Fabrication of 3D Bioprinted Bi-Phasic Scaffold for Bone–Cartilage Interface Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Bioink Preparation
2.3. Degradation Rate
2.4. Rheological Assessments
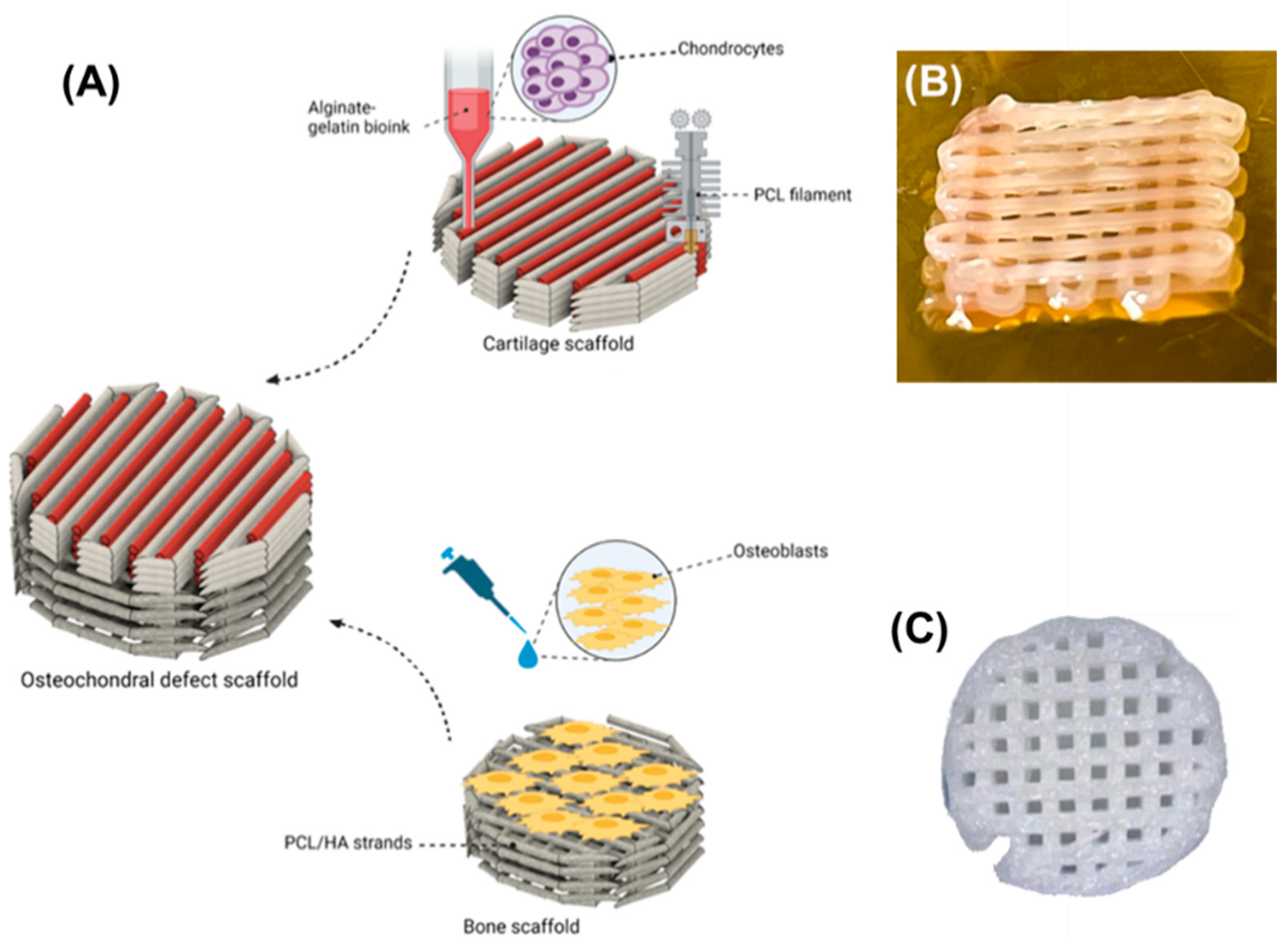
2.5. Scaffold Fabrication
2.6. Biological Characterisation
2.6.1. In Vitro Cell Culture
2.6.2. Scaffold Cytotoxicity
2.6.3. Viability Assessment
2.6.4. Proliferation Assessment
2.6.5. Cell Mineralization Assessment
2.6.6. Cell Morphology
2.7. Statistical Analysis
3. Results and Discussions
3.1. Physical Characterizations of Hydrogel Bioinks
3.2. Rheological Properties
3.3. Biological Properties
3.3.1. Bioink Cytotoxicity Assessment
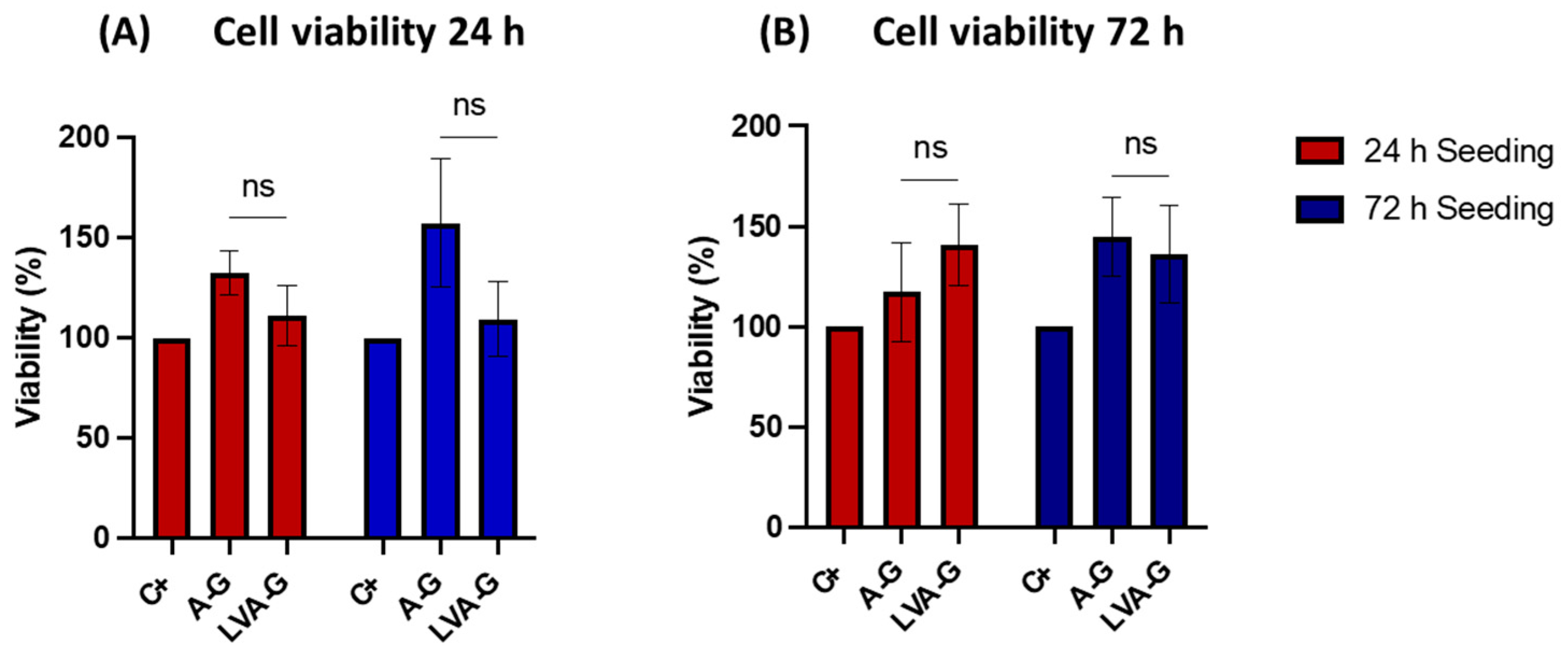
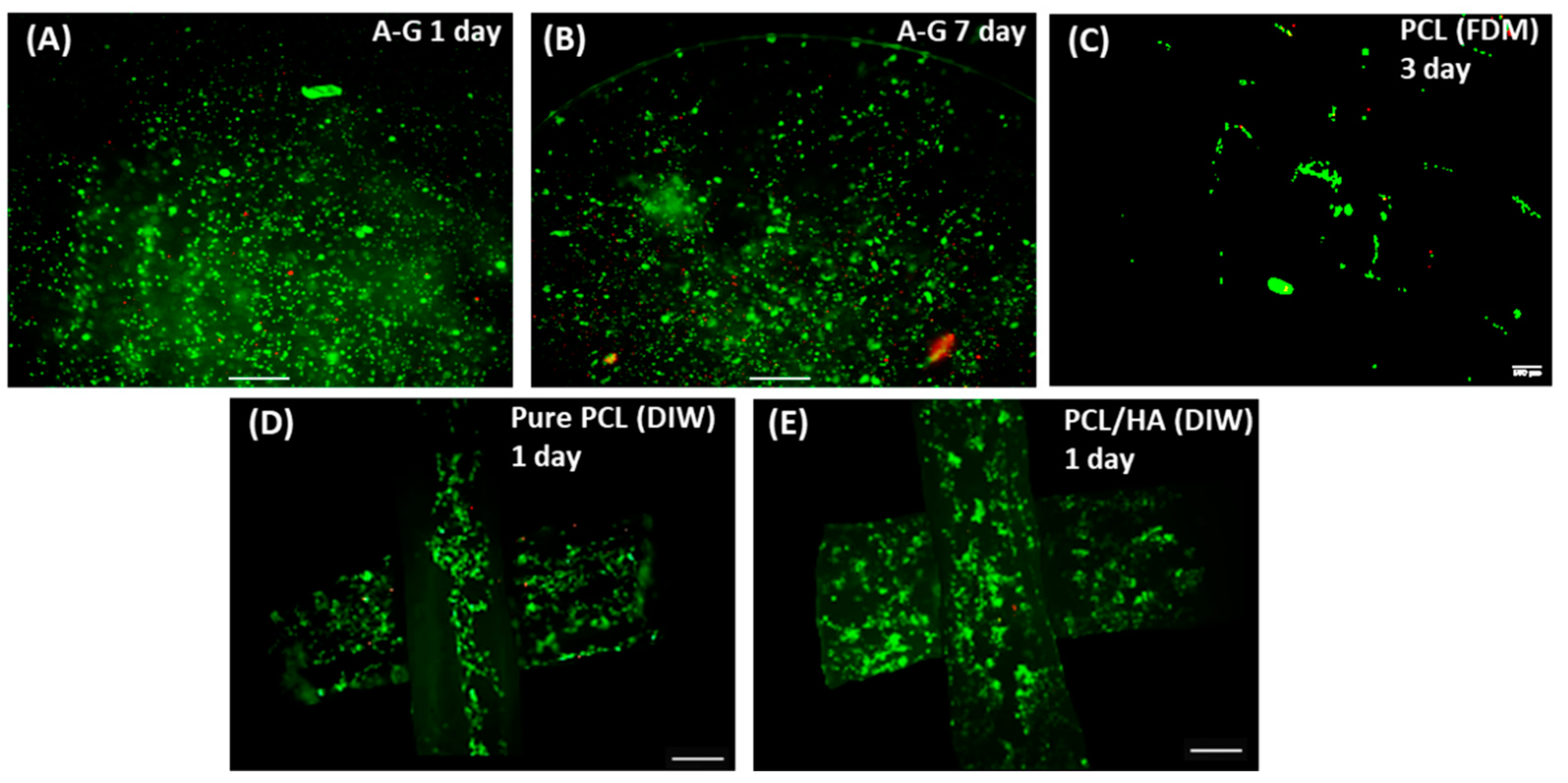
3.3.2. Cell Viability Assessments
3.3.3. Cell Proliferation
3.3.4. Mineralization Assessment
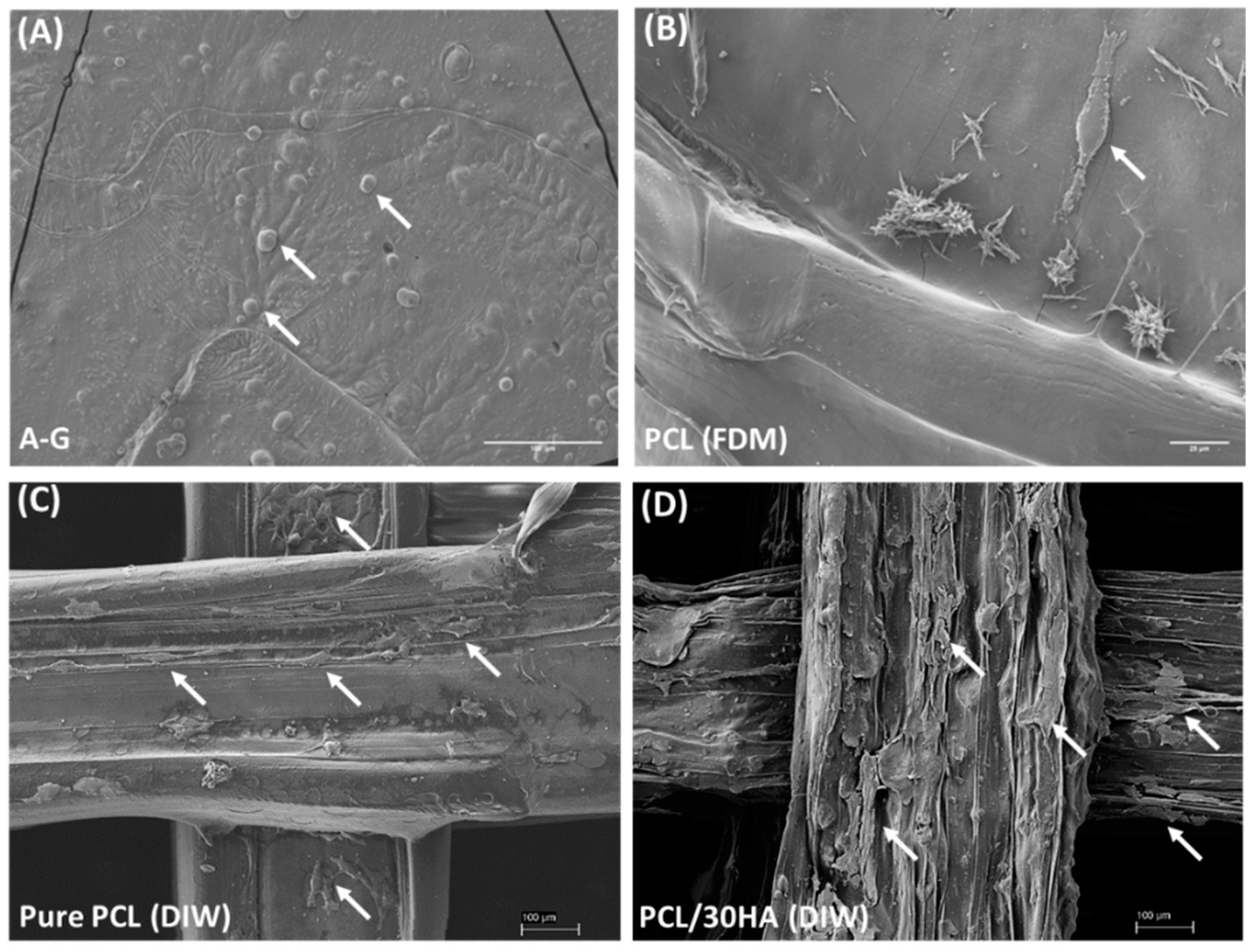
3.3.5. Cell Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sport. Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.; Goldring, M. Biology of the Normal Joint. In Kelley and Firestein's Textbook of Rheumatology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–19.e14. [Google Scholar] [CrossRef]
- Huang, J.; Lin, Y.W.; Fu, X.W.; Best, S.M.; Brooks, R.A.; Rushton, N.; Bonfield, W. Development of nano-sized hydroxyapatite reinforced composites for tissue engineering scaffolds. J. Mater. Sci. Mater. Med. 2007, 18, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; van Dijk, C.N.; Mueller-Gerbl, M. The basic science of the subchondral bone. Knee Surg. Sport. Traumatol. Arthrosc. 2010, 18, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, K.A.; Darling, E.M.; DuRaine, G.D.; Hu, J.C.; Reddi, A.H. Structure and Function of Cartilage. In Articular Cartilage; CRC Press: Boca Raton, FL, USA, 2013; pp. 1–50. [Google Scholar]
- Ansari, S.; Khorshidi, S.; Karkhaneh, A. Engineering of gradient osteochondral tissue: From nature to lab. Acta Biomater. 2019, 87, 41–54. [Google Scholar] [CrossRef]
- Ferguson, V.L.; Paietta, R.C. The Bone–Cartilage Interface. In Structural Interfaces and Attachments in Biology; Thomopoulos, S., Birman, V., Genin, G.M., Eds.; Springer: New York, NY, USA, 2013; pp. 91–118. [Google Scholar] [CrossRef]
- Ganesh, N.; Nair, S.; Nair, L.S. 13—Bone–cartilage interface. In Regenerative Engineering of Musculoskeletal Tissues and Interfaces; Nukavarapu, S.P., Freeman, J.W., Laurencin, C.T., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 327–343. [Google Scholar] [CrossRef]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike Bone, Cartilage Regeneration Remains Elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef]
- Clark, J.M. The structure of vascular channels in the subchondral plate. J. Anat. 1990, 171, 105. [Google Scholar]
- Newman, A.P. Articular cartilage repair. Am. J. Sport. Med. 1998, 26, 309–324. [Google Scholar] [CrossRef]
- Mow, V.C.; Guo, X.E. Mechano-Electrochemical Properties of Articular Cartilage: Their Inhomogeneities and Anisotropies. Annu. Rev. Biomed. Eng. 2002, 4, 175–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Tan, H.; Chen, G.; Guo, L.; Yang, L. Analysis of the Mineral Composition of the Human Calcified Cartilage Zone. Int. J. Med. Sci. 2012, 9, 353–360. [Google Scholar] [CrossRef]
- Antons, J.; Marascio, M.G.M.; Nohava, J.; Martin, R.; Applegate, L.A.; Bourban, P.E.; Pioletti, D.P. Zone-dependent mechanical properties of human articular cartilage obtained by indentation measurements. J. Mater. Sci. Mater. Med. 2018, 29, 57. [Google Scholar] [CrossRef]
- Kim, I.L.; Mauck, R.L.; Burdick, J.A. Hydrogel design for cartilage tissue engineering: A case study with hyaluronic acid. Biomaterials 2011, 32, 8771–8782. [Google Scholar] [CrossRef]
- Alford, J.W.; Cole, B.J. Cartilage Restoration, Part 1: Basic Science, Historical Perspective, Patient Evaluation, and Treatment Options. Am. J. Sport. Med. 2005, 33, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Emans, P.; Caron, M.; Rhijn, L.; Welting, T. Endochondral Bone Formation as Blueprint for Regenerative Medicine. In Tissue Regeneration; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Abbott, B. Noses and knees. Commun. Med. 2021, 1, 34. [Google Scholar] [CrossRef] [PubMed]
- Gikas, P.D.; Bayliss, L.; Bentley, G.; Briggs, T.W.R. An overview of autologous chondrocyte implantation. J. Bone Jt. Surg. Br. Vol. 2009, 91-B, 997–1006. [Google Scholar] [CrossRef]
- Haber, D.; Logan, C.; Murphy, C.; Sanchez, A.; LaPrade, R.; Provencher, M. Osteochondral Allograft Transplantation for the Knee: Post-Operative Rehabilitation. Int. J. Sport. Phys. Ther. 2019, 14, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Tamaddon, M.; Gilja, H.; Wang, L.; Oliveira, J.M.; Sun, X.; Tan, R.; Liu, C. Osteochondral scaffolds for early treatment of cartilage defects in osteoarthritic joints: From bench to clinic. Biomater. Transl. 2020, 1, 3–17. [Google Scholar] [CrossRef]
- Yousefi, A.-M.; Hoque, M.E.; Prasad, R.G.S.V.; Uth, N. Current strategies in multiphasic scaffold design for osteochondral tissue engineering: A review. J. Biomed. Mater. Res. Part A 2015, 103, 2460–2481. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Wei, W.; Ma, Y.; Yao, X.; Zhou, W.; Wang, X.; Li, C.; Lin, J.; He, Q.; Leptihn, S.; Ouyang, H. Advanced hydrogels for the repair of cartilage defects and regeneration. Bioact. Mater. 2021, 6, 998–1011. [Google Scholar] [CrossRef]
- Datta, S.; Barua, R.; Das, J. Importance of Alginate Bioink for 3D Bioprinting in Tissue Engineering and Regenerative Medicine. In Alginates—Recent Uses of This Natural Polymer; IntechOpen: London, UK, 2019; pp. 1–11. [Google Scholar]
- Pataky, K.; Braschler, T.; Negro, A.; Renaud, P.; Lutolf, M.P.; Brugger, J. Microdrop Printing of Hydrogel Bioinks into 3D Tissue-Like Geometries. Adv. Mater. 2012, 24, 391–396. [Google Scholar] [CrossRef]
- Santoro, M.; Tatara, A.M.; Mikos, A.G. Gelatin carriers for drug and cell delivery in tissue engineering. J. Control. Release 2014, 190, 210–218. [Google Scholar] [CrossRef]
- Zehnder, T.; Sarker, B.; Boccaccini, A.R.; Detsch, R. Evaluation of an alginate-gelatine crosslinked hydrogel for bioplotting. Biofabrication 2015, 7, 025001. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-S.; Sun Park, M.; Jeon, O.; Yong Choi, C.; Kim, B.-S. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Maroufkhani, M.; Mohammadzadeh-Komuleh, S.; Khoubi-Arani, Z. Chapter 7—Polymer nanocomposites for biomedical applications. In Fundamentals of Bionanomaterials; Barhoum, A., Jeevanandam, J., Danquah, M.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 175–215. [Google Scholar] [CrossRef]
- Motloung, M.P.; Mofokeng, T.G.; Ray, S.S. Viscoelastic, Thermal, and Mechanical Properties of Melt-Processed Poly (ε-Caprolactone) (PCL)/Hydroxyapatite (HAP) Composites. Materials 2022, 15, 104. [Google Scholar]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Abdul Haq, R.H.; Mohd Noh, H.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis method of hydroxyapatite: A review. Mater. Today Proc. 2020, 29, 233–239. [Google Scholar] [CrossRef]
- Poinern, G.E.; Brundavanam, R.K.; Mondinos, N.; Jiang, Z.-T. Synthesis and characterisation of nanohydroxyapatite using an ultrasound assisted method. Ultrason. Sonochemistry 2009, 16, 469–474. [Google Scholar] [CrossRef]
- Bajaj, P.; Schweller, R.M.; Khademhosseini, A.; West, J.L.; Bashir, R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014, 16, 247–276. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Khademhosseini, A. Modular Tissue Engineering: Engineering Biological Tissues from the Bottom Up. Soft Matter 2009, 5, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Bártolo, P.J. 3D bioprinting of photocrosslinkable hydrogel constructs. J. Appl. Polym. Sci. 2015, 132, 42458. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Akkineni, A.R.; Förster, Y.; Köhler, T.; Knaack, S.; Gelinsky, M.; Lode, A. Design and Fabrication of Complex Scaffolds for Bone Defect Healing: Combined 3D Plotting of a Calcium Phosphate Cement and a Growth Factor-Loaded Hydrogel. Ann. Biomed. Eng. 2017, 45, 224–236. [Google Scholar] [CrossRef]
- Bociaga, D.; Bartniak, M.; Grabarczyk, J.; Przybyszewska, K. Sodium Alginate/Gelatine Hydrogels for Direct Bioprinting-The Effect of Composition Selection and Applied Solvents on the Bioink Properties. Materials 2019, 12, 2669. [Google Scholar] [CrossRef]
- Shepherd, D.E.T.; Seedhom, B.B. Thickness of human articular cartilage in joints of the lower limb. Ann. Rheum. Dis. 1999, 58, 27–34. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Y.; Wang, J.; Yu, W.; Wang, W.; Ma, X. Monitoring of cell viability and proliferation in hydrogel-encapsulated system by resazurin assay. Appl. Biochem. Biotechnol. 2010, 162, 1996–2007. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Chen, H.; Fei, F.; Li, X.; Nie, Z.; Zhou, D.; Liu, L.; Zhang, J.; Zhang, H.; Fei, Z.; Xu, T. A facile, versatile hydrogel bioink for 3D bioprinting benefits long-term subaqueous fidelity, cell viability and proliferation. Regen. Biomater. 2021, 8, rbab026. [Google Scholar] [CrossRef]
- Mancha Sánchez, E.; Gómez-Blanco, J.C.; López Nieto, E.; Casado, J.G.; Macías-García, A.; Díaz Díez, M.A.; Carrasco-Amador, J.P.; Torrejón Martín, D.; Sánchez-Margallo, F.M.; Pagador, J.B. Hydrogels for Bioprinting: A Systematic Review of Hydrogels Synthesis, Bioprinting Parameters, and Bioprinted Structures Behavior. Front. Bioeng. Biotechnol. 2020, 6, 776. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Tseng, H.C.; Wong, S.W.; Wang, Z.; Deng, M.; Ko, C.-C. Dopaminergic effects on in vitro osteogenesis. Bone Res. 2015, 3, 15020. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, R.; Kassem, M.; Rattan, S.I.S. Heat Shock–Induced Enhancement of Osteoblastic Differentiation of hTERT-Immortalized Mesenchymal Stem Cells. Ann. N. Y. Acad. Sci. 2006, 1067, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Scogna, R.C.; Zhou, W.; Brand, S.; Fischer, J.E.; Winey, K.I. Nanotube Networks in Polymer Nanocomposites: Rheology and Electrical Conductivity. Macromolecules 2004, 37, 9048–9055. [Google Scholar] [CrossRef]



| Tissues | Cells | Main Chemical Compositions | Elastic/Young’s Modulus |
|---|---|---|---|
| Cartilage (superficial, middle, and deep zone) [1,7,8,10,11,12,13] | Chondrocytes | Dry weight: ~60 wt% Collagen (~90% Type II) ~35 wt% Proteoglycan (Water: 65–75 wt%) | 0.1–2.0 MPa |
| Cartilage (Calcified zone) [13,14] | Chondrocytes (hypertrophic) | Dry weight: ~ 20 wt% Collagen (Type II) ~65 wt% Hydroxyapatite | 6.44 ± 1.02 MPa |
| Subchondral bone [10,11,12,13,14,15,16] | Osteoblasts Osteoclasts Osteocytes Mesenchymal stem cells | Dry weight: ~ 80 wt% Hydroxyapatite ~ 10 wt% Collagen (>90% Type I) (Water: ~10 wt%) | 297–475 MPa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Gonnella, G.; Huang, J.; Di-Silvio, L. Fabrication of 3D Bioprinted Bi-Phasic Scaffold for Bone–Cartilage Interface Regeneration. Biomimetics 2023, 8, 87. https://doi.org/10.3390/biomimetics8010087
Chen H, Gonnella G, Huang J, Di-Silvio L. Fabrication of 3D Bioprinted Bi-Phasic Scaffold for Bone–Cartilage Interface Regeneration. Biomimetics. 2023; 8(1):87. https://doi.org/10.3390/biomimetics8010087
Chicago/Turabian StyleChen, Hongyi, Giovanni Gonnella, Jie Huang, and Lucy Di-Silvio. 2023. "Fabrication of 3D Bioprinted Bi-Phasic Scaffold for Bone–Cartilage Interface Regeneration" Biomimetics 8, no. 1: 87. https://doi.org/10.3390/biomimetics8010087
APA StyleChen, H., Gonnella, G., Huang, J., & Di-Silvio, L. (2023). Fabrication of 3D Bioprinted Bi-Phasic Scaffold for Bone–Cartilage Interface Regeneration. Biomimetics, 8(1), 87. https://doi.org/10.3390/biomimetics8010087








