Kinematic Behavior of an Untethered, Small-Scale Hydrogel-Based Soft Robot in Response to Magneto-Thermal Stimuli
Abstract
:1. Introduction
2. Materials and Methods
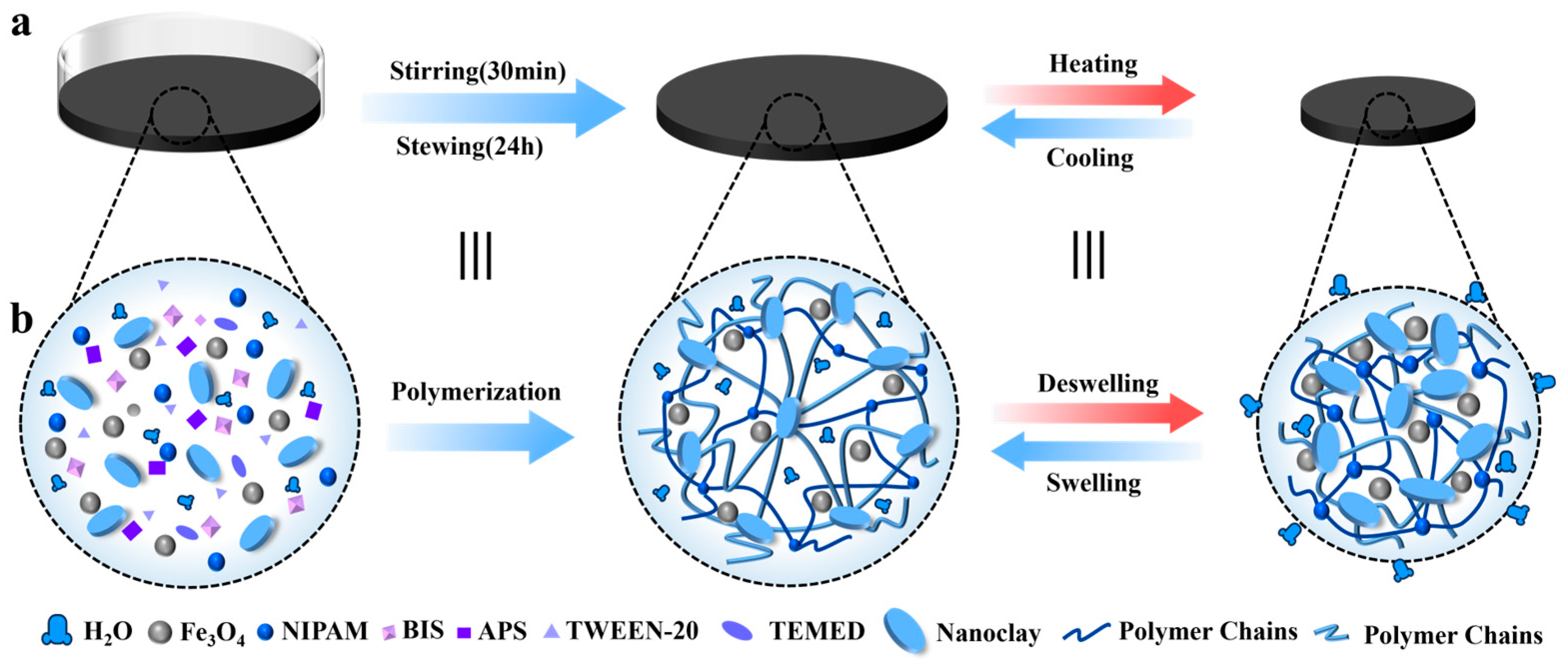
2.1. Preparation of the Magnetic, Temperature-Sensitive Hydrogel
2.2. Hydrogel-Based Soft Robot Design
2.3. Magnetic Application and Motion Platform
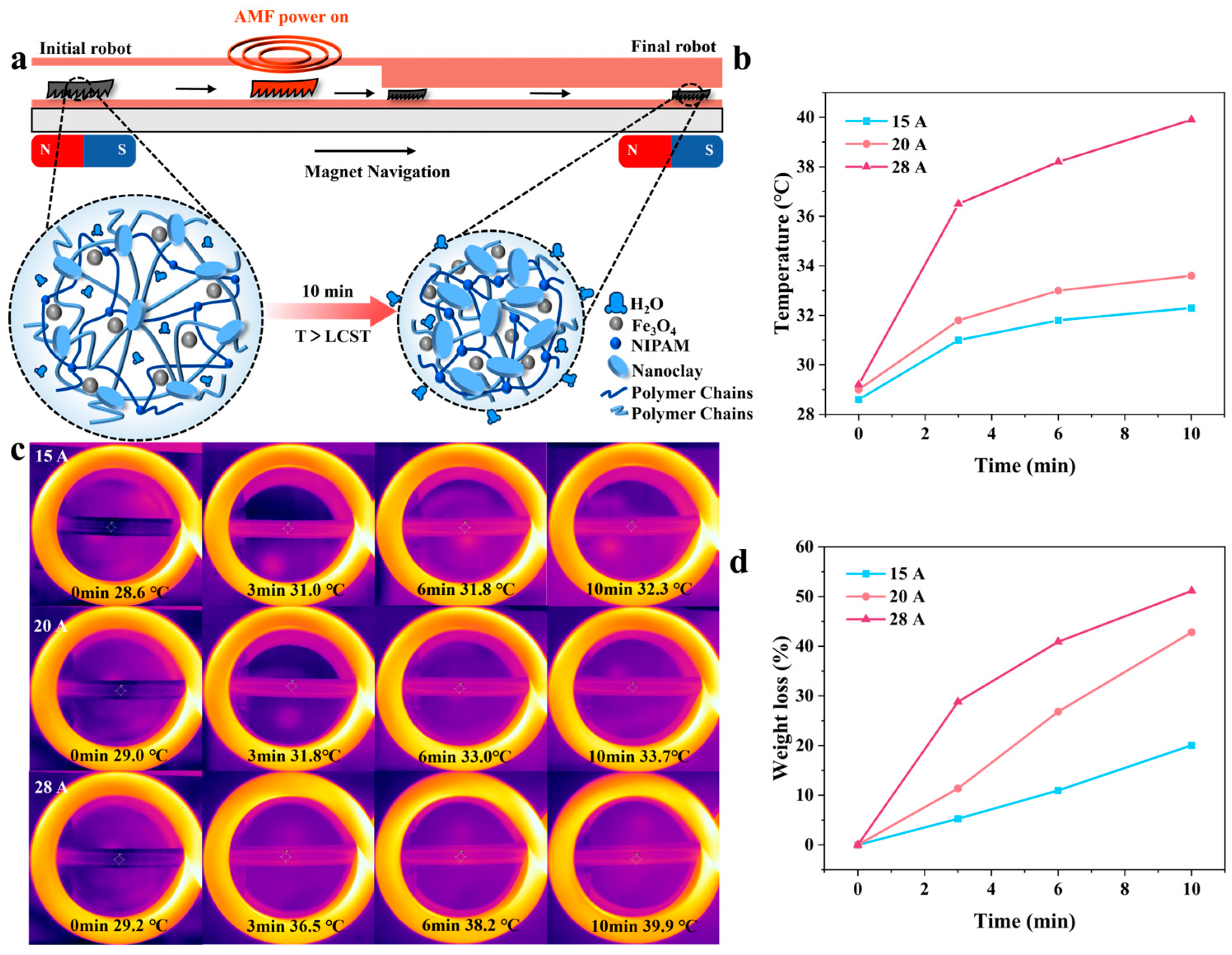
3. Results and Discussion
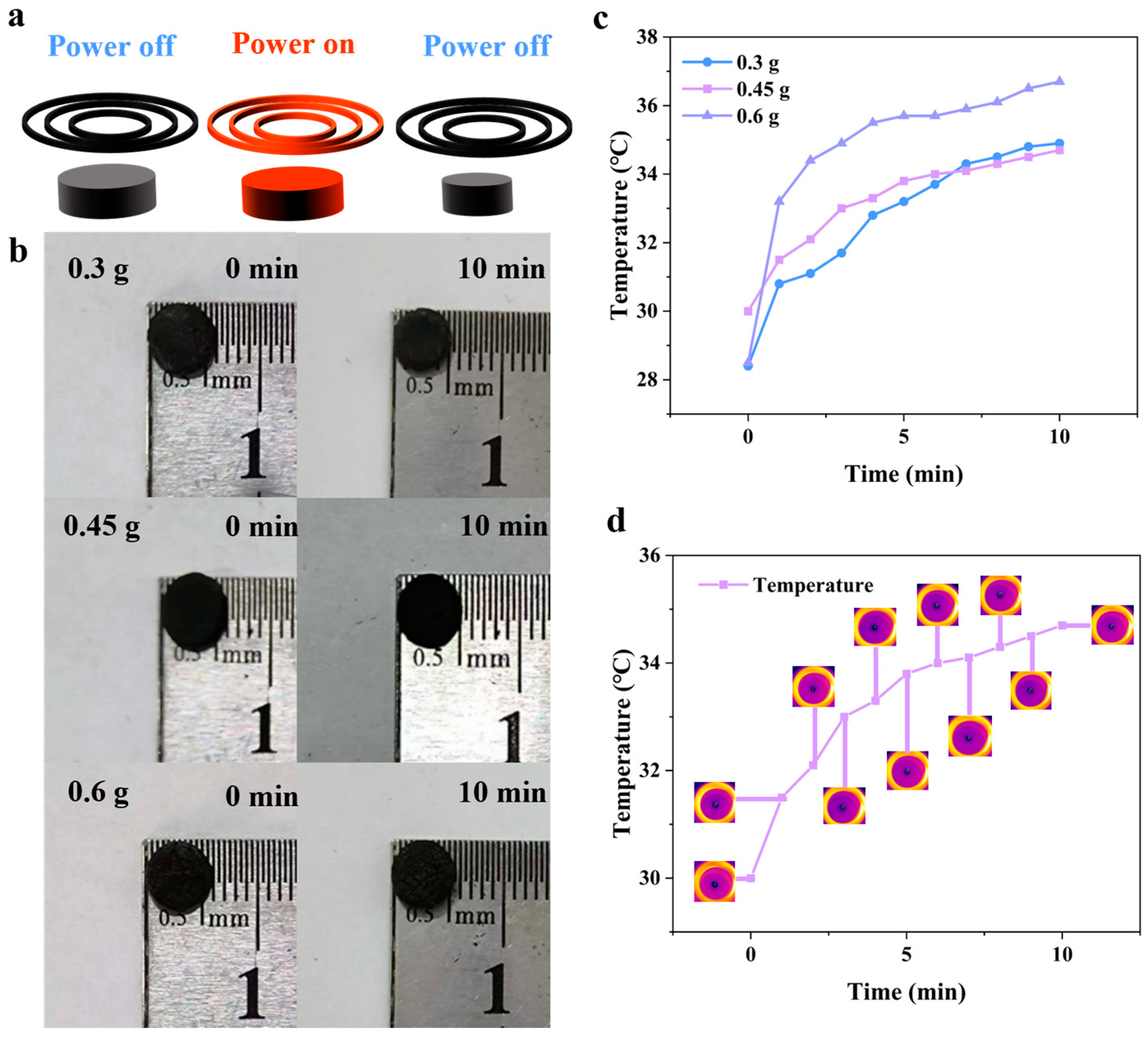
3.1. Magnetic, Temperature-Sensitive Hydrogel Gel Mechanism
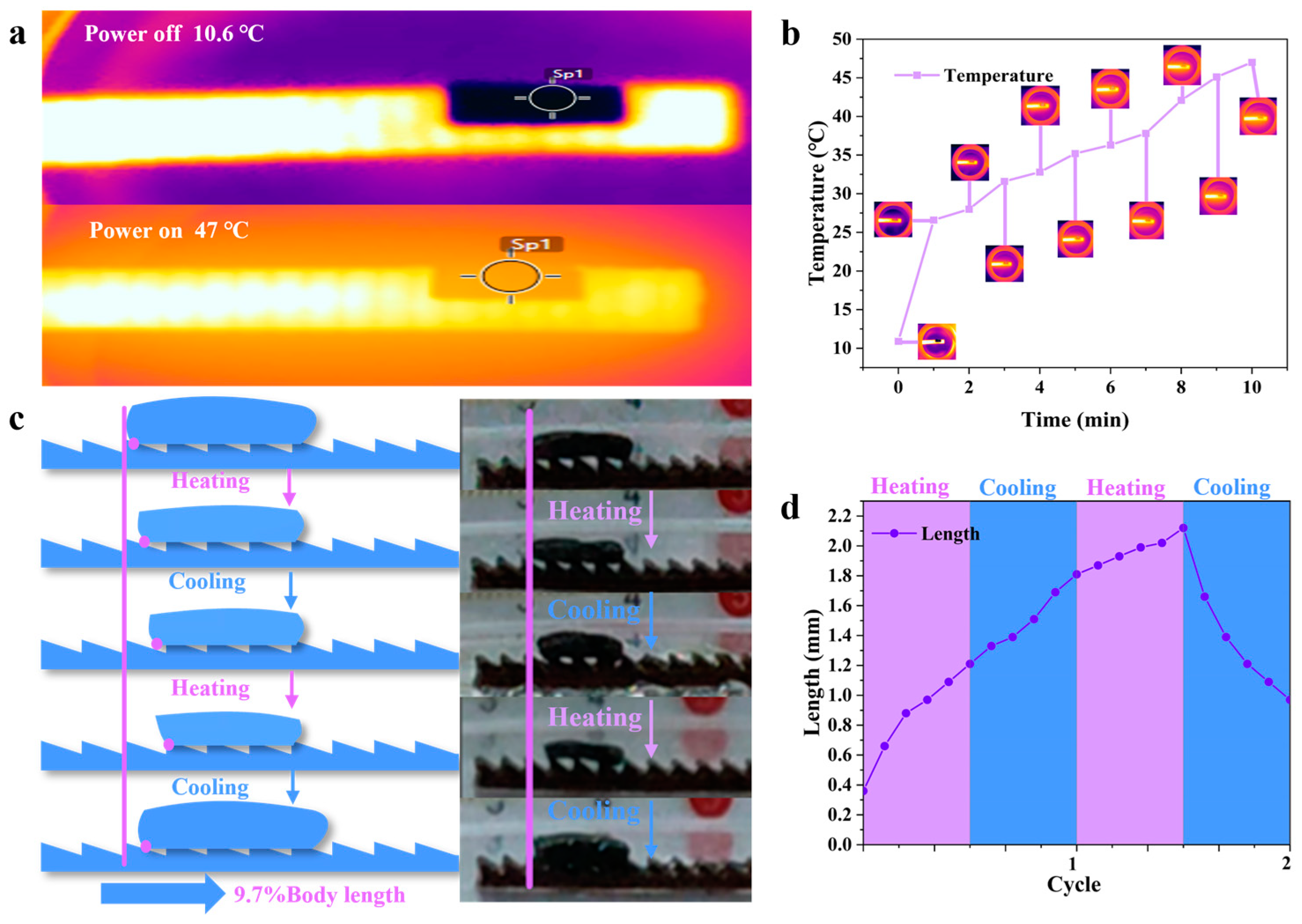
3.2. Hydrogel-Based Soft Robots without a Gastropod Microstructure
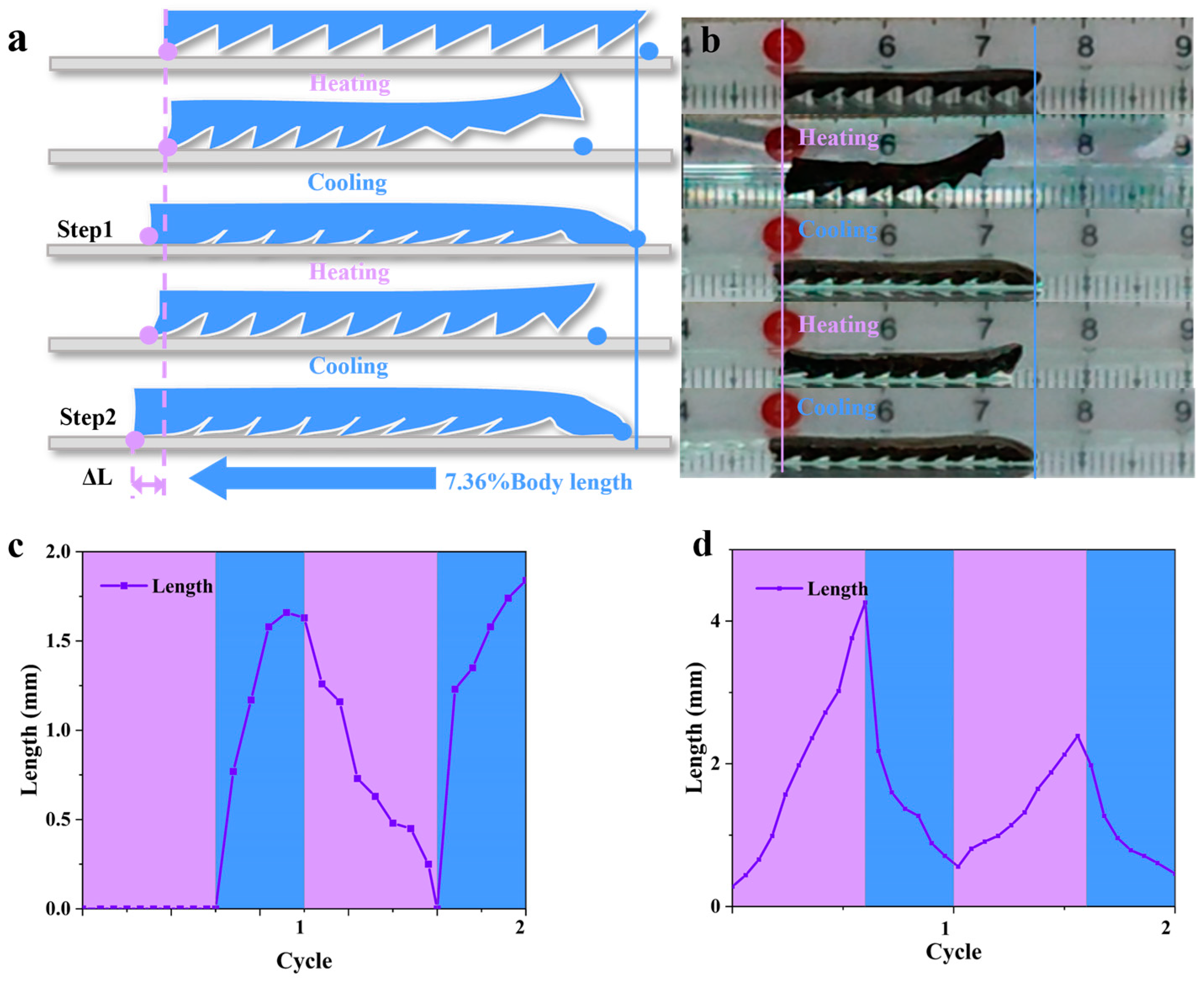
3.3. Hydrogel-Based Soft Robots with a Gastropod Microstructure
3.4. Magnetic Navigation and Water-Soluble-Drug Release Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, W.; Lum, G.Z.; Mastrangeli, M.; Sitti, M. Small-Scale Soft-Bodied Robot with Multimodal Locomotion. Nature 2018, 554, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, H.; Zhang, Z.; Yu, P.; Yang, L.; Du, J.; Niu, Y.; Jiang, J. Review of Bionic Crawling Micro-Robots. J. Intell. Robot. Syst. 2022, 105, 56. [Google Scholar] [CrossRef]
- Yeh, C.-Y.; Chou, S.-C.; Huang, H.-W.; Yu, H.-C.; Juang, J.-Y. Tube-Crawling Soft Robots Driven by Multistable Buckling Mechanics. Extrem. Mech. Lett. 2019, 26, 61–68. [Google Scholar] [CrossRef]
- Du, Z.; Fang, H.; Xu, J. Snake-Worm: A Bi-Modal Locomotion Robot. J. Bionic Eng. 2022, 19, 1272–1287. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Su, R. Design and Modeling of a Driving System for Soft Massage Robot. Mechatronics 2021, 79, 102640. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, Z.; Wang, X.; Cheng, G.; Zhang, Z.; Ding, J. Pneumatically Actuated Self-Healing Bionic Crawling Soft Robot. J. Intell. Robot. Syst. 2020, 100, 445–454. [Google Scholar] [CrossRef]
- Yu, M.; Liu, W.; Zhao, J.; Hou, Y.; Hong, X.; Zhang, H. Modeling and Analysis of a Composite Structure-Based Soft Pneumatic Actuators for Soft-Robotic Gripper. Sensors 2022, 22, 4851. [Google Scholar] [CrossRef]
- Xu, L.; Wagner, R.J.; Liu, S.; He, Q.; Li, T.; Pan, W.; Feng, Y.; Feng, H.; Meng, Q.; Zou, X.; et al. Locomotion of an Untethered, Worm-Inspired Soft Robot Driven by a Shape-Memory Alloy Skeleton. Sci. Rep. 2022, 12, 12392. [Google Scholar] [CrossRef]
- Ruth, D.J.S.; Sohn, J.-W.; Dhanalakshmi, K.; Choi, S.-B. Control Aspects of Shape Memory Alloys in Robotics Applications: A Review over the Last Decade. Sensors 2022, 22, 4860. [Google Scholar] [CrossRef]
- Pan, J.; Shi, Z.; Wang, T. Variable-Model SMA-Driven Spherical Robot. Sci. China Technol. Sci. 2019, 62, 1401–1411. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Z. Hydrogel and Machine Learning for Soft Robots’ Sensing and Signal Processing: A Review. J. Bionic Eng. 2023, 20, 845–857. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, L.; Liu, A.; He, S.; Shao, W. Ultrafast Thermo-Responsive Bilayer Hydrogel Actuator Assisted by Hydrogel Microspheres. Sens. Actuators B Chem. 2022, 357, 131434. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Chen, L.; Zhang, C.; Liao, L. Multi-Responsive Hydrogel Actuator with Photo-Switchable Color Changing Behaviors. Dye. Pigment. 2020, 174, 108042. [Google Scholar] [CrossRef]
- Jiao, D.; Zhu, Q.L.; Li, C.Y.; Zheng, Q.; Wu, Z.L. Programmable Morphing Hydrogels for Soft Actuators and Robots: From Structure Designs to Active Functions. Acc. Chem. Res. 2022, 55, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Song, W.J.; Sun, J.-Y. Hydrogel Soft Robotics. Mater. Today Phys. 2020, 15, 100258. [Google Scholar] [CrossRef]
- Gao, D.; Li, G.; Qu, X.; Li, X.; Xu, X.; Li, Y.; Zhou, Z. Thermo-Responsive Hydrogel with Tunable Transition Temperature for Effective Drug Release. Mater. Lett. 2022, 321, 132367. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, R.; Yuan, M.; Huang, X.; Ding, C.; Wu, H.; Wang, S.; Liu, A. Magnetically Driven PH-Responsive Composite Hydrogel for Controlled Drug Delivery. Funct. Mater. Lett. 2022, 15, 2250022. [Google Scholar] [CrossRef]
- Francis, W.; Dunne, A.; Delaney, C.; Florea, L.; Diamond, D. Spiropyran Based Hydrogels Actuators—Walking in the Light. Sens. Actuators B Chem. 2017, 250, 608–616. [Google Scholar] [CrossRef]
- Shen, T.; Font, M.G.; Jung, S.; Gabriel, M.L.; Stoykovich, M.P.; Vernerey, F.J. Remotely Triggered Locomotion of Hydrogel Mag-Bots in Confined Spaces. Sci. Rep. 2017, 7, 16178. [Google Scholar] [CrossRef]
- Tang, J.; Yin, Q.; Qiao, Y.; Wang, T. Shape Morphing of Hydrogels in Alternating Magnetic Field. ACS Appl. Mater. Interfaces 2019, 11, 21194–21200. [Google Scholar] [CrossRef]
- Kuo, J.-C.; Huang, H.-W.; Tung, S.-W.; Yang, Y.-J. A Hydrogel-Based Intravascular Microgripper Manipulated Using Magnetic Fields. Sens. Actuators A Phys. 2014, 211, 121–130. [Google Scholar] [CrossRef]
- Tang, J.; Yin, Q.; Shi, M.; Yang, M.; Yang, H.; Sun, B.; Guo, B.; Wang, T. Programmable Shape Transformation of 3D Printed Magnetic Hydrogel Composite for Hyperthermia Cancer Therapy. Extrem. Mech. Lett. 2021, 46, 101305. [Google Scholar] [CrossRef]
- Cezar, C.A.; Roche, E.T.; Vandenburgh, H.H.; Duda, G.N.; Walsh, C.J.; Mooney, D.J. Biologic-Free Mechanically Induced Muscle Regeneration. Proc. Natl. Acad. Sci. USA 2016, 113, 1534–1539. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Qiao, Y.; Chu, Y.; Tong, Z.; Zhou, Y.; Zhang, W.; Xie, S.; Hu, J.; Wang, T. Magnetic Double-Network Hydrogels for Tissue Hyperthermia and Drug Release. J. Mater. Chem. B 2019, 7, 1311–1321. [Google Scholar] [CrossRef]
- Yan, X.; Sun, T.; Song, Y.; Peng, W.; Xu, Y.; Luo, G.; Li, M.; Chen, S.; Fang, W.-W.; Dong, L.; et al. In Situ Thermal-Responsive Magnetic Hydrogel for Multidisciplinary Therapy of Hepatocellular Carcinoma. Nano Lett. 2022, 22, 2251–2260. [Google Scholar] [CrossRef]
- Xu, L.; Lamont, S.C.; Li, T.; Zhang, Y.; Pan, W.; Gao, C.; Zhu, C.; Chen, S.; Hu, H.; Ding, J.; et al. Nonlinear Viscoelasticity and Toughening Mechanisms in Nanoclay-PNIPAAm Double Network Hydrogels. ACS Macro Lett. 2023, 12, 549–554. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, W.; Gao, C.; Zhu, C.; Yang, Y.; Xu, L. Kinematic Behavior of an Untethered, Small-Scale Hydrogel-Based Soft Robot in Response to Magneto-Thermal Stimuli. Biomimetics 2023, 8, 379. https://doi.org/10.3390/biomimetics8040379
Pan W, Gao C, Zhu C, Yang Y, Xu L. Kinematic Behavior of an Untethered, Small-Scale Hydrogel-Based Soft Robot in Response to Magneto-Thermal Stimuli. Biomimetics. 2023; 8(4):379. https://doi.org/10.3390/biomimetics8040379
Chicago/Turabian StylePan, Wenlong, Chongyi Gao, Chen Zhu, Yabing Yang, and Lin Xu. 2023. "Kinematic Behavior of an Untethered, Small-Scale Hydrogel-Based Soft Robot in Response to Magneto-Thermal Stimuli" Biomimetics 8, no. 4: 379. https://doi.org/10.3390/biomimetics8040379
APA StylePan, W., Gao, C., Zhu, C., Yang, Y., & Xu, L. (2023). Kinematic Behavior of an Untethered, Small-Scale Hydrogel-Based Soft Robot in Response to Magneto-Thermal Stimuli. Biomimetics, 8(4), 379. https://doi.org/10.3390/biomimetics8040379






