Power Benefits of High-Altitude Flapping Wing Flight at the Monarch Butterfly Scale
Abstract
1. Introduction
2. Methods
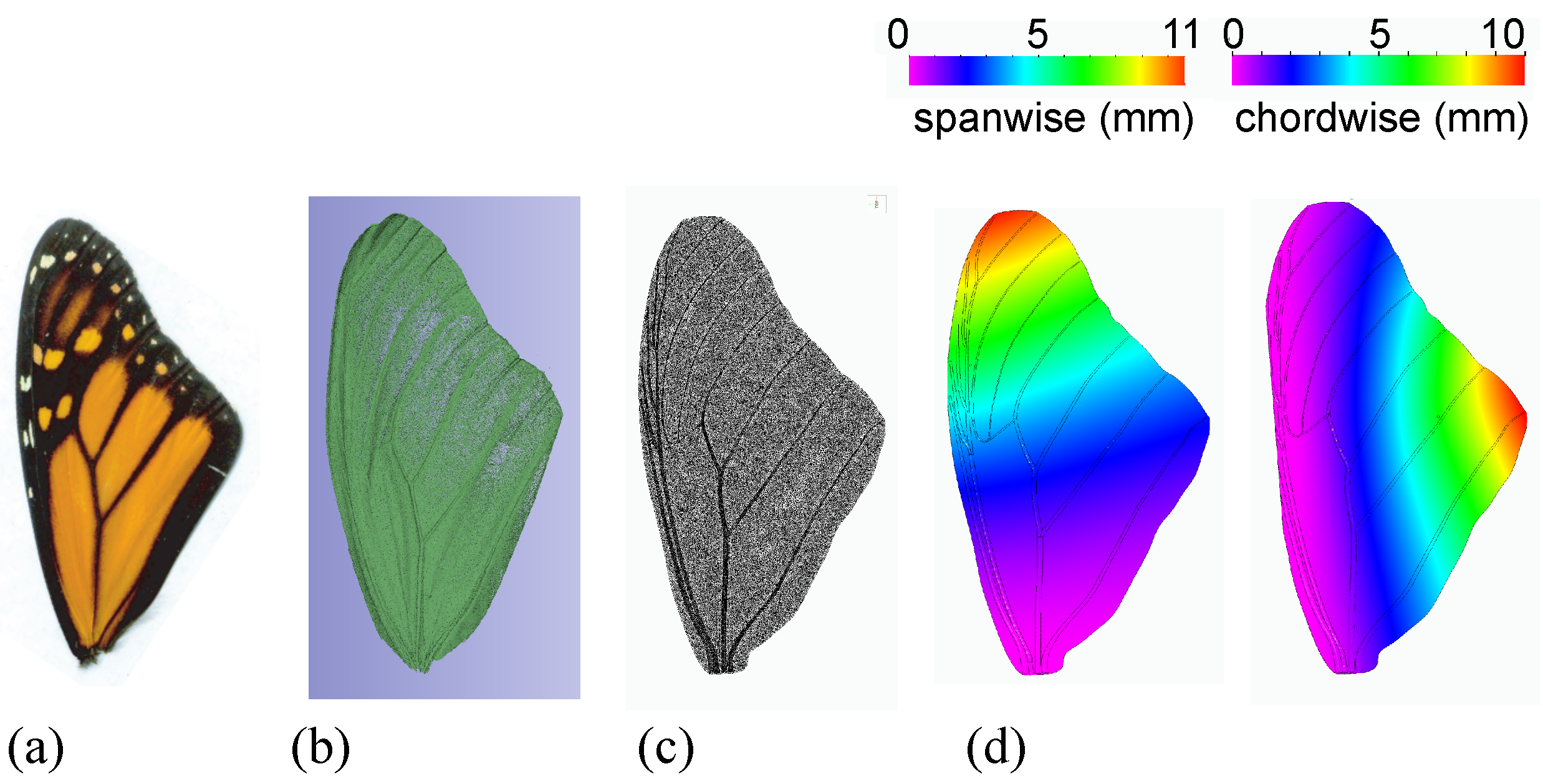
2.1. Experimental Measurements of the Structural Parameters of the Monarch Wing
2.1.1. Wing Density
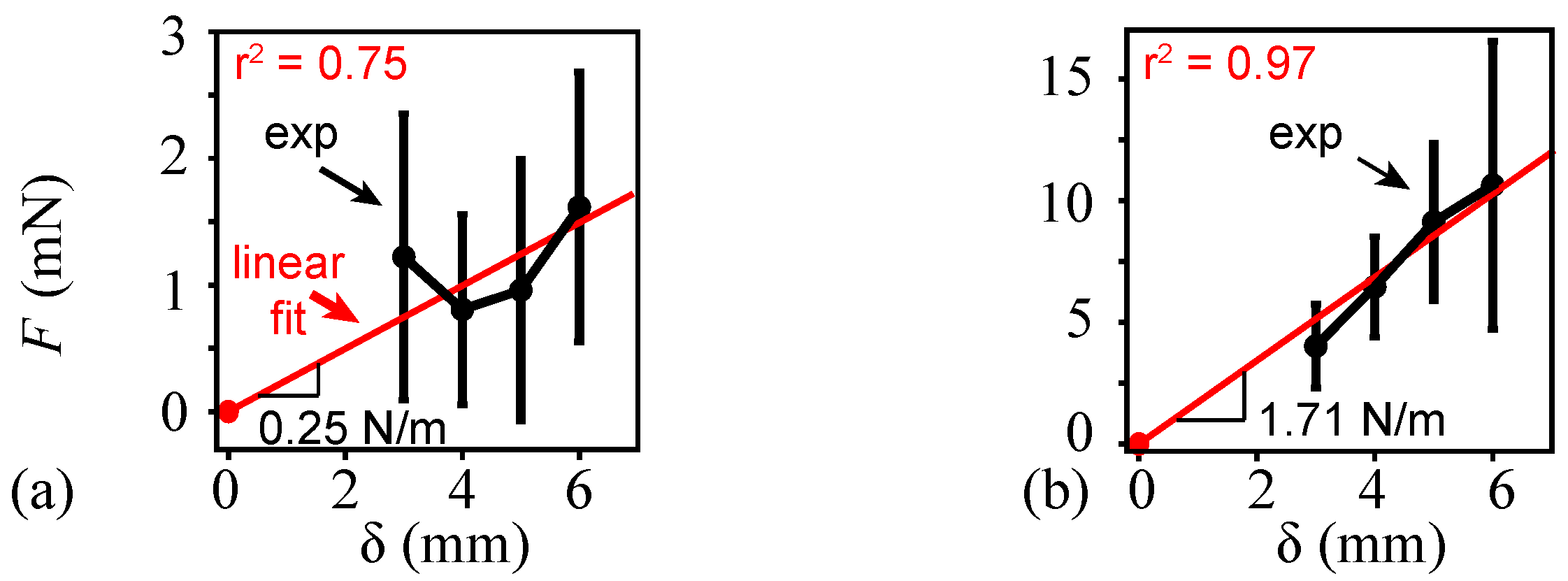
2.1.2. Deflection, Force, and Stiffness
2.2. Computational Modeling of Flexible Flapping Wings at the Monarch Scale
2.2.1. Wing Deformation and Motion
2.2.2. Governing Equations
2.2.3. Design Space
3. Results
3.1. Wing Density and Stiffness Measurements
3.2. Lift, Flight Speed, Thrust, and Power Variations with Altitude
3.3. Reynolds Number, Lift and Thrust Coefficients, and Stroke Plane Angle Variations with Altitude
4. Discussion
4.1. Measured Structural Parameters and Frequency Ratio
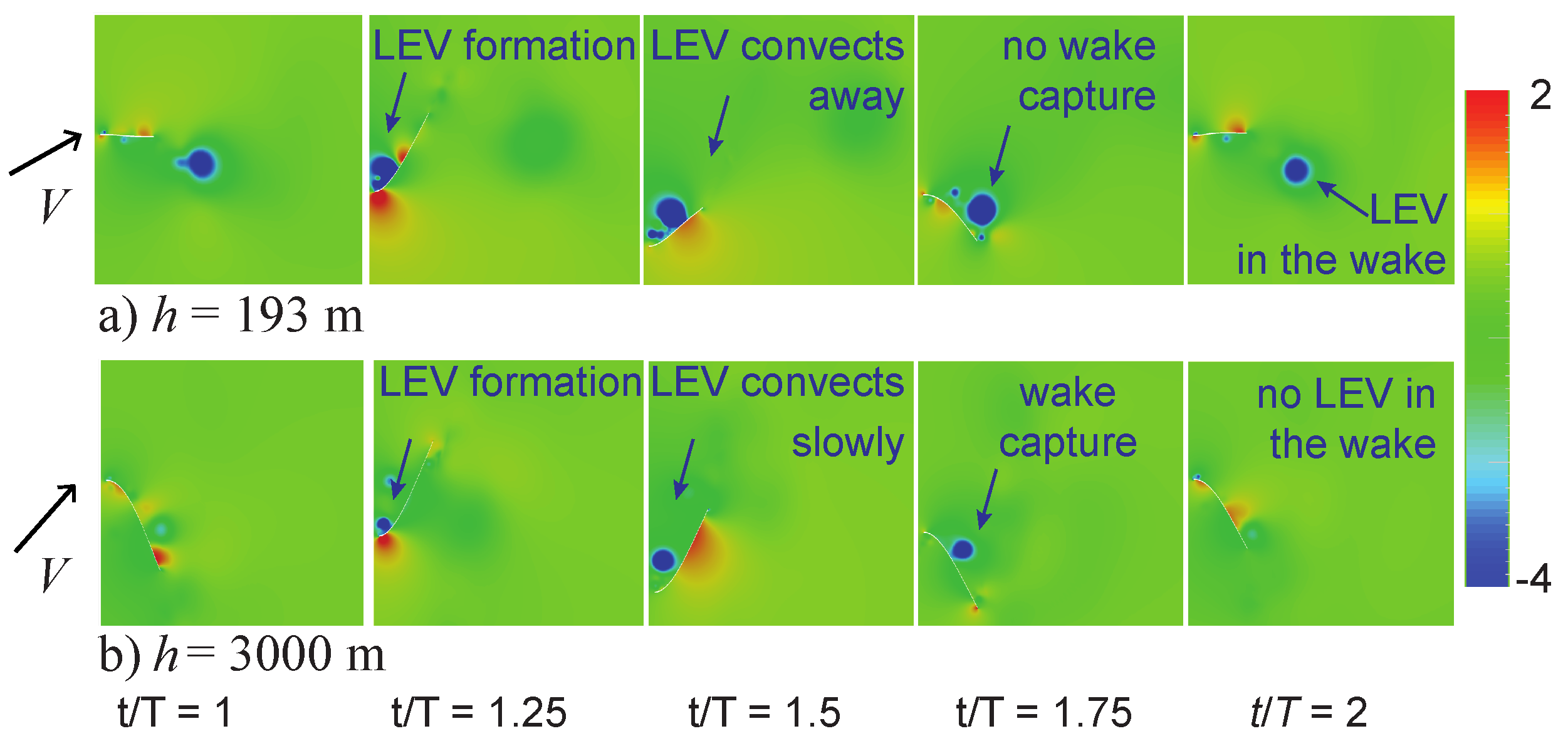
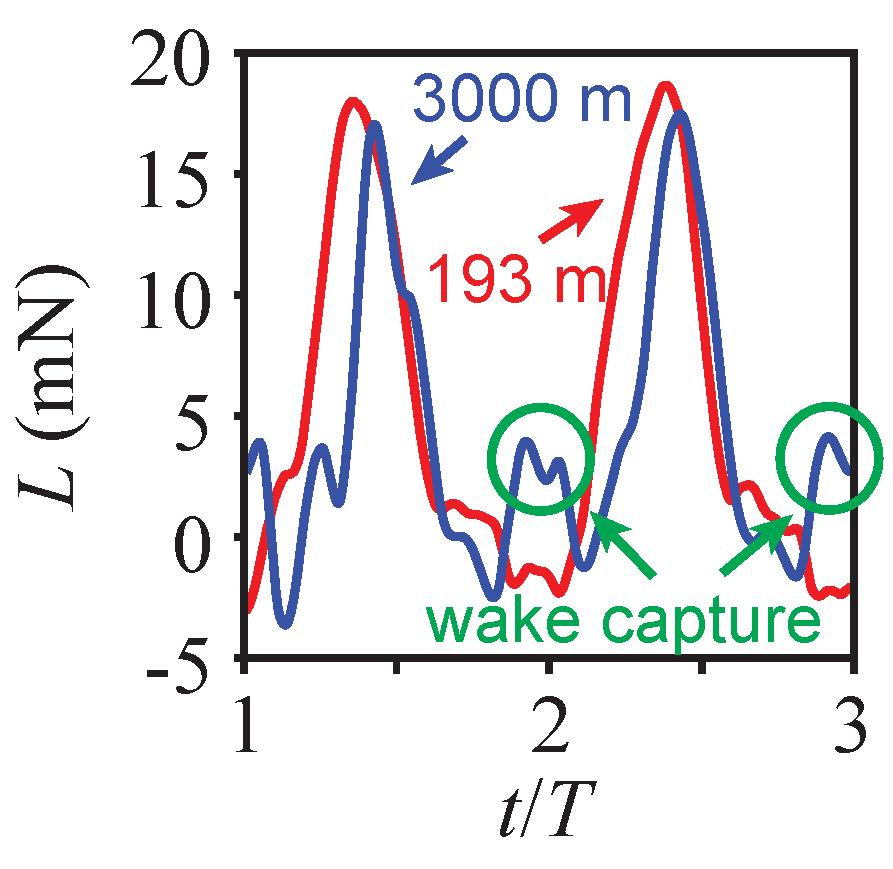
4.2. Wake-Capture Mechanism and Increased Efficiency in Thinner Air
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FEM | finite element model |
| FSI | fluid–structure interaction |
| LE | leading edge |
| LEV | leading edge vortex |
References
- Brower, L.P. Monarch Butterfly Orientation: Missing Pieces of a Magnificent Puzzle. J. Exp. Biol. 1996, 199, 93–103. [Google Scholar] [CrossRef]
- Brower, L.P.; Fink, L.S.; Walford, P. Fueling the Fall Migration of the Monarch Butterfly. Integr. Comp. Biol. 2006, 46, 1123–1142. [Google Scholar] [CrossRef] [PubMed]
- Slayback, D.A.; Brower, L.P.; Ramirez, M.I.; Fink, L.S. Establishing the Presence and Absence of Overwintering Colonies of the Monarch Butterfly in Mexico by the Use of Small Aircraft. Am. Entomol. 2007, 53, 28–40. [Google Scholar] [CrossRef][Green Version]
- Urquhart, F.A.; Urquhart, N.R. The Overwintering Site of the Eastern Population of the Monarch Butterfly (Danaus plexippus; Danaidae) in Southern Mexico. J. Lepid. Soc. 1976, 30, 153–158. [Google Scholar]
- Gibo, D.L. Altitudes Attained by Migrating Monarch Butterflies, Danaus plexippus (Lepidoptera: Danainae) as Reported by Glider Pilots. Can. J. Zool. 1981, 59, 571–572. [Google Scholar] [CrossRef]
- Wassenaar, L.I.; Hobson, K.A. Natal Origins of Migratory Monarch Butterflies at Wintering Colonies in Mexico: New Isotopic Evidence. Proc. Natl. Acad. Sci. USA 1998, 95, 15436–15439. [Google Scholar] [CrossRef]
- Alonso-Mejia, A.; Rendon-Salinas, E.; Montesinos-Patino, E.; Brower, L.P. Use of Lipid Reserves by Monarch Butterflies Overwintering in Mexico: Implications for Conservation. Ecol. Appl. 1997, 7, 934–947. [Google Scholar] [CrossRef]
- Urquhart, F.A. Found at Last; the Monarch’s Winter Home. Natl. Geogr. Mag. 1976, 150, 161–173. [Google Scholar]
- NASA Technical Reports Server. U.S. Standard Atmosphere, 1976; NASA-TM-X-74335; U.S. Government Printing Office: Washington, DC, USA, 1976.
- Sridhar, M.K.; Kang, C.K.; Landrum, D.B.; Aono, H.; Mathis, S.L.; Lee, T. Effects of Flight Altitude on the Lift Generation of Monarch Butterflies: From Sea Level to Overwintering Mountain. Bioinspiration Biomim. 2021, 16, 034002. [Google Scholar] [CrossRef]
- Shyy, W.; Aono, H.; Chimakurthi, S.K.; Trizila, P.; Kang, C.K.; Cesnik, C.E.S.; Liu, H. Recent Progress in Flapping Wing Aerodynamics and Aeroelasticity. Prog. Aerosp. Sci. 2010, 46, 284–327. [Google Scholar] [CrossRef]
- Kang, C.K.; Aono, H.; Cesnik, C.E.S.; Shyy, W. Effects of Flexibility on the Aerodynamic Performance of Flapping Wings. J. Fluid Mech. 2011, 689, 32–74. [Google Scholar] [CrossRef]
- Shyy, W.; Aono, H.; Kang, C.; Liu, H. An Introduction to Flapping Wing Aerodynamics; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Combes, S.A. Flexural Stiffness in Insect Wings I. Scaling and the Influence of Wing Venation. J. Exp. Biol. 2003, 206, 2979–2987. [Google Scholar] [CrossRef]
- Steppan, S. Flexural Stiffness Patterns of Butterfly Wings (Papilionoidea). J. Res 1996, 1996, 61–77. [Google Scholar] [CrossRef]
- Kang, C.K.; Shyy, W. Scaling Law and Enhancement of Lift Generation of an Insect-Size Hovering Flexible Wing. J. R. Soc. Interface 2013, 10, 20130361. [Google Scholar] [CrossRef] [PubMed]
- Combes, S.A.; Daniel, T.L. Flexural Stiffness in Insect Wings II. Spatial distribution and dynamic wing bending. J. Exp. Biol. 2003, 206, 2989–2997. [Google Scholar] [CrossRef]
- Chimakurthi, S.K.; Tang, J.; Palacios, R.; Cesnik, C.E.S.; Shyy, W. Computational Aeroelasticity Framework for Analyzing Flapping Wing Micro Air Vehicles. AIAA J. 2009, 47, 1865–1878. [Google Scholar] [CrossRef][Green Version]
- Bluman, J.E.; Sridhar, M.K.; Kang, C. Chordwise Wing Flexibility May Passively Stabilize Hovering Insects. J. R. Soc. Interface 2018, 15, 20180409. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.H.; Yang, J. Importance of Body Rotation during the Flight of a Butterfly. Phys. Rev. E 2016, 93, 033124. [Google Scholar] [CrossRef]
- Fang, Y.H.; Tang, C.H.; Lin, Y.J.; Yeh, S.I.; Yang, J.T. The Lift Effects of Chordwise Wing Deformation and Body Angle on Low-Speed Flying Butterflies. Biomimetics 2023, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Cranford, J.; Sridhar, M.K.; Kodali, D.; Landrum, D.B.; Slegers, N. Experimental Characterization of a Butterfly in Climbing Flight. AIAA J. 2018, 56, 15–24. [Google Scholar] [CrossRef]
- Tejaswi, K.; Sridhar, M.K.; Kang, C.K.; Lee, T. Effects of Abdomen Undulation in Energy Consumption and Stability for Monarch Butterfly. Bioinspiration Biomim. 2021, 16, 046003. [Google Scholar] [CrossRef]
- Twigg, R. Aeroelastic Characterization of Real and Bioinspired Artificial Butterfly Wings. Master’s Thesis, University of Alabama in Huntsville, Huntsville, AL, USA, 2020. [Google Scholar]
- Pohly, J.; Kang, C.; Lee, T.; Aono, H. Modeling Freely Flying Monarch Butterflies Using a Strongly Coupled High Fidelity Numerical Framework. In Proceedings of the 15th World Congress on Computational Mechanics (WCCM-XV) and 8th Asian Pacific Congress on Computational Mechanics (APCOM-VIII), Yokohama, Japan, 31 July–5 August 2022. [Google Scholar] [CrossRef]
- Sridhar, M.; Kang, C.K.; Landrum, D.B.; Aono, H. Fluid-Structure Interaction of Flexible Flapping Wings at High Altitude Conditions. In Proceedings of the 2020 AIAA Scitech Forum, Orlando, FL, USA, 6–10 January 2020. AIAA 2020–1781. [Google Scholar] [CrossRef]
- Tang, J.; Viieru, D.; Shyy, W. Effects of Reynolds Number and Flapping Kinematics on Hovering Aerodynamics. AIAA J. 2008, 46, 967–976. [Google Scholar] [CrossRef]
- Bluman, J.; Kang, C. Achieving Hover Equilibrium in Free Flight with a Flexible Flapping Wing. J. Fluids Struct. 2017, 75, 117–139. [Google Scholar] [CrossRef]
- de Boer, A.; van der Schoot, M.; Bijl, H. Mesh deformation based on radial basis function interpolation. Comput. Struct. 2007, 85, 784–795. [Google Scholar] [CrossRef]
- Sridhar, M. The Effects of Altitude on the Aerodynamic Performance of Monarch Butterflies. Ph.D. Thesis, University of Alabama in Huntsville, Huntsville, AL, USA, 2021. [Google Scholar]
- Twigg, R.; Sridhar, M.; Pohly, J.A.; Hildebrandt, N.; Kang, C.K.; Landrum, D.B.; Roh, K.H.; Salzwedel, S. Aeroelastic Characterization of Real and Artificial Monarch Butterfly Wings. In Proceedings of the 2020 AIAA Scitech Forum, Orlando, FL, USA, 6–10 January 2020. AIAA 2020–2002. [Google Scholar] [CrossRef]
- Tanaka, H.; Wood, R.J. Fabrication of Corrugated Artificial Insect Wings Using Laser Micromachined Molds. J. Micromechanics Microengineering 2010, 20, 75008. [Google Scholar] [CrossRef]
- Nakata, T.; Liu, H.; Tanaka, Y.; Nishihashi, N.; Wang, X.; Sato, A. Aerodynamics of a Bio-Inspired Flexible Flapping-Wing Micro Air Vehicle. Bioinspiration Biomim. 2011, 6, 45002. [Google Scholar] [CrossRef]
- Song, F.; Xiao, K.W.; Bai, K.; Bai, Y.L. Microstructure and Nanomechanical Properties of the Wing Membrane of Dragonfly. Mater. Sci. Eng. A 2007, 457, 254–260. [Google Scholar] [CrossRef]
- Wainwright, S.A.; Biggs, W.D.; Gosline, J.M.; Currey, J.D. Mechanical Design in Organisms; Princeton University Press: Princeton, NJ, USA, 1976. [Google Scholar]
- Jensen, M.; Weis-Fogh, T. Biology and Physics of Locust Flight. V. Strength and Elasticity of Locust Cuticle. Philos. Trans. R. Soc. Biol. Sci. 1962, 245, 137–169. [Google Scholar]
- Ramananarivo, S.; Godoy-Diana, R.; Thiria, B. Rather than Resonance, Flapping Wing Flyers May Play on Aerodynamics to Improve Performance. Proc. Natl. Acad. Sci. USA 2011, 108, 5964–5969. [Google Scholar] [CrossRef]
- Vanella, M.; Fitzgerald, T.; Preidikman, S.; Balaras, E.; Balachandran, B. Influence of Flexibility on the Aerodynamic Performance of a Hovering Wing. J. Exp. Biol. 2009, 212, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, L.; Zhou, C.; Wu, J.; Cheng, B. Effects of Reynolds number on leading-edge vortex formation dynamics and stability in revolving wings. J. Fluid Mech. 2022, 931, A13. [Google Scholar] [CrossRef]
- Jeong, J.; Hussain, F. On the identification of a vortex. J. Fluid Mech. 1995, 285, 69–94. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, C.-k.; Sridhar, M.; Twigg, R.; Pohly, J.; Lee, T.; Aono, H. Power Benefits of High-Altitude Flapping Wing Flight at the Monarch Butterfly Scale. Biomimetics 2023, 8, 352. https://doi.org/10.3390/biomimetics8040352
Kang C-k, Sridhar M, Twigg R, Pohly J, Lee T, Aono H. Power Benefits of High-Altitude Flapping Wing Flight at the Monarch Butterfly Scale. Biomimetics. 2023; 8(4):352. https://doi.org/10.3390/biomimetics8040352
Chicago/Turabian StyleKang, Chang-kwon, Madhu Sridhar, Rachel Twigg, Jeremy Pohly, Taeyoung Lee, and Hikaru Aono. 2023. "Power Benefits of High-Altitude Flapping Wing Flight at the Monarch Butterfly Scale" Biomimetics 8, no. 4: 352. https://doi.org/10.3390/biomimetics8040352
APA StyleKang, C.-k., Sridhar, M., Twigg, R., Pohly, J., Lee, T., & Aono, H. (2023). Power Benefits of High-Altitude Flapping Wing Flight at the Monarch Butterfly Scale. Biomimetics, 8(4), 352. https://doi.org/10.3390/biomimetics8040352





