Preservation of Mechanical and Morphological Properties of Porcine Cardiac Outflow Vessels after Decellularization and Wet Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Processing
2.2. Biocide Solution Preparation and Sample Storage
2.3. Mechanical Testing
2.4. Morphological Study
2.5. Scanning Electron Microscopy
2.6. Statistical Analysis
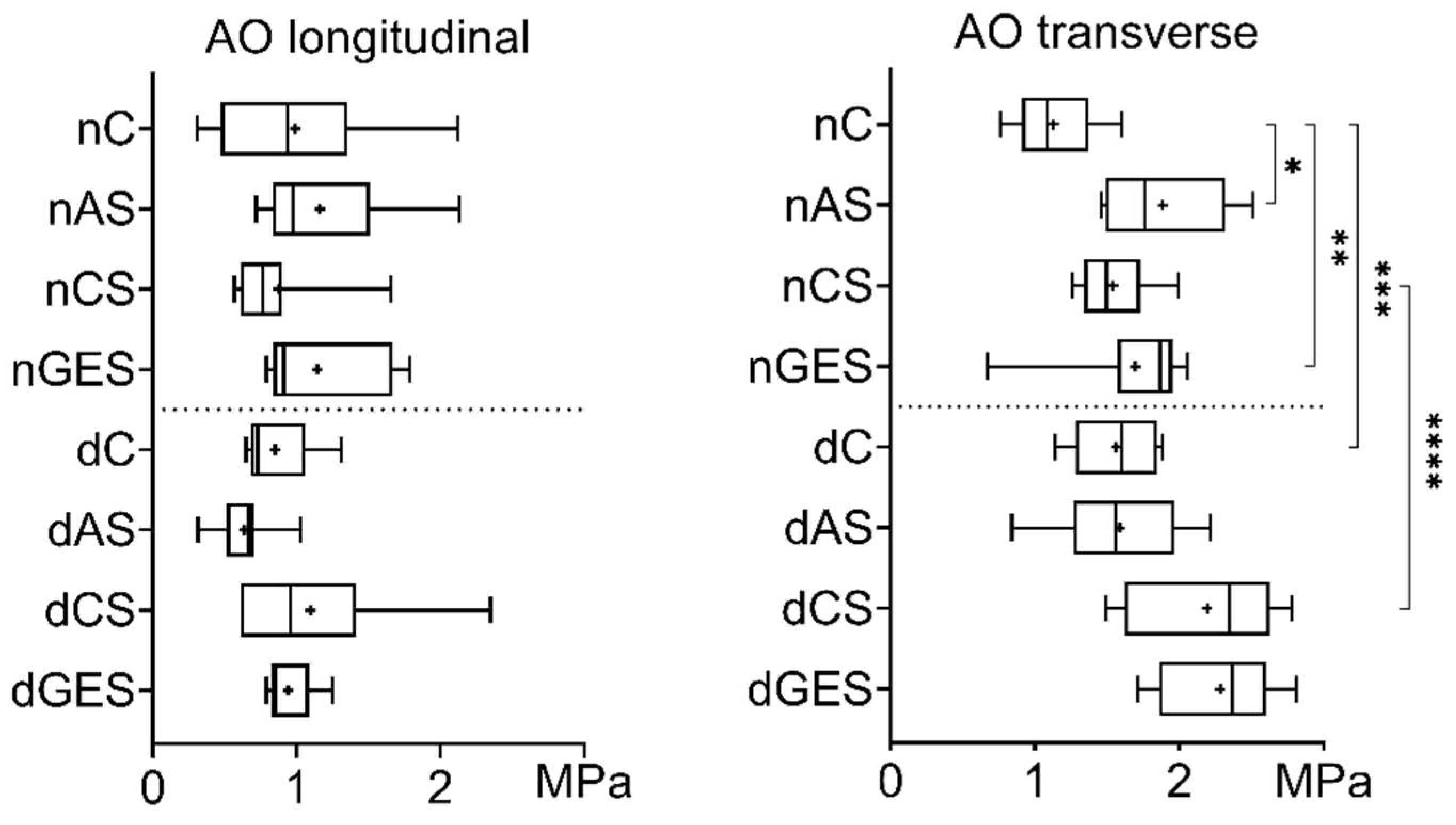
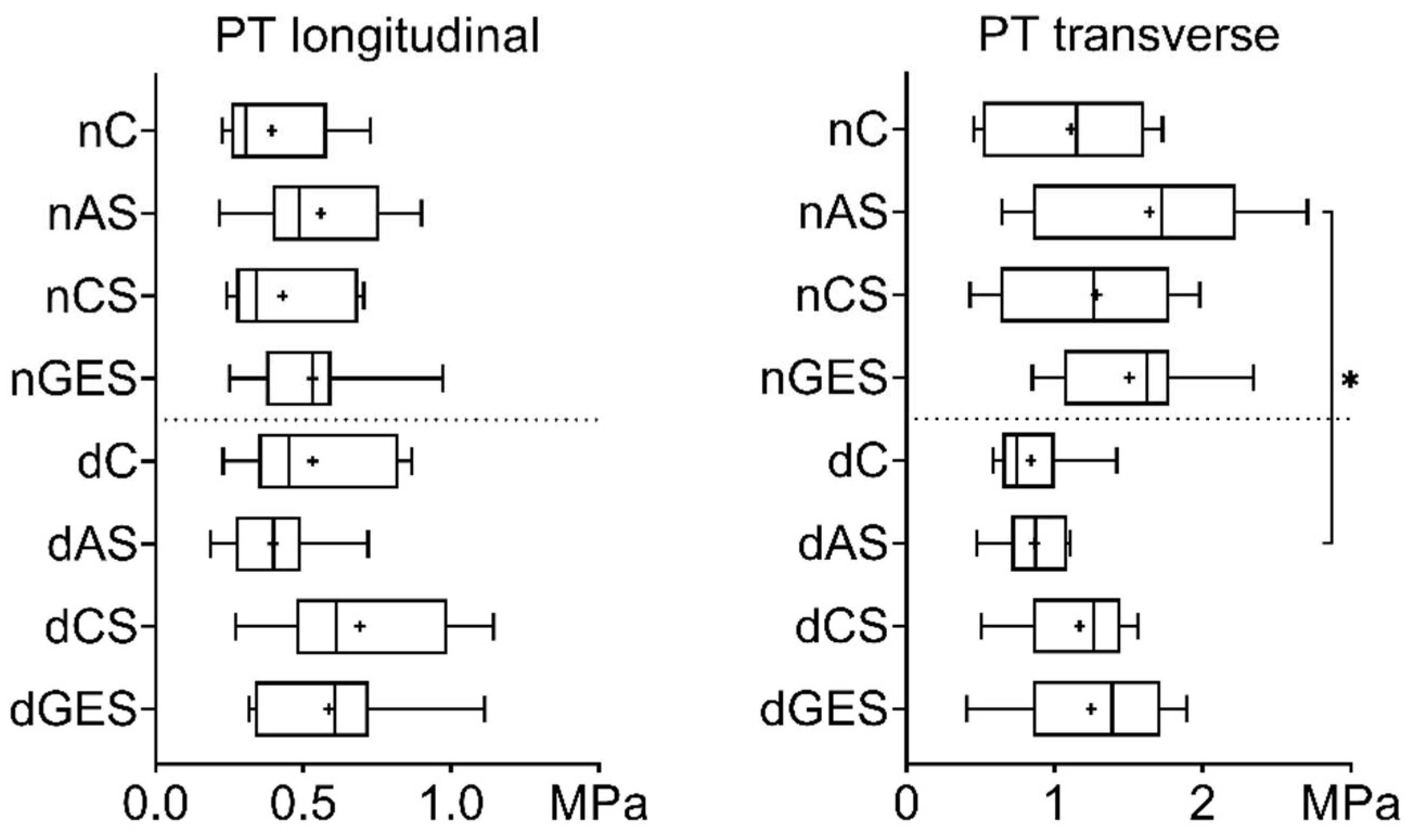
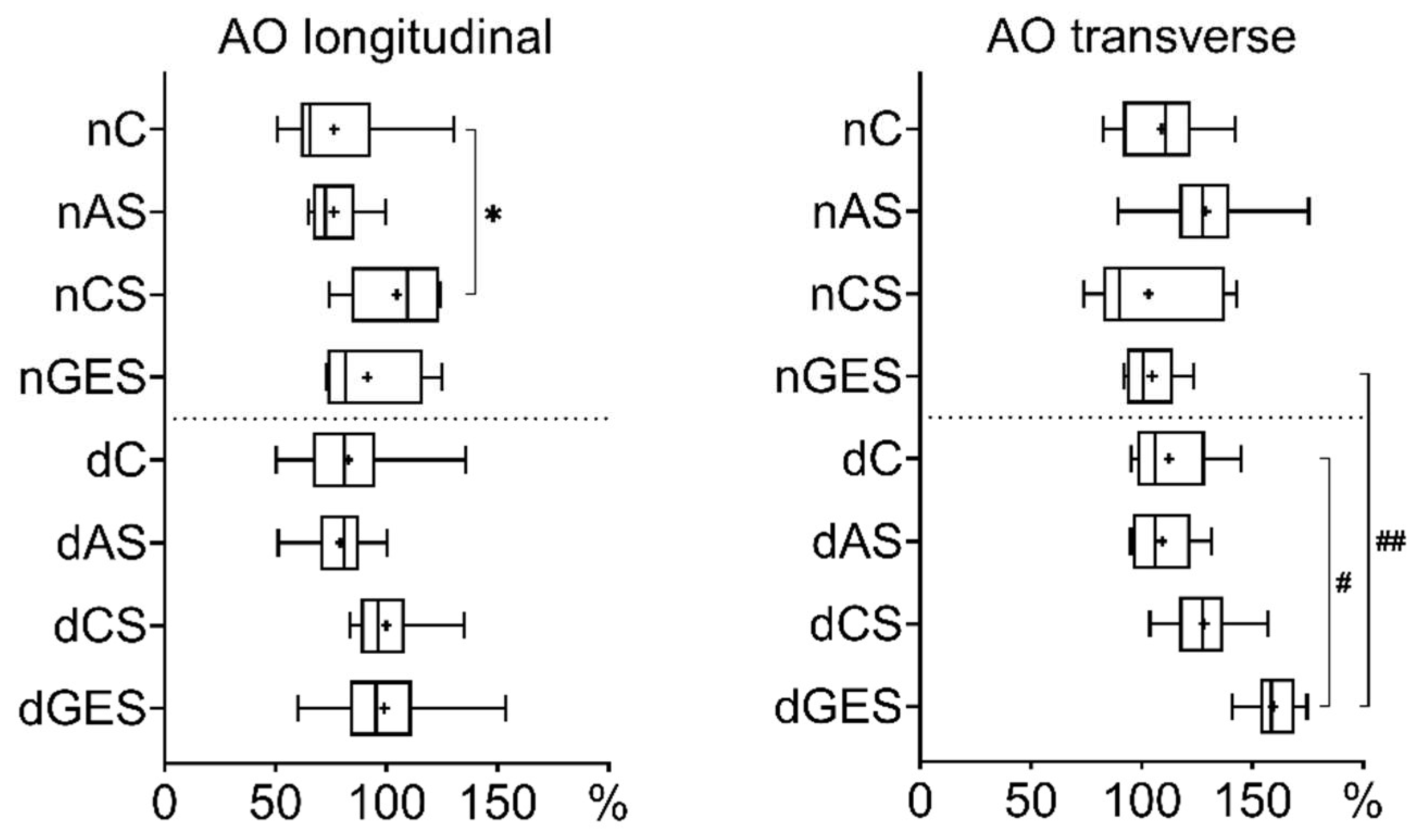
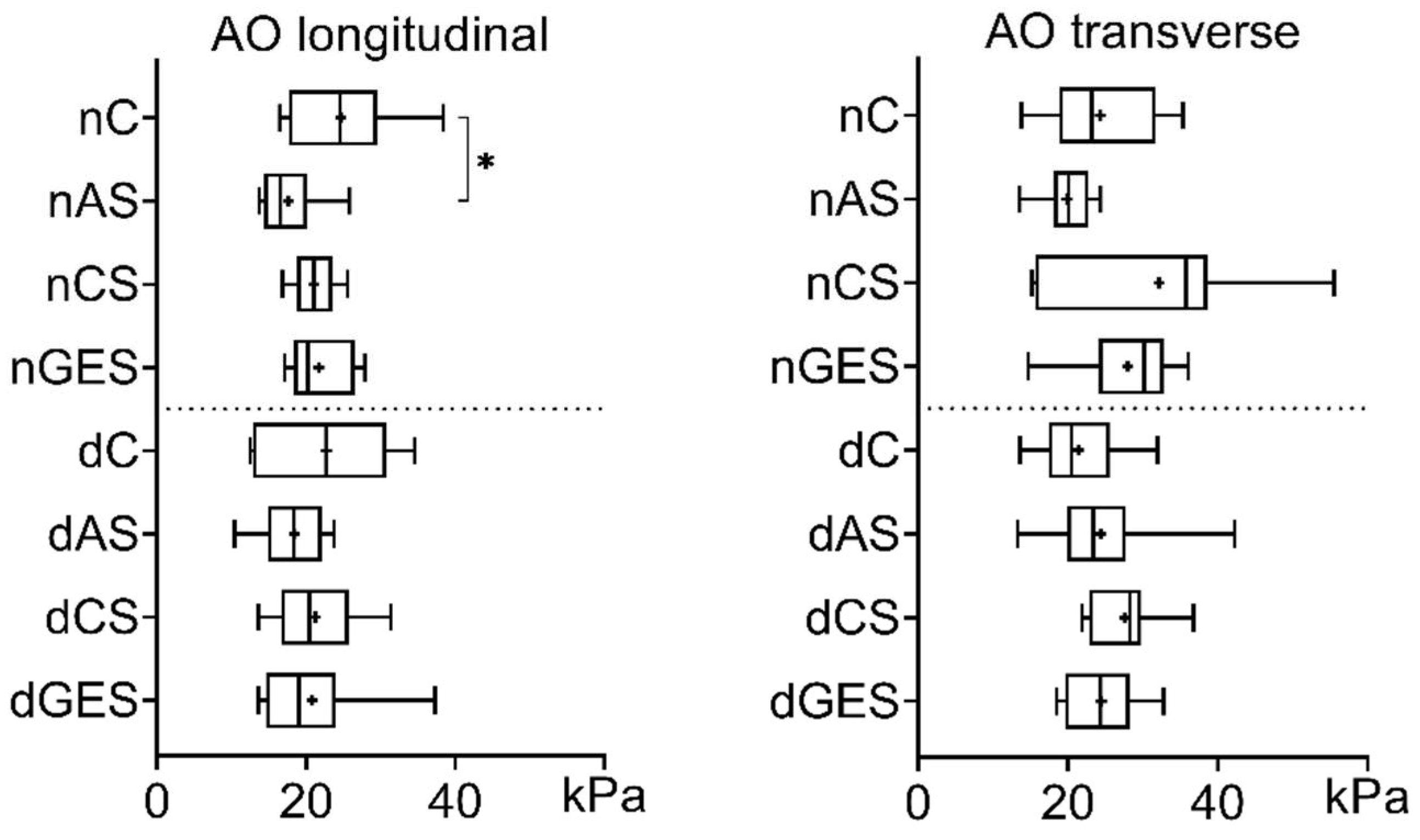
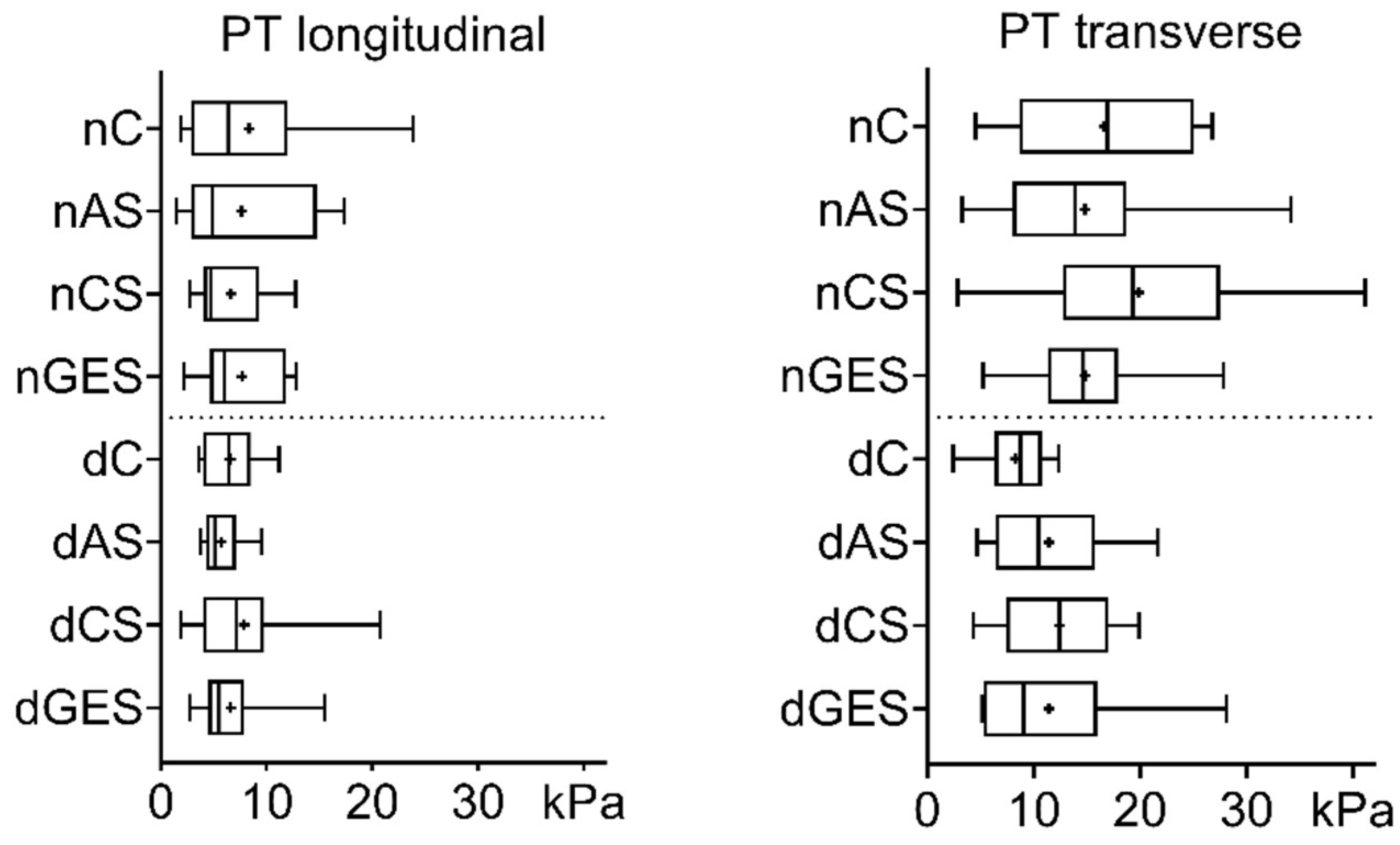
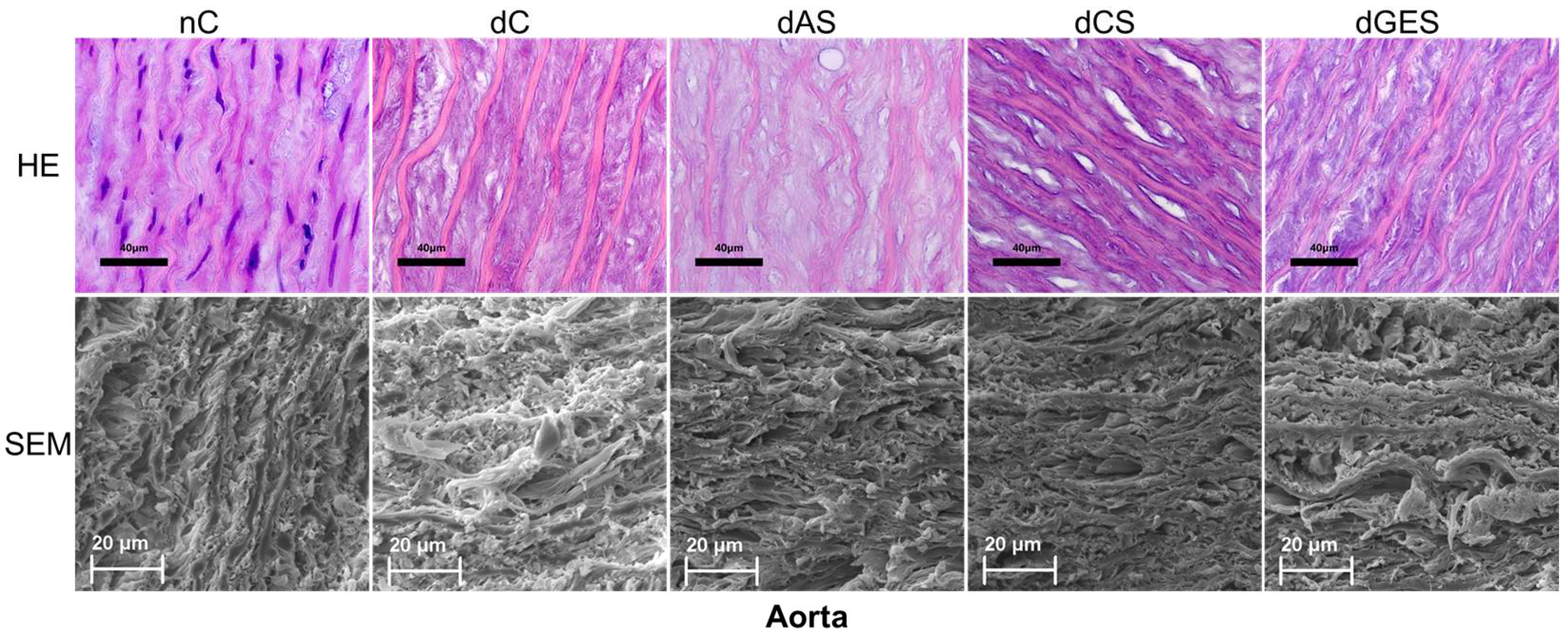
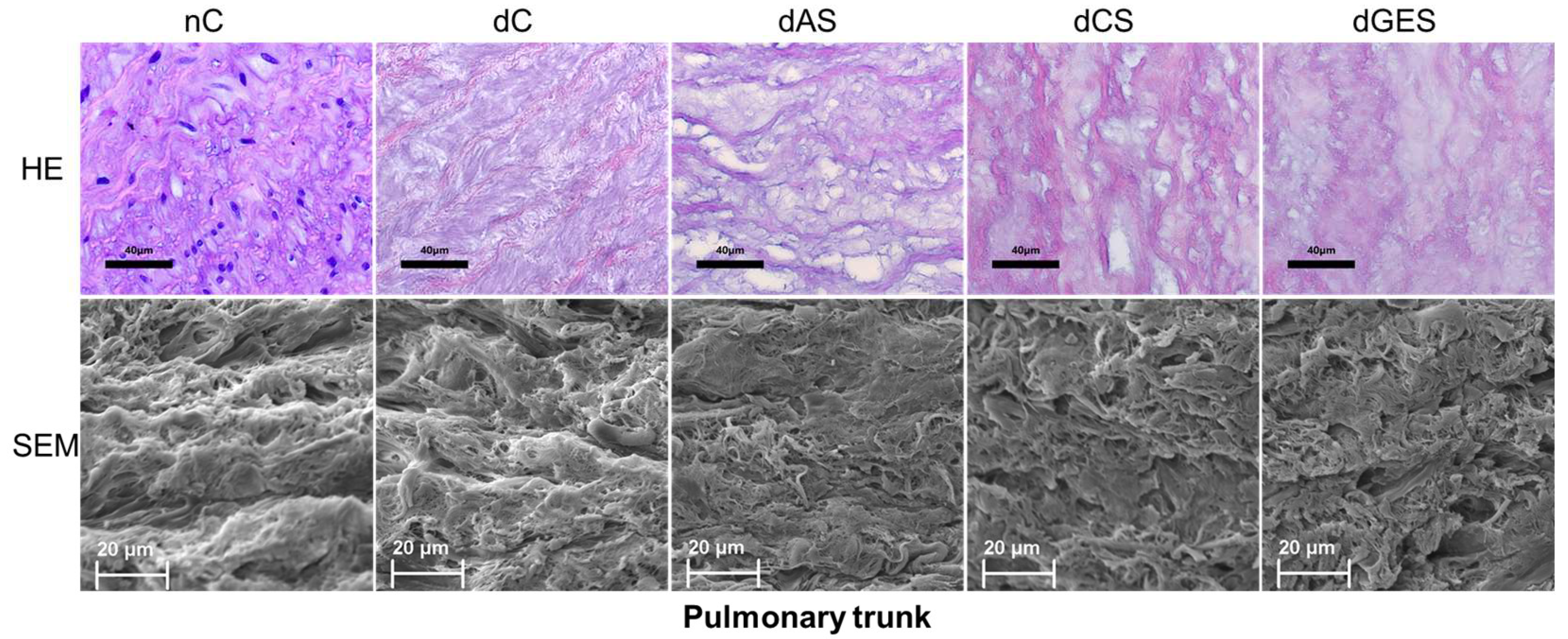
3. Results
3.1. Mechanical Properties
3.2. Microstructural Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horke, A.; Tudorache, I.; Laufer, G.; Andreas, M.; Pomar, J.L.; Pereda, D.; Quintana, E.; Sitges, M.; Meyns, B.; Rega, F.; et al. Early results from a prospective, single-arm European trial on decellularized allografts for aortic valve replacement: The ARISE study and ARISE Registry data. Eur. J. Cardiothorac. Surg. 2020, 58, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.S.; Hong, Z.N.; Sun, K.P.; Cao, H.; Chen, Q. Effect of the Different Mechanical Mitral Valve Sounds on the Patients’ Quality of Life. Thorac. Cardiovasc. Surg. 2020, 68, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Arabkhani, B.; Bekkers, J.A.; Andrinopoulou, E.-R.; Roos-Hesselink, J.W.; Takkenberg, J.J.M.; Bogers, A.J.J.C. Allografts in aortic position: Insights from a 27-year, single-center prospective study. J. Thorac. Cardiovasc. Surg. 2016, 152, 1572–1579.e3. [Google Scholar] [CrossRef]
- Demidov, D.P.; Astaspov, D.A.; Bogachev-Prokophiev, A.V.; Zheleznev, S.I. Quality of life after aortic valve replacement with biological prostheses in elderly patients. Patol. Krovoobrashcheniya Kardiokhirurgiya 2017, 21, 40–47. [Google Scholar] [CrossRef]
- Nappi, F.; Nenna, A.; Petitti, T.; Spadaccio, C.; Gambardella, I.; Lusini, M.; Chello, M.; Acar, C. Long-term outcome of cryopreserved allograft for aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2018, 156, 1357–1365.e6. [Google Scholar] [CrossRef]
- Fioretta, E.S.; von Boehmer, L.; Motta, S.E.; Lintas, V.; Hoerstrup, S.P.; Emmert, M.Y. Cardiovascular tissue engineering: From basic science to clinical application. Exp. Gerontol. 2019, 117, 1–12. [Google Scholar] [CrossRef]
- VeDepo, M.C.; Detamore, M.S.; Hopkins, R.A.; Converse, G.L. Recellularization of decellularized heart valves: Progress toward the tissue-engineered heart valve. J. Tissue Eng. 2017, 8, 204173141772632. [Google Scholar] [CrossRef]
- Cebotari, S.; Tudorache, I.; Ciubotaru, A.; Boethig, D.; Sarikouch, S.; Goerler, A.; Lichtenberg, A.; Cheptanaru, E.; Barnaciuc, S.; Cazacu, A.; et al. Use of Fresh Decellularized Allografts for Pulmonary Valve Replacement May Reduce the Reoperation Rate in Children and Young Adults. Circulation 2011, 124, S115–S123. [Google Scholar] [CrossRef]
- Argento, G.; Simonet, M.; Oomens, C.W.J.; Baaijens, F.P.T. Multi-scale mechanical characterization of scaffolds for heart valve tissue engineering. J. Biomech. 2012, 45, 2893–2898. [Google Scholar] [CrossRef]
- Human, P.; Zilla, P. The Neglected Villain of Bioprosthetic Degeneration: Inflammatory and Immune Processes. J. Long. Term. Eff. Med. Implants 2017, 27, 159–180. [Google Scholar] [CrossRef]
- Sergeevichev, D.; Subbotovskaya, A.; Dokuchayeva, A.; Sergeevicheva, V.; Vasiliev, V. CD28/CTLA-4 Expression in Peripheral Blood T-Cells Induced by Allogenic Heart Valves In Vitro. Tissue. Eng. Part A 2014, 20, O214. [Google Scholar] [CrossRef]
- Ariganello, M.B.; Simionescu, D.T.; Labow, R.S.; Lee, J.M. Macrophage differentiation and polarization on a decellularized pericardial biomaterial. Biomaterials 2011, 32, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Odarenko, Y.N.; Rutkovskaya, N.V.; Rogulina, N.V.; Stasev, A.N.; Kokorin, S.G.; Kagan, E.S.; Barbarash, L.S. Analysis of 23-year experience epoxy treated xenoaortic bioprosthesis in surgery mitral heart disease. Complex Issues Cardiovasc. Dis. 2015, 4, 17–25. [Google Scholar]
- Fiala, R.; Kochová, P.; Kubíková, T.; Cimrman, R.; Tonar, Z.; Špatenka, J.; Fabián, O.; Burkert, J. Mechanical and structural properties of human aortic and pulmonary allografts do not deteriorate in the first 10 years of cryopreservation and storage in nitrogen. Cell Tissue Bank. 2019, 20, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Lisy, M.; Kalender, G.; Schenke-Layland, K.; Brockbank, K.G.M.; Biermann, A.; Stock, U.A. Allograft Heart Valves: Current Aspects and Future Applications. Biopreserv. Biobank. 2017, 15, 148–157. [Google Scholar] [CrossRef]
- Brockbank, K.G.M.; Lightfoot, F.G.; Song, Y.C.; Taylor, M.J. Interstitial ice formation in cryopreserved homografts: A possible cause of tissue deterioration and calcification in vivo. J. Heart Valve Dis. 2000, 9, 200–206. [Google Scholar]
- Britikov, D.V.; Lauk-Dubitsky, S.E.; Serov, R.A.; Khugaev, G.A. Morphological evaluation of new method of valve and vascular allografts cryopreservation. Russ. Ann. Surg. 2019, 24, 16–23. [Google Scholar]
- Hickey, E.; Langley, S.M.; Allemby-Smith, O.; Livesey, S.A.; Monro, J.L. Subcoronary Allograft Aortic Valve Replacement: Parametric Risk-Hazard Outcome Analysis to a Minimum of 20 Years. Ann. Thorac. Surg. 2007, 84, 1564–1570. [Google Scholar] [CrossRef]
- Ostrovskij, J.P.; Judina, O.A.; Muratov, R.M. Manufacturing Technology and Technique of Using Cryopreserved Allografts in Surgery of Aortic Valve Defects; Belaruskaja Nauka: Minsk, Belarus, 2016. [Google Scholar]
- Wollmann, L.C.; Suss, P.H.; Kraft, L.; Ribeiro, V.S.; Noronha, L.; da Costa, F.D.A.; Tuon, F.F. Histological and Biomechanical Characteristics of Human Decellularized Allograft Heart Valves After Eighteen Months of Storage in Saline Solution. Biopreserv. Biobank. 2020, 18, 90–101. [Google Scholar] [CrossRef]
- Vyavahare, N.R.; Jones, P.L.; Hirsch, D.; Schoen, F.J.; Levy, R.J. Prevention of glutaraldehyde-fixed bioprosthetic heart valve calcification by alcohol pretreatment: Further mechanistic studies. J. Heart Valve Dis. 2000, 9, 561–566. [Google Scholar]
- Raghavan, D.; Simionescu, D.T.; Vyavahare, N.R. Neomycin prevents enzymemediated glycosaminoglycan degradation in bioprosthetic heart valves. Biomaterials 2007, 28, 2861–2868. [Google Scholar] [CrossRef]
- Hewitt, N.J.; Troutman, J.; Przibilla, J.; Schepky, A.; Ouédraogo, G.; Mahony, C.; Kenna, G.; Varçin, M.; Dent, M.P. Use of in vitro metabolism and biokinetics assays to refine predicted in vivo and in vitro internal exposure to the cosmetic ingredient, phenoxyethanol, for use in risk assessment. Regul. Toxicol. Pharmacol. 2022, 131, 105132. [Google Scholar] [CrossRef] [PubMed]
- Crosado, B.; Löffler, S.; Ondruschka, B.; Zhang, M.; Zwirner, J.; Hammer, N. Phenoxyethanol-Based Embalming for Anatomy Teaching: An 18 Years’ Experience with Crosado Embalming at the University of Otago in New Zealand. Anat. Sci. Educ. 2020, 13, 778–793. [Google Scholar] [CrossRef]
- Langer, S.; Sedigh Salakdeh, M.; Goertz, O.; Steinau, H.U.; Steinstraesser, L.; Homann, H.H. The impact of topical antiseptics on skin microcirculation. Eur. J. Med. Res. 2004, 9, 449–454. [Google Scholar]
- Vasilyeva, M.B.; Krasilnikova, A.A.; Kuznetsova, E.V.; Lunina, M.V. Alternative biocidal solutions for storage of allogeneic vascular grafts used for the replacement of cardiovascular elements. Patol. Krovoobrashcheniya Kardiokhirurgiya 2018, 22, 95. [Google Scholar] [CrossRef]
- Zhuravleva, I. Biocidal Composition for Aseptic Storage of Preserved Prosthetic Material Made of Tissues of Animal Origin. Patent RU2580621C1, 10 April 2016. [Google Scholar]
- Sergeevichev, D.; Vasiliyeva, M.; Kuznetsova, E.; Zhulkov, M.; Rusakova, Y. Morphological Post—Implantation Features of Aortic Conduits After Long—Term wet Storage. J. Med. Biol. Eng. 2023, 43, 185–194. [Google Scholar] [CrossRef]
- Jashari, R. Transplantation of cryopreserved human heart valves in Europe: 30 years of banking in Brussels and future perspectives. Cell Tissue Bank. 2021, 22, 519–537. [Google Scholar] [CrossRef] [PubMed]
- Cheung, D.T.; Weber, P.A.; Grobe, A.C.; Shomura, Y.; Choo, S.J.; Luo, H.H.; Marchion, D.C.; Duran, C.M.G. A new method for the preservation of aortic valve homografts. J. Heart Valve Dis. 2001, 10, 728–734; discussion 734–735. [Google Scholar]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Gilbert, T.W. Strategies for tissue and organ decellularization. J. Cell. Biochem. 2012, 113, 2217–2222. [Google Scholar] [CrossRef]
- Theodoridis, K.; Müller, J.; Ramm, R.; Findeisen, K.; Andrée, B.; Korossis, S.; Haverich, A.; Hilfiker, A. Effects of combined cryopreservation and decellularization on the biomechanical, structural and biochemical properties of porcine pulmonary heart valves. Acta Biomater. 2016, 43, 71–77. [Google Scholar] [CrossRef]
- Converse, G.L.; Armstrong, M.; Quinn, R.W.; Buse, E.E.; Cromwell, M.L.; Moriarty, S.J.; Lofland, G.K.; Hilbert, S.L.; Hopkins, R.A. Effects of cryopreservation, decellularization and novel extracellular matrix conditioning on the quasi-static and time-dependent properties of the pulmonary valve leaflet. Acta Biomater. 2012, 8, 2722–2729. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Oldenhof, H.; Dai, X.; Haverich, A.; Hilfiker, A.; Harder, M.; Wolkers, W.F. Protein stability in stored decellularized heart valve scaffolds and diffusion kinetics of protective molecules. Biochim. Biophys. Acta 2014, 1844, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Choi, S.-Y.; Sung, S.-C.; Lim, H.-G.; Park, S.; Kim, S.-H.; Kim, Y.J. Changes of the Structural and Biomechanical Properties of the Bovine Pericardium after the Removal of α-Gal Epitopes by Decellularization and α-Galactosidase Treatment. Korean J. Thorac. Cardiovasc. Surg. 2012, 45, 380–389. [Google Scholar] [CrossRef]
- McKee, C.T.; Last, J.A.; Russell, P.; Murphy, C.J. Indentation versus Tensile Measurements of Young’s Modulus for Soft Biological Tissues. Tissue Eng. Part B Rev. 2011, 17, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, N.; Gong, D.; Xia, C.; Xu, Z. Comparison of detergent-based decellularization protocols for the removal of antigenic cellular components in porcine aortic valve. Xenotransplantation 2018, 25, e12380. [Google Scholar] [CrossRef] [PubMed]
- Vasilieva, M.; Sergeevichev, D.; Yunoshev, A.; Larionov, P.M.; Novruzov, R.B.; Karaskov, A.M. Morphofunctional evaluation of enzymatic and detergent decellularization methods of cardiac allografts. Patol. Krovoobrashcheniya Kardiokhirurgiya 2012, 2, 77–80. [Google Scholar]
- Sergeevichev, D.S.; Sergeevicheva, V.V.; Subbotovskaya, A.I.; Vasilyeva, M.B.; Dokuchayeva, A.A.; Karaskov, A.M.; Kozlov, V.A. Decellularization as a prevention of immune response activation to allogeneic pulmonary valves. Cell. Transpl. Tissue Eng. 2013, 8, 55–60. [Google Scholar]
- Krasilnikova, A.A.; Sergeevichev, D.S.; Fomenko, V.V.; Korobeynikov, A.A.; Vasilyeva, M.B.; Yunoshev, A.S.; Karaskov, A.M.; Pokushalov, E.A. Globular chitosan treatment of bovine jugular veins: Evidence of anticalcification efficacy in the subcutaneous rat model. Cardiovasc. Pathol. 2018, 32, 1–7. [Google Scholar] [CrossRef]
- Sokolis, D.P.; Kefaloyannis, E.M.; Kouloukoussa, M.; Marinos, E.; Boudoulas, H.; Karayannacos, P.E. A structural basis for the aortic stress–strain relation in uniaxial tension. J. Biomech. 2006, 39, 1651–1662. [Google Scholar] [CrossRef]
- Mikhailova, I.P.; Manchenko, A.A.; Byzov, D.V.; Sandomirsky, B.P. Physical and mechanical properties of devitalized xenografts based on pericardium, aortic valve leaflets and arteries. Probl. Cryobiol. Cryomed. 2015, 25, 311–329. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Zhang, Y. Transmural variation in elastin fiber orientation distribution in the arterial wall. J. Mech. Behav. Biomed. Mater. 2018, 77, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Matsumoto, T. Multiphoton microscopy observations of 3D elastin and collagen fiber microstructure changes during pressurization in aortic media. Biomech. Model. Mechanobiol. 2017, 16, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Horvath, I.; Foran, D.J. Viscoelasticity of the Vessel Wall: The Role of Collagen and Elastic Fibers. Crit. Rev. Biomed. Eng. 2001, 29, 279–302. [Google Scholar] [CrossRef]
- Remi, E.; Khelil, N.; Di, I.; Roques, C.; Ba, M.; Medjahed-Hamidi, F.; Chaubet, F.; Letourneur, D.; Lansac, E.; Meddahi-Pelle, A. Pericardial Processing: Challenges, Outcomes and Future Prospects. In Biomaterials Science and Engineering; Rosario, P., Ed.; InTech: London, UK, 2011. [Google Scholar]
- Rezvova, M.A.; Kudrjavceva, J. Current approaches to chemical modification of proteins in biological tissues, implications and applications. Bioorg. Chem. 2018, 44, 22–37. [Google Scholar] [CrossRef]
- van Doormaal, T.P.C.; Sluijs, J.H.; Vink, A.; Tulleken, C.A.F.; van der Zwan, A. Comparing five simple vascular storage protocols. J. Surg. Res. 2014, 192, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Wilbring, M.; Tugtekin, S.M.; Zatschler, B.; Ebner, A.; Reichenspurner, H.; Kappert, U.; Matschke, K.; Deussen, A. Preservation of endothelial vascular function of saphenous vein grafts after long-time storage with a recently developed potassium-chloride and N-acetylhistidine enriched storage solution. Thorac. Cardiovasc. Surg. 2013, 61, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Fahner, P.J.; Idu, M.M.; Legemate, D.A.; Vanbavel, E.; Borstlap, J.; Pfaffendorf, M.; Van Marle, J.; Van Gulik, T.M. Morphological and Functional Alterations in Glycerol Preserved Rat Aortic Allografts. Int. J. Artif. Organs 2004, 27, 979–990. [Google Scholar] [CrossRef]

| Group | Description | Subgroups |
| C | Control | nC—native control samples |
| dC—decellularized control samples | ||
| AS | Antibiotic solution | nAS—native samples in AS |
| dAS—decellularized samples in AS | ||
| CS | Complex solution | nCS—native samples in CS |
| dCS—decellularized samples in CS | ||
| GES | Glycerol-ethanol solution | nGEM—native samples in GEM |
| dGEM—decellularized samples in GEM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergeevichev, D.; Vasiliyeva, M.; Kuznetsova, E.; Chelobanov, B. Preservation of Mechanical and Morphological Properties of Porcine Cardiac Outflow Vessels after Decellularization and Wet Storage. Biomimetics 2023, 8, 315. https://doi.org/10.3390/biomimetics8030315
Sergeevichev D, Vasiliyeva M, Kuznetsova E, Chelobanov B. Preservation of Mechanical and Morphological Properties of Porcine Cardiac Outflow Vessels after Decellularization and Wet Storage. Biomimetics. 2023; 8(3):315. https://doi.org/10.3390/biomimetics8030315
Chicago/Turabian StyleSergeevichev, David, Maria Vasiliyeva, Elena Kuznetsova, and Boris Chelobanov. 2023. "Preservation of Mechanical and Morphological Properties of Porcine Cardiac Outflow Vessels after Decellularization and Wet Storage" Biomimetics 8, no. 3: 315. https://doi.org/10.3390/biomimetics8030315
APA StyleSergeevichev, D., Vasiliyeva, M., Kuznetsova, E., & Chelobanov, B. (2023). Preservation of Mechanical and Morphological Properties of Porcine Cardiac Outflow Vessels after Decellularization and Wet Storage. Biomimetics, 8(3), 315. https://doi.org/10.3390/biomimetics8030315






