Formation of Hydroxyapatite-Based Hybrid Materials in the Presence of Platelet-Poor Plasma Additive
Abstract
1. Introduction
2. Materials and Methods
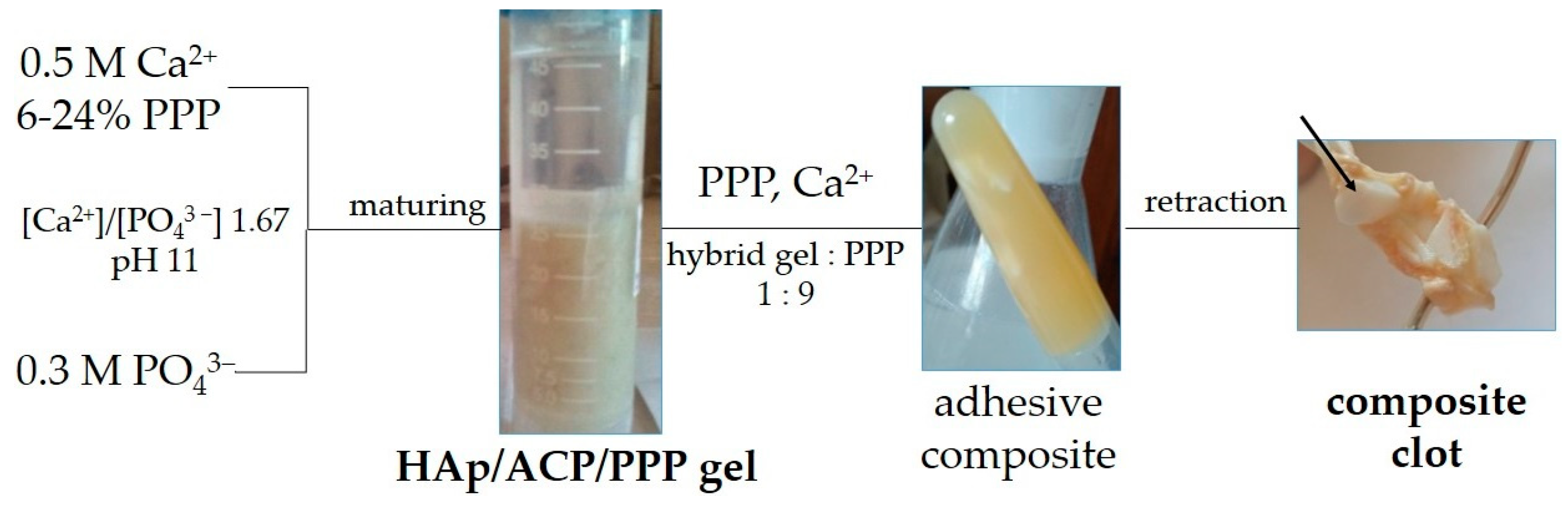
2.1. Reagents, Additives, and Sample Preparation
2.2. Characterization
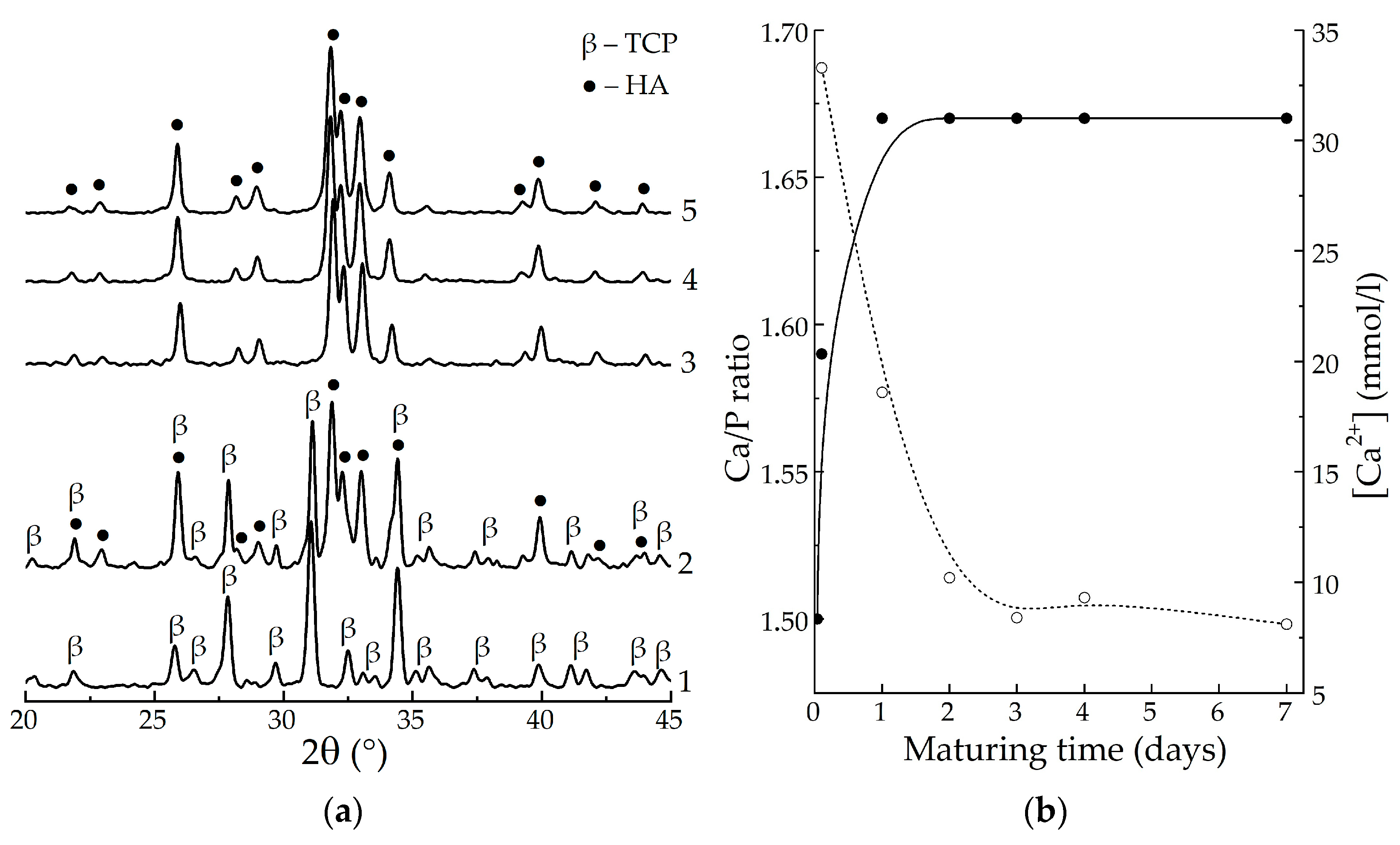
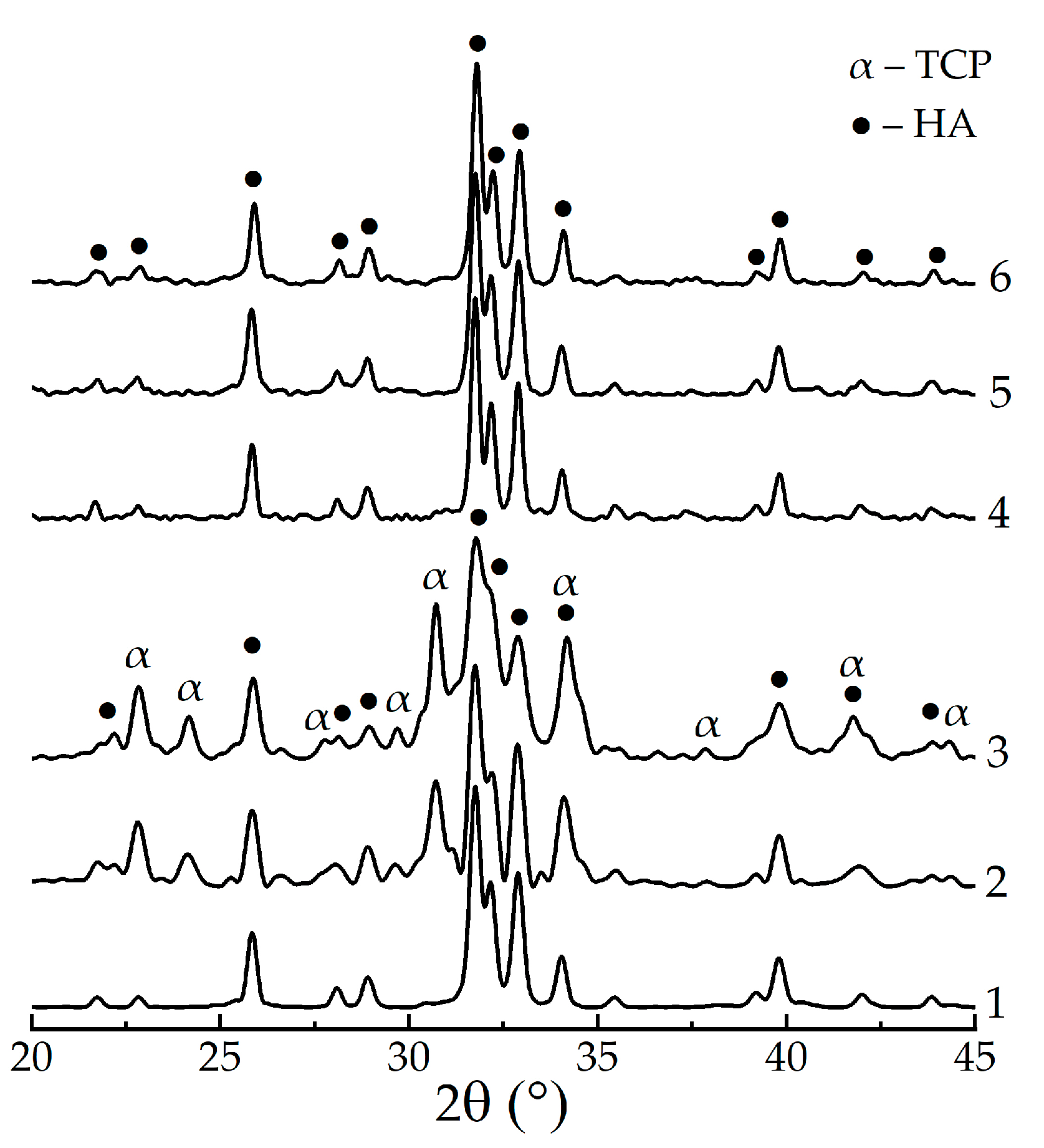
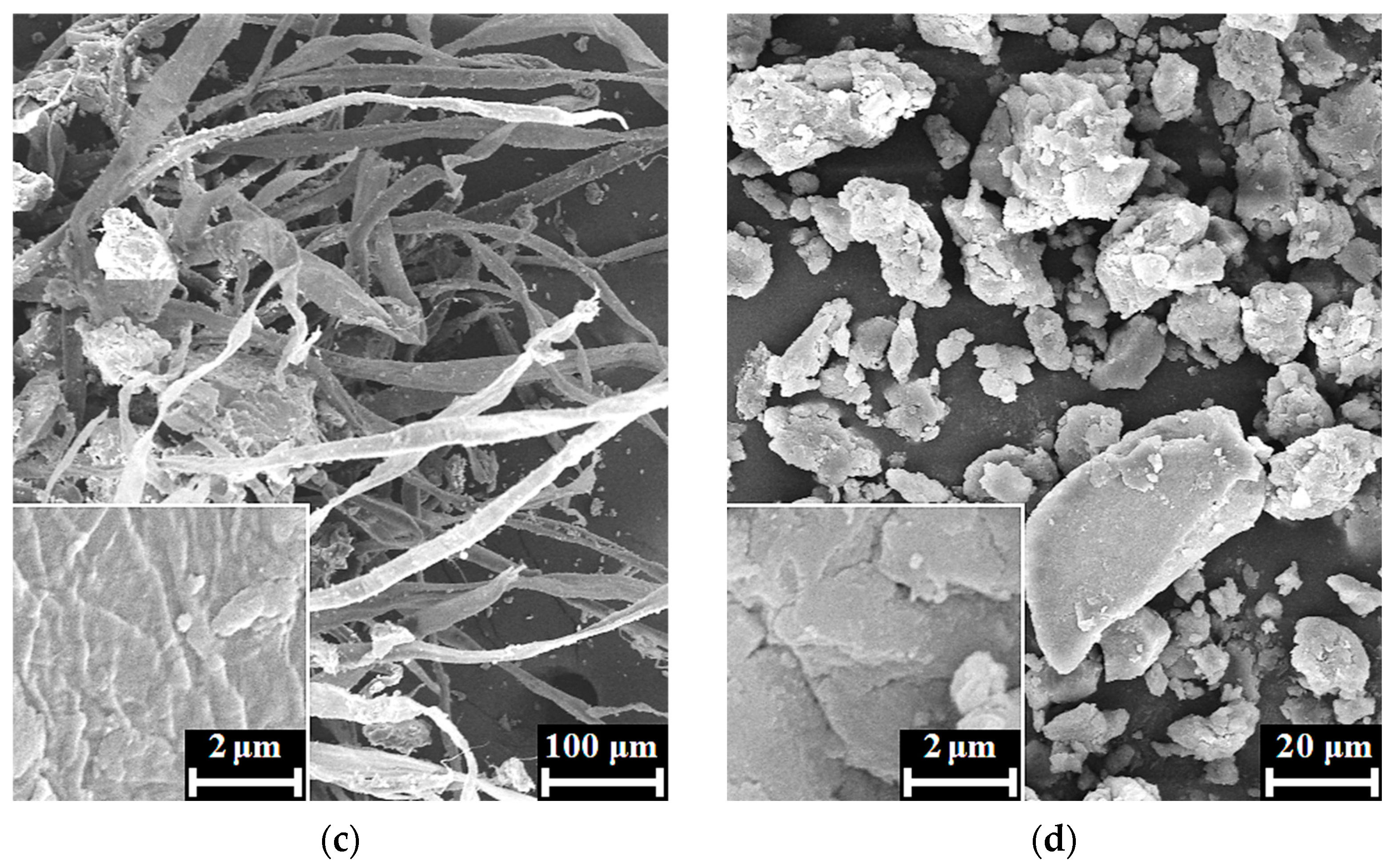
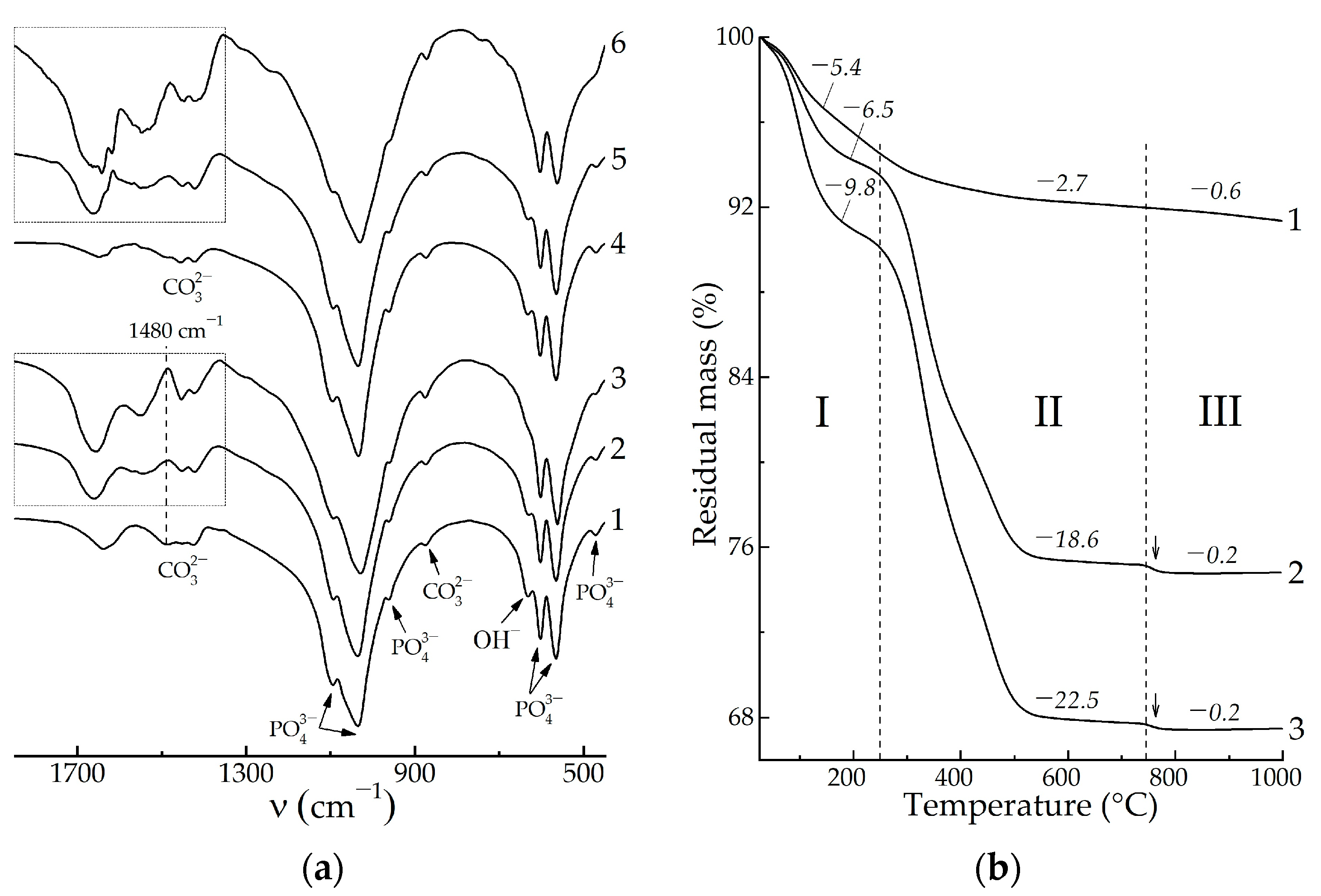
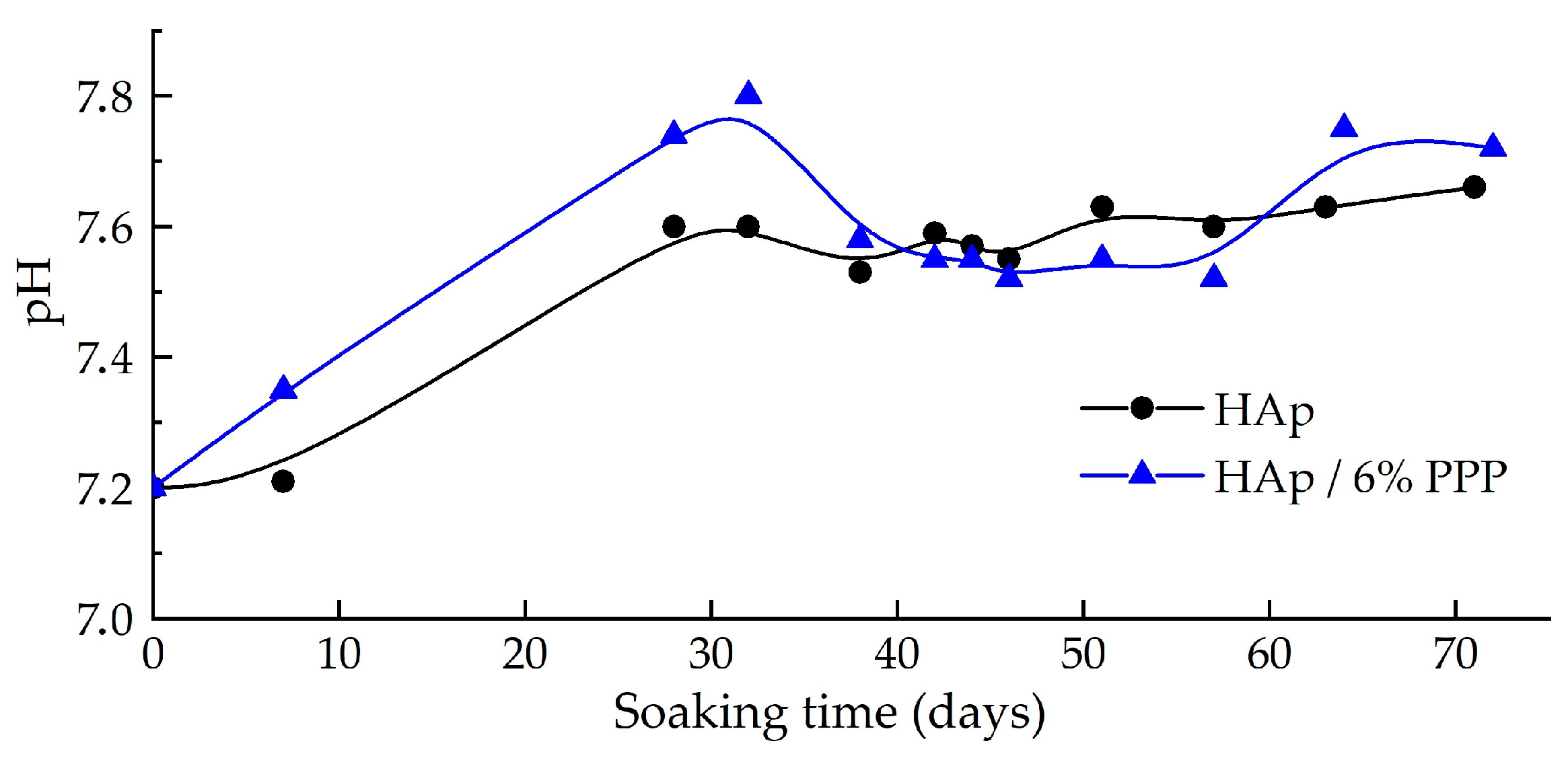
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, H.F.; Cengiz, I.F.; Silva, F.S.; Reis, R.L.; Oliveira, J.M. Scaffolds and coatings for bone regeneration. J. Mater. Sci. Mater. Med. 2020, 31, 27. [Google Scholar] [CrossRef] [PubMed]
- Safronova, T.V. Inorganic materials for regenerative medicine. Inorg. Mater. 2021, 57, 443–474. [Google Scholar] [CrossRef]
- Villa, T.; Brianza, S. Form and function of resorbable materials–based medical devices. In Bioresorbable Polymers for Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2017; pp. 95–100. [Google Scholar] [CrossRef]
- Hajebi, S.; Nasr, S.M.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Bioresorbable composite polymeric materials for tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 926–940. [Google Scholar] [CrossRef]
- Kazakova, G.; Safronova, T.; Golubchikov, D.; Shevtsova, O.; Rau, J.V. Resorbable Mg2+-containing phosphates for bone tissue repair. Materials 2021, 14, 4857. [Google Scholar] [CrossRef]
- Everts, V.; Jansen, I.D.C.; de Vries, T.J. Mechanisms of bone resorption. Bone 2022, 163, 116499. [Google Scholar] [CrossRef]
- Edén, M. Structure and formation of amorphous calcium phosphate and its role as surface layer of nanocrystalline apatite: Implications for bone mineralization. Materialia 2021, 17, 101107. [Google Scholar] [CrossRef]
- DileepKumar, V.G.; Sridhar, M.S.; Aramwit, P.; Krut’ko, V.K.; Musskaya, O.N.; Glazov, I.E.; Reddy, N. A Review on the Synthesis and Properties of Hydroxyapatite. J. Biomater. Sci. Polym. Ed. 2022, 33, 229–261. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Synthetic amorphous calcium phosphates (ACPs): Preparation, structure, properties, and biomedical applications. Biomater. Sci. 2021, 9, 7748–7798. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C.C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Biphasic, triphasic and multiphasic calcium orthophosphates. Acta Biomater. 2012, 8, 963–977. [Google Scholar] [CrossRef]
- Glazov, I.E.; Krut’ko, V.K.; Musskaya, O.N.; Kulak, A.I. Calcium Phosphate Apatites: Wet Formation, Thermal Transformations, Terminology, and Identification. Russ. J. Inorg. Chem. 2022, 67, 173–182. [Google Scholar] [CrossRef]
- Krebs, H.A. Chemical composition of blood plasma and serum. Ann. Rev. Biochem. 1950, 19, 409–430. [Google Scholar] [CrossRef]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Intern. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfest, D.M.D.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotech. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Pepkowitz, S.H.; Klapper, E. Platelet-rich plasma for the replenishment of bone. Curr. Osteopor. Rep. 2011, 9, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Mijiritsky, E.; Gardin, C.; Ferroni, L.; Lacza, Z.; Zavan, B. Albumin-impregnated bone granules modulate the interactions between mesenchymal stem cells and monocytes under in vitro inflammatory conditions. Mater. Sci. Eng. C 2020, 110, 110678. [Google Scholar] [CrossRef]
- Hunter, G.K. Role of osteopontin in modulation of hydroxyapatite formation. Calcif. Tiss. Int. 2013, 93, 348–354. [Google Scholar] [CrossRef]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef]
- Garnett, J.; Dieppe, P. The effects of serum and human albumin on calcium hydroxyapatite crystal growth. Biochem. J. 1990, 266, 863–868. [Google Scholar]
- Glazov, I.E.; Krut’ko, V.K.; Kulak, A.I.; Musskaya, O.N.; Vlasov, R.A.; Malakhovsky, P.O.; DileepKumar, V.G.; Surya, P.S.; Sridhar, M.S.; Reddy, N. Effect of platelet-poor plasma additive on the formation of biocompatible calcium phosphates. Mater. Today Commun. 2021, 47, 102224. [Google Scholar] [CrossRef]
- Suvarnapathaki, S.; Wu, X.; Lantigua, D.; Nguyen, M.A.; Camci-Unal, G. Hydroxyapatite-incorporated composite gels improve mechanical properties and bioactivity of bone scaffolds. Macromol. Biosci. 2020, 20, 2000176. [Google Scholar] [CrossRef] [PubMed]
- Spotnitz, W.D. Fibrin sealant: Past, present, and future: A brief review. World J. Surg. 2010, 34, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Ducheyne, P.; Radin, S. Determination of the Ca/P ratio in calcium-deficient hydroxyapatite using X-ray diffraction analysis. J. Mater. Sci. Mater. Med. 1993, 4, 165–168. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2015, 27, 2907–2915. [Google Scholar] [CrossRef]
- Bar-Yosef Ofir, P.; Govrin-Lippman, R.; Garti, N.; Füredi-Milhofer, H. The influence of polyelectrolytes on the formation and phase transformation of amorphous calcium phosphate. Cryst. Gr. Des. 2004, 4, 177–183. [Google Scholar] [CrossRef]
- Chandrasekar, A.; Sagadevan, S.; Dakshnamoorthy, A. Synthesis and characterization of nano-hydroxyapatite (n-HAP) using the wet chemical technique. Int. J. Phys. Sci. 2013, 8, 1639–1645. [Google Scholar]
- Pretorius, E.; Oberholzer, H.M.; van der Spuy, W.J.; Swanepoel, A.C.; Soma, P. Scanning electron microscopy of fibrin networks in rheumatoid arthritis: A qualitative analysis. Rheumatolog. Int. 2012, 32, 1611–1615. [Google Scholar] [CrossRef]
- Glazov, I.E.; Krut’ko, V.K.; Musskaya, O.N.; Kulak, A.I. Low-temperature formation and identification of biphasic calcium carbonate-phosphates. Russ. J. Inorg. Chem. 2022, 67, 1718–1730. [Google Scholar] [CrossRef]
- Litvinov, R.I.; Faizullin, D.A.; Zuev, Y.F.; Weisel, J.W. The α-helix to β-sheet transition in stretched and compressed hydrated fibrin clots. Biophys. J. 2012, 103, 1020–1027. [Google Scholar] [CrossRef]
- Krut’ko, V.K.; Kulak, A.I.; Lesnikovich, L.A.; Trofimova, I.V.; Musskaya, O.N.; Zhavnerko, G.K.; Paribok, I.V. Influence of the Dehydration Procedure on the Physicochemical Properties of Nanocrystalline Hydroxylapatite Xerogel. Russ. J. Gen. Chem. 2007, 77, 336–342. [Google Scholar] [CrossRef]
- Uskoković, V. The role of hydroxyl channel in defining selected physicochemical peculiarities exhibited by hydroxyapatite. RSC Adv. 2015, 46, 36614–36633. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Veis, A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem. Rev. 2008, 108, 4670–4693. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Lin, K.; Gou, Z.; Engqvist, H. Morphology control of hydroxyapatite crystal and its aggregates. Hydroxyap. Synth. Prop. App. 2012, 6, 243–265. [Google Scholar]
- Nawawi, N.; Alqap, A.; Sopyan, I. Recent progress on hydroxyapatite-based dense biomaterials for load bearing bone substitutes. Rec. Pat. Mater. Sci. 2011, 4, 63–80. [Google Scholar] [CrossRef]
- Peter, I.; Wu, K.; Diaz, R.; Borg-Stein, J. Platelet-rich plasma. Phys. Med. Rehabilit. Clin. 2016, 27, 825–853. [Google Scholar] [CrossRef]
- Skwarcz, S.; Bryzek, I.; Gregosiewicz, A.; Warda, E.; Gaweda, K.; Tarczynska, M.; Skwacz, J.; Nadulski, R.; Starek, A.; Sanford, J. The effect of activated platelet-rich plasma (PRP) on tricalcium hydroxyapatite phosphate healing in experimental, partial defects of long bone shafts in animal models. Pol. J. Vet. Sci. 2019, 22, 243–250. [Google Scholar] [CrossRef]
- Álvarez, C.; Rendueles, M.; Díaz, M. Alkaline hydrolysis of porcine blood haemoglobin: Applications for peptide and amino acid production. Anim. Prod. Sci. 2012, 53, 121–128. [Google Scholar] [CrossRef]
- Caudron, J.-M.; Ford, N.; Henkens, M.; Macé, C.; Kiddle-Monroe, R.; Pinel, J. Substandard medicines in resource-poor settings: A problem that can no longer be ignored. Trop. Med. Int. Health. 2008, 13, 1062–1072. [Google Scholar] [CrossRef]

| Sample | Maturing Time, Days | Content of Phases, % | Ca/P | |
|---|---|---|---|---|
| ACP (60 °C) 1 | α-TCP (800 °C) | |||
| HAp | 4 | – | – | 1.67 |
| HAp/6% PPP | 46 | 44 | 1.59 | |
| HAp/24% PPP | 47 | 45 | 1.59 | |
| HAp | 9 | – | – | 1.67 |
| HAp/6% PPP | – | – | 1.67 | |
| HAp/24% PPP | – | – | 1.67 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glazov, I.E.; Krut’ko, V.K.; Safronova, T.V.; Sazhnev, N.A.; Kil’deeva, N.R.; Vlasov, R.A.; Musskaya, O.N.; Kulak, A.I. Formation of Hydroxyapatite-Based Hybrid Materials in the Presence of Platelet-Poor Plasma Additive. Biomimetics 2023, 8, 297. https://doi.org/10.3390/biomimetics8030297
Glazov IE, Krut’ko VK, Safronova TV, Sazhnev NA, Kil’deeva NR, Vlasov RA, Musskaya ON, Kulak AI. Formation of Hydroxyapatite-Based Hybrid Materials in the Presence of Platelet-Poor Plasma Additive. Biomimetics. 2023; 8(3):297. https://doi.org/10.3390/biomimetics8030297
Chicago/Turabian StyleGlazov, Ilya E., Valentina K. Krut’ko, Tatiana V. Safronova, Nikita A. Sazhnev, Natalia R. Kil’deeva, Roman A. Vlasov, Olga N. Musskaya, and Anatoly I. Kulak. 2023. "Formation of Hydroxyapatite-Based Hybrid Materials in the Presence of Platelet-Poor Plasma Additive" Biomimetics 8, no. 3: 297. https://doi.org/10.3390/biomimetics8030297
APA StyleGlazov, I. E., Krut’ko, V. K., Safronova, T. V., Sazhnev, N. A., Kil’deeva, N. R., Vlasov, R. A., Musskaya, O. N., & Kulak, A. I. (2023). Formation of Hydroxyapatite-Based Hybrid Materials in the Presence of Platelet-Poor Plasma Additive. Biomimetics, 8(3), 297. https://doi.org/10.3390/biomimetics8030297









