Hypoxic Extracellular Matrix Preserves Its Competence after Expansion of Human MSCs under Physiological Hypoxia In Vitro
Abstract
:1. Introduction
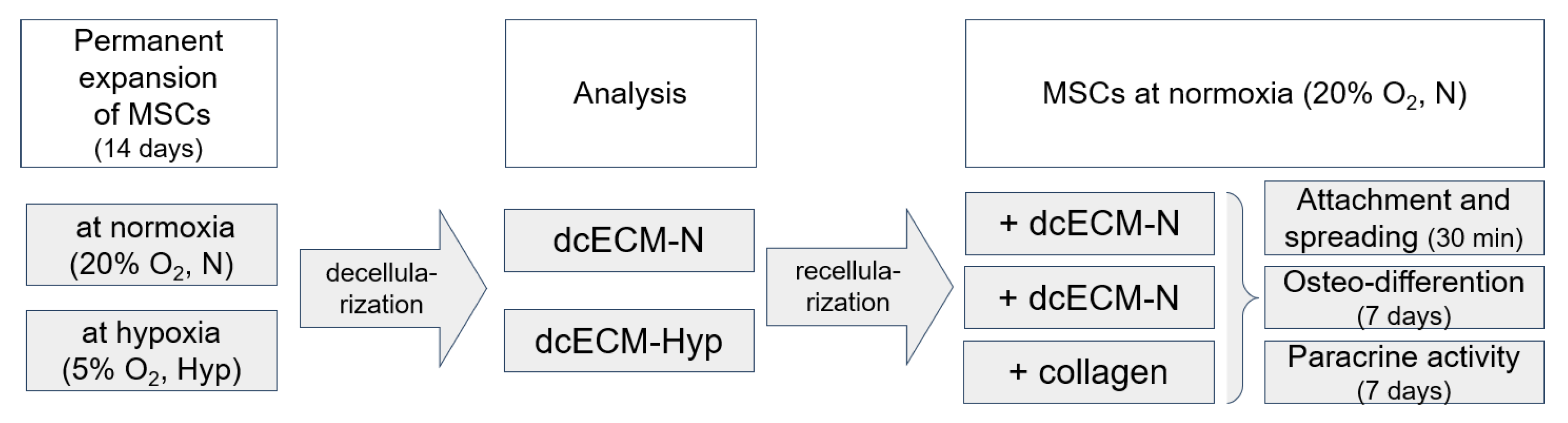
2. Materials and Methods
2.1. Cell Culture
2.2. Preparation of Decellularized Matrices
Analysis of the Decellularized Matrices
2.3. Recellularization of MSCs on Decellularized Matrices
2.3.1. Morphology and Actin Cytoskeleton of MSCs
2.3.2. Paracrine Activity of MSCs
2.4. Osteogenic Potential of MSCs
2.5. Microscopy
2.6. Statistical Analysis
3. Results and Discussion
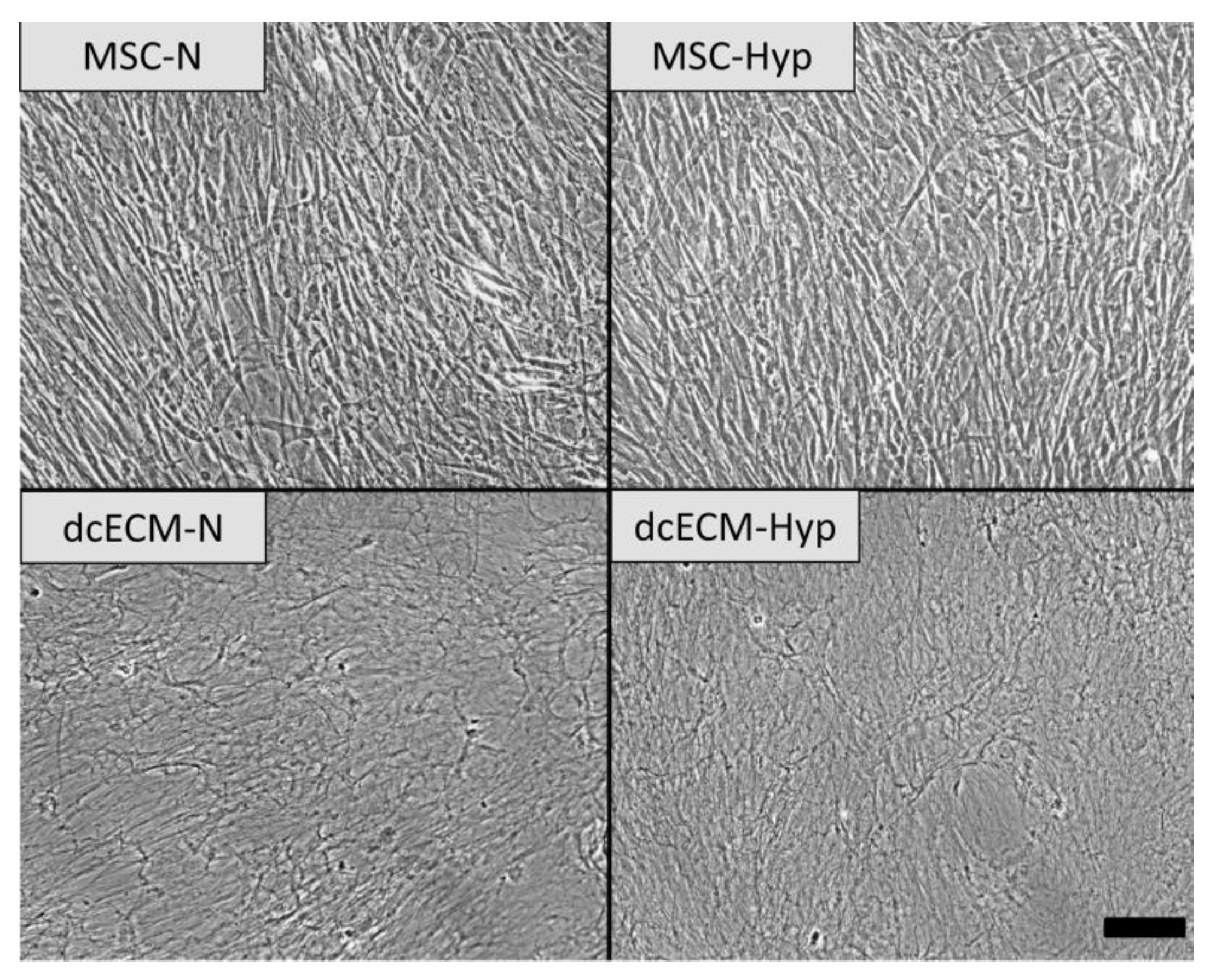
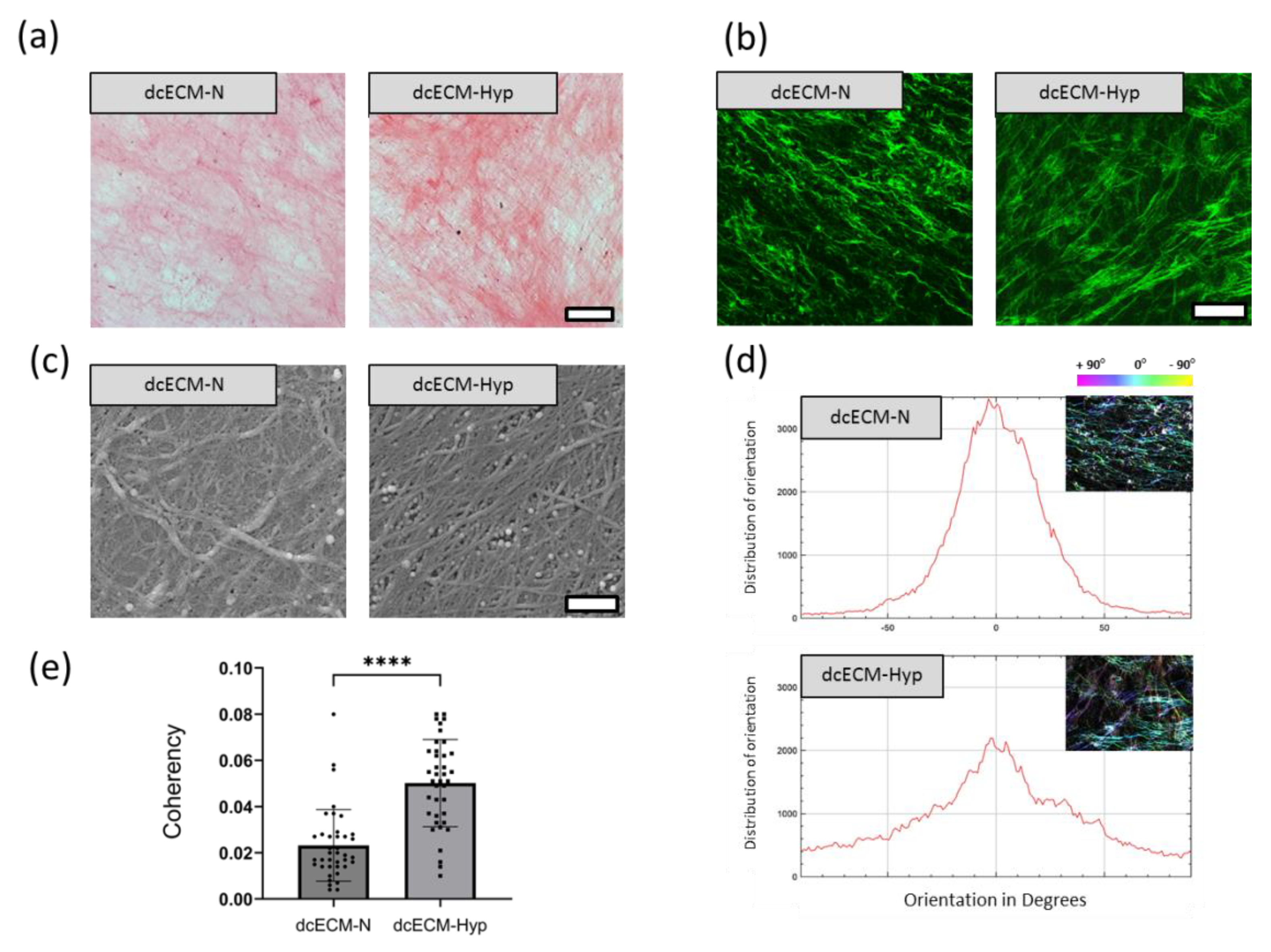
3.1. Decellularized Extracellular Matrices
Morphology of Decellularized Matrices
3.2. Recellularization of Decellularized Matrices
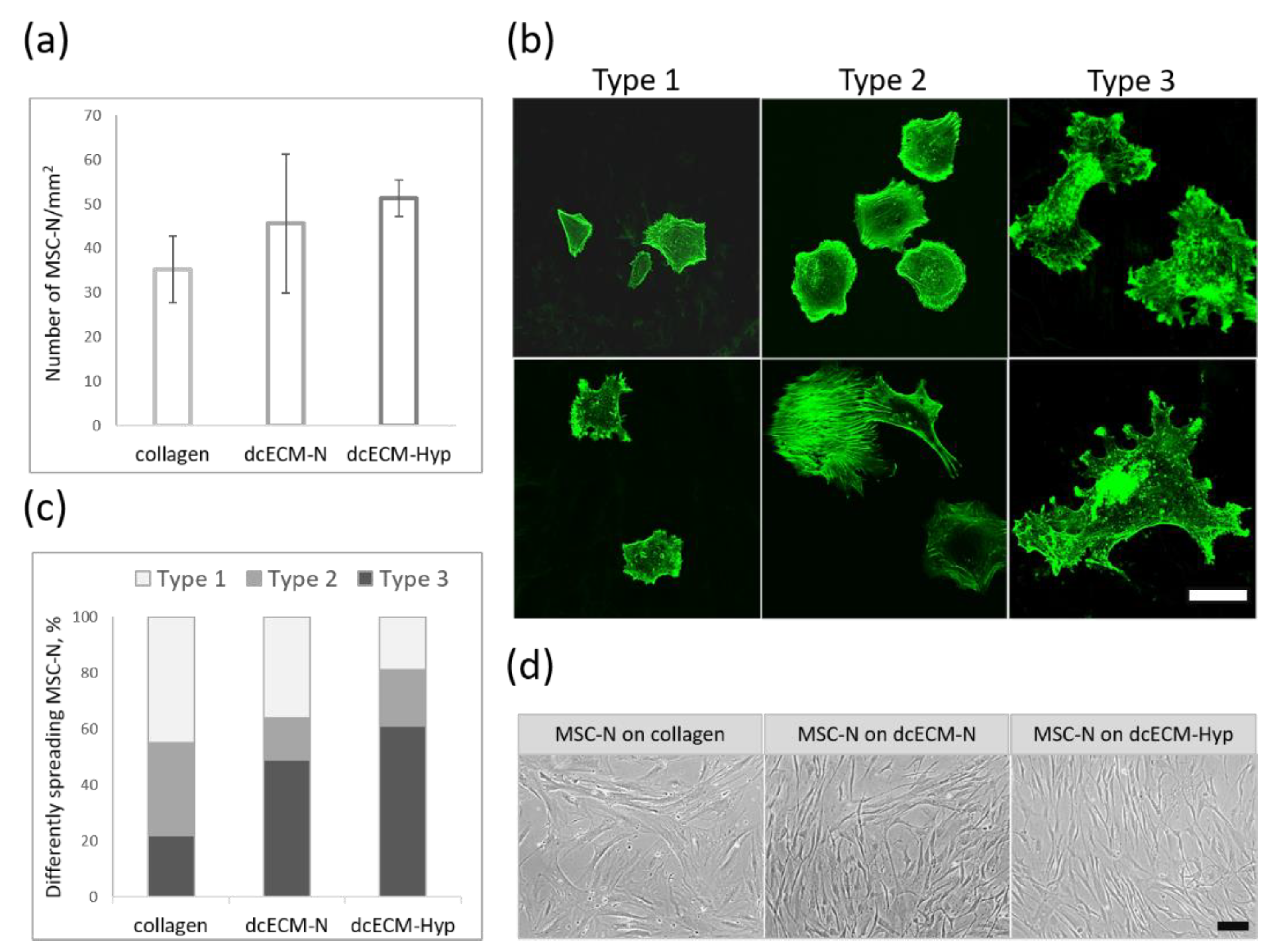
3.2.1. Attachment and Spreading of MSCs on Decellularized Matrices
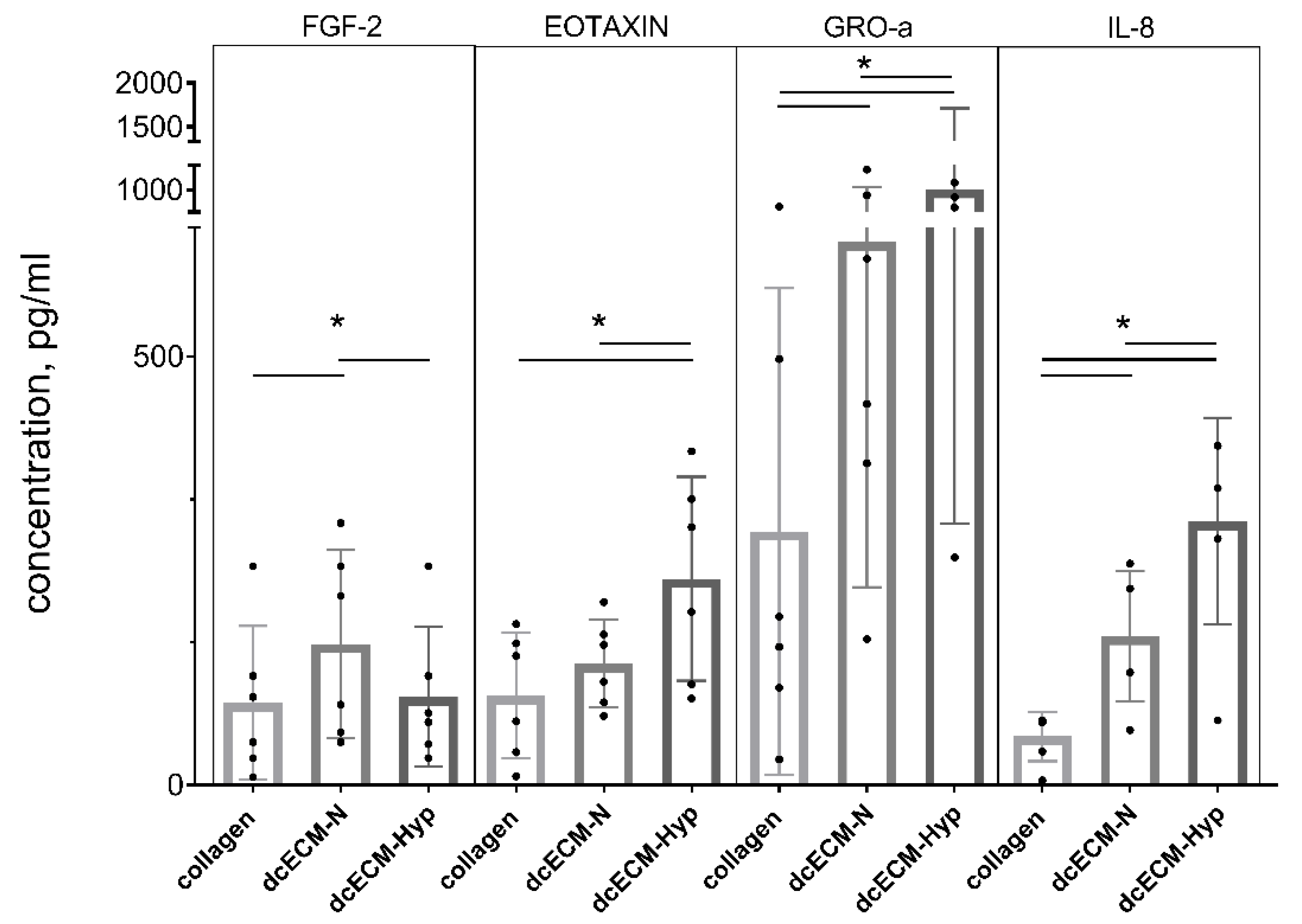
3.2.2. Paracrine Activity of MSCs on Decellularized Matrices
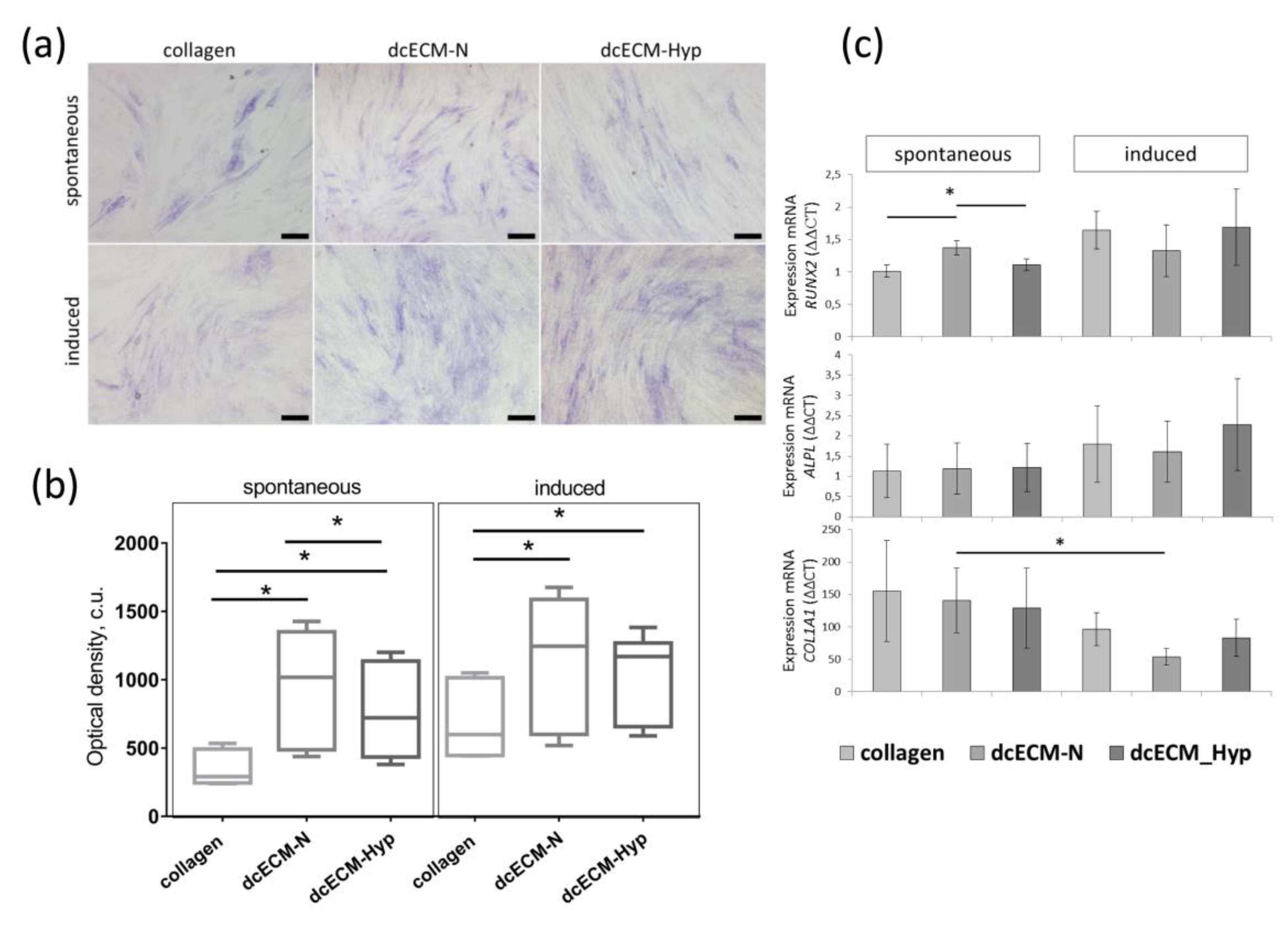
3.2.3. The Effect of Decellularized Matrices on the Osteogenic Potential of MSCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal Stem Cell Perspective: Cell Biology to Clinical Progress. Npj Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Costela-Ruiz, V.J.; Melguizo-Rodríguez, L.; Bellotti, C.; Illescas-Montes, R.; Stanco, D.; Arciola, C.R.; Lucarelli, E. Different Sources of Mesenchymal Stem Cells for Tissue Regeneration: A Guide to Identifying the Most Favorable One in Orthopedics and Dentistry Applications. Int. J. Mol. Sci. 2022, 23, 6356. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Fuentes, D.E.; Fernández-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldaña, H.A. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch. Med. Res. 2021, 52, 93–101. [Google Scholar] [CrossRef]
- Kumar, P.; Satyam, A.; Cigognini, D.; Pandit, A.; Zeugolis, D.I. Low Oxygen Tension and Macromolecular Crowding Accelerate Extracellular Matrix Deposition in Human Corneal Fibroblast Culture: Accelerated Extracellular Matrix Deposition in Human Corneal Fibroblast Culture. J. Tissue Eng. Regen. Med. 2018, 12, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Tokhanbigli, S.; Baghaei, K.; Asadirad, A.; Hashemi, S.M.; Asadzadeh-Aghdaei, H.; Zali, M.R. Immunoregulatory Impact of Human Mesenchymal-Conditioned Media and Mesenchymal Derived Exosomes on Monocytes. Mol. Biol. Res. Commun. 2019, 8, 1397. [Google Scholar] [CrossRef]
- Asgari Taei, A.; Nasoohi, S.; Hassanzadeh, G.; Kadivar, M.; Dargahi, L.; Farahmandfar, M. Enhancement of Angiogenesis and Neurogenesis by Intracerebroventricular Injection of Secretome from Human Embryonic Stem Cell-derived Mesenchymal Stem Cells in Ischemic Stroke Model. Biomed. Pharmacother. 2021, 140, 111709. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. The MSC: An Injury Drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular Matrix: A Dynamic Microenvironment for Stem Cell Niche. Biochim. Biophys. Acta BBA Gen. Subj. 2014, 1840, 2506–2519. [Google Scholar] [CrossRef]
- De Castro Brás, L.E.; Frangogiannis, N.G. Extracellular Matrix-Derived Peptides in Tissue Remodeling and Fibrosis. Matrix Biol. 2020, 91–92, 176–187. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A Guide to the Composition and Functions of the Extracellular Matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Wells, J.M.; Gaggar, A.; Blalock, J.E. MMP Generated Matrikines. Matrix Biol. 2015, 44–46, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Brassart-Pasco, S.; Brézillon, S.; Brassart, B.; Ramont, L.; Oudart, J.-B.; Monboisse, J.C. Tumor Microenvironment: Extracellular Matrix Alterations Influence Tumor Progression. Front. Oncol. 2020, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S.; Vallet, S.D. Fragments Generated upon Extracellular Matrix Remodeling: Biological Regulators and Potential Drugs. Matrix Biol. 2019, 75–76, 170–189. [Google Scholar] [CrossRef]
- Popov, A.; Kozlovskaya, E.; Rutckova, T.; Styshova, O.; Vakhrushev, A.; Kupera, E.; Tekutyeva, L. Antitumor Properties of Matrikines of Different Origins: Prospects and Problems of Their Application. Int. J. Mol. Sci. 2023, 24, 9502. [Google Scholar] [CrossRef]
- Pourjafar, M.; Saidijam, M.; Mansouri, K.; Ghasemibasir, H.; Karimi Dermani, F.; Najafi, R. All-Trans Retinoic Acid Preconditioning Enhances Proliferation, Angiogenesis and Migration of Mesenchymal Stem Cell in Vitro and Enhances Wound Repair In Vivo. Cell Prolif. 2017, 50, e12315. [Google Scholar] [CrossRef] [PubMed]
- Pei, M. Environmental Preconditioning Rejuvenates Adult Stem Cells’ Proliferation and Chondrogenic Potential. Biomaterials 2017, 117, 10–23. [Google Scholar] [CrossRef]
- Noronha, N.D.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming Approaches to Improve the Efficacy of Mesenchymal Stromal Cell-Based Therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef]
- Hao, D.; He, C.; Ma, B.; Lankford, L.; Reynaga, L.; Farmer, D.L.; Guo, F.; Wang, A. Hypoxic Preconditioning Enhances Survival and Proangiogenic Capacity of Human First Trimester Chorionic Villus-Derived Mesenchymal Stem Cells for Fetal Tissue Engineering. Stem Cells Int. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Ivanovic, Z. Hypoxia or in Situ Normoxia: The Stem Cell Paradigm. J. Cell. Physiol. 2009, 219, 271–275. [Google Scholar] [CrossRef]
- Carreau, A.; Hafny-Rahbi, B.E.; Matejuk, A.; Grillon, C.; Kieda, C. Why Is the Partial Oxygen Pressure of Human Tissues a Crucial Parameter? Small Molecules and Hypoxia. J. Cell. Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef]
- Ivanovic, Z.; Vlaski-Lafarge, M. Anaerobiosis and Stemness: An Evolutionary Paradigm; Elsevier/Academic Press: Amsterdam, The Netherlands, 2016; ISBN 978-0-12-800540-8. [Google Scholar]
- Chow, D.C.; Wenning, L.A.; Miller, W.M.; Papoutsakis, E.T. Modeling pO2 Distributions in the Bone Marrow Hematopoietic Compartment. I. Krogh’s Model. Biophys. J. 2001, 81, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Wu, J.; Runnels, J.M.; Zaher, W.; Mortensen, L.J.; Alt, C.; Turcotte, R.; et al. Direct Measurement of Local Oxygen Concentration in the Bone Marrow of Live Animals. Nature 2014, 508, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Pasarica, M.; Sereda, O.R.; Redman, L.M.; Albarado, D.C.; Hymel, D.T.; Roan, L.E.; Rood, J.C.; Burk, D.H.; Smith, S.R. Reduced Adipose Tissue Oxygenation in Human Obesity. Diabetes 2009, 58, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Buravkova, L.B.; Andreeva, E.R.; Gogvadze, V.; Zhivotovsky, B. Mesenchymal Stem Cells and Hypoxia: Where Are We? Mitochondrion 2014, 19, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Pavlacky, J.; Polak, J. Technical Feasibility and Physiological Relevance of Hypoxic Cell Culture Models. Front. Endocrinol. 2020, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, E.R.; Udartseva, O.O.; Zhidkova, O.V.; Buravkov, S.V.; Ezdakova, M.I.; Buravkova, L.B. IFN-gamma Priming of Adipose-derived Stromal Cells at “Physiological” Hypoxia. J. Cell. Physiol. 2018, 233, 1535–1547. [Google Scholar] [CrossRef]
- Buravkova, L.B.; Rylova, Y.V.; Andreeva, E.R.; Kulikov, A.V.; Pogodina, M.V.; Zhivotovsky, B.; Gogvadze, V. Low ATP Level Is Sufficient to Maintain the Uncommitted State of Multipotent Mesenchymal Stem Cells. Biochim. Biophys. Acta BBA Gen. Subj. 2013, 1830, 4418–4425. [Google Scholar] [CrossRef]
- Matveeva, D.K.; Andreeva, E.R.; Novikov, N.N.; Pustovoy, V.I.; Buravkova, L.B. Structural Organization and Composition of Extracellular Matrix of Multipotent Mesenchymal Stromal Cells under Different Oxygen Levels in Vitro. Clin. Exp. Morphol. 2020, 9, 57–63. [Google Scholar] [CrossRef]
- Corrado, C.; Fontana, S. Hypoxia and HIF Signaling: One Axis with Divergent Effects. Int. J. Mol. Sci. 2020, 21, 5611. [Google Scholar] [CrossRef]
- Dekker, Y.; Le Dévédec, S.E.; Danen, E.H.J.; Liu, Q. Crosstalk between Hypoxia and Extracellular Matrix in the Tumor Microenvironment in Breast Cancer. Genes. 2022, 13, 1585. [Google Scholar] [CrossRef]
- Petrova, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Amelio, I. The Hypoxic Tumour Microenvironment. Oncogenesis 2018, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, D.K.; Andreeva, E.R.; Buravkova, L.B. Selection of the Optimal Protocol for Preparation of a Decellularized Extracellular Matrix of Human Adipose Tissue-Derived Mesenchymal Stromal Cells. Mosc. Univ. Biol. Sci. Bull. 2019, 74, 235–239. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Chun, S.Y.; Ha, Y.-S.; Kim, D.H.; Kim, J.; Song, P.H.; Kim, H.T.; Yoo, E.S.; Kim, B.S.; Kwon, T.G. Hypoxia Enhances Cell Properties of Human Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2017, 14, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Cox, T.R.; Erler, J.T. The Rationale for Targeting the LOX Family in Cancer. Nat. Rev. Cancer 2012, 12, 540–552. [Google Scholar] [CrossRef]
- Cox, T.R.; Bird, D.; Baker, A.-M.; Barker, H.E.; Ho, M.W.-Y.; Lang, G.; Erler, J.T. LOX-Mediated Collagen Crosslinking Is Responsible for Fibrosis-Enhanced Metastasis. Cancer Res. 2013, 73, 1721–1732. [Google Scholar] [CrossRef]
- James, D.S.; Brereton, C.J.; Davies, D.E.; Jones, M.G.; Campagnola, P.J. Examining Lysyl Oxidase-like Modulation of Collagen Architecture in 3D Spheroid Models of Idiopathic Pulmonary Fibrosis via Second-Harmonic Generation Microscopy. J. Biomed. Opt. 2021, 26, 066501. [Google Scholar] [CrossRef]
- Hieda, M. Signal Transduction across the Nuclear Envelope: Role of the LINC Complex in Bidirectional Signaling. Cells 2019, 8, 124. [Google Scholar] [CrossRef]
- Muncie, J.M.; Weaver, V.M. The Physical and Biochemical Properties of the Extracellular Matrix Regulate Cell Fate. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 130, pp. 1–37. ISBN 978-0-12-809802-8. [Google Scholar]
- Lin, H.; Yang, G.; Tan, J.; Tuan, R.S. Influence of Decellularized Matrix Derived from Human Mesenchymal Stem Cells on Their Proliferation, Migration and Multi-Lineage Differentiation Potential. Biomaterials 2012, 33, 4480–4489. [Google Scholar] [CrossRef]
- Aleksandrova, S.A.; Pinaev, G.P. Reorganization of actin cytoskeleton in the initial stage of transendothelial migration of bone marrow multipotent mesenchymal stromal cells. Biofizika 2014, 59, 913–918. [Google Scholar] [PubMed]
- Sakhenberg, E.I.; Nikolaenko, N.S.; Pinaev, G.P. Actin cytoskeleton organization and spreading of bone marrow stromal cells and cartilage cells during their combined and independent cultivation on different extracellular matrix proteins. Tsitologiia 2014, 56, 708–716. [Google Scholar]
- Wang, Z.; Guo, Y.; Zhang, P. A Rapid Quantitation of Cell Attachment and Spreading Based on Digital Image Analysis: Application for Cell Affinity and Compatibility Assessment of Synthetic Polymers. Mater. Sci. Eng. C 2021, 128, 112267. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.; Redmann, A.-L.; Terentjev, E.M. Universal Kinetics of the Onset of Cell Spreading on Substrates of Different Stiffness. Biophys. J. 2019, 116, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 763. [Google Scholar] [CrossRef]
- Peng, K.-Y.; Liu, Y.-H.; Li, Y.-W.; Yen, B.L.; Yen, M.-L. Extracellular Matrix Protein Laminin Enhances Mesenchymal Stem Cell (MSC) Paracrine Function through Avβ3/CD61 Integrin to Reduce Cardiomyocyte Apoptosis. J. Cell. Mol. Med. 2017, 21, 1572–1583. [Google Scholar] [CrossRef]
- Prewitz, M.C.; Seib, F.P.; Von Bonin, M.; Friedrichs, J.; Stißel, A.; Niehage, C.; Müller, K.; Anastassiadis, K.; Waskow, C.; Hoflack, B.; et al. Tightly Anchored Tissue-Mimetic Matrices as Instructive Stem Cell Microenvironments. Nat. Methods 2013, 10, 788–794. [Google Scholar] [CrossRef]
- Deng, M.; Tan, J.; Dai, Q.; Luo, F.; Xu, J. Macrophage-Mediated Bone Formation in Scaffolds Modified With MSC-Derived Extracellular Matrix Is Dependent on the Migration Inhibitory Factor Signaling Pathway. Front. Cell Dev. Biol. 2021, 9, 714011. [Google Scholar] [CrossRef]
- Cuesta-Gomez, N.; Graham, G.J.; Campbell, J.D.M. Chemokines and Their Receptors: Predictors of the Therapeutic Potential of Mesenchymal Stromal Cells. J. Transl. Med. 2021, 19, 156. [Google Scholar] [CrossRef]
- Hagmann, S.; Moradi, B.; Frank, S.; Dreher, T.; Kämmerer, P.W.; Richter, W.; Gotterbarm, T. FGF -2 Addition during Expansion of Human Bone Marrow-derived Stromal Cells Alters MSC Surface Marker Distribution and Chondrogenic Differentiation Potential. Cell Prolif. 2013, 46, 396–407. [Google Scholar] [CrossRef]
- Ma, Y.; Kakudo, N.; Morimoto, N.; Lai, F.; Taketani, S.; Kusumoto, K. Fibroblast Growth Factor-2 Stimulates Proliferation of Human Adipose-Derived Stem Cells via Src Activation. Stem Cell Res. Ther. 2019, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ma, T. Autocrine Fibroblast Growth Factor 2-Mediated Interactions between Human Mesenchymal Stem Cells and the Extracellular Matrix under Varying Oxygen Tension. J. Cell. Biochem. 2013, 114, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhyan, R.K.; Gerasimov, U.V. Bone Marrow Osteogenic Stem Cells: In Vitro Cultivation and Transplantation in Diffusion Chambers. Cell Prolif. 1987, 20, 263–272. [Google Scholar] [CrossRef]
- Zhang, P.; Ha, N.; Dai, Q.; Zhou, S.; Yu, C.; Jiang, L. Hypoxia Suppresses Osteogenesis of Bone Mesenchymal Stem Cells via the Extracellular Signal-Regulated 1/2 and P38-Mitogen Activated Protein Kinase Signaling Pathways. Mol. Med. Rep. 2017, 16, 5515–5522. [Google Scholar] [CrossRef] [PubMed]
- Nowwarote, N.; Petit, S.; Ferre, F.C.; Dingli, F.; Laigle, V.; Loew, D.; Osathanon, T.; Fournier, B.P.J. Extracellular Matrix Derived From Dental Pulp Stem Cells Promotes Mineralization. Front. Bioeng. Biotechnol. 2022, 9, 740712. [Google Scholar] [CrossRef]
- Sart, S.; Jeske, R.; Chen, X.; Ma, T.; Li, Y. Engineering Stem Cell-Derived Extracellular Matrices: Decellularization, Characterization, and Biological Function. Tissue Eng. Part. B Rev. 2020, 26, 402–422. [Google Scholar] [CrossRef]
- Van, S.Y.; Noh, Y.K.; Kim, S.W.; Oh, Y.M.; Kim, I.H.; Park, K. Human Umbilical Cord Blood Mesenchymal Stem Cells Expansion via Human Fibroblast-Derived Matrix and Their Potentials toward Regenerative Application. Cell Tissue Res. 2019, 376, 233–245. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, L.; Chen, X.; Liu, T.; Pan, G.; Cui, W.; Li, M.; Luo, Z.-P.; Pei, M.; Yang, H.; et al. Culturing on Decellularized Extracellular Matrix Enhances Antioxidant Properties of Human Umbilical Cord-Derived Mesenchymal Stem Cells. Mater. Sci. Eng. C 2016, 61, 437–448. [Google Scholar] [CrossRef]
- Xiong, Y.; He, J.; Zhang, W.; Zhou, G.; Cao, Y.; Liu, W. Retention of the Stemness of Mouse Adipose-Derived Stem Cells by Their Expansion on Human Bone Marrow Stromal Cell-Derived Extracellular Matrix. Tissue Eng. Part. A 2015, 21, 1886–1894. [Google Scholar] [CrossRef]
- Selvaraj, S.; Rupert, S.; Nandabalan, S.K.; Anbalagan, C.; Rajaram, P.S.; Satyanesan, J.; Vennila, R.; Rajagopal, S. Effect of Cell Derived Matrices on Growth and Differentiation of Human Wharton’s Jelly Derived Mesenchymal Stem Cells. Cells Tissues Organs, 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Mathews, S.; Bhonde, R.; Gupta, P.K.; Totey, S. Extracellular Matrix Protein Mediated Regulation of the Osteoblast Differentiation of Bone Marrow Derived Human Mesenchymal Stem Cells. Differentiation 2012, 84, 185–192. [Google Scholar] [CrossRef]
- Dos Santos, F.; Andrade, P.Z.; Boura, J.S.; Abecasis, M.M.; Da Silva, C.L.; Cabral, J.M.S. Ex Vivo Expansion of Human Mesenchymal Stem Cells: A More Effective Cell Proliferation Kinetics and Metabolism under Hypoxia. J. Cell. Physiol. 2009, 223, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Marinkovic, M.; Tran, O.N.; Block, T.J.; Rakian, R.; Gonzalez, A.O.; Dean, D.D.; Yeh, C.-K.; Chen, X.-D. Native Extracellular Matrix, Synthesized Ex Vivo by Bone Marrow or Adipose Stromal Cells, Faithfully Directs Mesenchymal Stem Cell Differentiation. Matrix Biol. Plus 2020, 8, 100044. [Google Scholar] [CrossRef] [PubMed]
- Rao Pattabhi, S.; Martinez, J.S.; Keller, T.C.S. Decellularized ECM Effects on Human Mesenchymal Stem Cell Stemness and Differentiation. Differentiation 2014, 88, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Volkmer, E.; Kallukalam, B.C.; Maertz, J.; Otto, S.; Drosse, I.; Polzer, H.; Bocker, W.; Stengele, M.; Docheva, D.; Mutschler, W.; et al. Hypoxic Preconditioning of Human Mesenchymal Stem Cells Overcomes Hypoxia-Induced Inhibition of Osteogenic Differentiation. Tissue Eng. Part A 2010, 16, 153–164. [Google Scholar] [CrossRef]
- Yang, D.-C.; Yang, M.-H.; Tsai, C.-C.; Huang, T.-F.; Chen, Y.-H.; Hung, S.-C. Hypoxia Inhibits Osteogenesis in Human Mesenchymal Stem Cells through Direct Regulation of RUNX2 by TWIST. PLoS ONE 2011, 6, e23965. [Google Scholar] [CrossRef]
- Kindstedt, E.; Holm, C.K.; Sulniute, R.; Martinez-Carrasco, I.; Lundmark, R.; Lundberg, P. CCL11, a Novel Mediator of Inflammatory Bone Resorption. Sci. Rep. 2017, 7, 5334. [Google Scholar] [CrossRef]
- Mussano, F.; Genova, T.; Petrillo, S.; Roato, I.; Ferracini, R.; Munaron, L. Osteogenic Differentiation Modulates the Cytokine, Chemokine, and Growth Factor Profile of ASCs and SHED. Int. J. Mol. Sci. 2018, 19, 1454. [Google Scholar] [CrossRef]
- Park, M.S.; Kim, Y.H.; Jung, Y.; Kim, S.H.; Park, J.C.; Yoon, D.S.; Kim, S.-H.; Lee, J.W. In Situ Recruitment of Human Bone Marrow-Derived Mesenchymal Stem Cells Using Chemokines for Articular Cartilage Regeneration. Cell Transplant. 2015, 24, 1067–1083. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matveeva, D.; Buravkov, S.; Andreeva, E.; Buravkova, L. Hypoxic Extracellular Matrix Preserves Its Competence after Expansion of Human MSCs under Physiological Hypoxia In Vitro. Biomimetics 2023, 8, 476. https://doi.org/10.3390/biomimetics8060476
Matveeva D, Buravkov S, Andreeva E, Buravkova L. Hypoxic Extracellular Matrix Preserves Its Competence after Expansion of Human MSCs under Physiological Hypoxia In Vitro. Biomimetics. 2023; 8(6):476. https://doi.org/10.3390/biomimetics8060476
Chicago/Turabian StyleMatveeva, Diana, Sergey Buravkov, Elena Andreeva, and Ludmila Buravkova. 2023. "Hypoxic Extracellular Matrix Preserves Its Competence after Expansion of Human MSCs under Physiological Hypoxia In Vitro" Biomimetics 8, no. 6: 476. https://doi.org/10.3390/biomimetics8060476
APA StyleMatveeva, D., Buravkov, S., Andreeva, E., & Buravkova, L. (2023). Hypoxic Extracellular Matrix Preserves Its Competence after Expansion of Human MSCs under Physiological Hypoxia In Vitro. Biomimetics, 8(6), 476. https://doi.org/10.3390/biomimetics8060476





