Investigation of Inclusion States of Silicate and Carbonate Ions in Hydroxyapatite Particles Prepared under the Presence of Sodium Silicate
Abstract
:1. Introduction
2. Materials and Methods
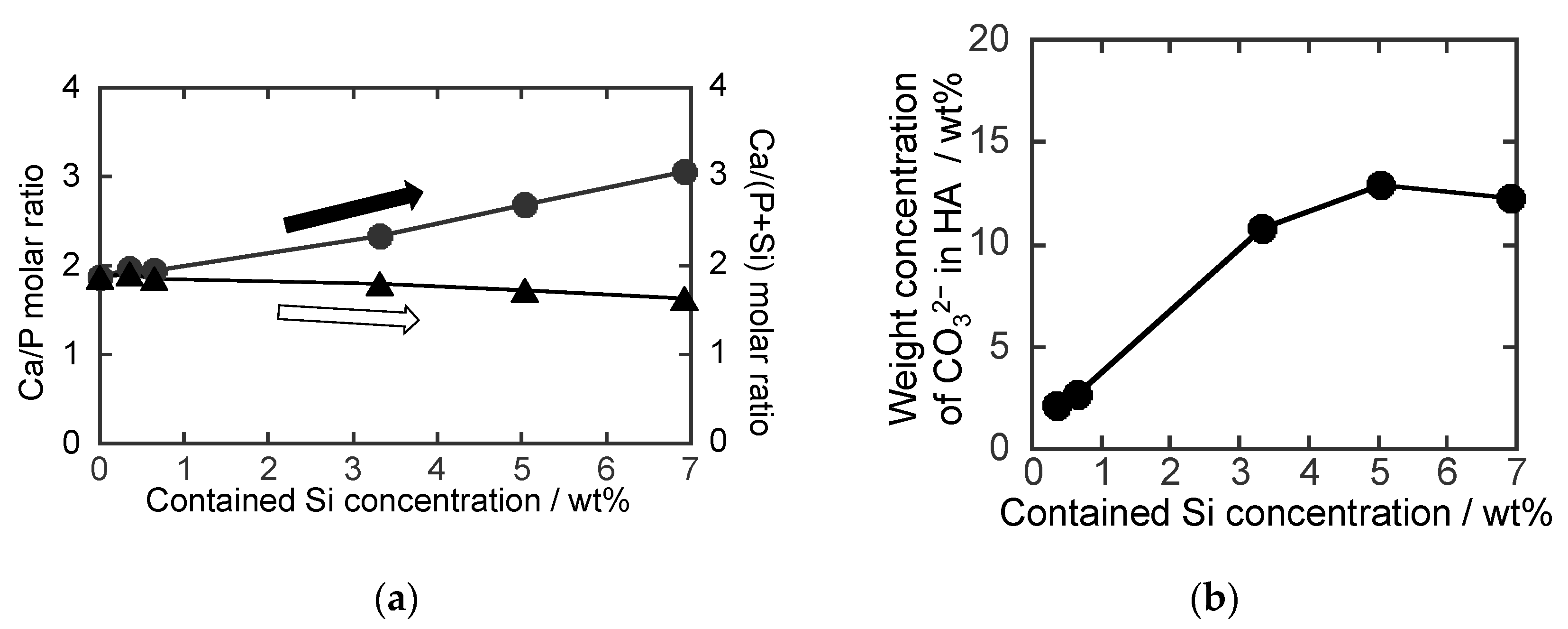
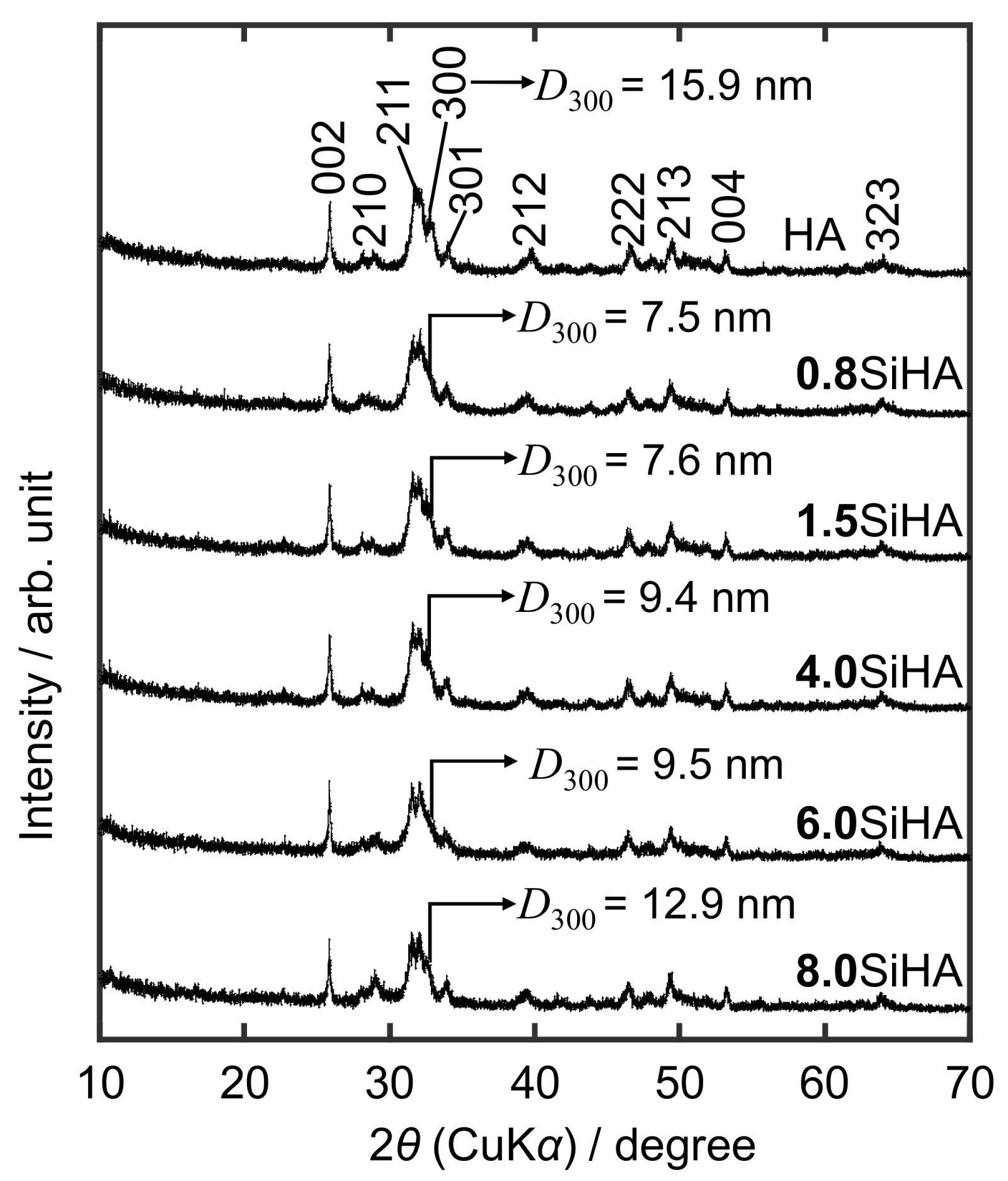
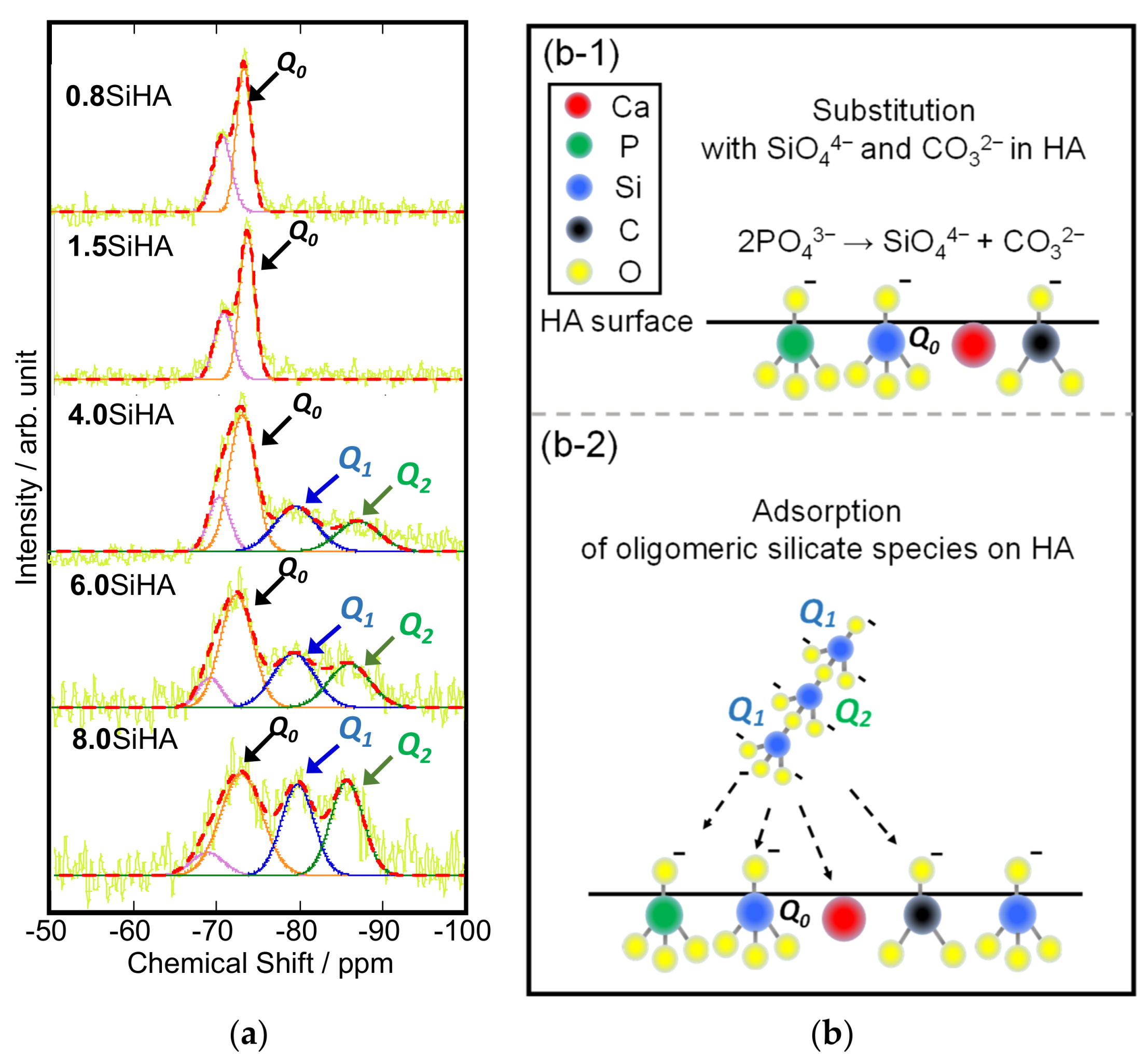
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palard, M.; Champion, E.; Foucaud, S. Synthesis of silicated hydroxyapatite Ca10(PO4)6−x(SiO4)x(OH)2−x. J. Solid State Chem. 2008, 181, 1950–1960. [Google Scholar] [CrossRef]
- Solonenko, A.P. Biomaterials based on mixtures of calcium phosphates and silicates: Investigation of possible production by precipitation from water solutions. Glas. Ceram. 2017, 73, 386–389. [Google Scholar] [CrossRef]
- Galindo, T.G.P.; Chai, Y.; Tagaya, M. Hydroxyapatite nanoparticle coating on polymer for constructing effective biointeractive interfaces. J. Nanomater. 2019, 2019, 6495239. [Google Scholar] [CrossRef] [Green Version]
- Posner, A.S.; Betts, F. Synthetic Amorphous Calcium Phosphate and Its Relation to Bone Mineral Structure. Acc. Chem. Res. 1975, 8, 273–281. [Google Scholar] [CrossRef]
- Patel, K.D.; Singh, R.K.; Lee, J.H.; Kim, H.W. Electrophoretic coatings of hydroxyapatite with various nanocrystal shapes. Mater. Lett. 2019, 234, 148–154. [Google Scholar] [CrossRef]
- Singh, R.K.; Kim, T.H.; Patel, K.D.; Kim, J.J.; Kim, H.W. Development of biocompatible apatite nanorod-based drug-delivery system with in situ fluorescence imaging capacity. J. Mater. Chem. B 2014, 2, 2039–2050. [Google Scholar] [CrossRef]
- Solonenko, A.P.; Golovanova, O.A. Silicate-substituted carbonated hydroxyapatite powders prepared by precipitation from aqueous solutions. Russ. J. Inorg. Chem. 2014, 59, 1228–1236. [Google Scholar] [CrossRef]
- Solonenko, A.P.; Blesman, A.I.; Polonyankin, D.A.; Bel’skaya, L.V. Effect of sodium silicate on the nature of crystallization products in calcium phosphate systems. Russ. J. Inorg. Chem. 2017, 62, 1286–1292. [Google Scholar] [CrossRef]
- Sindu, P.A.; Kolanthai, E.; Suganthi, R.V.; Arul, K.T.; Manikandan, E.; Catalani, L.H.; Kalkura, S.N. Green synthesis of Si-incorporated hydroxyapatite using sodium metasilicate as silicon precursor and in vitro antibiotic release studies. J. Photochem. Photobiol. B Biol. 2017, 175, 163–172. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef]
- Tagaya, M.; Ikoma, T.; Takeguchi, M.; Hanagata, N.; Tanaka, J. Interfacial serum protein effect on biological apatite growth. J. Phys. Chem. C 2011, 115, 22523–22533. [Google Scholar] [CrossRef]
- Sprio, S.; Tampieri, A.; Landi, E.; Sandri, M.; Martorana, S.; Celotti, G.; Logroscino, G. Physico-chemical properties and solubility behaviour of multi-substituted hydroxyapatite powders containing silicon. Mater. Sci. Eng. C 2008, 28, 179–187. [Google Scholar] [CrossRef]
- Gibson, I.R.; Best, S.M.; Bonfield, W. Chemical characterization of silicon-substituted hydroxyapatite. J. Biomed. Mater. Res. 1999, 44, 422–428. [Google Scholar] [CrossRef]
- Hijón, N.; Cabañas, M.V.; Peña, J.; Vallet-Regí, M. Dip coated silicon-substituted hydroxyapatite films. Acta Biomater. 2006, 2, 567–574. [Google Scholar] [CrossRef]
- Xu, J.L.; Khor, K.A. Chemical analysis of silica doped hydroxyapatite biomaterials consolidated by a spark plasma sintering method. J. Inorg. Biochem. 2007, 101, 187–195. [Google Scholar] [CrossRef]
- Suetsugu, Y.; Takahashi, Y.; Okamura, F.P.; Tanaka, J. Structure Analysis of A-Type Carbonate Apatite by a Single-Crystal X-ray Diffraction Method. J. Solid State Chem. 2000, 155, 292–297. [Google Scholar] [CrossRef]
- Fleet, M.E.; Liu, X. Coupled substitution of type A and B carbonate in sodium-bearing apatite. Biomaterials 2007, 28, 916–926. [Google Scholar] [CrossRef]
- Bang, L.T.; Long, B.D.; Othman, R. Carbonate hydroxyapatite and silicon-substituted carbonate hydroxyapatite: Synthesis, mechanical properties, and solubility evaluations. Sci. World J. 2014, 2014, 969876. [Google Scholar] [CrossRef]
- Ibrahim, D.M.; Mostafa, A.A.; Korowash, S.I. Chemical characterization of some substituted hydroxyapatites. Chem. Cent. J. 2011, 5, 74. [Google Scholar] [CrossRef] [Green Version]
- Bang, L.T.; Ramesh, S.; Purbolaksono, J.; Ching, Y.C.; Long, B.D.; Chandran, H.; Othman, R. Effects of silicate and carbonate substitution on the properties of hydroxyapatite prepared by aqueous co-precipitation method. Mater. Des. 2015, 87, 788–796. [Google Scholar] [CrossRef]
- Reffitt, D.M.; Ogston, N.; Jugdaohsingh, R.; Cheung, H.F.J.; Evans, B.A.J.; Thompson, R.P.H.; Powell, J.J.; Hampson, G.N. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 2003, 32, 127–135. [Google Scholar] [CrossRef]
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 105, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Biedrzycka, A.; Skwarek, E.; Hanna, U.M. Hydroxyapatite with magnetic core: Synthesis methods, properties, adsorption and medical applications. Adv. Colloid Interface Sci. 2021, 291, 102401. [Google Scholar] [CrossRef] [PubMed]
- Skwarek, E.; Gładysz-Płaska, A.; Choromańska, J.B.; Broda, E. Adsorption of uranium ions on nano-hydroxyapatite and modified by Ca and Ag ions. Adsorption 2019, 25, 639–647. [Google Scholar] [CrossRef] [Green Version]
- Skwarek, E.; Gładysz–Płaska, A.; Bolbukh, Y. Adsorption of Uranyl Ions at the Nano-hydroxyapatite and Its Modification, Nanosca. Res. Lett. 2017, 12, 278. [Google Scholar]
- Grunenwald, A.; Keyser, C.; Sautereau, A.M.; Crubézy, E.; Ludes, B.; Drouet, C. Revisiting carbonate quantification in apatite (bio)minerals: A validated FTIR methodology. J. Archaeol. Sci. 2014, 49, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Tagaya, M.; Abe, S.; Motozuka, S.; Shiba, K.; Takemura, T.; Hayashi, I.; Sakaguchi, Y. Surface-engineered mesoporous silica particles with luminescent, cytocompatible and targeting properties for cancer cell imaging. RSC Adv. 2017, 7, 13643–13652. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Tagaya, M.; Yamada, S.; Motozuka, S. Synthesis of nanostructured silica/hydroxyapatite hybrid particles containing amphiphilic triblock copolymer for effectively controlling hydration layer structures with cytocompatibility. J. Mater. Chem. B 2020, 8, 1524–1537. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Aminian, A.; Solati-Hashjin, M.; Samadikuchaksaraei, A.; Bakhshi, F.; Gorjipour, F.; Farzadi, A.; Moztarzadeh, F.; Schmücker, M. Synthesis of silicon-substituted hydroxyapatite by a hydrothermal method with two different phosphorous sources. Ceram. Int. 2011, 37, 1219–1229. [Google Scholar] [CrossRef]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Kanaya, T.; Tsuru, K.; Shirosaki, Y.; Osaka, A.; Fujii, E.; Kawabata, K.; Gasqueres, G.; Bonhomme, C.; Babonneau, F.; et al. Heterogeneous structure and in vitro degradation behavior of wet-chemically derived nanocrystalline silicon-containing hydroxyapatite particles. Acta Biomater. 2013, 9, 4856–4867. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, N.Y.; Hassan, H.M.; Abd Elkader, O.H. Preparation and Characterization of Na+, SiO44−, and CO32−Co-Substituted Hydroxyapatite. J. Am. Ceram. Soc. 2011, 94, 1584–1590. [Google Scholar] [CrossRef]
- Kröger, F.A.; Vink, H.J. Relations between the Concentrations of Imperfections in Crystalline Solids. Solid State Phys. 1956, 3, 307–435. [Google Scholar]
- Targonska, S.; Wiglusz, R.J. Investigation of physicochemical properties of the structurally modified nanosized silicate-substituted hydroxyapatite co-doped with eu3+ and sr2+ ions. Nanomaterials 2021, 11, 27. [Google Scholar] [CrossRef]
- Gibson, I.R.; Bonfield, W. Novel synthesis and characterization of an AB-type carbonate-substituted hydroxyapatite. J. Biomed. Mater. Res. 2001, 59, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Pietak, A.M.; Reid, J.W.; Stott, M.J.; Sayer, M. Silicon substitution in the calcium phosphate bioceramics. Biomaterials 2007, 28, 4023–4032. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.L.; Xiao, X.F.; Liu, R.F. Structural characterization of silicon-substituted hydroxyapatite synthesized by a hydrothermal method. Mater. Lett. 2005, 59, 3841–3846. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peñaflor Galindo, T.G.; Sugimoto, K.; Yamada, S.; Sugibuchi, T.; Liu, Z.; Tagaya, M. Investigation of Inclusion States of Silicate and Carbonate Ions in Hydroxyapatite Particles Prepared under the Presence of Sodium Silicate. Biomimetics 2022, 7, 40. https://doi.org/10.3390/biomimetics7020040
Peñaflor Galindo TG, Sugimoto K, Yamada S, Sugibuchi T, Liu Z, Tagaya M. Investigation of Inclusion States of Silicate and Carbonate Ions in Hydroxyapatite Particles Prepared under the Presence of Sodium Silicate. Biomimetics. 2022; 7(2):40. https://doi.org/10.3390/biomimetics7020040
Chicago/Turabian StylePeñaflor Galindo, Tania Guadalupe, Kazuto Sugimoto, Shota Yamada, Taito Sugibuchi, Zizhen Liu, and Motohiro Tagaya. 2022. "Investigation of Inclusion States of Silicate and Carbonate Ions in Hydroxyapatite Particles Prepared under the Presence of Sodium Silicate" Biomimetics 7, no. 2: 40. https://doi.org/10.3390/biomimetics7020040
APA StylePeñaflor Galindo, T. G., Sugimoto, K., Yamada, S., Sugibuchi, T., Liu, Z., & Tagaya, M. (2022). Investigation of Inclusion States of Silicate and Carbonate Ions in Hydroxyapatite Particles Prepared under the Presence of Sodium Silicate. Biomimetics, 7(2), 40. https://doi.org/10.3390/biomimetics7020040




