The Role of Process-Directing Agents on Enamel Lesion Remineralization: Fluoride Boosters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tooth Preparation
2.2. Bacteria-Induced Enamel Demineralization
2.3. Remineralization Experiments
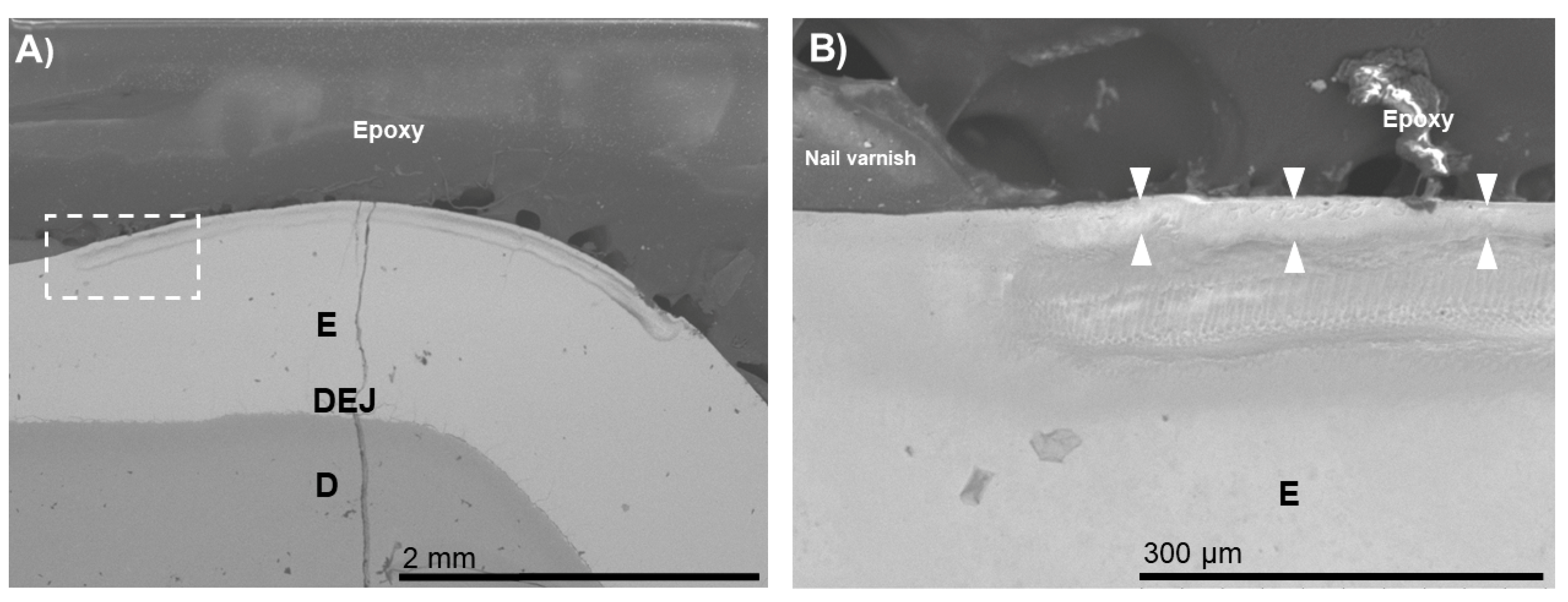
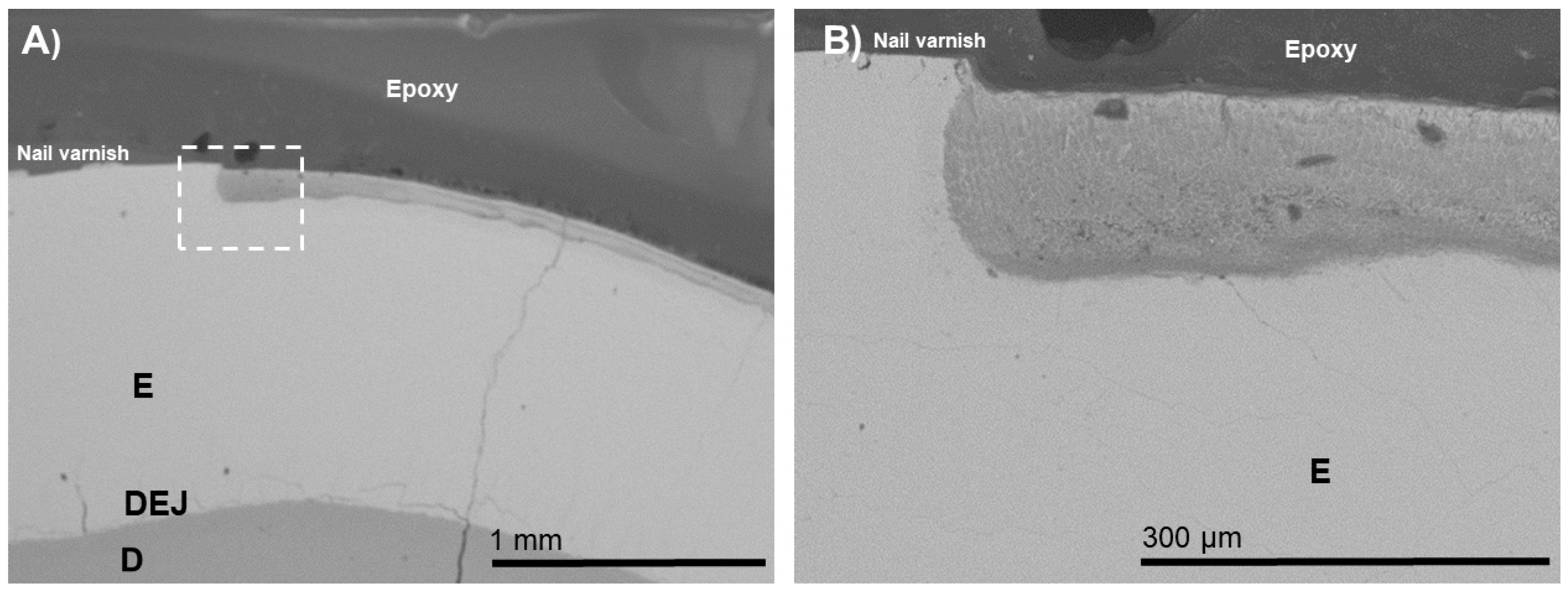
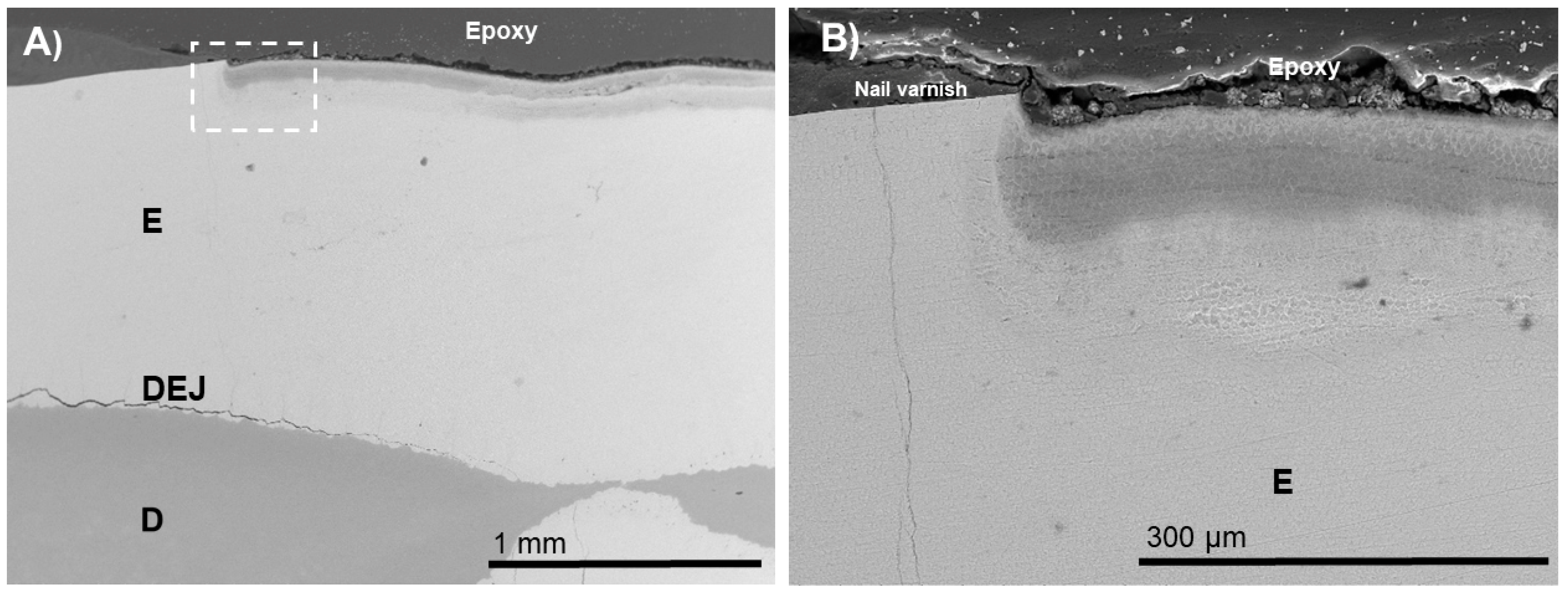
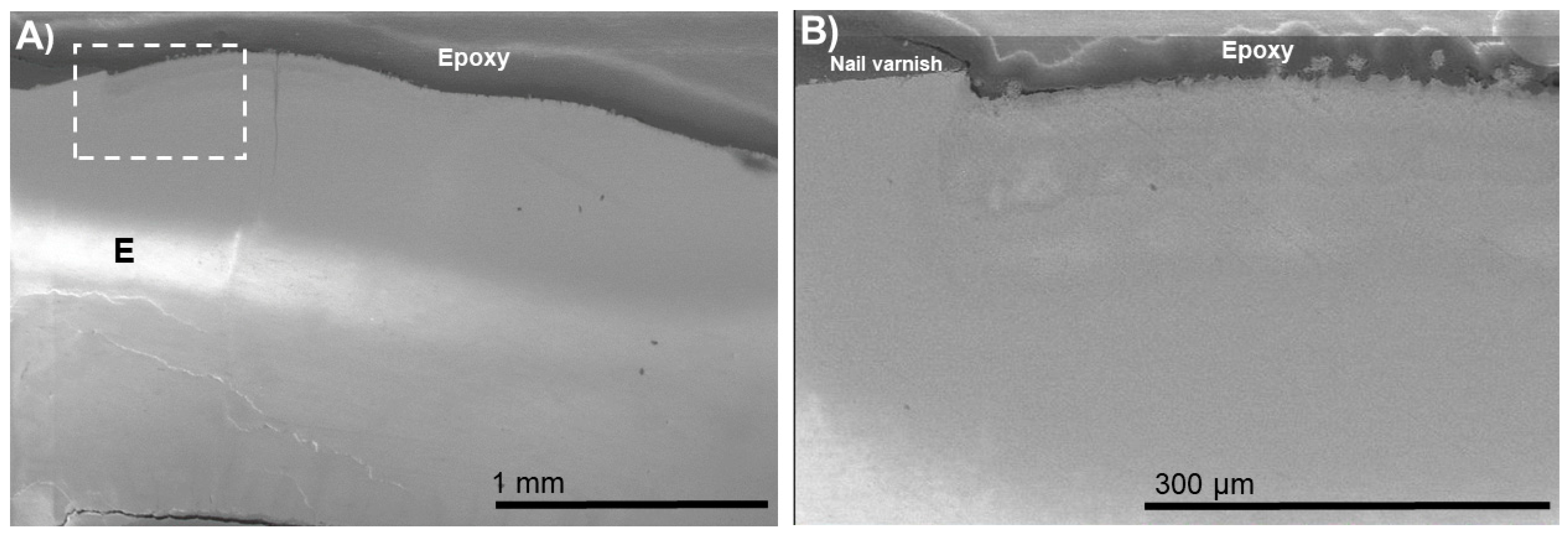
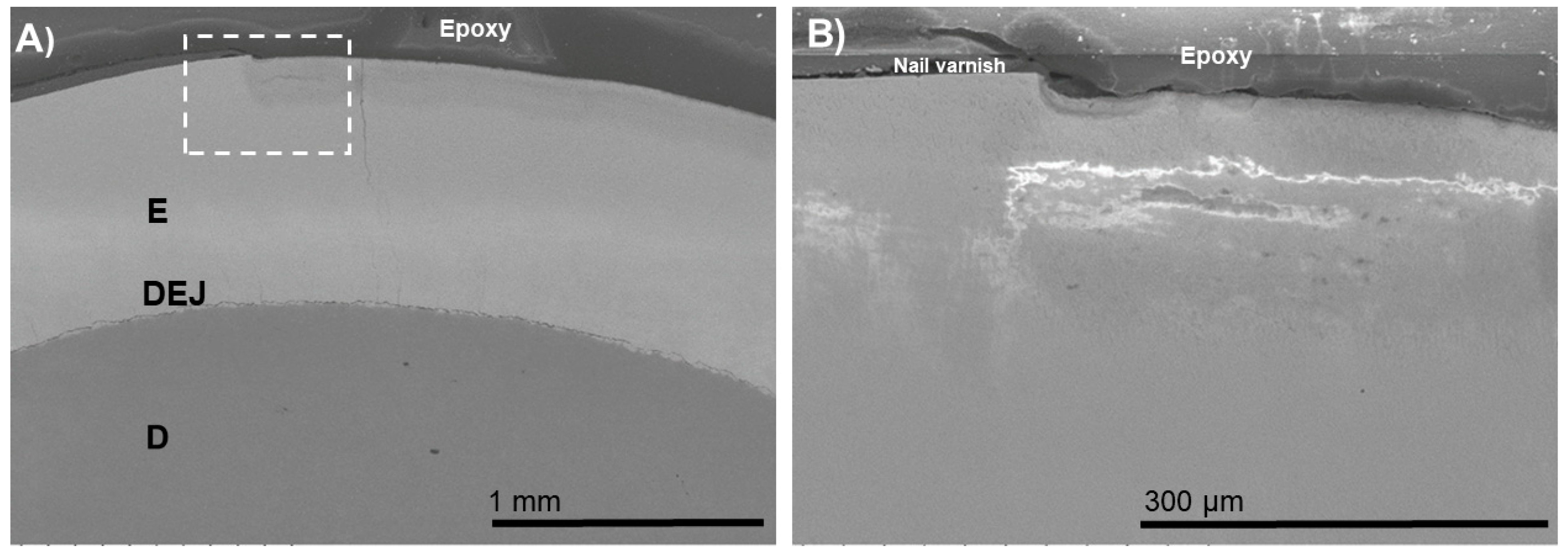
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Featherstone, J.D.B.; Chaffee, B.W. The Evidence for Caries Management by Risk Assessment (CAMBRA®). Adv. Dent. Res. 2018, 29, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, N.J.; Cai, F.; Huq, N.L.; Burrow, M.F.; Reynolds, E.C. New approaches to enhanced remineralization of tooth enamel. J. Dent. Res. 2010, 89, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M. Enhancing Fluoride: Clinical Human Studies of Alternatives or Boosters for Caries Management. Caries Res. 2016, 50, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Babaie, E.; Bacino, M.; White, J.; Nurrohman, H.; Marshall, G.W.; Saeki, K.; Habelitz, S. Polymer-Induced Liquid Precursor (PILP) remineralization of artificial and natural dentin carious lesions evaluated by nanoindentation and microcomputed tomography. J. Dent. 2021, 109, 103659. [Google Scholar] [CrossRef] [PubMed]
- Nurrohman, H.; Habelitz, S.; Saeki, K.; Sadr, A.; Gower, L.B.; Pazdernik, V.; Tagami, J.; Marshall, S.J.; Marshall, G.W. Enhanced silver diamine fluoride therapy using the PILP method -A nanoindentation study. Dent. Mater. J. 2020, 39, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Bacino, M.; Girn, V.; Nurrohman, H.; Saeki, K.; Marshall, S.J.; Gower, L.; Saeed, E.; Stewart, R.; Le, T.; Marshall, G.W.; et al. Integrating the PILP-mineralization process into a restorative dental treatment. Dent. Mater. 2019, 35, 53–63. [Google Scholar] [CrossRef]
- Saxena, N.; Cremer, M.A.; Dolling, E.S.; Nurrohman, H.; Habelitz, S.; Marshall, G.W.; Gower, L.B. Influence of fluoride on the mineralization of collagen via the polymer-induced liquid-precursor (PILP) process. Dent. Mater. 2018, 34, 1378–1390. [Google Scholar] [CrossRef]
- Nurrohman, H.; Carneiro, K.M.M.; Hellgeth, J.; Saeki, K.; Marshall, S.J.; Marshall, G.W.; Habelitz, S. The role of protease inhibitors on the remineralization of demineralized dentin using the PILP method. PLoS ONE 2017, 12, e0188277. [Google Scholar] [CrossRef] [Green Version]
- Nurrohman, H.; Saeki, K.; Carneiro, K.M.M.; Chien, Y.C.; Djomehri, S.; Ho, S.P.; Qin, C.; Marshall, S.J.; Gower, L.B.; Marshall, G.W.; et al. Repair of dentin defects from DSPP knockout mice by PILP mineralization. J. Mater. Res. 2016, 31, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Moradian-Oldak, J.; George, A. Biomineralization of Enamel and Dentin Mediated by Matrix Proteins. J. Dent. Res. 2021, 100, 1020–1029. [Google Scholar] [CrossRef]
- Sodek, J.; Ganss, B.; McKee, M.D. Osteopontin. Crit. Rev. Oral. Biol. Med. 2000, 11, 279–303. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.W.; Torchia, D.A.; Fohr, B.; Young, M.F.; Fedarko, N.S. Flexible Structures of SIBLING Proteins, Bone Sialoprotein, and Osteopontin. Biochem. Biophys. Res. Commun. 2001, 280, 460–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, K.J.; Huq, N.L.; Reynolds, E.C. Protein dynamics of bovine dentin phosphophoryn. J. Pept. Res. 2005, 66, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Platzer, G.; Schedlbauer, A.; Chemelli, A.; Ozdowy, P.; Coudevylle, N.; Auer, R.; Kontaxis, G.; Hartl, M.; Miles, A.G.; Wallace, B.A.; et al. The metastasis-associated extracellular matrix protein osteopontin forms transient structure in ligand interaction sites. Biochemistry 2011, 50, 6113–6124. [Google Scholar] [CrossRef] [PubMed]
- Gericke, A.; Qin, C.; Spevak, L.; Fujimoto, Y.; Butler, W.T.; Sorensen, E.S.; Boskey, A.L. Importance of phosphorylation for osteopontin regulation of biomineralization. Calcif. Tissue Int. 2005, 77, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinho, V.C.; Chong, L.Y.; Worthington, H.V.; Walsh, T. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2016, 7, CD002284. [Google Scholar]
- Shahid, M. Regular supervised fluoride mouthrinse use by children and adolescents associated with caries reduction. Evid. Based Dent. 2017, 18, 11–12. [Google Scholar] [CrossRef]
- Zohoori, F.V.; Maguire, A. Are there good reasons for fluoride labelling of food and drink? Br. Dent. J. 2018, 224, 215–217. [Google Scholar] [CrossRef]
- Mirabello, G.; Ianiro, A.; Bomans, P.H.H.; Yoda, T.; Arakaki, A.; Friedrich, H.; de With, G.; Sommerdijk, N.A.J.M. Crystallization by particle attachment is a colloidal assembly process. Nat. Mater. 2020, 19, 391–396. [Google Scholar] [CrossRef]
- Bai, Y.; Yu, Z.; Ackerman, L.; Zhang, Y.; Bonde, J.; Li, W.; Habelitz, S. Protein nanoribbons template enamel mineralization. Proc. Natl. Acad. Sci. USA 2020, 117, 19201–19208. [Google Scholar] [CrossRef]
- Habelitz, S.; Bai, Y. Mechanisms of Enamel Mineralization Guided by Amelogenin Nanoribbons. J. Dent. Res. 2021, 100, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Imani, M.M.; Safaei, M.; Afnaniesfandabad, A.; Moradpoor, H.; Sadeghi, M.; Golshah, A.; Sharifi, R.; Mozaffari, H.R. Efficacy of CPP-ACP and CPP-ACPF for Prevention and Remineralization of White Spot Lesions in Orthodontic Patients: A Systematic Review of Randomized Controlled Clinical Trials. Acta Inform. Med. 2019, 27, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Sharda, S.; Gupta, A.; Goyal, A.; Gauba, K. Remineralization potential and caries preventive efficacy of CPP-ACP/Xylitol/Ozone/Bioactive glass and topical fluoride combined therapy versus fluoride mono-therapy—A systematic review and meta-analysis. Acta Odontol. Scand. 2021, 79, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Rechmann, P.; Bekmezian, S.; Rechmann, B.M.T.; Chaffee, B.W.; Featherstone, J.D.B. MI Varnish and MI Paste Plus in a caries prevention and remineralization study: A randomized controlled trial. Clin. Oral. Investig. 2018, 22, 2229–2239. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Roloff-Chiang, B.; Mills, B.E.; Shalchi, S.; Spiekerman, C.; Korpak, A.M.; Starrett, J.L.; Greenlee, G.M.; Drangsholt, R.J.; Matunas, J.C. Effectiveness of MI Paste Plus and PreviDent fluoride varnish for treatment of white spot lesions: A randomized controlled trial. Am. J. Orthod. Dentofacial. Orthop. 2013, 143, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Esparza-Villalpando, V.; Fernandez-Hernandez, E.; Rosales-Berber, M.; Torre-Delgadillo, G.; Garrocho-Rangel, A.; Pozos-Guillén, A. Clinical Efficacy of Two Topical Agents for the Remineralization of Enamel White Spot Lesions in Primary Teeth. Pediatr. Dent. 2021, 43, 95–101. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurrohman, H.; Carter, L.; Barnes, N.; Zehra, S.; Singh, V.; Tao, J.; Marshall, S.J.; Marshall, G.W. The Role of Process-Directing Agents on Enamel Lesion Remineralization: Fluoride Boosters. Biomimetics 2022, 7, 54. https://doi.org/10.3390/biomimetics7020054
Nurrohman H, Carter L, Barnes N, Zehra S, Singh V, Tao J, Marshall SJ, Marshall GW. The Role of Process-Directing Agents on Enamel Lesion Remineralization: Fluoride Boosters. Biomimetics. 2022; 7(2):54. https://doi.org/10.3390/biomimetics7020054
Chicago/Turabian StyleNurrohman, Hamid, Logan Carter, Noah Barnes, Syeda Zehra, Vineet Singh, Jinhui Tao, Sally J. Marshall, and Grayson W. Marshall. 2022. "The Role of Process-Directing Agents on Enamel Lesion Remineralization: Fluoride Boosters" Biomimetics 7, no. 2: 54. https://doi.org/10.3390/biomimetics7020054
APA StyleNurrohman, H., Carter, L., Barnes, N., Zehra, S., Singh, V., Tao, J., Marshall, S. J., & Marshall, G. W. (2022). The Role of Process-Directing Agents on Enamel Lesion Remineralization: Fluoride Boosters. Biomimetics, 7(2), 54. https://doi.org/10.3390/biomimetics7020054







