing: The role of growth factors. Drugs Today 2003, 39, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Desmouliere, A.; Redard, M.; Darby, I.; Gabbiani, G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am. J. Pathol. 1995, 146, 56. [Google Scholar]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Boo, S.; Dagnino, L. Integrins as modulators of transforming growth factor beta signaling in dermal fibroblasts during skin regeneration after injury. Adv. Wound Care 2013, 2, 238–246. [Google Scholar] [CrossRef]
- Smith, P.C.; Martínez, C.; Martínez, J.; McCulloch, C.A. Role of fibroblast populations in periodontal wound healing and tissue remodeling. Front. Physiol. 2019, 10, 270. [Google Scholar] [CrossRef]
- Muiznieks, L.D.; Keeley, F.W. Molecular assembly and mechanical properties of the extracellular matrix: A fibrous protein perspective. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 866–875. [Google Scholar] [CrossRef]
- Mescher, A.L. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration 2017, 4, 39–53. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Landis, C. Electrical phenomena of the skin. Psychol. Bull. 1932, 29, 693–752. [Google Scholar] [CrossRef]
- Burnette, R.R.; Ongpipattanakul, B. Characterization of the Pore Transport Properties and Tissue Alteration of Excised Human Skin during Iontophoresis. J. Pharm. Sci. 1988, 77, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Miklavčič, D.; Pavšelj, N.; Hart, F.X. Electric properties of tissues. In Wiley Encyclopedia of Biomedical Engineering; Wiley: Chichester, UK, 2006. [Google Scholar]
- Meng, S.; Rouabhia, M.; Zhang, Z.; De, D.; De, F.; Laval, U. Electrical stimulation in tissue regeneration. Appl. Biomed. Eng. 2011, 3, 37–62. [Google Scholar]
- Stewart, S.; Rojas-Muñoz, A.; Belmonte, J.C.I. Bioelectricity and epimorphic regeneration. Bioessays 2007, 29, 1133–1137. [Google Scholar] [CrossRef]
- Nuccitelli, R. Endogenous electric fields in embryos during development, regeneration and wound healing. Radiat. Prot. Dosim. 2003, 106, 375–383. [Google Scholar] [CrossRef]
- Lansdown, A.B. Calcium: A potential central regulator in wound healing in the skin. Wound Repair Regen. 2002, 10, 271–285. [Google Scholar] [CrossRef]
- Lee, S.H.; Elias, P.M.; Proksch, E.; Menon, G.K.; Mao-Quiang, M.; Feingold, K.R. Calcium and potassium are important regulators of barrier homeostasis in murine epidermis. J. Clin. Investig. 1992, 89, 530–538. [Google Scholar] [CrossRef]
- Denda, M.; Hosoi, J.; Asida, Y. Visual imaging of ion distribution in human epidermis. Biochem. Biophys. Res. Commun. 2000, 272, 134–137. [Google Scholar] [CrossRef]
- Mauro, T.; Elias, P.; Cullander, C.; Bench, G.; Sidderas-Haddad, E.; Feingold, K. Acute barrier perturbation abolishes the Ca2+ and K+ gradients in murine epidermis: Quantitative measurement using PIXE. J. Investig. Dermatol. 1998, 111, 1198–1201. [Google Scholar] [CrossRef]
- Menon, G.K.; Price, L.F.; Bommannan, B.; Elias, P.M.; Feingold, K.R. Selective obliteration of the epidermal calcium gradient leads to enhanced lamellar body secretion. J. Investig. Dermatol. 1994, 102, 789–795. [Google Scholar] [CrossRef]
- Haller, T.; Auktor, K.; Frick, M.; Mair, N.; Dietl, P. Threshold calcium levels for lamellar body exocytosis in type II pneumocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 1999, 277, L893–L900. [Google Scholar] [CrossRef] [PubMed]
- Nuccitelli, R.; Nuccitelli, P.; Ramlatchan, S.; Sanger, R.; Smith, P.J. Imaging the electric field associated with mouse and human skin wounds. Wound Repair Regen. 2008, 16, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Foulds, I.; Barker, A. Human skin battery potentials and their possible role in wound healing. Br. J. Dermatol. 1983, 109, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.; Vaughan, J.A. Human eccrine sweat gland activity and palmar electrical skin resistance. J. Appl. Physiol. 1965, 20, 980–983. [Google Scholar] [CrossRef] [PubMed]
- Freedman, L.W.; Scerbo, A.S.; Dawson, M.E.; Raine, A.; McClure, W.O.; Venables, P.H. The relationship of sweat gland count to electrodermal activity. Psychophysiology 1994, 31, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.Y.; Isseroff, R.R.; Nuccitelli, R. Human keratinocytes migrate to the negative pole in direct current electric fields comparable to those measured in mammalian wounds. J. Cell Sci. 1996, 109, 199–207. [Google Scholar] [CrossRef]
- Tavernier, A.; Dierickx, M.; Hinsenkamp, M. Tensors of dielectric permittivity and conductivity of in vitro human dermis and epidermis. Bioelectrochem. Bioenerg. 1993, 30, 65–72. [Google Scholar] [CrossRef]
- Tagami, H. Location-related differences in structure and function of the stratum corneum with special emphasis on those of the facial skin. Int. J. Cosmet. Sci. 2008, 30, 413–434. [Google Scholar] [CrossRef]
- Wen, J.; Tang, J.; Ning, H.; Hu, N.; Zhu, Y.; Gong, Y.; Xu, C.; Zhao, Q.; Jiang, X.; Hu, X. Multifunctional ionic skin with sensing, UV-filtering, water-retaining, and anti-freezing capabilities. Adv. Funct. Mater. 2021, 31, 2011176. [Google Scholar] [CrossRef]
- Tamura, T.; Tenhunen, M.; Lahtinen, T.; Repo, T.; Schwan, H. Modelling of the dielectric properties of normal and irradiated skin. Phys. Med. Biol. 1994, 39, 927. [Google Scholar] [CrossRef]
- Tagami, H.; Ohi, M.; Iwatsuki, K.; Kanamaru, Y.; Yamada, M.; Ichijo, B. Evaluation of the skin surface hydration in vivo by electrical measurement. J. Investig. Dermatol. 1980, 75, 500–507. [Google Scholar] [CrossRef]
- Raven, M.S. Experimental measurements of the skin effect and internal inductance at low frequencies. Acta Technica 2015, 60, 51–69. [Google Scholar]
- Weaver, J.C.; Vaughan, T.E.; Chizmadzhev, Y. Theory of electrical creation of aqueous pathways across skin transport barriers. Adv. Drug Deliv. Rev. 1999, 35, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Pikal, M.J. The role of electroosmotic flow in transdermal iontophoresis. Adv. Drug Deliv. Rev. 1992, 9, 201–237. [Google Scholar] [CrossRef]
- Darlenski, R.; Sassning, S.; Tsankov, N.; Fluhr, J. Non-invasive in vivo methods for investigation of the skin barrier physical properties. Eur. J. Pharm. Biopharm. 2009, 72, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Vanable, J. Integumentary potentials and wound healing. Electr. Fields Vertebr. Repair 1989, 171–224. [Google Scholar] [CrossRef]
- Messerli, M.A.; Graham, D.M. Extracellular electrical fields direct wound healing and regeneration. Biol. Bull. 2011, 221, 79–92. [Google Scholar] [CrossRef]
- Pietak, A.; Levin, M. Exploring instructive physiological signaling with the bioelectric tissue simulation engine. Front. Bioeng. Biotechnol. 2016, 4, 55. [Google Scholar] [CrossRef]
- Bikle, D.D. Role of vitamin D and calcium signaling in epidermal wound healing. J. Endocrinol. Investig. 2023, 46, 205–212. [Google Scholar] [CrossRef]
- Kowalczyk, A.P.; Green, K.J. Structure, function, and regulation of desmosomes. Prog. Mol. Biol. Transl. Sci. 2013, 116, 95–118. [Google Scholar]
- Du Bois-Reymond, E.H. Vorläufiger Abriss einer Untersuchung über den Sogenannten Froschstrom und über die Elektromotorischen Fische; Wiley: Chichester, UK, 1843. [Google Scholar]
- Illingworth, C.M.; Barker, A.T. Measurement of electrical currents emerging during the regeneration of amputated finger tips in children. Clin. Phys. Physiol. Meas. 1980, 1, 87. [Google Scholar] [CrossRef]
- Nuccitelli, R.; Nuccitelli, P.; Li, C.; Narsing, S.; Pariser, D.M.; Lui, K. The electric field near human skin wounds declines with age and provides a noninvasive indicator of wound healing. Wound Repair Regen. 2011, 19, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Obedencio, G.P.; Isseroff, R.R.; Nuccitelli, R. Involucrin-Positive Keratinocytes Demonstrate Decreased Migration Speed but Sustained Directional Migration in a DC Electric Field. J. Investig. Dermatol. 1999, 113, 851–855. [Google Scholar] [CrossRef]
- Yan, T.; Jiang, X.; Lin, G.; Tang, D.; Zhang, J.; Guo, X.; Zhang, D.; Zhang, Q.; Jia, J.; Huang, Y. Autophagy is required for the directed motility of keratinocytes driven by electric fields. FASEB J. 2019, 33, 3922–3935. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Sun, H.; Liu, J.; Guo, X.; Huang, J.; Jiang, X.; Zhang, Y.; Huang, Y.; Fan, D.; Zhang, J. Keratinocyte electrotaxis induced by physiological pulsed direct current electric fields. Bioelectrochemistry 2019, 127, 113–124. [Google Scholar] [CrossRef]
- Guo, X.; Jiang, X.; Ren, X.; Sun, H.; Zhang, D.; Zhang, Q.; Zhang, J.; Huang, Y. The galvanotactic migration of keratinocytes is enhanced by hypoxic preconditioning. Sci. Rep. 2015, 5, 10289. [Google Scholar] [CrossRef]
- Levy, L.; Broad, S.; Diekmann, D.; Evans, R.D.; Watt, F.M. β1 integrins regulate keratinocyte adhesion and differentiation by distinct mechanisms. Mol. Biol. Cell 2000, 11, 453–466. [Google Scholar] [CrossRef]
- Erickson, C.A.; Nuccitelli, R. Embryonic fibroblast motility and orientation can be influenced by physiological electric fields. J. Cell Biol. 1984, 98, 296–307. [Google Scholar] [CrossRef]
- McLeod, K.J.; Lee, R.C.; Ehrlich, H.P. Frequency dependence of electric field modulation of fibroblast protein synthesis. Science 1987, 236, 1465–1469. [Google Scholar] [CrossRef]
- Jennings, J.; Chen, D.; Feldman, D. Transcriptional response of dermal fibroblasts in direct current electric fields. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2008, 29, 394–405. [Google Scholar] [CrossRef]
- Hoare, J.I.; Rajnicek, A.M.; McCaig, C.D.; Barker, R.N.; Wilson, H.M. Electric fields are novel determinants of human macrophage functions. J. Leucoc. Biol. 2016, 99, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Clementi, G.; Cottone, F.; Di Michele, A.; Gammaitoni, L.; Mattarelli, M.; Perna, G.; López-Suárez, M.; Baglio, S.; Trigona, C.; Neri, I. Review on Innovative Piezoelectric Materials for Mechanical Energy Harvesting. Energies 2022, 15, 6227. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Li, Y.; Du, J.; Hao, X.; Huang, H. A high-power wearable triboelectric nanogenerator prepared from self-assembled electrospun poly(vinylidene fluoride) fibers with a heart-like structure. J. Mater. Chem. A 2019, 7, 11724–11733. [Google Scholar] [CrossRef]
- Zhang, J.H.; Li, Z.; Xu, J.; Li, J.; Yan, K.; Cheng, W.; Xin, M.; Zhu, T.; Du, J.; Chen, S.; et al. Versatile self-assembled electrospun micropyramid arrays for high-performance on-skin devices with minimal sensory interference. Nat. Commun. 2022, 13, 5839. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Song, J. Piezoelectric Nanogenerators Based on Zinc Oxide Nanowire Arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-I.; Son, J.H.; Hwang, G.-T.; Jeong, C.K.; Ryu, J.; Koo, M.; Choi, I.; Lee, S.H.; Byun, M.; Wang, Z.L.; et al. Highly-Efficient, Flexible Piezoelectric PZT Thin Film Nanogenerator on Plastic Substrates. Adv. Mater. 2014, 26, 2514–2520. [Google Scholar] [CrossRef]
- Park, K.-I.; Xu, S.; Liu, Y.; Hwang, G.-T.; Kang, S.-J.L.; Wang, Z.L.; Lee, K.J. Piezoelectric BaTiO3 Thin Film Nanogenerator on Plastic Substrates. Nano Lett. 2010, 10, 4939–4943. [Google Scholar] [CrossRef]
- Lee, J.-H.; Yoon, H.-J.; Kim, T.Y.; Gupta, M.K.; Lee, J.H.; Seung, W.; Ryu, H.; Kim, S.-W. Micropatterned P(VDF-TrFE) Film-Based Piezoelectric Nanogenerators for Highly Sensitive Self-Powered Pressure Sensors. Adv. Funct. Mater. 2015, 25, 3203–3209. [Google Scholar] [CrossRef]
- Clementi, G.; Lombardi, G.; Margueron, S.; Suarez, M.A.; Lebrasseur, E.; Ballandras, S.; Imbaud, J.; Lardet-Vieudrin, F.; Gauthier-Manuel, L.; Dulmet, B.; et al. LiNbO3 films—A low-cost alternative lead-free piezoelectric material for vibrational energy harvesters. Mech. Syst. Signal Process. 2021, 149, 107171. [Google Scholar] [CrossRef]
- Ryu, H.; Yoon, H.-J.; Kim, S.-W. Hybrid Energy Harvesters: Toward Sustainable Energy Harvesting. Adv. Mater. 2019, 31, 1802898. [Google Scholar] [CrossRef]
- Ryu, H.; Lee, J.-H.; Kim, T.-Y.; Khan, U.; Lee, J.H.; Kwak, S.S.; Yoon, H.-J.; Kim, S.-W. High-Performance Triboelectric Nanogenerators Based on Solid Polymer Electrolytes with Asymmetric Pairing of Ions. Adv. Energy Mater. 2017, 7, 1700289. [Google Scholar] [CrossRef]
- Bhang, S.H.; Jang, W.S.; Han, J.; Yoon, J.-K.; La, W.-G.; Lee, E.; Kim, Y.S.; Shin, J.-Y.; Lee, T.-J.; Baik, H.K.; et al. Zinc Oxide Nanorod-Based Piezoelectric Dermal Patch for Wound Healing. Adv. Funct. Mater. 2017, 27, 1603497. [Google Scholar] [CrossRef]
- Assimacopoulos, D. Wound healing promotion by the use of negative electric current. Am. Surg. 1968, 34, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-H.; Lee, Y.; Lee, M.-G.; Song, W.J.; Park, J.-U.; Sun, J.-Y. Accelerated wound healing with an ionic patch assisted by a triboelectric nanogenerator. Nano Energy 2021, 79, 105463. [Google Scholar] [CrossRef]
- Meng, X.; Xiao, X.; Jeon, S.; Kim, D.; Park, B.-J.; Kim, Y.-J.; Rubab, N.; Kim, S.; Kim, S.-W. An Ultrasound-Driven Bioadhesive Triboelectric Nanogenerator for Instant Wound Sealing and Electrically Accelerated Healing in Emergencies. Adv. Mater. 2023, 35, 2209054. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Zhou, N.; Gao, Y.; Xie, G.; Du, H.; Jiang, H.; Zhang, L.; Tao, J.; Zhu, J. Bioinspired hybrid patches with self-adhesive hydrogel and piezoelectric nanogenerator for promoting skin wound healing. Nano Res. 2020, 13, 2525–2533. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Y.; Zhao, Y.; Ye, M.; Zhang, S.; Gong, S. Ultrasound-activable piezoelectric membranes for accelerating wound healing. Biomater. Sci. 2022, 10, 692–701. [Google Scholar] [CrossRef]
- Liang, J.; Zeng, H.; Qiao, L.; Jiang, H.; Ye, Q.; Wang, Z.; Liu, B.; Fan, Z. 3D Printed Piezoelectric Wound Dressing with Dual Piezoelectric Response Models for Scar-Prevention Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 30507–30522. [Google Scholar] [CrossRef]
- Fu, S.; Yi, S.; Ke, Q.; Liu, K.; Xu, H. A Self-Powered Hydrogel/Nanogenerator System Accelerates Wound Healing by Electricity-Triggered On-Demand Phosphatase and Tensin Homologue (PTEN) Inhibition. ACS Nano 2023, 17, 19652–19666. [Google Scholar] [CrossRef]
- Barman, S.R.; Chan, S.W.; Kao, F.C.; Ho, H.Y.; Khan, I.; Pal, A.; Huang, C.C.; Lin, Z.H. A self-powered multifunctional dressing for active infection prevention and accelerated wound healing. Sci. Adv. 2023, 9, eadc8758. [Google Scholar] [CrossRef]
- Luo, B.; Zhou, Q.; Chen, W.; Sun, L.; Yang, L.; Guo, Y.; Liu, H.; Wu, Z.; Neisiany, R.E.; Qin, X.; et al. Nonadjacent Wireless Electrotherapy for Tissue Repair by a 3D-Printed Bioresorbable Fully Soft Triboelectric Nanogenerator. Nano Lett. 2023, 23, 2927–2937. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Ke, Q.; Chen, Q.; Zhang, X.; Su, J.; Ning, C.; Fang, L. Flexible, Breathable, and Self-Powered Patch Assembled of Electrospun Polymer Triboelectric Layers and Polypyrrole-Coated Electrode for Infected Chronic Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 17641–17652. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Suo, H.; Xie, G.; Lyu, Q.; Mo, M.; Xie, Z.; Zhou, N.; Zhang, L.; Tao, J.; Zhu, J. Self-powered and photothermal electronic skin patches for accelerating wound healing. Nano Energy 2022, 93, 106906. [Google Scholar] [CrossRef]
- Sharma, A.; Panwar, V.; Mondal, B.; Prasher, D.; Bera, M.K.; Thomas, J.; Kumar, A.; Kamboj, N.; Mandal, D.; Ghosh, D. Electrical stimulation induced by a piezo-driven triboelectric nanogenerator and electroactive hydrogel composite, accelerate wound repair. Nano Energy 2022, 99, 107419. [Google Scholar] [CrossRef]
- Li, H.; Xu, F.; Guan, T.; Li, Y.; Sun, J. Mechanically and environmentally stable triboelectric nanogenerator based on high-strength and anti-compression self-healing ionogel. Nano Energy 2021, 90, 106645. [Google Scholar] [CrossRef]
- Hou, K.-X.; Dai, X.; Zhao, S.-P.; Huang, L.-B.; Li, C.-H. A damage-tolerant, self-healing and multifunctional triboelectric nanogenerator. Nano Energy 2023, 116, 108739. [Google Scholar] [CrossRef]
- Lee, K.Y.; Gupta, M.K.; Kim, S.-W. Transparent flexible stretchable piezoelectric and triboelectric nanogenerators for powering portable electronics. Nano Energy 2015, 14, 139–160. [Google Scholar] [CrossRef]
- Veeralingam, S.; Badhulika, S. Lead-Free Transparent Flexible Piezoelectric Nanogenerator for Self-Powered Wearable Electronic Sensors and Energy Harvesting through Rainwater. ACS Appl. Energy Mater. 2022, 5, 12884–12896. [Google Scholar] [CrossRef]
- Lu, S.-H.; Samandari, M.; Li, C.; Li, H.; Song, D.; Zhang, Y.; Tamayol, A.; Wang, X. Multimodal sensing and therapeutic systems for wound healing and management: A review. Sens. Actuators Rep. 2022, 4, 100075. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, T.; Wang, N. From Piezoelectric Nanogenerator to Non-Invasive Medical Sensor: A Review. Biosensors 2023, 13, 113. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.-J.; Tiruneh, D.M.; Ryu, H.; Yoon, J.-K. Piezoelectric and Triboelectric Nanogenerators for Enhanced Wound Healing. Biomimetics 2023, 8, 517. https://doi.org/10.3390/biomimetics8070517
Jang H-J, Tiruneh DM, Ryu H, Yoon J-K. Piezoelectric and Triboelectric Nanogenerators for Enhanced Wound Healing. Biomimetics. 2023; 8(7):517. https://doi.org/10.3390/biomimetics8070517
Chicago/Turabian StyleJang, Hye-Jeong, Daniel Manaye Tiruneh, Hanjun Ryu, and Jeong-Kee Yoon. 2023. "Piezoelectric and Triboelectric Nanogenerators for Enhanced Wound Healing" Biomimetics 8, no. 7: 517. https://doi.org/10.3390/biomimetics8070517
APA StyleJang, H.-J., Tiruneh, D. M., Ryu, H., & Yoon, J.-K. (2023). Piezoelectric and Triboelectric Nanogenerators for Enhanced Wound Healing. Biomimetics, 8(7), 517. https://doi.org/10.3390/biomimetics8070517





