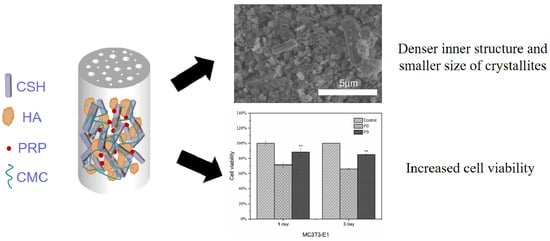
Effect of Platelet-Rich Plasma Addition on the Chemical Properties and Biological Activity of Calcium Sulfate Hemihydrate Bone Cement
Abstract
1. Introduction
2. Materials and Methods
2.1. PRP Isolation
2.2. Materials
2.3. Samples Preparation
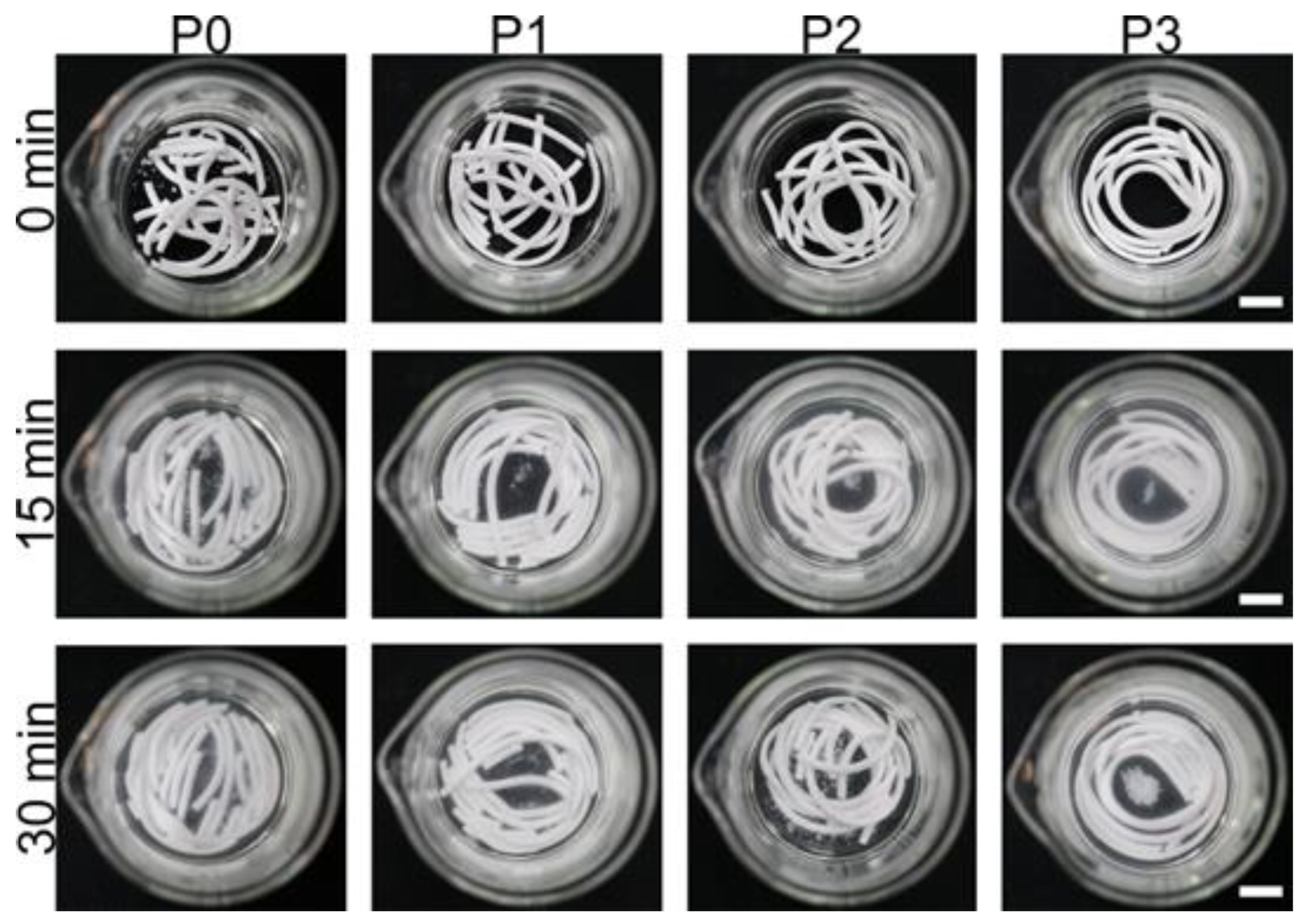
2.4. Washout Resistance Testing and Injectability Testing
2.5. Setting Time
2.6. Mechanical Strength Testing
2.7. Characterization of Bone Cement
2.8. Surface Zeta Potential and Protein Adsorption of Bone Cement
2.9. pH of Bone Cement
2.10. In Vitro Degradation Analysis
2.11. Hemolytic Rate Measurement
2.12. In Vitro Cytotoxicity
2.13. ALP Assays and Alizarin Red Staining (ARS)
2.14. qRT-PCR Assay
2.15. Western Blot Assay
2.16. Statistical Analysis
3. Results
3.1. Platelet Concentration
3.2. Washout Resistance Testing and Injectability Testing
3.3. Setting Time and Compressive Strength
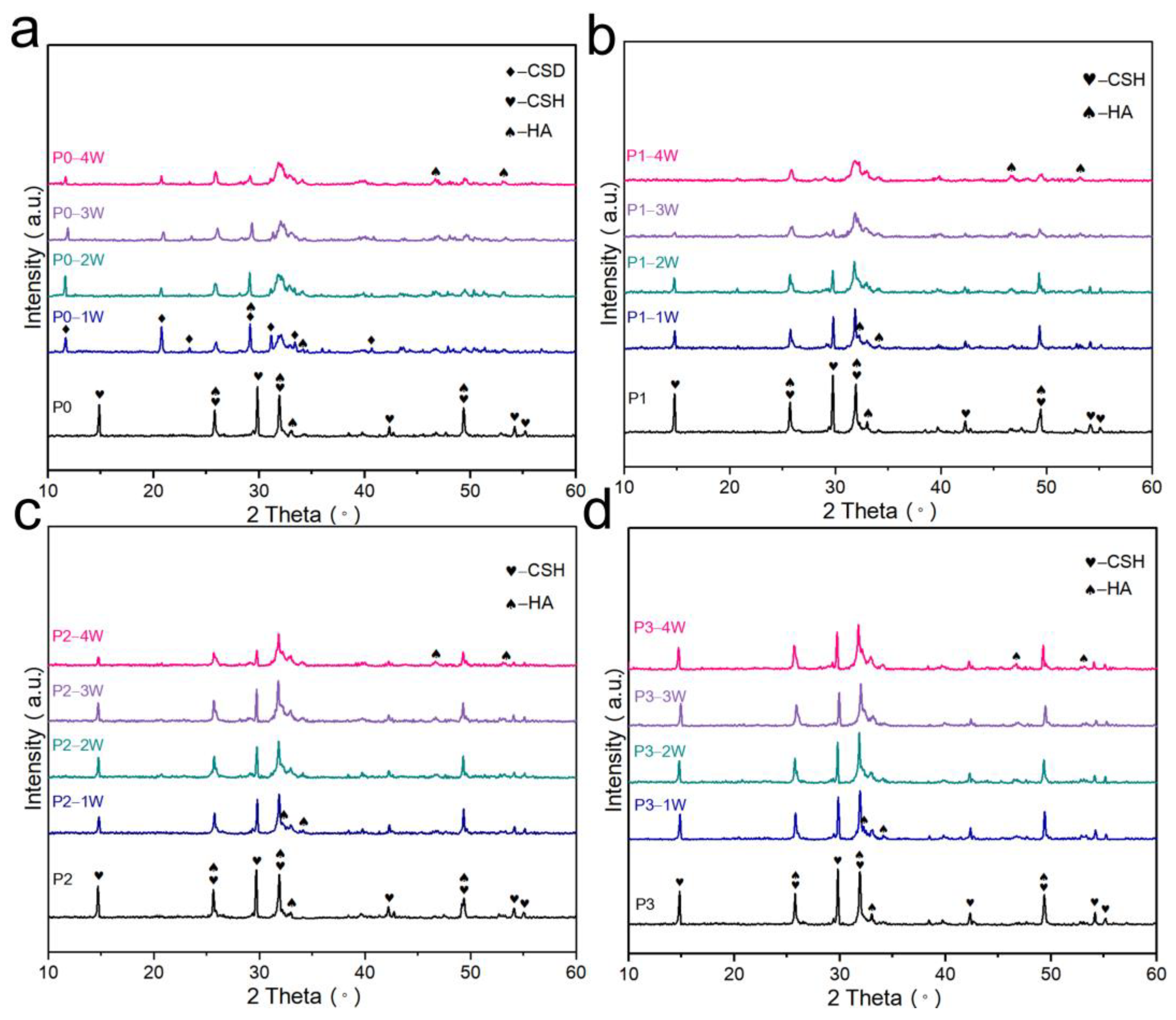
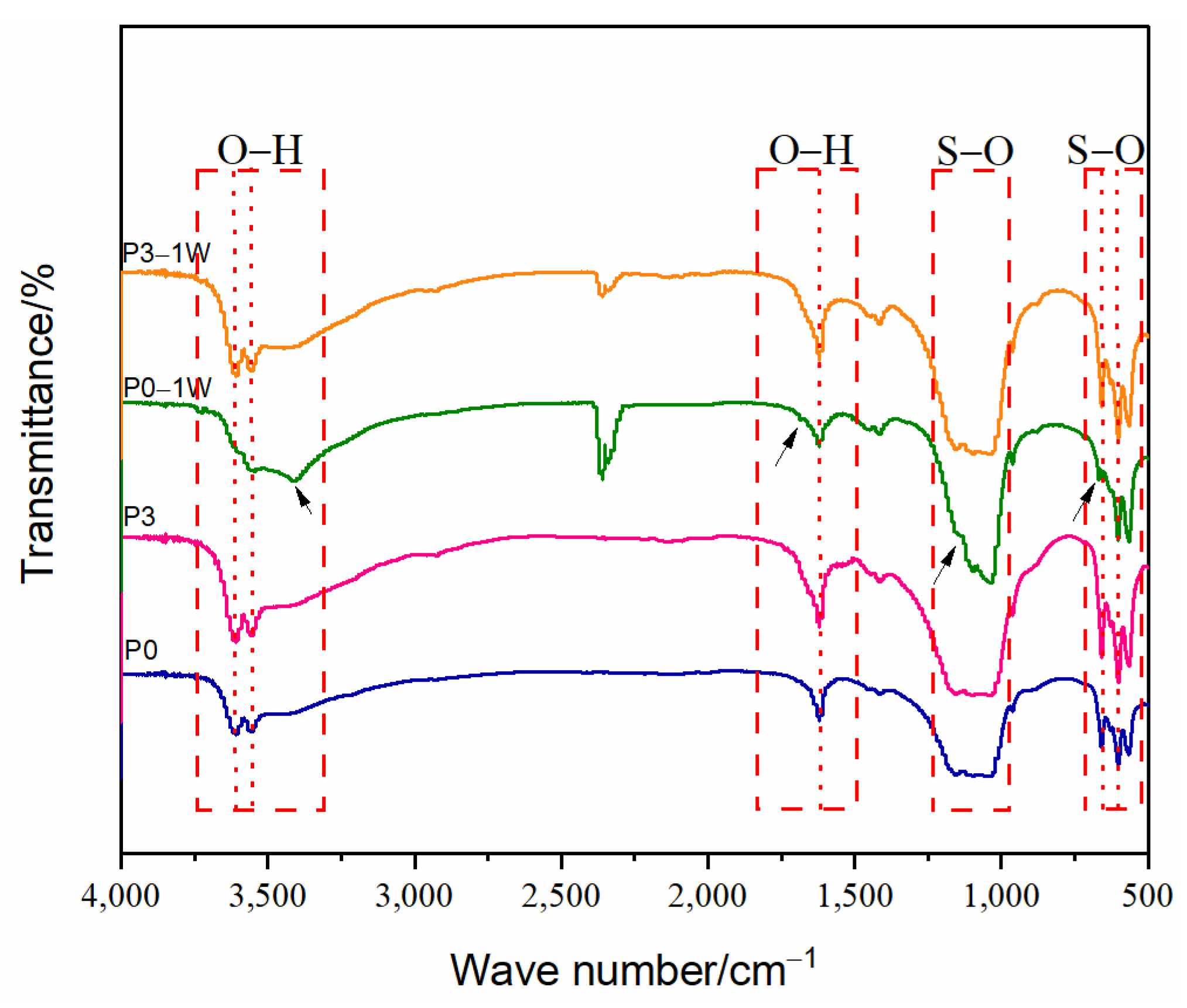
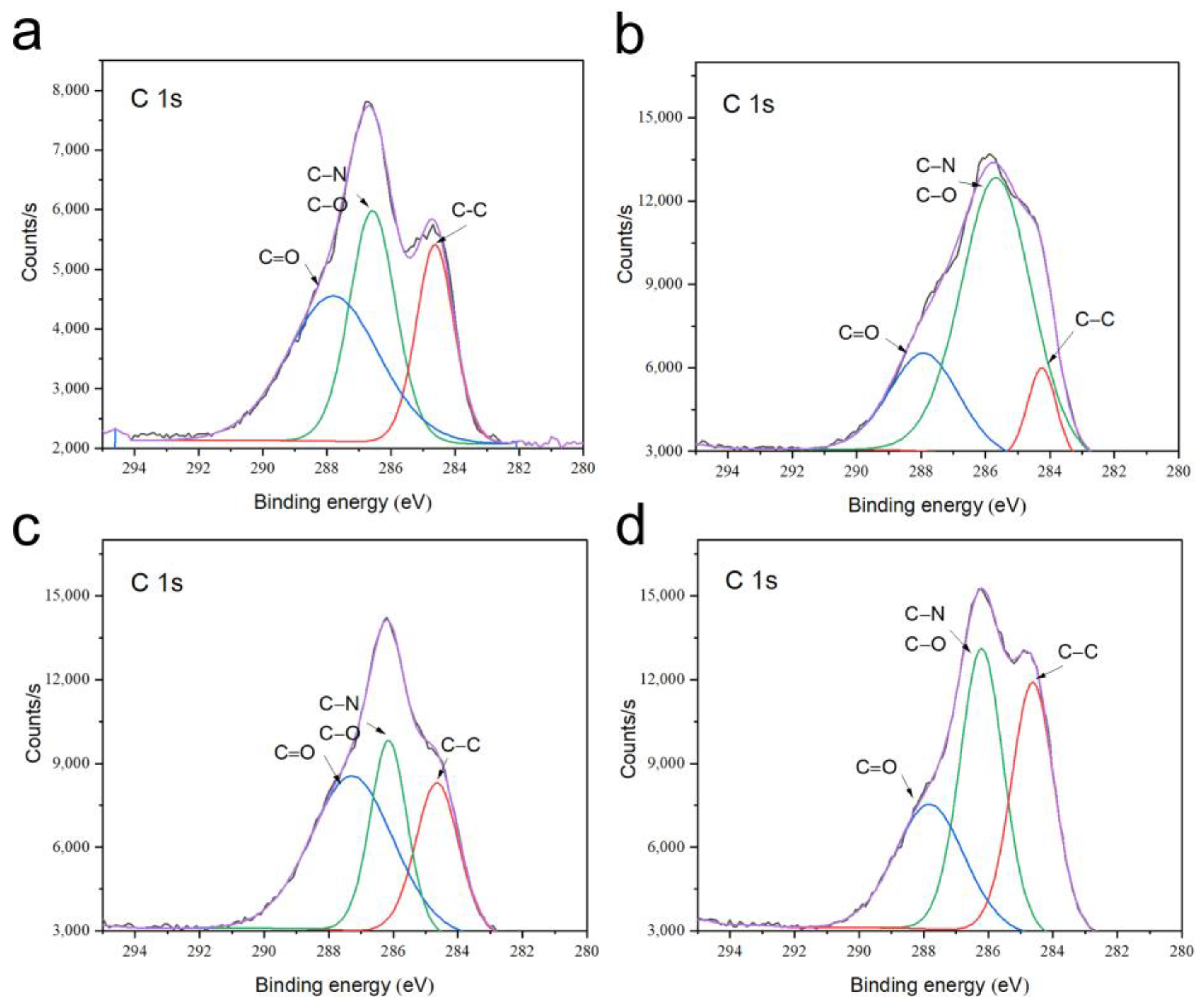
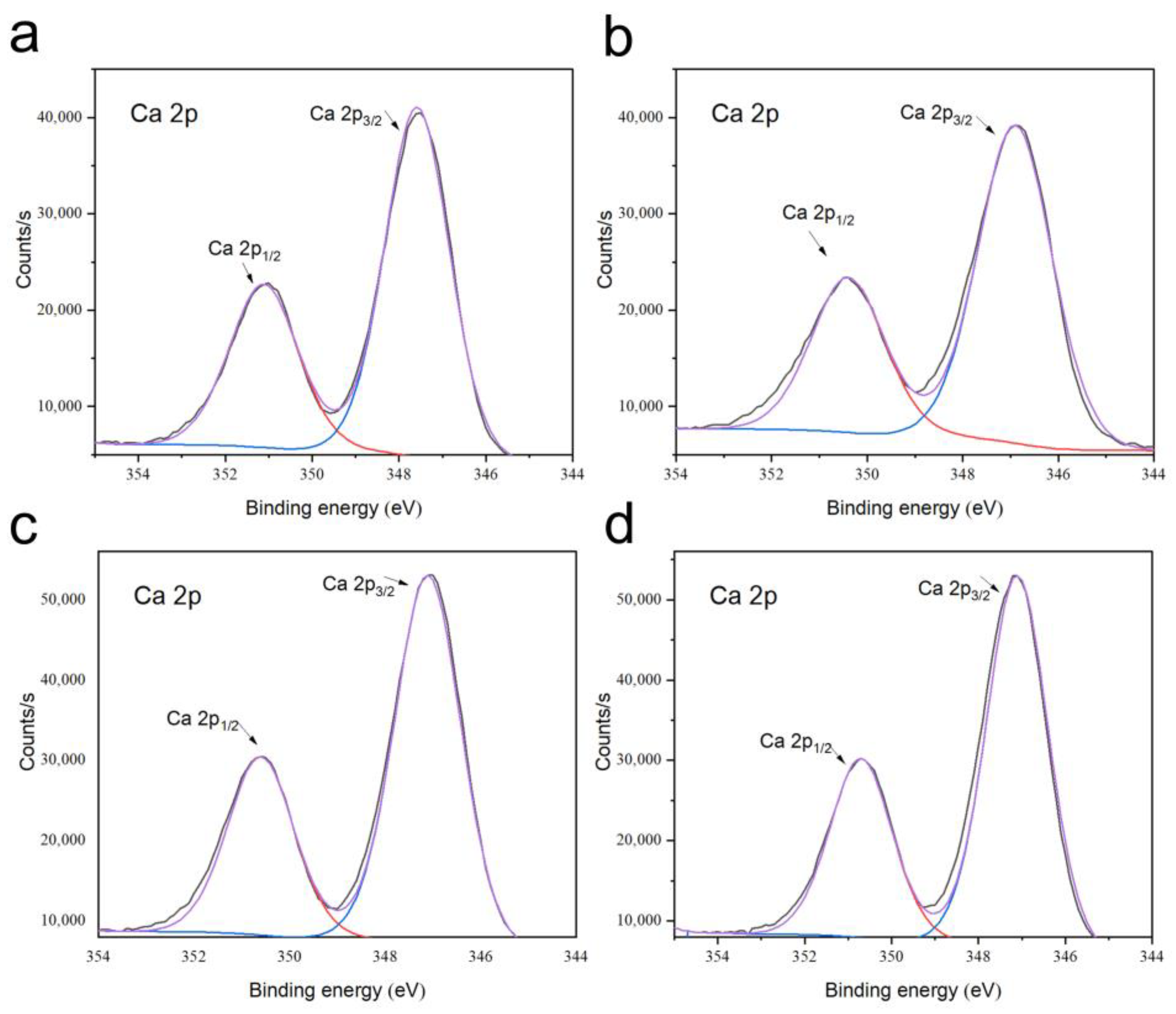
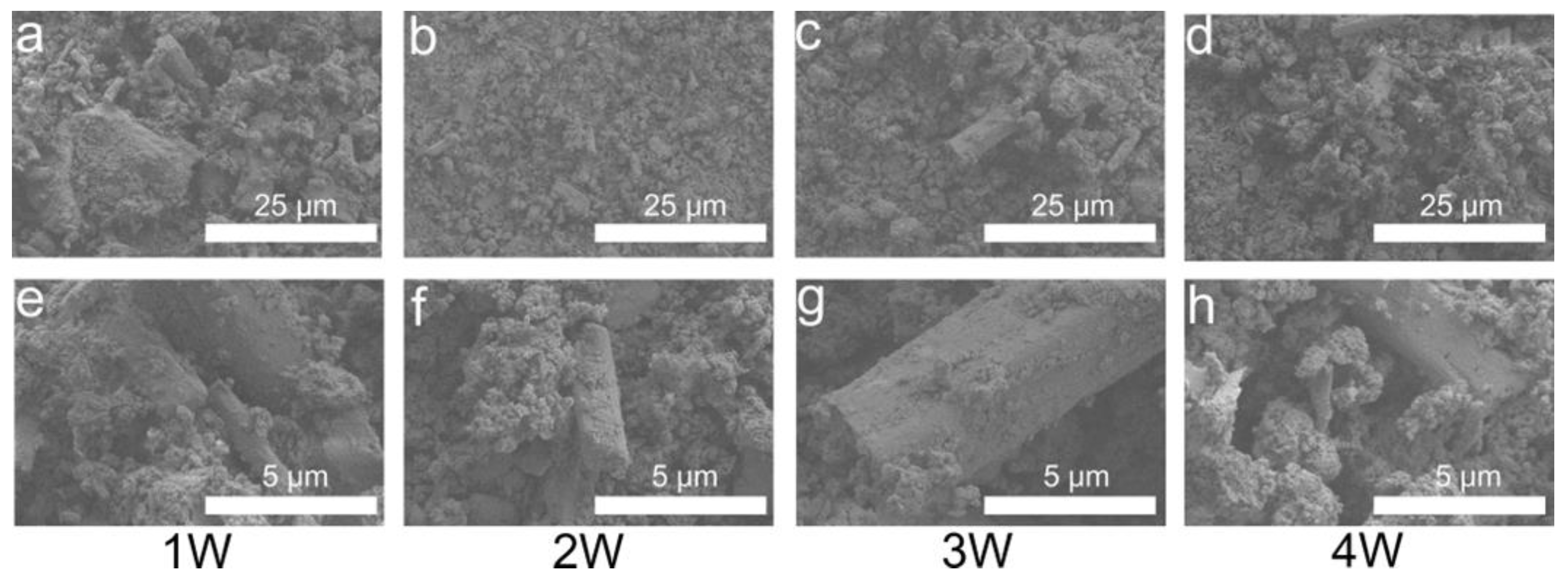
3.4. Phase and Microstructural Analysis
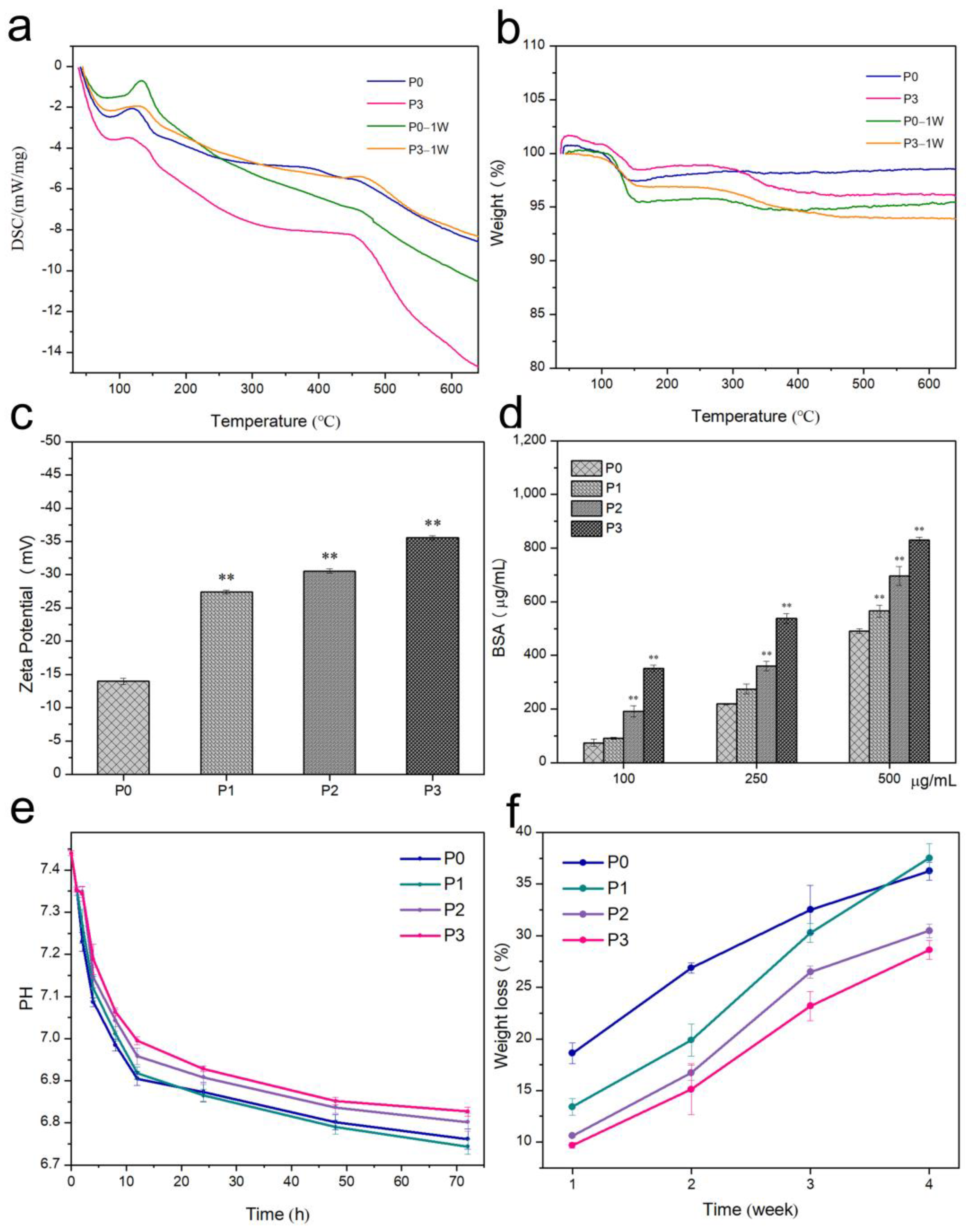
3.5. Thermal Stability
3.6. Surface Zeta Potential and Protein Adsorption of Bone Cement
3.7. pH of Bone Cement
3.8. In Vitro Degradation
3.9. Hemolytic Rate and In Vitro Biocompatibility
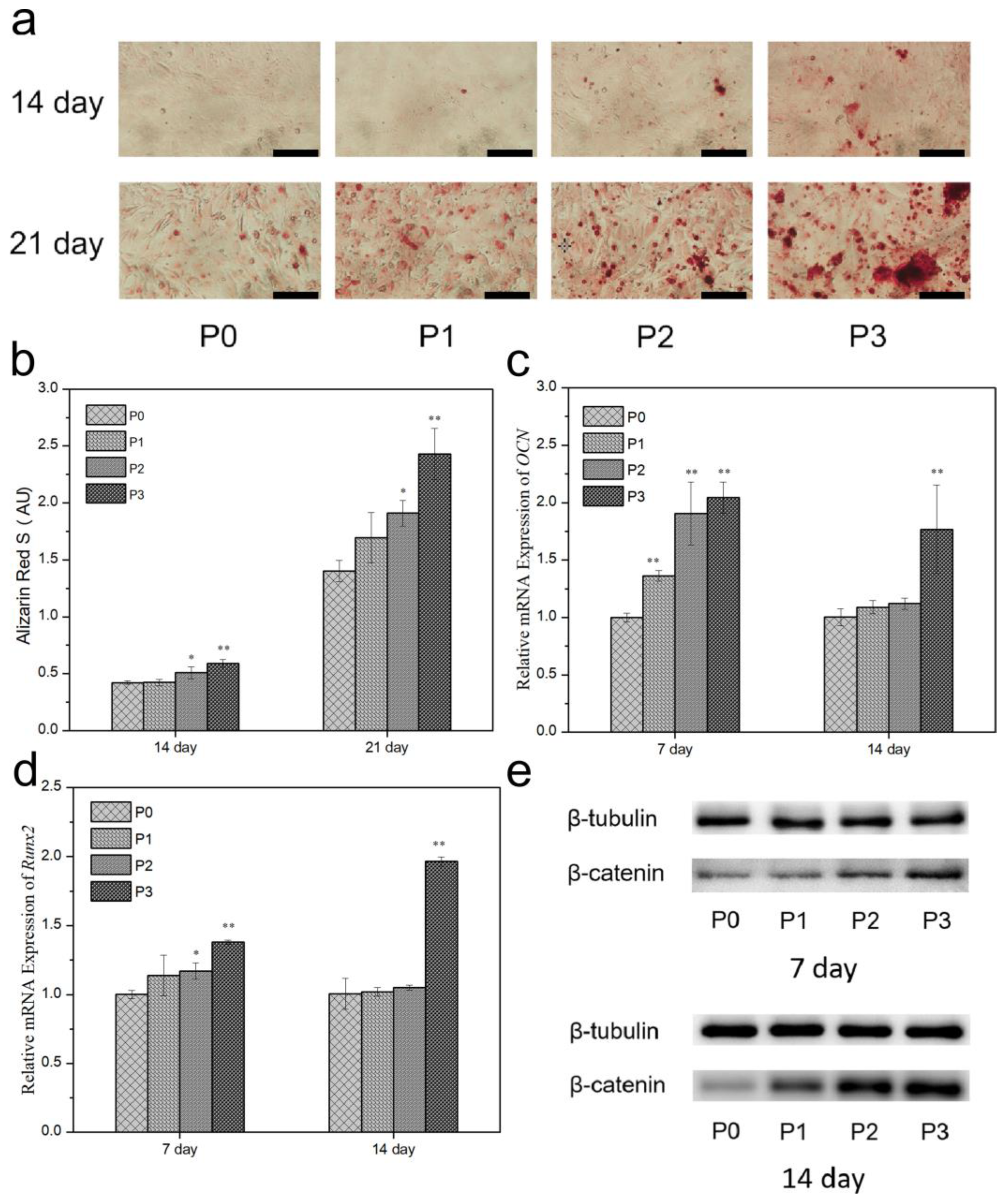
3.10. ALP and Mineralization
3.11. Gene Expression
3.12. Protein Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, Y.; Ma, P.; Wang, Y. Construction and properties of an osteogenic-antibacterial functionalised drug delivery system based on hydroxyapatite microspheres. Inorg. Chem. Commun. 2022, 140, 109419. [Google Scholar] [CrossRef]
- Czechowska, J.; Zima, A.; Siek, D.; Ślósarczyk, A. The importance of chitosan and nano-TiHA in cement-type composites on the basis of calcium sulfate. Ceram. Int. 2016, 42, 15559–15567. [Google Scholar] [CrossRef]
- Fillingham, Y.; Jacobs, J. Bone grafts and their substitutes. Bone Jt. J. 2016, 98, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, H.; Liu, X.; Cui, F.-Z. Injectable calcium sulfate/mineralized collagen-based bone repair materials with regulable self-setting properties. J. Biomed. Mater. Res. A 2011, 99, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yu, B.; Pei, P.; Ding, H.; Yu, B.; Zhu, Y. 3D printing of pearl/CaSO4 composite scaffolds for bone regeneration. J. Mater. Chem. B 2018, 6, 499–509. [Google Scholar] [CrossRef]
- Favvas, E.P.; Stefanopoulos, K.L.; Vordos, N.C.; Drosos, G.I.; Mitropoulos, A.C. Structural characterization of calcium sulfate bone graft substitute cements. Mater. Res. 2016, 19, 1108–1113. [Google Scholar] [CrossRef]
- Fleiter, N.; Walter, G.; Bösebeck, H.; Vogt, S.; Büchner, H.; Hirschberger, W.; Hoffmann, R. Clinical use and safety of a novel gentamicin-releasing resorbable bone graft substitute in the treatment of osteomyelitis/osteitis. Bone Jt. Res. 2014, 3, 223–229. [Google Scholar] [CrossRef]
- Shuai, C.; Zhou, J.; Wu, P.; Gao, C.; Feng, P.; Xiao, T.; Deng, Y.; Peng, S. Enhanced stability of calcium sulfate scaffolds with 45S5 bioglass for bone repair. Materials 2015, 8, 7498–7510. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Yao, D.; Xu, Q.; Liu, L.; Tian, Z.; Zhu, Y. Effects of mesoporous bioglass on physicochemical and biological properties of calcium sulfate bone cements. Appl. Mater. Today 2017, 9, 612–621. [Google Scholar] [CrossRef]
- Chen, C.; Wang, C.; Hsueh, N.; Ding, S. Improvement of in vitro physicochemical properties and osteogenic activity of calcium sulfate cement for bone repair by dicalcium silicate. J. Alloys Compd. 2014, 585, 25–31. [Google Scholar] [CrossRef]
- Huan, Z.; Chang, J. Self-setting properties and in vitro bioactivity of calcium sulfate hemihydrate-tricalcium silicate composite bone cements. Acta Biomater. 2007, 3, 952–960. [Google Scholar] [CrossRef]
- Kolekar, T.V.; Bandgarc, S.S.; Yadav, H.M.; Kim, D.; Magalad, V.T. Hemolytic and biological assessment of lithium substituted hydroxyapatite nanoparticles for L929 and Hela cervical cancer cells. Inorg. Chem. Commun. 2022, 137, 109172. [Google Scholar] [CrossRef]
- Murugesan, V.; Vaiyapuri, M.; Murugeasan, A. Fabrication and characterization of strontium substituted chitosan modify hydroxyapatite for biomedical applications. Inorg. Chem. Commun. 2022, 142, 109653. [Google Scholar] [CrossRef]
- Pupilli, F.; Ruffini, A.; Dapporto, M.; Tavoni, M.; Tampieri, A.; Sprio, S. Design strategies and biomimetic approaches for calcium phosphate scaffolds in bone tissue regeneration. Biomimetics 2022, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Zheng, M.H.; Tägil, M. The composite of hydroxyapatite and calcium sulphate: A review of preclinical evaluation and clinical applications. Expert Rev. Med. Devic. 2013, 10, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Hesaraki, S.; Moztarzadeh, F.; Nemati, R.; Nezafati, N. Preparation and characterization of calcium sulfate-biomimetic apatite nanocomposites for controlled release of antibiotics. J. Biomed. Mater. Res. B 2009, 91, 651–661. [Google Scholar] [CrossRef]
- Reynolds, M.A.; Aichelmann-Reidy, M.E.; Kassolis, J.D.; Prasad, H.S.; Rohrer, M.D. Calcium sulfate-carboxymethylcellulose bone graft binder: Histologic and morphometric evaluation in a critical size defect. J. Biomed. Mater. Res. B 2007, 2, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Ravoor, J. A study on retention of MWCNT in robocasted MWCNT-HAP scaffold structures using vacuum sintering technique and their characteristics. Ceram. Int. 2022, 48, 31289–31298. [Google Scholar] [CrossRef]
- Agis, H.; Beirer, B.; Watzek, G.; Gruber, R. Effects of carboxymethylcellulose and hydroxypropylmethylcellulose on the differentiation and activity of osteoclasts and osteoblasts. J. Biomed. Mater. Res. A 2010, 95, 504–509. [Google Scholar] [CrossRef]
- Hu, M.; Chu, P.; Huang, S.; Shih, B.; Ko, C.; Hu, J.; Chen, W. Injectability, processability, drug loading, and antibacterial activity of gentamicin-impregnated mesoporous bioactive glass composite calcium phosphate bone cement in vitro. Biomimetics 2022, 7, 121. [Google Scholar] [CrossRef]
- Collins, T.; Alexander, D.; Barkatali, B. Platelet-rich plasma: A narrative review. Gen. Orthop. 2021, 6, 225–235. [Google Scholar] [CrossRef]
- Hasan, M.L.; Taz, M.; Lee, B. Effects of platelet-rich plasma on biological activity and bone regeneration of brushite-based calcium phosphate cement. J. Biomed. Mater. Res. B 2018, 106, 2316–2325. [Google Scholar] [CrossRef]
- Wang, F.; Guo, Y.; Lv, R.; Xu, W.; Wang, W. Development of nano-tricalcium phosphate/polycaprolactone/platelet-rich plasma biocomposite for bone defect regeneration. Arab. J. Chem. 2020, 13, 7160–7169. [Google Scholar] [CrossRef]
- Ko, C.; Chen, W.; Chen, J.; Wang, Y.; Shih, C.; Tyan, Y.; Hung, C.; Wang, J. Properties of osteoconductive biomaterials: Calcium phosphate cement with different ratios of platelet-rich plasma as identifiers. Mat. Sci. Eng. C 2013, 33, 3537–3544. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Shi, Z.; Xu, H.H.K.; Yang, B.; Weir, M.D.; Li, G.; Song, Y.; Wang, J.; Hu, K.; Wang, P.; et al. Bone regeneration in minipigs via calcium phosphate cement scaffold delivering autologous bone marrow mesenchymal stem cells and platelet-rich plasma. J. Tissue Eng. Regen. Med. 2017, 18, 937–948. [Google Scholar] [CrossRef]
- Latifi, M.; Sani, M.; Salmannejad, M.; Kabir-Salmani, M.; Bavanati, H.B.; Talaei-Khozani, T. Synergistic impact of platelet rich plasma-heparin sulfate with hydroxyapatite/zirconia on the osteoblast differentiation potential of adipose-derived mesenchymal stem cells. Cell Tissue Bank 2022, 23, 669–683. [Google Scholar] [CrossRef]
- Intini, G.; Andreana, S.; Margarone, J.E.; Bush, P.J.; Dziak, R. Engineering a bioactive matrix by modifications of calcium sulfate. Tissue Eng. 2004, 8, 997–1008. [Google Scholar] [CrossRef]
- Intini, G.; Andreana, S.; Intini, F.E.; Buhite, R.J.; Bobek, L.A. Calcium sulfate and platelet-rich plasma make a novel osteoinductive biomaterial for bone regeneration. J. Transl. Med. 2007, 5, 13. [Google Scholar] [CrossRef]
- Chen, H.; Ji, X.; Zhang, Q.; Tian, X.; Zhang, B.; Tang, P. Effects of calcium sulfate combined with platelet-rich plasma on restoration of long bone defect in rabbits. Chin. Med. J. 2016, 129, 557–561. [Google Scholar] [CrossRef]
- Syam, S.; Chang, C.; Lan, W.; Ou, K.; Huang, B.; Lin, Y.; Saito, T.; Tsai, H.; Chuo, Y.; Yang, T.; et al. An innovative bioceramic bone graft with platelet-rich plasma for rapid bone healing and regeneration in a rabbit model. Appl. Sci. 2021, 11, 5271. [Google Scholar] [CrossRef]
- Shin, H.-S.; Woo, H.-M.; Kang, B.-J. Optimisation of a double-centrifugation method for preparation of canine platelet-rich plasma. BMC Vet. Res. 2017, 13, 198. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Ye, J.; He, F. Setting behavior, mechanical property and biocompatibility of anti-washout wollastonite/calcium phosphate composite cement. Ceram. Int. 2016, 42, 13670–13681. [Google Scholar] [CrossRef]
- Li, X.; He, F.; Ye, J. Preparation, characterization and in vitro cell performance of anti-washout calcium phosphate cement modified by sodium polyacrylate. RSC Adv. 2017, 7, 32842–32849. [Google Scholar] [CrossRef]
- Weibrich, G.; Hansen, T.; Kleis, W.; Buch, R.; Hitzler, W.E. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone 2004, 34, 665–671. [Google Scholar] [CrossRef]
- Mellier, C.; Lefèvre, F.; Fayon, F.; Montouillout, V.; Despas, C.; Ferrec, M.L.; Boukhechba, F.; Walcarius, A.; Janvier, P.; Dutilleul, M.; et al. A straightforward approach to enhance the textural, mechanical and biological properties of injectable calcium phosphate apatitic cements (CPCs): CPC/blood composites, a comprehensive study. Acta Biomater. 2017, 62, 328–339. [Google Scholar] [CrossRef]
- Mandal, P.K.; Mandal, T.K. Anion water in gypsum (CaSO4·2H2O) and hemihydrate (CaSO4·1/2H2O). Cement Concr. Res. 2002, 32, 313–316. [Google Scholar] [CrossRef]
- Yin, S.; Yang, L. α or β-hemihydrates transformed from dihydrate calcium sulfate in a salt-mediated glycerol–water solution. J. Cryst. Growth 2020, 550, 125885. [Google Scholar] [CrossRef]
- Tsigkou, O.; Jones, J.R.; Polak, J.M.; Stevens, M.M. Differentiation of fetal osteoblasts and formation of mineralized bone nodules by 45S5 bioglass conditioned medium in the absence of osteogenic supplements. Biomaterials 2009, 30, 3542–3550. [Google Scholar] [CrossRef]
- Mirza, E.H.; Khan, A.A.; Al-Khureif, A.A.; Saadaldin, S.A.; Mohamed, B.A.; Fareedi, F.; Khan, M.M.; Alfayez, M.; Al-Fotawi, R.; Vallittu, P.K.; et al. Characterization of osteogenic cells grown over modified graphene-oxide-biostable polymers. Biomed. Mater. 2019, 14, 065004. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I.; Halleux, C.; Keller, H.; Pegurri, M.; Gooi, J.H.; Weber, P.B.; Feng, J.Q.; Bonewald, L.F.; Kneissel, M. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol. Cell Biol. 2010, 30, 3071–3085. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.P.; Traykova, T.; Planell, J.A. Calcium phosphate cements as bone drug delivery systems: A review. J. Control Release 2006, 113, 102–110. [Google Scholar] [CrossRef]
- Paluszkiewicz, C.; Czechowska, J.; Ślósarczyk, A.; Paszkiewicz, Z. Evaluation of a setting reaction pathway in the novel composite TiHA-CSD bone cement by FT-Raman and FT-IR spectroscopy. J. Mol. Struct. 2013, 1034, 289–295. [Google Scholar] [CrossRef]
- Lewis, K.N.; Thomas, M.V.; Puleo, D.A. Mechanical and degradation behavior of polymer-calcium sulfate composites. J. Mater. Sci. Mater. Med. 2006, 17, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Lioliou, M.G.; Paraskeva, C.A.; Koutsoukos, P.G.; Payatakes, A.C. Calcium sulfate precipitation in the presence of water-soluble polymers. J. Colloid Interface Sci. 2006, 303, 164–170. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Chen, Z.; Cui, F.; Liu, H.; Mao, K.; Wang, Y. Injectable bone cement based on mineralized collagen. J. Biomed. Mater. Res. B 2010, 94, 72–79. [Google Scholar] [CrossRef]
- Latifi, M.; Talaei-Khozani, T.; Mehraban-Jahromi, H.; Sani, M.; Sadeghi-Atabadi, M.; Fazel-Anvari, A.; Kabir-Salmani, M. Fabrication of platelet-rich plasma heparin sulfate/hydroxyapatite/zirconia scaffold. Bioinspir. Biomim. Nanobiomater. 2018, 7, 122–130. [Google Scholar] [CrossRef]
- Khan, A.A.; Mirza, E.H.; Mohamed, B.A.; El-Sharawy, M.A.; Hasil Al-Asmari., M.; Abdullah Al-Khureif, A.; Ahmad, D.M.; Vallittu, P.K. Static and dynamic mechanical properties of graphene oxide-based bone cementing agents. J. Compos. Mater. 2019, 53, 2297–2304. [Google Scholar] [CrossRef]
- Chen, R.; Wang, J.; Liu, C. Biomaterials act as enhancers of growth factors in bone regeneration. Adv. Funct. Mater. 2016, 26, 8810–8823. [Google Scholar] [CrossRef]
- Zhou, J.; Yuan, F.; Peng, S.; Xie, H.; Wu, P.; Feng, P.; Gao, C.; Yang, Y.; Guo, W.; Lai, D.; et al. Tunable degradation rate and favorable bioactivity of porous calcium sulfate scaffolds by introducing nano-hydroxyapatite. Appl. Sci. 2016, 6, 411. [Google Scholar] [CrossRef]
- Aquino-Martínez, R.; Angelo, A.P.; Pujol, F.V. Calcium-containing scaffolds induce bone regeneration by regulating mesenchymal stem cell differentiation and migration. Stem Cell Res. Ther. 2017, 8, 265. [Google Scholar] [CrossRef]
- Gustavsson, J.; Ginebra, M.P.; Engel, E.; Planell, J. Ion reactivity of calcium-deficient hydroxyapatite in standard cell culture media. Acta Biomater. 2011, 7, 4242–4252. [Google Scholar] [CrossRef] [PubMed]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cells Mater. 2014, 28, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Alliston, T.; Choy, L.; Ducy, P.; Karsenty, G.; Derynck, R. TGF-beta-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J. 2001, 20, 2254–2272. [Google Scholar] [CrossRef] [PubMed]



| Cement Type | Solid Phase Composition (g/g) | Liquid Phase Composition (Normal Saline/PRP [mL/mL]) | Final Liquid/Solid Ratio (mL/g) |
|---|---|---|---|
| P0 | HA (49%) a+ CSH (49%) b + CMC (2%) c | 5:0 | 0.9 |
| P1 | HA (49%) a+ CSH (49%) b + CMC (2%) c | 4:1 | 0.9 |
| P2 | HA (49%) a+ CSH (49%) b + CMC (2%) c | 3:2 | 0.9 |
| P3 | HA (49%) a+ CSH (49%) b + CMC (2%) c | 2:3 | 0.9 |
| Gene | 5′-3′ | Primers |
|---|---|---|
| OCN | forward | GAACCAAGAAGGCACAGACAGA |
| reverse | GGCGGGACACCTACTCTCAT | |
| Runx2 | forward | AGCAGGAGGGCAATAAGGTAGT |
| reverse | TCGTCACAAGCAGGGTTAAGC |
| Group | PLTs Count in Whole Blood (109/L) | PLTs Count in PRP (109/L) |
|---|---|---|
| 1 | 343.33 ± 25.42 | 1508.33 ± 35.31 |
| 2 | 381.00 ± 13.14 | 1534.00 ± 33.95 |
| 3 | 330.00 ± 16.39 | 1466.00 ± 42.06 |
| Cement Type | Injectability (%) |
|---|---|
| P0 | 96.51 ± 0.24 |
| P1 | 96.73 ± 0.12 |
| P2 | 96.95 ± 0.11 * |
| P3 | 97.01 ± 0.07 * |
| Cement Type | Initial Setting Time (min) | Final Setting Time (min) |
|---|---|---|
| P0 | 22.78 ± 0.76 | 89.25 ± 1.31 |
| P1 | 29.78 ± 0.41 ** | 120.06 ± 1.38 ** |
| P2 | 34.46 ± 0.52 ** | 122.57 ± 0.51 ** |
| P3 | 37.85 ± 1.11 ** | 133.23 ± 0.21 ** |
| Cement Type | Compressive Strength (MPa) | ||||
|---|---|---|---|---|---|
| 0 Week | 1 Week | 2 Weeks | 3 Weeks | 4 Weeks | |
| P0 | 5.06 ± 0.43 | 1.99 ± 0.34 | 0.91 ± 0.14 | 0.79 ± 0.06 | 0.76 ± 0.04 |
| P1 | 5.96 ± 0.26 ** | 2.28 ± 0.19 | 1.70 ± 0.33 ** | 1.19 ± 0.21 | 0.88 ± 0.15 |
| P2 | 6.61 ± 0.22 ** | 3.59 ± 0.16 ** | 2.59 ± 0.35 ** | 2.20 ± 0.30 * | 1.65 ± 0.12 ** |
| P3 | 6.84 ± 0.31 ** | 3.72 ± 0.26 ** | 2.74 ± 0.37 ** | 2.74 ± 0.37 ** | 1.81 ± 0.28 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Wang, Y.; Liang, Y.; Zhu, S.; Jiang, H.; Wu, S.; Ge, X.; Li, Z. Effect of Platelet-Rich Plasma Addition on the Chemical Properties and Biological Activity of Calcium Sulfate Hemihydrate Bone Cement. Biomimetics 2023, 8, 262. https://doi.org/10.3390/biomimetics8020262
Liu J, Wang Y, Liang Y, Zhu S, Jiang H, Wu S, Ge X, Li Z. Effect of Platelet-Rich Plasma Addition on the Chemical Properties and Biological Activity of Calcium Sulfate Hemihydrate Bone Cement. Biomimetics. 2023; 8(2):262. https://doi.org/10.3390/biomimetics8020262
Chicago/Turabian StyleLiu, Jingyu, Yifan Wang, Yanqin Liang, Shengli Zhu, Hui Jiang, Shuilin Wu, Xiang Ge, and Zhaoyang Li. 2023. "Effect of Platelet-Rich Plasma Addition on the Chemical Properties and Biological Activity of Calcium Sulfate Hemihydrate Bone Cement" Biomimetics 8, no. 2: 262. https://doi.org/10.3390/biomimetics8020262
APA StyleLiu, J., Wang, Y., Liang, Y., Zhu, S., Jiang, H., Wu, S., Ge, X., & Li, Z. (2023). Effect of Platelet-Rich Plasma Addition on the Chemical Properties and Biological Activity of Calcium Sulfate Hemihydrate Bone Cement. Biomimetics, 8(2), 262. https://doi.org/10.3390/biomimetics8020262