Abstract
In this review, the current status of the influence of added ions (i.e., SiO44−, CO32−, etc.) and surface states (i.e., hydrated and non-apatite layers) on the biocompatibility nature of hydroxyapatite (HA, Ca10(PO4)6(OH)2) is discussed. It is well known that HA is a type of calcium phosphate with high biocompatibility that is present in biological hard tissues such as bones and enamel. This biomedical material has been extensively studied due to its osteogenic properties. The chemical composition and crystalline structure of HA change depending on the synthetic method and the addition of other ions, thereby affecting the surface properties related to biocompatibility. This review illustrates the structural and surface properties of HA substituted with ions such as silicate, carbonate, and other elemental ions. The importance of the surface characteristics of HA and its components, the hydration layers, and the non-apatite layers for the effective control of biomedical function, as well as their relationship at the interface to improve biocompatibility, has been highlighted. Since the interfacial properties will affect protein adsorption and cell adhesion, the analysis of their properties may provide ideas for effective bone formation and regeneration mechanisms.
1. Introduction
Bone grafting is one of the surgical treatments for bone defects caused by osteoporosis, bone tumors, and bone atrophy in dental implants, and is generally performed by using autologous or allogeneic bone grafts, in which the bone is taken from the patient’s own or a compatible donor, and then transplanted into the defect site. However, it presents some problems, such as increased burden on patients due to surgery and limitation of the amount of bone used [1,2,3,4]. Synthetic bone has recently been produced as an alternative to autogenous bone to address these problems. Calcium phosphate (CP), hydroxyapatite (HA), ceramics, CP cements, and bioactive glass are generally known as synthetic bone materials [5,6,7]. Synthetic bone has the advantages of high biocompatibility, fewer and minimal requirements for surgery, and low risk of inflammation; however, it is unable to induce cells associated with bone metabolism from the surrounding tissue after replacing a bone defect. Accordingly, the improvement of the ability to form new bone has been researched. Therefore, it is important to elucidate the mechanism of biological bone formation and to create synthetic bone that resembles biological bone.
Biological bone is composed of an organic matrix, reinforced by the deposition of inorganic salts consisting mainly of Ca2+ and PO43−, with 30 wt.% of organic matrix and 70 wt.% of inorganic salts. Biological bone is metabolically active, with “bone resorption”, the destruction of old bone, and “bone formation”, the creation of new bone. This cycle of bone metabolism is physiologically important, and its functions are to replace brittle old bone and to strengthen areas subjected to load [8,9]. Figure 1 shows the bone formation process. Osteocytes are derived from osteoblasts and are formed by the incorporation of osteoblasts into the bone matrix [10]. Osteocytes act as mechanosensory cells because they build networks by joining small tubules together [11]. Due to their function, they are thought to be the cells that detect the mechanical loading of bone and are involved in the activity and generation of osteoblasts and osteoclasts [12]. Bone resorption is carried out by phagocytic multinucleated cells called osteoclasts. These cells originate from monocytes or monocyte-like cells produced in the bone marrow [13]. Bone resorption occurs when osteoclasts come into contact with bone. During this process, proteolytic enzymes released from osteoclast lysosomes dissolve the organic substrate of bone. This mechanism releases several acids, including citric and lactic acids, from mitochondria and secretory vesicles. This phenomenon results in the dissolution of bone composed of Ca2+ and PO43− [14]. On the other hand, osteoblasts are responsible for bone formation. The mechanism of bone formation begins with the secretion of collagen molecules and substrate substances by osteoblasts. Subsequently, osteoblasts form collagen fibers from the collagen molecules to form allogeneic bone, which incorporates some of the osteoblasts to become osteocytes [15]. Within a few days of the formation of the analogous bone, amorphous calcium phosphate (ACP) begins to precipitate on the surface of the collagen fibers. The precipitated ACP is converted to HA over a period of weeks to months, during which atoms are added or replaced [16,17]. A few percent of HA remains amorphous and is more rapidly absorbed when Ca2+ is needed in the extracellular fluid. This is the process of bone formation. Also, in focusing on cells, they have cell-adhesive proteins called integrins on their surfaces. In these cells, the adhesion function of integrins would be carried out by peptide ligands [18]. Arg-Gly-Asp (RGD) is a type of peptide ligand that has been shown to promote osteoblast adhesion [19,20]. In the case of the RGD modified with hyaluronan, it has been reported to stimulate cell adhesion [21]. Glycine-histidine-lysine (GHK) is also the peptide ligand found in osteonectin that has been reported to enhance osteogenic differentiation [22]. Bioceramics that could facilitate this process have not yet been synthesized, which is a challenge in the development of synthetic bone.
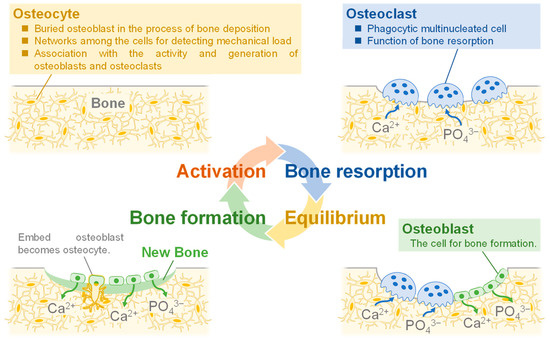
Figure 1.
Illustration of the process of bone formation in vivo.
As mentioned above, for preparing specific materials that promote bone formation and are compatible with both bone replacement and therapy, the following conditions are required:
- (1)
- Bioceramics that resemble biological bone [23].
- (2)
- Bioceramics with low crystallinity [24].
- (3)
- Bioceramics of nanocrystalline structure [25,26,27].
Bioceramics that fulfil these conditions can be used to replace bone defects without toxicity to the bone, and their high bioactivity can elute bone-like components at the interface between the bone and the bioceramics to promote rapid adhesion between the bone and the bioceramics, thus facilitating treatment and allowing early treatment of bone defects [28].
In this review, the current status and issues of bone defect sites in the human body are explained based on examples of conventional HA, and the necessity of regenerative functions in addition to bone defect sites for biomedical applications is proposed. The characteristics and properties of HA substituted with heterogeneous ions such as silicate, carbonate, and other elemental ions are also explained. The importance of the surface characteristics of HA and its components, the hydration layers, and the non-apatitic layers for the effective control of biomedical function, as well as their relationship at the interface to improve biocompatibility, are highlighted. The possibility of a surface layer being formed between HA and its components is also proposed.
2. Hydroxyapatite (HA) Substituted with Other Ions
2.1. Characteristics of HA
CP is composed of Ca2+ and PO43− or P2O74− and is classified as a bioceramic. Table 1 shows the classification of CP compounds. It is classified according to the Ca/P molar ratio, which is the ratio of Ca2+ to PO43−. Six types are present in hard tissues in vivo, with Ca/P molar ratios in the range of 1.0–1.67 [29]. The bioactivity of it is related to their crystalline structure, porosity, and dissolution rate, which are controlled by changing the parameters for various biomedical applications. In particular, the porosity of HA containing nanoparticles or nanopores is expected to function as a drug delivery system [30,31]. The controlled parameters are known to be dominantly influenced by the Ca/P molar ratio. Among the CPs, HA is the major component of bone and has been widely studied by controlling the parameters.

Table 1.
Classification of the calcium phosphate compounds.
Figure 2 shows the crystalline structure of HA, with (a) the overall view and (b) the view from [001]. HA has a Ca/P molar ratio of 1.67 and a chemical composition of Ca10(PO4)6(OH)2. The crystalline structure is hexagonal, the space group is P63/m, and the unit cell size is a = 0.94 nm and c = 0.69 nm. The calcium in the structure is classified into Ca(I) (columnar Ca) and Ca(II) (axis Ca), where the Ca(I) is aligned parallel to the c-axis and the Ca(II) surrounds the c-axis at the four corners of each unit cell where hydroxyl groups are present [32]. It is also known that HA has a high ion exchange capacity. The Ca2+ is substituted by Na+, K+, Mg2+, etc.; the PO43− by SiO44−, CO32−, etc.; and the OH− by F−, Cl−, etc. [33]. These ions are thought to exist within the crystal and on the surface. They exist by substitution with Ca2+, PO43−, and/or OH− when they are present inside the crystal and by reaction with functional groups such as P-OH and Ca-OH exposed on the surface when they are present on the surface.
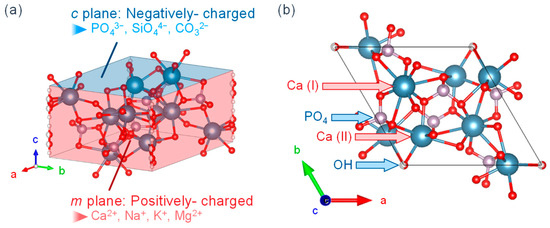
Figure 2.
HA crystalline structure from the views of (a) overall and (b) [001].
Amorphous HA can be synthesized by some synthetic methods; highly crystalline and stable HA can be obtained by calcination of amorphous HA. HA is known to have high biocompatibility since it is contained in biological hard tissues. Therefore, it is used in biomaterials such as artificial bones and implants, and as a fixation layer for chromatography due to its high protein adsorption capacity [34,35,36]. Recently, its biocompatibility and protein adsorption capacity have been investigated for use as a drug delivery system [37,38].
The composition, structure, and morphology of HA vary greatly depending on the synthetic method. The techniques for synthesizing HA include the hydrothermal method, chemical precipitation method, emulsion method, and solid phase reaction method [39,40,41,42,43]. In human bodies, HA is synthesized by the biomineralization process. Recent studies on biomineralization described the biological formation of HA and its nucleation in body fluids to finally be self-assembled into complex structures such as teeth and bones [44]. The HA obtained by the biomineralization process promotes osteoblast adhesion, proliferation, and osseointegration [45,46]. The method for precipitating HA by simulating the biological fluid environment has been studied. Among these synthetic methods of producing HA, except for the hydrothermal method, calcium-deficient HA (CDHA) is easily obtained. Furthermore, ACP can be easily precipitated in aqueous solutions of Ca2+ and PO43− at pH values above 9 for synthetic HA. The obtained ACP can be converted to HA by the recrystallization method [33,47,48].
2.2. Synthesis of HA to Enhance Its Biological Functions: Ion Substitution with Different Elements
HA in biological hard tissues is substituted with various elemental ions, such as carbonate and silicate ions, that work on the concentration, size, and type of action on the HA crystal lattice to enhance its physicochemical properties by altering its electron density and surface conditions [49]. Table 2 shows the different elemental ions that can be substituted in HA. These substitutions can be a cationic ion (with Ca2+) or an anionic ion (with PO43− and OH−). Ionic substitution affects lattice parameters, crystallinity, surface charge, and morphology. The Ca2+ sites are mainly replaced by alkali metals and alkaline earth metals, and partially by transition metals. The lattice constants of the HA crystal structure are changed by these substitution ions, which is mainly attributed to the deficiency of OH− and Ca2+ and the change of ionic radius. For example, Mg2+, Sr2+, Mn2+, and Zn2+ ions increase the a-axis and the c-axis, while Na+, SiO44−, CO32− (type B), and F− ions decrease the a-axis and increase the c-axis, CO32− (type A) and Cl− increase the a-axis and decrease the c-axis, and K+ decreases the a and c-axis [49].

Table 2.
Different ionic elements substituted into the HA structure for improving its functions.
The functions performed by the other elemental ions that are substituting in HA in biological bone affect various components of the bone metabolic process. The important phenomena in bone formation and implantation, namely osteoinduction, osteoconduction, and osseointegration, have been investigated in the previous study [50,51]. Osteoinduction is the process by which primitive, undifferentiated pluripotent cells are stimulated in some way to grow into the osteogenic cell lineage and induce osteogenesis [51,52]. In addition to osteoblasts and osteoclasts, the bones and surrounding tissues contain many undifferentiated cells. These undifferentiated cells have been reported to develop into osteocytes over time and are crucially important for bone healing and implant fixation [53]. The other elemental ions have been reported to promote bone formation and other processes by regulating the expression of genes and proteins involved in various stages of osteogenic differentiation [54,55,56,57]. This is a fundamental biological mechanism that occurs regularly in bone defect treatment and implantation. Osteoconduction refers to the ability of biomaterial surfaces to grow bone and is the process of inducing the adaptation of different biomaterial surfaces to the biological body. Since bone growth at the implant surface is dependent on the action of differentiated osteocytes, it can be considered that osteoconduction is dependent on osteoinduction. Moreover, various types of bone growth factors are required for bone formation and bone growth, including osteoconduction, which cannot occur without an adequate blood supply [58]. Osteoconduction in implants also depends on the biomaterial used and its response. In the case of metallic materials, there are reports showing that osteoconduction is not possible with Ag and Cu while it is possible with Ti and other materials [50]. Osseointegration is defined as the direct contact between bone and implant at the order-level by an optical microscope, as well as between the implant and bone via the cells or other biological tissues, with the result that the bone tissue can be formed at the bone-implant interface and the implant is directly fixed [59,60]. Osseointegration is not an isolated phenomenon; it depends on bone induction and osteogenesis. Therefore, materials that cannot promote bone growth cannot undergo osseointegration. These phenomena are interrelated, and the development of biomaterials that contribute to these phenomena is considered very important in the field of implants.
The substitution of other elemental ions in HA can enhance osteoblast differentiation, osteoinductive, osteoconductive, and osseointegration functions, such as the release of substituted ions due to reduced solubility of HA. Substitution of Cu2+, Mg2+, and SiO44− enhances osteoinduction, whereas substitution of Na+, CO32−, and Cl− enhances osteoinduction [61,62,63]. Furthermore, HA substituted with Zn2+ and Sr2+ has various biological functions, such as enhancement of bone formation and suppression of bone resorption and osteoporosis. It has been reported that SiO44−-substituted HA improves osseointegration properties and dissolution rate. F−-substituted HA was used to treat osteoporosis; however, excessive amounts of F− may cause osteosclerosis and other diseases [64,65,66,67,68,69,70]. The combination of these different elemental ion substitutions is considered important for the synthesis of HA, which retains its beneficial properties for bone formation.
2.3. Structure and Properties of HA with Silicate Ion Substitution
Studies have found that the human body contains about 1–2 g of silicon (Si), and biological bone contains 36 ppm [71,72]. In mice and rats, 0.5 wt.% Si is present in the active growth points of bones [73], and a lack of Si in the diets of mice and rats leads to abnormal bone growth and cranial deformation [74]. The presence of Si affects bone growth. In vitro studies demonstrated that the ingestion of Si, in the form of orthosilicate, enhanced collagen I synthesis and promoted osteoblast differentiation [75]. On the other hand, in synthetic bioactive ceramics, such as bio-glasses and apatite-wollastonite containing SiO2, the reactivity of the crystalline surface containing Si affects its bioactivity [76]. SiO44−-substituted HA particles have also been produced in various forms and used as coatings for titanium implants, showing improved osseointegration properties [66,68,77]. A synthetic porous SiO44−-substituted HA with the trade name Actifuse TM has been successfully used as a bone replacement material in patients with level 1–2 lumbar degenerative disease and was found to be as effective as an autologous bone graft [78]. Figure 3 shows the crystal structures of previously described SiO44−-substituted HA [76]. Silicic acid was present in HA in the form of an anionic substitution. Gibson et al. successfully synthesized the single-phase SiO44−-substituted HA with SiO44− substitution (0.4 wt.%) into the PO43− site of HA with a precipitation reaction using calcium hydroxide as the calcium source, orthophosphoric acid as the phosphoric acid source, and silicon tetraacetate as the SiO44− source. The compositional formula was proposed as shown in Equation (1) [76].
Ca10(PO4)6−x(SiO4)x(OH)2−x
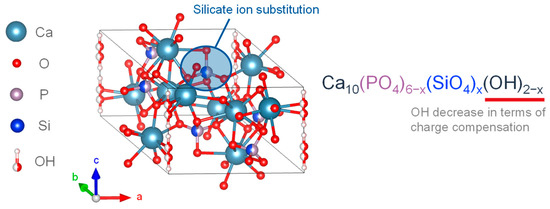
Figure 3.
Silicon-substituted HA crystalline structure.
The experimental results of SiO44− substitution of HA showed that the OH− group decreased with the SiO44− substitution, and the SiO44−substitution caused changes in the crystal structure and chemical composition, including a decrease in the a-axis and an increase in the c-axis [76]. Bianco et al. synthesized SiO44−-substituted α-TCP in a mixed phase with 1.26 wt.% SiO44− and CO32− with a precipitation reaction using calcium hydroxide as the calcium source, orthophosphoric acid as the phosphoric acid source, and tetraethoxysilane (TEOS) as the SiO44− source. As a result, these particles were found to have an increased a-axis and c-axis [79]. In an example of synthesizing SiO44−-substituted HA by varying the added amount of SiO44− from 0.8–5.0 wt.%, it was confirmed that the incorporated concentration of SiO44− was up to 1.5 wt.%, and the SiO44−-substituted HA exceeding 1.5 wt.% contained ACP with amorphous silica [80].
2.4. Structures and Properties of Carbonate Ion Substituted HA
Carbonate ion (CO32−) is present in biological hard tissues, such as biological bone and dentin tissue, in an amount of 2.3−8.0 wt.%, in the form of substitution with PO43− and OH− in HA [81,82]. The crystalline structure of CO32−-substituted HA is similar to that of HA in biological hard tissues. It is suggested to play an important role in bone metabolism and is expected to be used as a bone replacement material [81,82,83,84,85].
Figure 4 shows the crystalline structure of two types of CO32−-substituted HA. It is an anionic substitution and has two different forms depending on the substitution site [86,87,88]. The A-type carbonate HA is in Figure 4a. It is called carbonate apatite (CAP), and is the substitution form in which CO32− is substituted with the OH site of HA. The chemical composition is expressed by the formula in Equation (2).
Ca10(PO4)6−x(OH)2−2x(CO3)x
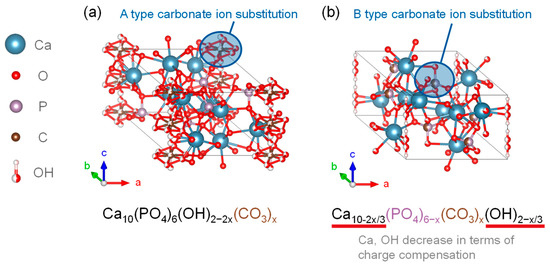
Figure 4.
Carbonate-substituted hydroxyapatite crystalline structures of (a) CAP and (b) CHA.
In general, CAP can be synthesized by heating HA to approximately 1000 °C in a carbon dioxide atmosphere [89]. The lattice parameters of CAP prepared by Walleyes showed an increase in the a-axis and a decrease in the c-axis in X-ray diffraction. It was reported that for every 1 wt.% substitution of CO32− in CAP, the a-axis of the lattice parameter was lengthened by 0.0025 nm, with an upper limit of up to 4.4 wt.% [90,91].
The B-type carbonate-substituted HA (CHA) shown in Figure 4b is a substitutional form in which CO32− was substituted at the PO43− site of HA, and the chemical compositions of the substitutional formulas are given in Equations (3) and (4) [92].
Ca10−2x/3(PO4)6−x(CO3)x(OH)2−x/3
Ca10−x/2(PO4)6−x(CO3)x(OH)2
CHA remains charge neutral due to the loss of Ca2+ and OH− upon CO32− substitution. For each 1 wt.% substitution of CO32−, the a-axis of the lattice parameter was shortened by 0.00006 nm and the c-axis was elongated, which could be up to the maximum inclusion content of 22.2 wt.% [91]. The AB-type carbonate-ion-substituted HA, in which carbonate ions were substituted at both the PO43− and OH− sites of the HA crystalline structure, was also present [93]. The HA showed enhanced dissolution properties in vitro and in vivo, and had higher osteoconductivity than the stoichiometric HA. The increased CO32− content in HA results in the formation of CaO and β-TCP phases by calcination at temperatures where CO32− decomposes, and the mechanical tests showed that CAP had similar strength to that of stoichiometric HA [93].
In dentistry, it was observed that an increase in CO32− content increases the dissolution characteristics of HA in weak acids. This property has been reported to preferentially dissolve tooth enamel, the surface material of teeth, in the initial stages of dental caries, leading to the progression of caries [94,95]. Depending on the progression of caries, secondary caries and tooth erosion may occur; therefore, proper control of CO32− ion substitution is important [96,97]. Thus, the changes caused by HA dissolution in bone due to CO32− ions should be studied from the viewpoints related to bone remodelling.
3. Surface Layers on HA
3.1. Hydration Layer
As the bioceramics come into the contact with water molecules, the water molecules are adsorbed onto the surfaces and the hydration layers are formed. The formation of the hydration layer is caused by various factors, including the interactions between the bioceramic surfaces, ions, and water molecules. The interactive states play an essential role in the subsequent biological reactions [98,99,100].
Figure 5 shows the three hydration layers on the bioceramic surface. In most of the studies, the hydration layer consists of three layers: unfrozen water, intermediate water, and free water, and it has been thought that the cell-adhesive proteins are affected by retaining these layers [101]. Non-freezing and intermediate water are unable to form hydrogen bonds due to strong interactions with the bioceramic surface, thus, water molecules remain in a state where they can move and are difficult to freeze. Free water does not directly interact with the bioceramic surfaces and behave similarly to bulk water, forming hydrogen bonds with the surrounding H2O. Below 0 °C it freezes, thus stopping the molecular motion. The difference between the three layers was evaluated in terms of the heat balance during the freezing and melting of water by differential scanning calorimetry (DSC) and the mobility of water molecules by 1H-NMR [102]. The difference between the three layers was due to their interaction acting on the bioceramic surfaces, which modifies the melting point and the mobility. Based on the thermal value at each transition of DSC, non-freezing water was defined as water that does not freeze at −100 °C; intermediate water was water that froze at temperatures lower than 0 °C during the temperature increase process; and free water was water that froze at temperatures below 0 °C and crystallized at −100 °C [102]. The layer structure was defined as water that was crystallized at temperatures below 0 °C and crystallizes at −100 °C [103]. The structure of the hydration layer rearranges on femto- to picosecond time scales, and liquid water is an amorphous structure with a disordered network on very short time scales; however, it has the randomness of a liquid on longer time scales. In 1H-NMR measurements, the relaxation times of non-freezing water, intermediate water, and free water were 10−8–10−6 s, 10−10–10−9 s, and 10−12–10−11 s, respectively, indicating stronger interactions with the bioceramics as well as lower mobility [102]. In the FT-IR spectra, the layer state could be evaluated by separating the absorption bands of the stretching vibration of the hydroxyl groups (3600 cm−1, 3400 cm−1, 3200 cm−1) corresponding to non-freezing water, intermediate water, and free water, respectively [102]. A variety of other analytical methods were used to evaluate the hydration structure (i.e., hydrogen molecular bonding state and mobility). However, the layer on the HA surface has not been studied in detail, thus it is necessary to evaluate the layer bonded to the surface as an integral part.
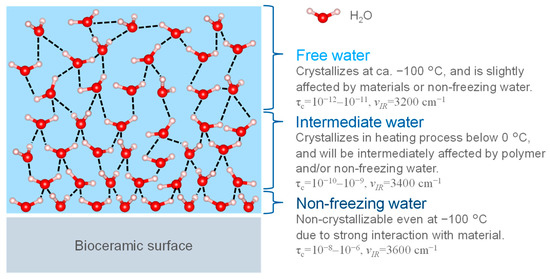
Figure 5.
The three types of hydration layers. τc: relaxation time of water molecule motion by solid state NMR measurement. νIR: IR absorption wavenumber.
3.2. Non-Apatitic Layer on HA Surface
Figure 6 shows a model of the surface layer bounded with the HA. Biological apatite in naturally calcified tissue is formed in vivo in an aqueous environment at room temperature. Thus, recent studies of HA have focused on HA prepared by wet methods, which can synthesize HA similar to the in vivo environment in order to mimic biological apatite. The detailed surface structure of the HA synthesized by wet methods has not been fully elucidated; however, it is predicted that it will be a low-crystalline surface, and such a structure is being studied by spectroscopic methods such as FT-IR and NMR, which are sensitive to perturbations of the local ionic environment. FT-IR spectra show an absorption band in the 680–480 cm−1 region corresponding to the non-apatitic layer on the HA surface, which is assigned to PO43− and HPO42− [103]. The coordination environment of phosphate and calcium ions and the interaction of these ions were investigated by NMR spectroscopy, indicating that the HA particles are composed of a highly crystalline core and a non-apatite layer composed of ACP [104]. The layer is highly reactive due to its unstable structure composed of divalent ions such as Ca2+, HPO42−, and CO32−, etc. In particular, it has been shown from ab initio calculations that the reaction with H2O exhibits moderate Lewis acidity due to the strong bonding of the exposed ions in the non-apatitic layer (e.g., Ca2+, HPO42−, CO32−, etc.) due to electrostatic interactions with the H2O [105,106]. The ions in the layer are organized in a geometric configuration and are stabilized by a structured hydrogen bonding network of the hydration layer [106]. In addition to this reaction, the layer is believed to contribute to the growth of HA nuclei, ion exchange, and the adsorption of organic molecules. The reactivity of the layer is thought to play a significant role in biocompatibility, and further research is needed.
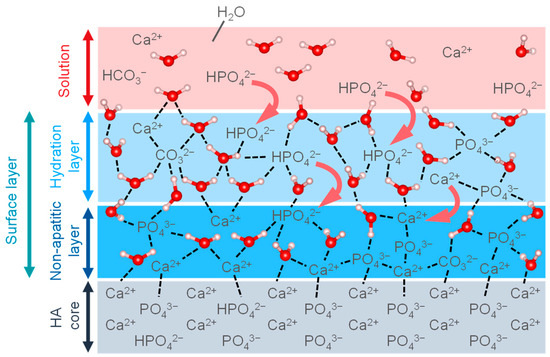
Figure 6.
Illustration of the surface layers on HA core.
3.3. Relationship between the Surface Layer and Biocompatibility
The biocompatibility of HA is highly related to the cell adhesion behavior, and the cell-bioceramic interfaces are formed in vivo, as shown in Figure 7 [107,108]. Firstly, the cell adhesion occurs through three processes upon implantation in the body. In the process, H2O and ions in body fluid adsorb on the surfaces, forming the hydration layer. Here, the amount of H2O, the concentration and type of ions in the vicinity of the bioceramics are important factors for the next processes. In the second process, the proteins are adsorbed onto the hydration layer, and the protein adsorption is saturated to form the protein adsorption layer. The type and orientation structure of the adsorbed protein in this process determine the function of the attached cells. In the third process, the cells are adhered to the adsorbed protein layer and proliferate with their spreading. The layers formed by the first and second processes determine the cell behavior. Thus, it is important to control the first process and then evaluate the protein adsorption layer for the desired cell adhesion properties in order to consider biocompatibility [109,110]. The highly-reactive surface layer on HA is known to interact strongly with the substances in surrounding aqueous solutions [111,112,113]. The interactions are supported by ion mobility, ion exchange capacity, and molecular adsorption [114,115,116]. The interfacial phenomena induce the combination between HA surface layer and water, such as the dissolution and deposition of ions and the solid dissolution of organic and mineral phases [117,118,119]. In other words, the interfacial phenomena are considered to be the formation process of the HA surface layer that affects the hydration layer and cell adhesion. In particular, the inclusion of SiO44− and CO32− ions in HA induces structural defects and increases its dissolution rate in vivo [120,121,122]. This promotes hydrogen bonding networks, increases cell and bone attachment rates, and activates osteoblasts, leading to the activation of surrounding tissues based on increased rates of ion solubilization and diffusion. This phenomenon is related to the ion exchange behavior at the non-apatitic layer of the HA surface, where the other elemental ions that have leached and diffused into the layer by the ion exchange increase the genetic markers and proteins for inducing osteogenesis, and some reports suggest the increase in the activity of osteoblasts [54,55,56,57,58]. These surface phenomena of ion-substituted HA affect the hydration layer, leading to significant changes in the dissolution properties of HA and contributing to its biocompatibility, resulting in improved protein adsorption, cell adhesion, and osteogenesis.
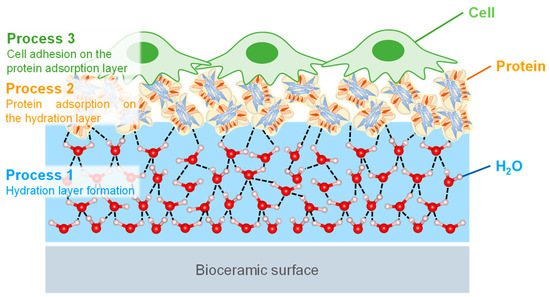
Figure 7.
Illustration of the cell adhesion processes of (1) the hydration layer formed on the bioceramic surface, (2) protein adsorption on the hydration layer, and (3) cells that recognize and adhere to the protein adsorption layer.
4. Conclusions
In this review, the current status of the effect of substitution ions on the surface properties and biocompatibility of HA was discussed. Changing the chemical composition and crystalline structure of HA depending on the synthesis method and added ions was emphasized, which improved the surface properties of HA and thus affected its biocompatibility. In particular, the ability of HA to be substituted by silicate and carbonate is remarkable for its use as a bioceramic. Furthermore, the characteristics of the HA surface layers (i.e., hydrated and non-apatite layers) that affect subsequent biological responses were clearly summarized and categorized. Since the surface layers are important for both protein adsorption and cell adhesion, analysis of their properties may provide important clues to gain insight into the efficient mechanisms of bone formation.
Author Contributions
Writing of the manuscript, K.S.; review and editing, Y.Z., T.G.P.G. and R.K.; supervision, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bauer, T.W.; Muschler, G.F. Bone Graft Materials. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Younger, E.M.; Chapman, M.W. Morbidity at Bone Graft Donor Sites. J. Orthop. Trauma 1989, 3, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Banwart, J.C.; Asher, M.A.; Hassanein, R.S. Iliac Crest Bone Graft Harvest Donor Site Morbidity. Spine 1995, 20, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Arrington, E.D.; Smith, W.J.; Chambers, H.G.; Bucknell, A.L.; Davino, N.A. Complications of iliac crest bone graft harvesting. Clin. Orthop. Relat. Res. 1996, 329, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, A.S.; Boden, S.D.; Goldberg, V.M.; Khan, Y.; Laurencin, C.T.; Rosier, R.N. Bone-Graft Substitutes: Facts, Fictions, and Applications. J. Bone Jt. Surg. 2001, 83, 98–103. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Yeung, K.W.K.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R Rep. 2014, 80, 1–36. [Google Scholar]
- Zwingenberger, S.; Nich, C.; Valladares, R.D.; Yao, Z.; Stiehler, M.; Goodman, S.B. Recommendations and Considerations for the Use of Biologics in Orthopedic Surgery. BioDrugs 2012, 26, 245–256. [Google Scholar]
- Guyton, A.C.; Hall, J.E. Text Book of Medical Physiology (Guyton Physiology), 11th ed.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Parfitt, A.M. Osteonal and hemi-osteonal remodeling: The spatial and temporal framework for signal traffic in adult human bone. J. Cell. Biochem. 1994, 55, 273–286. [Google Scholar] [CrossRef]
- Aarden, E.M.; Nijiwede, P.J.; Burger, E.H. Function of osteocytes in bone. J. Cell. Biochem. 2003, 55, 287–299. [Google Scholar] [CrossRef]
- Bonewald, L.F. The Amazing Osteocyte. J. Bone Miner. Res. 2010, 26, 229–238. [Google Scholar] [CrossRef]
- Bellido, T. Osteocyte-Driven Bone Remodeling. Calcif. Tissue Int. 2014, 94, 25–34. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone Resorption by Osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.C. How the osteoclast degrades bone. BioEssays 1998, 20, 837–846. [Google Scholar] [CrossRef]
- Anderson, H.C. Mechanism of mineral formation in bone. Lab. Investig. 1989, 60, 320–330. [Google Scholar] [PubMed]
- Cölfen, H. A crystal-clear view. Nat. Mater. 2010, 9, 960–961. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.E. Role of matrix vesicles in biomineralization. Biochim. Biophys. Acta-Gen. Subj. 2009, 1790, 1592–1598. [Google Scholar]
- Pierschbacher, M.D.; Ruoslahti, E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 1984, 309, 30–33. [Google Scholar] [CrossRef]
- Schaffner, P.; Dard, M.M. Structure and function of RGD peptides involved in bone biology. Cell. Mol. Life Sci. 2003, 60, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K. Osteoblast adhesion to biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar]
- Balasundaram, G.; Sato, M.; Webster, T.J. Using hydroxyapatite nanoparticles and decreased crystallinity to promote osteoblast adhesion similar to functionalizing with RGD. Biomaterials 2006, 27, 2798–2805. [Google Scholar] [CrossRef]
- Klontzas, M.E.; Reakasame, S. Oxidized alginate hydrogels with the GHK peptide enhance cord blood mesenchymal stem cell osteogenesis: A paradigm for metabolomics-based evaluation of biomaterial design. Acta Biomater. 2019, 88, 224–240. [Google Scholar] [CrossRef]
- Ogiso, M.; Kaneda, H.; Arasaki, J.; Ishida, K.; Tabata, T. Epithelial attachment to hy-droxyapatite ceramics implant. J. Dent. Res. 1980, 59, 941. [Google Scholar]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Stupp, S.I.; Ciegler, G.W. Organoapatites: Materials for artificial bone. I. Synthesis and microstructure. J. Biomed. Mater. Res. 1992, 26, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Webster, T. Enhanced osteoclast-like cell functions on nanophase ceramics. Biomaterials 2001, 22, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Best, S.M.; Bonfield, W.; Brooks, R.A.; Rushton, N.; Jayasinghe, S.N.; Edirisinghe, M.J. In vitro assessment of the biological response to nanosized hydroxyapatite. J. Mater. Sci. Mater. Med. 2004, 15, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Mocquot, C.; Attik, N.; Pradelle-Plasse, N.; Grosgogeat, B.; Colon, P. Bioactivity assessment of bioactive glasses for dental applications: A critical review. Dent. Mater. 2020, 36, 1116–1143. [Google Scholar] [CrossRef]
- Tung, M.S. Calcium Phosphates: Structure, Composition, Solubility, and Stability. In Calcium Phosphates in Biological and Industrial Systems; Springer: Berlin/Heidelberg, Germany, 1998; pp. 1–19. [Google Scholar]
- Munir, M.U.; Salman, S. Nano-hydroxyapatite as a delivery system: Overview and advancements. Artif. Cells Nanomed. Biotechnol. 2021, 49, 717–727. [Google Scholar] [CrossRef]
- Salètes, M.; Vartin, M.; Mocquot, C.; Chevalier, C.; Grosgogeat, B.; Colon, P.; Attik, N. Mesoporous Bioactive Glasses Cytocompatibility Assessment: A Review of In Vitro Studies. Biomimetics 2021, 6, 9. [Google Scholar] [CrossRef]
- Kay, M.I.; Young, R.A.; Posner, A.S. Crystal Structure of Hydroxyapatite. Nature 1964, 204, 1050–1052. [Google Scholar] [CrossRef]
- Elliott, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Brown, W.E.; Eidelman, N.; Tomazic, B. Octacalcium Phosphate as a Precursor in Biomineral Formation. Adv. Dent. Res. 1987, 1, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T. Theory of chromatography on hydroxyapatite columns with small loads. J. Chromatogr. A 1978, 157, 7–42. [Google Scholar] [CrossRef]
- Otsuka, M.; Matsuda, Y.; Yu, D.; Wong, J.; Fox, J.L.; Higuchi, W.I. A novel skeletal drug delivery system for anti-bacterial drugs using self-setting hydroxyapatite cement. Chem. Pharm. Bull. 1990, 38, 3500–3502. [Google Scholar] [CrossRef] [PubMed]
- Shinto, Y.; Uchida, A.; Korkusuz, F.; Araki, N.; Ono, K. Calcium hydroxyapatite ceramic used as a delivery system for antibiotics. J. Bone Jt. Surg. Br. 1992, 74-B, 600–604. [Google Scholar] [CrossRef]
- Treboux, G.; Kanzaki, N.; Onuma, K.; Ito, A. Energy-Preeminent Isomer of the Ca3(PO4)2 Cluster. J. Phys. Chem. A 1999, 103, 8118–8120. [Google Scholar] [CrossRef]
- Feng, W.; Mu-sen, L.; Yu-peng, L.; Yong-xin, Q. A simple sol–gel technique for preparing hydroxyapatite nanopowders. Mater. Lett. 2005, 59, 916–919. [Google Scholar] [CrossRef]
- Kuriakose, T.A.; Kalkura, S.N.; Palanichamy, M.; Arivuoli, D.; Dierks, K.; Bocelli, G.; Betzel, C. Synthesis of stoichiometric nano crystalline hydroxyapatite by ethanol-based sol–gel technique at low temperature. J. Cryst. Growth 2004, 263, 517–523. [Google Scholar] [CrossRef]
- Liu, J.; Ye, X.; Wang, H.; Zhu, M.; Wang, B.; Yan, H. The influence of pH and temperature on the morphology of hydroxyapatite synthesized by hydrothermal method. Ceram. Int. 2003, 29, 629–633. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Gong, H.; Jiang, X.; Wang, H.; Li, K. Effects of synthesis conditions on the morphology of hydroxyapatite nanoparticles produced by wet chemical process. Powder Technol. 2010, 203, 315–321. [Google Scholar] [CrossRef]
- Bose, S.; Saha, S.K. Synthesis and Characterization of Hydroxyapatite Nanopowders by Emulsion Technique. Chem. Mater. 2003, 15, 4464–4469. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Nanometric calcium orthophosphates (CaPO4): Preparation, properties and biomedical applications. Adv. Nano-BioMater. Devices 2019, 3, 422–513. [Google Scholar]
- Cai, Y.; Liu, Y.; Yan, W.; Hu, Q.; Tao, J.; Zhang, M.; Shi, Z.; Tang, R. Role of hydroxyapatite nanoparticle size in bone cell proliferation. J. Mater. Chem. 2007, 17, 3780–3787. [Google Scholar] [CrossRef]
- Mondal, S.; Maharta, S.; Kundu, S.; Mondal, B.l. Development of biocompatible sinterable hydroxyapatite from fish scale. Mater. Sci. Eng. 2007, C27, 441–449. [Google Scholar]
- LeGeros, R.Z. Calcium phosphates in oral biology and medicine. Monogr. Oral Sci. 1991, 15, 154–171. [Google Scholar]
- Dorozhkin, S.V. Calcium Orthophosphate Bioceramics. Eurasian Chem. J. 2010, 12, 247. [Google Scholar] [CrossRef]
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1285–1299. [Google Scholar]
- Johansson, C.B.; Han, C.H.; Wennerberg, A.; Albrektsson, T. A Quantitative Comparison of Machined Commercially Pure Titanium and TitaniumAluminum-Vanadium Implants in Rabbit Bone. Int. J. Oral Maxillofac. Implant. 1998, 13, 315–321. [Google Scholar]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar]
- Bruder, S.R.; Jaiswal, N.; Ricalton, N.S.; Mosca, J.D.; Kraus, K.H.; Kadiyala, S. Mesenchy ma1 Stem Cells in Osteobiology and Applied Bone Regeneration. Clin. Orthop. Relat. Res. 1998, 355, S247–S256. [Google Scholar] [CrossRef]
- Young, R.W. Nucleic acids, protein synthesis and bone. Clin. Orthop. Relat. Res. 1963, 26, 147–160. [Google Scholar] [CrossRef]
- Jung, G.Y.; Park, Y.J.; Han, J.S. Effects of HA released calcium ion on osteoblast differentiation. J. Mater. Sci. Mater. Med. 2010, 21, 1649–1654. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Zeng, X.; Ma, L.L.; Weng, W.; Yan, W.; Qian, M. Osteoblastic cell response on fluoridated hydroxyapatite coatings. Acta Biomater. 2007, 3, 191–197. [Google Scholar] [CrossRef]
- Ni, G.X.; Yao, Z.P.; Huang, G.T.; Liu, W.G.; Lu, W.W. The effect of strontium incorporation in hydroxyapatite on osteoblasts in vitro. J. Mater. Sci. Mater. Med. 2011, 22, 961–967. [Google Scholar] [CrossRef]
- Qi, T.; Weng, J.; Yu, F.; Zhang, W.; Li, G.; Qin, H.; Tan, Z.; Zeng, H. Insights into the Role of Magnesium Ions in Affecting Osteogenic Differentiation of Mesenchymal Stem Cells. Biol. Trace Elem. Res. 2021, 199, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T. The healing of autologous bone grafts after varying degrees of surgical trauma. A microscopic and histochemical study in the rabbit. J. Bone Jt. Surg. Br. 1980, 62-B, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Lindström, J. Osseointegrated Titanium Implants: Requirements for Ensuring a Long-Lasting, Direct Bone-to-Implant Anchorage in Man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Attik, N.; Phantarasmy, M.; Abouelleil, H.; Chevalier, C.; Barraco, A.; Grosgogeat, B.; Lafon, A. Comparison of the Biological Behavior and Topographical Surface Assessment of a Minimally Invasive Dental Implant and a Standard Implant: An In Vitro Study. Materials 2022, 15, 7540. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Yoo, D.S.; Chung, Y.-C.; Rhee, S.-H. Enhanced bioactivity and osteoconductivity of hydroxyapatite through chloride substitution. J. Biomed. Mater. Res. Part A 2014, 102, 455–469. [Google Scholar] [CrossRef]
- Veljovic, D.; Matic, T.; Stamenic, T.; Kojic, V.; Dimitrijevic-Brankovic, S.; Lukic, M.J.; Jevtic, S.; Radovanovic, Z.; Petrovic, R.; Janackovic, D. Mg/Cu co-substituted hydroxyapatite—Biocompatibility, mechanical properties and antimicrobial activity. Ceram. Int. 2019, 45, 22029–22039. [Google Scholar] [CrossRef]
- Xu, T.; He, X.; Chen, Z.; He, L.; Lu, M.; Ge, J.; Weng, J.; Mu, Y.; Duan, K. Effect of magnesium particle fraction on osteoinduction of hydroxyapatite sphere-based scaffolds. J. Mater. Chem. B 2019, 7, 5648–5660. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E.; Capuccini, C.; Gazzano, M. Strontium-substituted hydroxyapatite nanocrystals. Inorg. Chim. Acta 2007, 360, 1009–1016. [Google Scholar] [CrossRef]
- Gerstenfeld, S.; Hinojosa, L.; JL, F.M.; Marchante, J.M.; JI, G.A.; Sanz-Medel, A.; JB, C.A. Effect of strontium on bone metabolism in hemodialysis patients. Nefrologia 2003, 23 (Suppl. S2), 52–56. [Google Scholar] [PubMed]
- Kishi, S.; Yamaguchi, M. Inhibitory effect of zinc compounds on osteoclast-like cell formation in mouse marrow cultures. Biochem. Pharmacol. 1994, 48, 1225–1230. [Google Scholar] [CrossRef]
- Baeza, A.; Izquierdo-Barba, I.; Vallet-Regí, M. Biotinylation of silicondoped hydroxyapatite: A new approach to protein fixation for bone tissue regeneration. Acta Biomater. 2010, 6, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Hahn, B.D.; Lee, J.M.; Park, D.S.; Choi, J.J.; Ryu, J.; Yoon, W.H.; Lee, B.K.; Shin, D.S.; Kim, H.E. Aerosol deposition of silicon-substituted hydroxyapatite coatings for biomedical applications. Thin Solid Film. 2010, 518, 2194–2199. [Google Scholar] [CrossRef]
- Cheng, K.; Shen, G.; Weng, W.; Han, G.; Ferreira, J.M.; Yang, J. Synthesis of hydroxyapatite/fluoroapatite solid solution by a sol–gel method. Mater. Lett. 2001, 51, 37–41. [Google Scholar] [CrossRef]
- Porter, A.E.; Patel, N.; Skepper, J.N.; Best, S.M.; Bonfield, W. Effect of sintered silicate-substituted hydroxyapatite on remodelling processes at the bone–implant interface. Biomaterials 2004, 25, 3303–3314. [Google Scholar] [CrossRef]
- Jugdaohsingh, R. Silicon and bone health. J. Nutr. Health Aging 2007, 11, 99–110. [Google Scholar]
- Whiting, S.J.; Draper, H.H. Effect of a Chronic Acid Load as Sulfate or Sulfur Amino Acids on Bone Metabolism in Adult Rats. J. Nutr. 1981, 111, 1721–1726. [Google Scholar] [CrossRef]
- Carlisle, E.M. Silicon as an essential trace element in animal nutrition. Ciba Found. Symp. 1986, 121, 123–139. [Google Scholar]
- Carlisle, E.M. Silicon: An Essential Element for the Chick. Science 1972, 178, 619–621. [Google Scholar] [CrossRef]
- Reffitt, D.M.; Ogston, N.; Jugdaohsingh, R.; Cheung, H.F.J.; Evans, B.A.J.; Thompson, R.P.H.; Powell, J.J.; Hampson, G.N. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 2003, 32, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Gibson, I.R.; Best, S.M.; Bonfield, W. Chemical characterization of silicon-substituted hydroxyapatite. J. Biomed. Mater. Res. 1999, 44, 422–428. [Google Scholar]
- Li, D.-H.; Lin, J.; Lin, D.-Y.; Wang, X.-X. Synthesized siliconsubstituted hydroxyapatite coating on titanium substrate by electrochemical deposition. J. Mater. Sci. Mater. Med. 2011, 22, 1205–1211. [Google Scholar] [CrossRef]
- Jenis, L.G.; Banco, R.J. Efficacy of Silicate-Substituted Calcium Phosphate Ceramic in Posterolateral Instrumented Lumbar Fusion. Spine 2010, 35, E1058–E1063. [Google Scholar] [CrossRef]
- Bianco, A.; Cacciotti, I.; Lombardi, M.; Montanaro, L. Si-substituted hydroxyapatite nanopowders: Synthesis, thermal stability and sinterability. Mater. Res. Bull. 2009, 44, 345–354. [Google Scholar] [CrossRef]
- Dong, G.; Zheng, Y.; He, L.; Wu, G.; Deng, C. The effect of silicon doping on the transformation of amorphous calcium phosphate to siliconsubstituted α-tricalcium phosphate by heat treatment. Ceram. Int. 2016, 42, 883–890. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Ripamonti, A.; Roveri, N.; Romanello, M.; Suarez, K.N.; Moro, L. Chemical and structural characterization of the mineral phase from cortical and trabecular bone. J. Inorg. Biochem. 1997, 68, 45–51. [Google Scholar] [CrossRef]
- Driessens, F.C.M. The mineral in bone, dentin and tooth enamel. Bull. Des. Soc. Chim. Belges. 2010, 89, 663–689. [Google Scholar] [CrossRef]
- Sroka-Bartnicka, A.; Borkowski, L.; Ginalska, G.; Ślósarczyk, A.; Kazarian, S.G. Structural transformation of synthetic hydroxyapatite under simulated in vivo conditions studied with ATR-FTIR spectroscopic imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 171, 155–161. [Google Scholar] [CrossRef]
- Doi, Y. Sintered carbonate apatites as bone substitutes. Cells Mater. 1997, 7, 111–122. [Google Scholar]
- Montel, G.; Bonel, G.; Heughebaert, J.C.; Trombe, J.C.; Rey, C. New concepts in the composition, crystallization and growth of the mineral component of calcified tissues. J. Cryst. Growth 1981, 53, 74–99. [Google Scholar]
- Jarcho, M.; Kay, J.F.; Gumaer, K.I.; Doremus, R.H.; Drobeck, H.P. Tissue, cellular and subcellular events at a bone-ceramic hydroxylapatite interface. J. Bioeng. 1977, 1, 79–92. [Google Scholar] [PubMed]
- LeGeros, R.Z. Properties of Osteoconductive Biomaterials: Calcium Phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Hayek, E.; Böhler, W.; Lechleitner, J.; Petter, H. Hydrothermalsynthese von Calcium-Apatiten. Zeitschrift für Anorg. Und Allg. Chem. 1958, 295, 241–246. [Google Scholar] [CrossRef]
- Young, R.A.; Bartlett, M.L.; Spooner, S.; Mackie, P.E.; Bonel, G. Reversible high temperature exchange of carbonate and hydroxyl ions in tooth enamel and synthetic hydroxyapatite. J. Biol. Phys. 1981, 9, 1–26. [Google Scholar] [CrossRef]
- Young, R.A. Biological Apatite vs Hydroxyapatite at the Atomic Level. Clin. Orthop. Relat. Res. 1975, 113, 249–262. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Trautz, O.R.; Klein, E.; LeGeros, J.P. Two types of carbonate substitution in the apatite structure. Experientia 1969, 25, 5–7. [Google Scholar] [CrossRef]
- Landi, E.; Celotti, G.; Logroscino, G.; Tampieri, A. Carbonated hydroxyapatite as bone substitute. J. Eur. Ceram. Soc. 2003, 23, 2931–2937. [Google Scholar] [CrossRef]
- Merry, J.C.; Gibson, I.R.; Best, S.M.; Bonfield, W. Synthesis and characterization of carbonate hydroxyapatite. J. Mater. Sci. Mater. Med. 1998, 9, 779–783. [Google Scholar] [CrossRef]
- Ingram, G.S. The Role of Carbonate in Dental Mineral. Caries Res. 1973, 7, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Hallsworth, A.S.; Weatherell, J.A.; Robinson, C. Loss of Carbonate during the First Stages of Enamel Caries. Caries Res. 1973, 7, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Askar, H.; Krois, J.; Göstemeyer, G.; Bottenberg, P.; Zero, D.; Banerjee, A.; Schwendicke, F. Secondary caries: What is it, and how it can be controlled, detected, and managed? Clin. Oral Investig. 2020, 24, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.C.; Wiegand, A.; Rios, D.; Honório, H.M.; Buzalaf, M.A.R. Insights into preventive measures for dental erosion. J. Appl. Oral Sci. 2009, 17, 75–86. [Google Scholar] [CrossRef]
- Frauenfelder, H.; Fenimore, P.; McMahon, B. Hydration, slaving and protein function. Biophys. Chem. 2002, 98, 35–48. [Google Scholar] [CrossRef]
- Bagchi, B. Water dynamics in the hydration layer around proteins and micelles. Chem. Rev. 2005, 105, 3197–3219. [Google Scholar] [CrossRef]
- Yang, A.H.C.; Hou, J.; Chen, V.; Xu, Z.K. Surface and interface engineering for organic–inorganic composite membranes. J. Mater. Chem. A 2016, 4, 9716–9729. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, K. Thermal Characterization of Novel Polymers for Biomedical Applications. Netsusokutei = Calorim. Therm. Anal. 2012, 39, 151–157. [Google Scholar]
- Tanaka, M.; Sato, K.; Kitakami, E.; Kobayashi, S.; Hoshiba, T.; Fukushima, K. Design of biocompatible and biodegradable polymers based on intermediate water concept. Polym. J. 2015, 47, 114–121. [Google Scholar] [CrossRef]
- Gómez-Morales, J.; Iafisco, M.; Delgado-López, J.M.; Sarda, S.; Drouet, C. Progress on the preparation of nanocrystalline apatites and surface characterization: Overview of fundamental and applied aspects. Prog. Cryst. Growth Charact. Mater. 2013, 59, 1–46. [Google Scholar]
- Rossi, A.M.; Prado da Silva, M.H.; Ramirez, A.J.; Biggemann, D.; Caraballo, M.M.; Mascarenhas, Y.P.; Eon, J.G.; Moure, G.T. Structural Properties of Hydroxyapatite with Particle Size Less Than 10 Nanometers. Key Eng. Mater. 2007, 330, 255–258. [Google Scholar] [CrossRef]
- Delgado-López, J.M.; Iafisco, M.; Rodríguez, I.; Tampieri, A.; Prat, M.; Gómez-Morales, J. Crystallization of bioinspired citrate-functionalized nanoapatite with tailored carbonate content. J. Acta Biomater. 2012, 8, 3491–3499. [Google Scholar] [CrossRef] [PubMed]
- Bolis, V.; Busco, C.; Martra, G.; Bertinetti, L.; Sakhno, Y.; Ugliengo, P.; Chiatti, F.; Corno, M.; Roveri, N. Coordination chemistry of Ca sites at the surface of nanosized hydroxyapatite: Interaction with H2O and CO. Phil. Trans. R. Soc. A 2012, 370, 1313–1336. [Google Scholar] [CrossRef] [PubMed]
- Tagaya, M. In situ QCM-D study of nano-bio interfaces with enhanced biocompatibility. Polym. J. 2015, 47, 599–608. [Google Scholar] [CrossRef]
- Kasemo, B. Biological surface science. Surf. Sci. 2002, 500, 656–677. [Google Scholar] [CrossRef]
- El-Ghannam, A.; Ducheyne, P.; Shapiro, I.M. Effect of serum proteins on osteoblast adhesion to surface-modified bioactive glass and hydroxyapatite. J. Orthop. Res. 1999, 17, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Rouahi, M.; Champion, E.; Gallet, O.; Jada, A.; Anselme, K. Physico-chemical characteristics and protein adsorption potential of hydroxyapatite particles: Influence on in vitro biocompatibility of ceramics after sintering. Colloids Surf. B Biointerfaces 2006, 47, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Panda, R.N.; Hsieh, M.F.; Chung, R.J.; Chin, T.S. FTIR, XRD, SEM and Solid State NMR Investigations of Carbonate-containing Hydroxyapatite Nano-particles Synthesized by Hydroxide-gel Technique. J. Phys. Chem. Solids 2003, 64, 193–199. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Sfihi, H.; Barroug, A. Physico-chemical properties of nanocrystalline apatites: Implications for biominerals and biomaterials. Mater. Sci. Eng. 2007, 27, 198–205. [Google Scholar] [CrossRef]
- Bertinetti, L.; Drouet, C.; Combes, C.; Rey, C.; Tampieri, A.; Coluccia, S.; Martra, G. Surface Characteristics of Nanocrystalline Apatites: Effect of Mg Surface Enrichment on Morphology, Surface Hydration Species, and Cationic Environments. Langmuir 2009, 25, 5647–5654. [Google Scholar] [CrossRef]
- Cazalbou, S.; Eichert, D.; Ranz, X.; Drouet, C.; Combes, C.; Harmand, M.F.; Rey, C. Ion exchanges in apatites for biomedical application. J. Mater. Sci. Mater. Med. 2005, 16, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Drouet, C.; Carayon, M.T.; Combes, C.; Rey, C. Exchange of biologically relevant ions on nanocrystalline apatites. Geochim. Cosmochim. Acta 2005, 69, A69. [Google Scholar]
- Autefage, H.; Briand-Mésange, F.; Cazalbou, S.; Drouet, C.; Fourmy, D.; Gonçalvès, S.; Salles, J.P.; Combes, C.; Swider, P.; Rey, C. Adsorption and release of BMP-2 on nanocrystalline apatite-coated and uncoated hydroxyapatite/beta-tricalcium phosphate porous ceramics. J. Biomed. Mater. Res. Part B 2009, 91, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Bruijn, J.D.; Bovell, Y.P.; van Blitterswijk, C.A. Structural arrangements at the interface between plasma sprayed calcium phosphates and bone. Biomaterials 1994, 15, 543–550. [Google Scholar] [CrossRef]
- Daculsi, G.; LeGeros, R.Z.; Nery, E.; Lynch, K.; Kerebel, B. Transformation of biphasic calcium phosphate ceramics in vivo: Ultrastructural and physicochemical characterization. J. Biomed. Mater. Res. 1989, 23, 883–894. [Google Scholar] [CrossRef]
- Schepers, E.; Clercq, M.D.; Ducheyne, P.; Kempeneers, R. Bioactive glass particulate material as a filler for bone lesions. J. Oral Rehabil. 1991, 18, 439–452. [Google Scholar] [CrossRef]
- Porter, A.E.; Patel, N.; Skepper, J.N.; Best, S.M.; Bonfield, W. Comparison of in vivo dissolution processes in hydroxyapatite and silicon-substituted hydroxyapatite bioceramics. Biomaterials 2003, 24, 4609–4620. [Google Scholar] [CrossRef]
- Qiu, Z.; Noh, I.; Zhang, S. Silicate-doped hydroxyapatite and its promotive effect on bone mineralization. Front. Mater. Sci. 2013, 7, 40–50. [Google Scholar]
- Tang, P.F.; Li, G.; Wang, J.F.; Zheng, Q.J.; Wang, Y. Development, Characterization, and Validation of Porous Carbonated Hydroxyapatite Bone Cement. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 886–893. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).