Biomimetic Aspects of Oral and Dentofacial Regeneration
Abstract
1. Introduction
2. Biomimetics in Restorative Dentistry
2.1. Enamel Biomimetics
2.1.1. Physiochemical Synthesis
2.1.2. Protein-Matrix-Guided Synthesis
2.1.3. Enamel Surface Mineralisation
2.1.4. Cell and Tissue Culture Systems for Enamel Organ Engineering
2.2. Biomimetic Aspects of Dentin and Dentin-Pulp-Complex Regeneration
2.3. Dentin-Pulp Complex Regeneration
3. Biomimetics in Oral and Maxillofacial Regeneration
3.1. Biomimetics in Bone Regeneration
3.1.1. Bone, a Complex Hub, and a Multitasker
3.1.2. Determinants of Biomimetics for Bone Regeneration
3.1.3. Bone Grafts and Scaffolds
3.1.4. Cell Therapy
3.1.5. Cell-Free Therapies
3.2. Biomimetics in Mucosal Repair
3.2.1. Oral Mucosa
3.2.2. Determinants of Biomimetics
3.2.3. Mucosal Grafts
4. Biomimetics of Periodontal Tissue Engineering and Regeneration
4.1. Periodontal Regeneration
4.1.1. Cell-Based Therapies
4.1.2. Cell-Free Therapies
4.1.3. Guided Tissue Regeneration
4.2. Implant BIOMIMETICS
4.2.1. Surface Modification and Alternative Materials for Implant Osseointegration
4.2.2. Antimicrobial/Anti-Inflammatory Aspects of Oral Implantology
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Robinson, R. The organic constituent of enamel. Tufts Dent. Outlook 1945, 19, 5. [Google Scholar]
- Featherstone, J.; Chaffee, B. The evidence for caries management by risk assessment (CAMBRA®). Adv. Dent. Res. 2018, 29, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Pandya, M.; Diekwisch, T.G.H. Enamel biomimetics-fiction or future of dentistry. Int. J. Oral Sci. 2019, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Clarkson, B.H.; Sun, K.; Mansfield, J.F. Self-assembly of synthetic hydroxyapatite nanorods into an enamel prism-like structure. J. Colloid Interface Sci. 2005, 288, 97–103. [Google Scholar] [CrossRef]
- Ren, F.; Ding, Y.; Ge, X.; Lu, X.; Wang, K.; Leng, Y. Growth of one-dimensional single-crystalline hydroxyapatite nanorods. J. Cryst. Growth 2012, 349, 75–82. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, Z.; Yang, J.; Lu, D.; Kishen, A.; Li, Y.; Chen, Z.; Que, K.; Zhang, Q.; Deng, X.; et al. Oriented and Ordered Biomimetic Remineralization of the Surface of Demineralized Dental Enamel Using HAP@ACP Nanoparticles Guided by Glycine. Sci. Rep. 2017, 7, 40701. [Google Scholar] [CrossRef]
- Atsawasuwan, P.; Lu, X.; Ito, Y.; Chen, Y.; Gopinathan, G.; Evans, C.; Kulkarni, A.; Gibson, C.; Luan, X.; Diekwisch, T. Expression and function of enamel-related gene products in calvarial development. J. Dent. Res. 2013, 92, 622–628. [Google Scholar] [CrossRef]
- Lijima, M.; Moriwaki, Y.; Wen, H.B.; Fincham, A.G.; Moradian-Oldak, J. Elongated Growth of Octacalcium Phosphate Crystals in Recombinant Amelogenin Gels under Controlled Ionic Flow. J. Dent. Res. 2002, 81, 69–73. [Google Scholar] [CrossRef]
- Fan, D.; Iijima, M.; Bromley, K.M.; Yang, X.; Mathew, S.; Moradian-Oldak, J. The Cooperation of Enamelin and Amelogenin in Controlling Octacalcium Phosphate Crystal Morphology. Cells Tissues Organs 2011, 194, 194–198. [Google Scholar] [CrossRef]
- Pandya, M.; Lin, T.; Li, L.; Allen, M.J.; Jin, T.; Luan, X.; Diekwisch, T.G.H. Posttranslational Amelogenin Processing and Changes in Matrix Assembly during Enamel Development. Front. Physiol. 2017, 8, 790. [Google Scholar] [CrossRef]
- Li, Q.-L.; Ning, T.-Y.; Cao, Y.; Zhang, W.-b.; Mei, M.L.; Chu, C.H. A novel self-assembled oligopeptide amphiphile for biomimetic mineralization of enamel. BMC Biotechnol. 2014, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.; Ruan, Q.; Mukherjee, K.; Nutt, S.; Moradian-Oldak, J. The Presence of MMP-20 Reinforces Biomimetic Enamel Regrowth. J. Dent. Res. 2018, 97, 84–90. [Google Scholar] [CrossRef]
- Shen, P.; Walker, G.D.; Yuan, Y.; Reynolds, C.; Stanton, D.P.; Fernando, J.R.; Reynolds, E.C. Importance of bioavailable calcium in fluoride dentifrices for enamel remineralization. J. Dent. 2018, 78, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lin, X.; Zhong, T.; Xie, F. Evaluation of the efficacy of casein phosphopeptide-amorphous calcium phosphate on remineralization of white spot lesions in vitro and clinical research: A systematic review and meta-analysis. BMC Oral Health 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fernando, J.R.; Shen, P.; Sim, C.P.C.; Chen, Y.Y.; Walker, G.D.; Yuan, Y.; Reynolds, C.; Stanton, D.P.; MacRae, C.M.; Reynolds, E.C. Self-assembly of dental surface nanofilaments and remineralisation by SnF2 and CPP-ACP nanocomplexes. Sci. Rep. 2019, 9, 1285. [Google Scholar] [CrossRef] [PubMed]
- Bossu, M.; Saccucci, M.; Salucci, A.; Di Giorgio, G.; Bruni, E.; Uccelletti, D.; Sarto, M.S.; Familiari, G.; Relucenti, M.; Polimeni, A. Enamel remineralization and repair results of Biomimetic Hydroxyapatite toothpaste on deciduous teeth: An effective option to fluoride toothpaste. J. Nanobiotechnol. 2019, 17, 17. [Google Scholar] [CrossRef]
- Bakry, A.S.; Marghalani, H.Y.; Amin, O.A.; Tagami, J. The effect of a bioglass paste on enamel exposed to erosive challenge. J. Dent. 2014, 42, 1458–1463. [Google Scholar] [CrossRef]
- Taha, A.A.; Patel, M.P.; Hill, R.G.; Fleming, P.S. The effect of bioactive glasses on enamel remineralization: A systematic review. J. Dent. 2017, 67, 9–17. [Google Scholar] [CrossRef]
- Kohda, N.; Iijima, M.; Kawaguchi, K.; Toshima, H.; Muguruma, T.; Endo, K.; Mizoguchi, I. Inhibition of enamel demineralization and bond-strength properties of bioactive glass containing 4-META/MMA-TBB-based resin adhesive. Eur. J. Oral Sci. 2015, 123, 202–207. [Google Scholar] [CrossRef]
- Manfred, L.; Covell, D.A.; Crowe, J.J.; Tufekci, E.; Mitchell, J.C. A novel biomimetic orthodontic bonding agent helps prevent white spot lesions adjacent to brackets. Angle Orthod. 2013, 83, 97–103. [Google Scholar] [CrossRef]
- Liu, H.; Yan, X.; Pandya, M.; Luan, X.; Diekwisch, T.G.H. 4Daughters of the Enamel Organ: Development, Fate, and Function of the Stratum Intermedium, Stellate Reticulum, and Outer Enamel Epithelium. Stem Cells Dev. 2016, 25, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- DenBesten, P.K.; Gao, C.; Li, W.; Mathews, C.H.E.; Gruenert, D.C. Development and characterization of an SV40 immortalized porcine ameloblast-like cell line. Eur. J. Oral Sci. 1999, 107, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, M.; Ishikawa, M.; Nakamura, T.; Iwamoto, T.; Yamada, A.; Fukumoto, E.; Saito, M.; Otsu, K.; Harada, H.; Yamada, Y. Role of epithelial-stem cell interactions during dental cell differentiation. J. Biol. Chem. 2012, 287, 10590–10601. [Google Scholar] [CrossRef]
- Tjäderhane, L.; Carrilho, M.R.; Breschi, L.; Tay, F.R.; Pashley, D.H. Dentin basic structure and composition—An overview. Endod. Top. 2009, 20, 3–29. [Google Scholar] [CrossRef]
- Fernando, D.; Attik, N.; Pradelle-Plasse, N.; Jackson, P.; Grosgogeat, B.; Colon, P. Bioactive glass for dentin remineralization: A systematic review. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 1369–1377. [Google Scholar] [CrossRef]
- Niu, L.-n.; Zhang, W.; Pashley, D.H.; Breschi, L.; Mao, J.; Chen, J.-H.; Tay, F.R. Biomimetic remineralization of dentin. Dent. Mater. 2014, 30, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Sauro, S.; Osorio, R.; Watson, T.F.; Toledano, M. Therapeutic effects of novel resin bonding systems containing bioactive glasses on mineral-depleted areas within the bonded-dentine interface. J. Mater. Sci. Mater. Med. 2012, 23, 1521–1532. [Google Scholar] [CrossRef]
- Tezvergil-Mutluay, A.; Seseogullari-Dirihan, R.; Feitosa, V.P.; Cama, G.; Brauer, D.S.; Sauro, S. Effects of Composites Containing Bioactive Glasses on Demineralized Dentin. J. Dent. Res. 2017, 96, 999–1005. [Google Scholar] [CrossRef]
- Jun, S.-K.; Yang, S.-A.; Kim, Y.-J.; El-Fiqi, A.; Mandakhbayar, N.; Kim, D.-S.; Roh, J.; Sauro, S.; Kim, H.-W.; Lee, J.-H.; et al. Multi-functional nano-adhesive releasing therapeutic ions for MMP-deactivation and remineralization. Sci. Rep. 2018, 8, 5663. [Google Scholar] [CrossRef]
- Profeta, A.C.; Mannocci, F.; Foxton, R.; Watson, T.F.; Feitosa, V.P.; De Carlo, B.; Mongiorgi, R.; Valdré, G.; Sauro, S. Experimental etch-and-rinse adhesives doped with bioactive calcium silicate-based micro-fillers to generate therapeutic resin–dentin interfaces. Dent. Mater. 2013, 29, 729–741. [Google Scholar] [CrossRef]
- Braga, R.R. Calcium phosphates as ion-releasing fillers in restorative resin-based materials. Dent. Mater. 2019, 35, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.; Cabello, I.; Toledano, M. Bioactivity of zinc-doped dental adhesives. J. Dent. 2014, 42, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Yamauti, M.; Ruiz-Requena, M.E.; Osorio, R. A ZnO-doped adhesive reduced collagen degradation favouring dentine remineralization. J. Dent. 2012, 40, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.-S.; Kim, J.; Kim, Y.K.; Liu, Y.; Dickens, S.H.; Pashley, D.H.; Ling, J.-Q.; Tay, F.R. A chemical phosphorylation-inspired design for Type I collagen biomimetic remineralization. Dent. Mater. 2010, 26, 1077–1089. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H. Guided tissue remineralisation of partially demineralised human dentine. Biomaterials 2008, 29, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Olszta, M.J.; Odom, D.J.; Douglas, E.P.; Gower, L.B. A New Paradigm for Biomineral Formation: Mineralization via an Amorphous Liquid-Phase Precursor. Connect. Tissue Res. 2003, 44, 326–334. [Google Scholar] [CrossRef]
- Liu, Y.; Li, N.; Qi, Y.; Niu, L.-n.; Elshafiy, S.; Mao, J.; Breschi, L.; Pashley, D.H.; Tay, F.R. The use of sodium trimetaphosphate as a biomimetic analog of matrix phosphoproteins for remineralization of artificial caries-like dentin. Dent. Mater. 2011, 27, 465–477. [Google Scholar] [CrossRef]
- Liu, Y.; Li, N.; Qi, Y.-p.; Dai, L.; Bryan, T.E.; Mao, J.; Pashley, D.H.; Tay, F.R. Intrafibrillar Collagen Mineralization Produced by Biomimetic Hierarchical Nanoapatite Assembly. Adv. Mater. 2011, 23, 975–980. [Google Scholar] [CrossRef]
- Kim, Y.K.; Gu, L.-s.; Bryan, T.E.; Kim, J.R.; Chen, L.; Liu, Y.; Yoon, J.C.; Breschi, L.; Pashley, D.H.; Tay, F.R. Mineralisation of reconstituted collagen using polyvinylphosphonic acid/polyacrylic acid templating matrix protein analogues in the presence of calcium, phosphate and hydroxyl ions. Biomaterials 2010, 31, 6618–6627. [Google Scholar] [CrossRef]
- Borén, D.L.; Jonasson, P.; Kvist, T. Long-term survival of endodontically treated teeth at a public dental specialist clinic. J. Endod. 2015, 41, 176–181. [Google Scholar] [CrossRef]
- Fristad, I.; Molven, O.; Halse, A. Nonsurgically retreated root filled teeth–radiographic findings after 20–27 years. Int. Endod. J. 2004, 37, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Prati, C.; Pirani, C.; Zamparini, F.; Gatto, M.; Gandolfi, M. A 20-year historical prospective cohort study of root canal treatments. A Multilevel analysis. Int. Endod. J. 2018, 51, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shen, H.; Suh, B.I. Bioactive dental restorative materials: A review. Am. J. Dent. 2013, 26, 219. [Google Scholar] [PubMed]
- Hilton, T.J. Keys to Clinical Success with Pulp Capping: A Review of the Literature. Oper. Dent. 2009, 34, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Komabayashi, T.; Zhu, Q.; Eberhart, R.; Imai, Y. Current status of direct pulp-capping materials for permanent teeth. Dent. Mater. J. 2016, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.-M.; Jeon, S.H.; Park, J.-Y.; Chung, J.-H.; Choung, Y.-H.; Choung, P.-H. Dental Stem Cell Therapy with Calcium Hydroxide in Dental Pulp Capping. Tissue Eng. Part A 2010, 16, 1823–1833. [Google Scholar] [CrossRef]
- Sangwan, P.; Sangwan, A.; Duhan, J.; Rohilla, A. Tertiary dentinogenesis with calcium hydroxide: A review of proposed mechanisms. Int. Endod. J. 2013, 46, 3–19. [Google Scholar] [CrossRef]
- Natale, L.C.; Rodrigues, M.C.; Xavier, T.A.; Simões, A.; de Souza, D.N.; Braga, R.R. Ion release and mechanical properties of calcium silicate and calcium hydroxide materials used for pulp capping. Int. Endod. J. 2015, 48, 89–94. [Google Scholar] [CrossRef]
- Tawil, P.Z.; Duggan, D.J.; Galicia, J.C. Mineral trioxide aggregate (MTA): Its history, composition, and clinical applications. Compend. Contin. Educ. Dent. (Jamesburg NJ 1995) 2015, 36, 247–252; quiz 254, 264. [Google Scholar]
- Ford, T.R.P.; Torabinejad, M.; Abedi, H.R.; Bakland, L.K.; Kariyawasam, S.P. Using mineral trioxide aggregate as a pulp-capping material. J. Am. Dent. Assoc. 1996, 127, 1491–1494. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Casey, J.A.; VanderWeele, R.A.; Vandewalle, K.S. Mechanical properties of new dental pulp-capping materials. Gen. Dent. 2016, 64, 44–48. [Google Scholar] [PubMed]
- Norrby, K. Mast cells and angiogenesis. APMIS 2002, 110, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Oguntebi, B.; Clark, A.; Wilson, J. Pulp capping with Bioglass® and autologous demineralized dentin in miniature swine. J. Dent. Res. 1993, 72, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Mocquot, C.; Colon, P.; Fernando, D.; Jackson, P.; Pradelle-Plasse, N.; Grosgogeat, B.; Attik, N. The infuence of experimental bioactive glasses on pulp cells behavior in vitro. Dent. Mater. 2020, 36, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Kohli, M.R.; Yu, Q.; Kim, S.; Qu, T.; He, W.-X. Biodentine Induces Human Dental Pulp Stem Cell Differentiation through Mitogen-activated Protein Kinase and Calcium-/Calmodulin-dependent Protein Kinase II Pathways. J. Endod. 2014, 40, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Laurent, P.; Camps, J.; About, I. BiodentineTM induces TGF-β1 release from human pulp cells and early dental pulp mineralization. Int. Endod. J. 2012, 45, 439–448. [Google Scholar] [CrossRef]
- Asgary, S.; Nazarian, H.; Khojasteh, A.; Shokouhinejad, N. Gene Expression and Cytokine Release during Odontogenic Differentiation of Human Dental Pulp Stem Cells Induced by 2 Endodontic Biomaterials. J. Endod. 2014, 40, 387–392. [Google Scholar] [CrossRef]
- Lutfi, A.; Kannan, T.; Fazliah, M.; Jamaruddin, M.; Saidi, J. Proliferative activity of cells from remaining dental pulp in response to treatment with dental materials. Aust. Dent. J. 2010, 55, 79–85. [Google Scholar] [CrossRef]
- Dammaschke, T.; Stratmann, U.; Fischer, R.-J.; Sagheri, D.; Schäfer, E. Proliferation of rat molar pulp cells after direct pulp capping with dentine adhesive and calcium hydroxide. Clin. Oral Investig. 2011, 15, 577–587. [Google Scholar] [CrossRef]
- Tran-Hung, L.; Laurent, P.; Camps, J.; About, I. Quantification of angiogenic growth factors released by human dental cells after injury. Arch. Oral Biol. 2008, 53, 9–13. [Google Scholar] [CrossRef]
- Guven, E.P.; Yalvac, M.E.; Sahin, F.; Yazici, M.M.; Rizvanov, A.A.; Bayirli, G. Effect of Dental Materials Calcium Hydroxide–containing Cement, Mineral Trioxide Aggregate, and Enamel Matrix Derivative on Proliferation and Differentiation of Human Tooth Germ Stem Cells. J. Endod. 2011, 37, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, S.R.; Carnes , D.L., Jr.; Cochran, D.L. In Vitro Effects of Enamel Matrix Derivative on Microvascular Cells. J. Periodontol. 2007, 78, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Olsson, H.; Davies, J.R.; Holst, K.E.; Schröder, U.; Petersson, K. Dental pulp capping: Effect of Emdogain Gel on experimentally exposed human pulps. Int. Endod. J. 2005, 38, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Morotomi, T.; Washio, A.; Kitamura, C. Current and future options for dental pulp therapy. Jpn. Dent. Sci. Rev. 2019, 55, 5–11. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.-M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.-Y.; Shi, S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-J.; Gronthos, S.; Shi, S. Critical reviews in oral biology & medicine: Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792. [Google Scholar]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T.-J. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Yamaguchi, S.; Amagasa, T. Multilineage cells from apical pulp of human tooth with immature apex. Oral Sci. Int. 2007, 4, 45–58. [Google Scholar] [CrossRef]
- Koyama, N.; Okubo, Y.; Nakao, K.; Bessho, K. Evaluation of pluripotency in human dental pulp cells. J. Oral Maxillofac. Surg. 2009, 67, 501–506. [Google Scholar] [CrossRef]
- d’Aquino, R.; Graziano, A.; Sampaolesi, M.; Laino, G.; Pirozzi, G.; De Rosa, A.; Papaccio, G. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: A pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007, 14, 1162–1171. [Google Scholar] [CrossRef]
- Arthur, A.; Rychkov, G.; Shi, S.; Koblar, S.A.; Gronthos, S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells 2008, 26, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Lindroos, B.; Mäenpää, K.; Ylikomi, T.; Oja, H.; Suuronen, R.; Miettinen, S. Characterisation of human dental stem cells and buccal mucosa fibroblasts. Biochem. Biophys. Res. Commun. 2008, 368, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Walboomers, X.F.; Shi, S.; Fan, M.; Jansen, J.A. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006, 12, 2813–2823. [Google Scholar] [CrossRef]
- Zhang, W.; Walboomers, X.F.; Van Kuppevelt, T.H.; Daamen, W.F.; Van Damme, P.A.; Bian, Z.; Jansen, J.A. In vivo evaluation of human dental pulp stem cells differentiated towards multiple lineages. J. Tissue Eng. Regen. Med. 2008, 2, 117–125. [Google Scholar] [CrossRef]
- Shi, S.; Bartold, P.; Miura, M.; Seo, B.; Robey, P.; Gronthos, S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod. Craniofacial Res. 2005, 8, 191–199. [Google Scholar] [CrossRef]
- Sakai, V.; Zhang, Z.; Dong, Z.; Neiva, K.; Machado, M.; Shi, S.; Santos, C.; Nör, J. SHED differentiate into functional odontoblasts and endothelium. J. Dent. Res. 2010, 89, 791–796. [Google Scholar] [CrossRef]
- Nakamura, S.; Yamada, Y.; Katagiri, W.; Sugito, T.; Ito, K.; Ueda, M. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J. Endod. 2009, 35, 1536–1542. [Google Scholar] [CrossRef]
- Alipour, R.; Sadeghi, F.; Hashemi-Beni, B.; Zarkesh-Esfahani, S.H.; Heydari, F.; Mousavi, S.B.; Adib, M.; Narimani, M.; Esmaeili, N. Phenotypic characterizations and comparison of adult dental stem cells with adipose-derived stem cells. Int. J. Prev. Med. 2010, 1, 164. [Google Scholar] [PubMed]
- Nourbakhsh, N.; Soleimani, M.; Taghipour, Z.; Karbalaie, K.; Mousavi, S.-B.; Talebi, A.; Nadali, F.; Tanhaei, S.; Kiyani, G.-A.; Nematollahi, M. Induced in vitro differentiation of neural-like cells from human exfoliated deciduous teeth-derived stem cells. Int. J. Dev. Biol. 2011, 55, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Handa, K.; Saito, M.; Tsunoda, A.; Yamauchi, M.; Hattori, S.; Sato, S.; Toyoda, M.; Teranaka, T.; Narayanan, A.S. Progenitor cells from dental follicle are able to form cementum matrix in vivo. Connect. Tissue Res. 2002, 43, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Kémoun, P.; Laurencin-Dalicieux, S.; Rue, J.; Farges, J.-C.; Gennero, I.; Conte-Auriol, F.; Briand-Mesange, F.; Gadelorge, M.; Arzate, H.; Narayanan, A.S. Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res. 2007, 329, 283–294. [Google Scholar] [CrossRef]
- Völlner, F.; Ernst, W.; Driemel, O.; Morsczeck, C. A two-step strategy for neuronal differentiation in vitro of human dental follicle cells. Differentiation 2009, 77, 433–441. [Google Scholar] [CrossRef]
- Yalvac, M.E.; Ramazanoglu, M.; Gumru, O.Z.; Sahin, F.; Palotás, A.; Rizvanov, A.A. Comparison and optimisation of transfection of human dental follicle cells, a novel source of stem cells, with different chemical methods and electro-poration. Neurochem. Res. 2009, 34, 1272–1277. [Google Scholar] [CrossRef]
- Lin, N.H.; Gronthos, S.; Bartold, P. Stem cells and periodontal regeneration. Aust. Dent. J. 2008, 53, 108–121. [Google Scholar] [CrossRef]
- Trevino, E.G.; Patwardhan, A.N.; Henry, M.A.; Perry, G.; Dybdal-Hargreaves, N.; Hargreaves, K.M.; Diogenes, A. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J. Endod. 2011, 37, 1109–1115. [Google Scholar] [CrossRef]
- Jadhav, G.; Shah, N.; Logani, A. Revascularization with and without Platelet-rich Plasma in Nonvital, Immature, Anterior Teeth: A Pilot Clinical Study. J. Endod. 2012, 38, 1581–1587. [Google Scholar] [CrossRef]
- Chrepa, V.; Pitcher, B.; Henry, M.A.; Diogenes, A. Survival of the Apical Papilla and Its Resident Stem Cells in a Case of Advanced Pulpal Necrosis and Apical Periodontitis. J. Endod. 2017, 43, 561–567. [Google Scholar] [CrossRef]
- Dianat, O.; Mashhadi Abas, F.; Paymanpour, P.; Eghbal, M.J.; Haddadpour, S.; Bahrololumi, N. Endodontic repair in immature dogs’ teeth with apical periodontitis: Blood clot vs. plasma rich in growth factors scaffold. Dent. Traumatol. 2017, 33, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Turman, M. Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: A case report. J. Endod. 2011, 37, 265–268. [Google Scholar] [CrossRef]
- Athirasala, A.; Tahayeri, A.; Thrivikraman, G.; França, C.M.; Monteiro, N.; Tran, V.; Ferracane, J.; Bertassoni, L.E. A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication 2018, 10, 024101. [Google Scholar] [CrossRef] [PubMed]
- Lambricht, L.; De Berdt, P.; Vanacker, J.; Leprince, J.; Diogenes, A.; Goldansaz, H.; Bouzin, C.; Préat, V.; Dupont-Gillain, C.; Des Rieux, A. The type and composition of alginate and hyaluronic-based hydrogels influence the viability of stem cells of the apical papilla. Dent. Mater. 2014, 30, e349–e361. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, L.; Gardin, C.; Sivolella, S.; Brunello, G.; Berengo, M.; Piattelli, A.; Bressan, E.; Zavan, B. A hyaluronan-based scaffold for the in vitro construction of dental pulp-like tissue. Int. J. Mol. Sci. 2015, 16, 4666–4681. [Google Scholar] [CrossRef] [PubMed]
- Pardue, E.L.; Ibrahim, S.; Ramamurthi, A. Role of hyaluronan in angiogenesis and its utility to angiogenic tissue engineering. Organogenesis 2008, 4, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Friedman, P.M.; Mafong, E.A.; Kauvar, A.N.; Geronemus, R.G. Safety data of injectable nonanimal stabilized hyaluronic acid gel for soft tissue augmentation. Dermatol. Surg. 2002, 28, 491–494. [Google Scholar] [PubMed]
- Souto, G.D.; Farhane, Z.; Casey, A.; Efeoglu, E.; McIntyre, J.; Byrne, H.J. Evaluation of cytotoxicity profile and intracellular localisation of doxorubicin-loaded chitosan nanoparticles. Anal. Bioanal. Chem. 2016, 408, 5443–5455. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Diogenes, A.; Kishen, A. Temporal-controlled release of bovine serum albumin from chitosan nanoparticles: Effect on the regulation of alkaline phosphatase activity in stem cells from apical papilla. J. Endod. 2014, 40, 1349–1354. [Google Scholar] [CrossRef]
- Shrestha, S.; Torneck, C.D.; Kishen, A. Dentin conditioning with bioactive molecule releasing nanoparticle system enhances adherence, viability, and differentiation of stem cells from apical papilla. J. Endod. 2016, 42, 717–723. [Google Scholar] [CrossRef]
- Ishimatsu, H.; Kitamura, C.; Morotomi, T.; Tabata, Y.; Nishihara, T.; Chen, K.-K.; Terashita, M. Formation of dentinal bridge on surface of regenerated dental pulp in dentin defects by controlled release of fibroblast growth factor–2 from gelatin hydrogels. J. Endod. 2009, 35, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.E.; Nuge, T.; Yeow, T.K.; Nordin, N.; Prasad, R. Gelatin based scaffolds for tissue engineering-a review. Polym. Res. J. 2015, 9, 15–32. [Google Scholar]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Helenius, G.; Bäckdahl, H.; Bodin, A.; Nannmark, U.; Gatenholm, P.; Risberg, B. In vivo biocompatibility of bacterial cellulose. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2006, 76, 431–438. [Google Scholar] [CrossRef]
- Gong, T.; Heng, B.C.; Lo, E.C.M.; Zhang, C. Current advance and future prospects of tissue engineering approach to dentin/pulp regenerative therapy. Stem Cells Int. 2016, 2016, 9204574. [Google Scholar] [CrossRef]
- Chang, B.; Ahuja, N.; Ma, C.; Liu, X. Injectable scaffolds: Preparation and application in dental and craniofacial regeneration. Mater. Sci. Eng. R Rep. 2017, 111, 1–26. [Google Scholar] [CrossRef]
- Nosrat, A.; Kolahdouzan, A.; Khatibi, A.H.; Verma, P.; Jamshidi, D.; Nevins, A.J.; Torabinejad, M. Clinical, radiographic, and histologic outcome of regenerative endodontic treatment in human teeth using a novel collagen-hydroxyapatite scaffold. J. Endod. 2019, 45, 136–143. [Google Scholar] [CrossRef]
- Kim, T.G.; Wikesjö, U.M.; Cho, K.S.; Chai, J.K.; Pippig, S.D.; Siedler, M.; Kim, C.K. Periodontal wound healing/regeneration following implantation of recombinant human growth/differentiation factor-5 (rhGDF-5) in an absorbable collagen sponge carrier into one-wall intrabony defects in dogs: A dose-range study. J. Clin. Periodontol. 2009, 36, 589–597. [Google Scholar] [CrossRef]
- Sumita, Y.; Honda, M.J.; Ohara, T.; Tsuchiya, S.; Sagara, H.; Kagami, H.; Ueda, M. Performance of collagen sponge as a 3-D scaffold for tooth-tissue engineering. Biomaterials 2006, 27, 3238–3248. [Google Scholar] [CrossRef]
- Nune, M.; Kumaraswamy, P.; Maheswari Krishnan, U.; Sethuraman, S. Self-assembling peptide nanofibrous scaffolds for tissue engineering: Novel approaches and strategies for effective functional regeneration. Curr. Protein Pept. Sci. 2013, 14, 70–84. [Google Scholar] [CrossRef]
- Aligholi, H.; Rezayat, S.M.; Azari, H.; Mehr, S.E.; Akbari, M.; Mousavi, S.M.M.; Attari, F.; Alipour, F.; Hassanzadeh, G.; Gorji, A. Preparing neural stem/progenitor cells in PuraMatrix hydrogel for transplantation after brain injury in rats: A comparative methodological study. Brain Res. 2016, 1642, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, W.L.; Hargreaves, K.M.; Jin, L.; Samaranayake, L.P.; Zhang, C. The interplay of dental pulp stem cells and endothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue Eng. Part A 2015, 21, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, B.N.; Zeitlin, B.D.; Nör, J.E. A hydrogel scaffold that maintains viability and supports differentiation of dental pulp stem cells. Dent. Mater. 2013, 29, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dang, M.; Zhang, Z.; Hu, J.; Eyster, T.W.; Ni, L.; Ma, P.X. Dentin regeneration by stem cells of apical papilla on injectable nanofibrous microspheres and stimulated by controlled BMP-2 release. Acta Biomater. 2016, 36, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Presta, R.; Benedetti, L.; Cusella De Angelis, M.G.; Lupi, S.M.; Rodriguez y Baena, R. Emerging perspectives in scaffold for tissue engineering in oral surgery. Stem Cells Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Shiehzadeh, V.; Aghmasheh, F.; Shiehzadeh, F.; Joulae, M.; Kosarieh, E.; Shiehzadeh, F. Healing of large periapical lesions following delivery of dental stem cells with an injectable scaffold: New method and three case reports. Indian J. Dent. Res. 2014, 25, 248. [Google Scholar] [CrossRef]
- Pérez, R.A.; Won, J.-E.; Knowles, J.C.; Kim, H.-W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef]
- Moussa, D.G.; Aparicio, C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 58–75. [Google Scholar] [CrossRef]
- Tabarsi, B.; Pourghasem, M.; Moghaddamnia, A.; Shokravi, M.; Ehsani, M.; Ahmadyar, M.; Asgary, S. Comparison of skin test reactivity of two endodontic biomaterials in rabbits. Pak. J. Biol. Sci. 2012, 15, 250–254. [Google Scholar]
- Nosrat, A.; Seifi, A.; Asgary, S. Pulpotomy in caries-exposed immature permanent molars using calcium-enriched mixture cement or mineral trioxide aggregate: A randomized clinical trial. Int. J. Paediatr. Dent. 2013, 23, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Azimi, S.; Fazlyab, M.; Sadri, D.; Saghiri, M.A.; Khosravanifard, B.; Asgary, S. Comparison of pulp response to mineral trioxide aggregate and a bioceramic paste in partial pulpotomy of sound human premolars: A randomized controlled trial. Int. Endod. J. 2014, 47, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Paula, A.B.; Laranjo, M.; Marto, C.M.; Paulo, S.; Abrantes, A.M.; Casalta-Lopes, J.; Marques-Ferreira, M.; Botelho, M.F.; Carrilho, E. Direct Pulp Capping: What is the Most Effective Therapy?-Systematic Review and Meta-Analysis. J. Evid. Dent. Pract. 2018, 18, 298–314. [Google Scholar] [CrossRef] [PubMed]
- da Rosa, W.L.O.; Cocco, A.R.; Silva, T.M.D.; Mesquita, L.C.; Galarca, A.D.; Silva, A.F.D.; Piva, E. Current trends and future perspectives of dental pulp capping materials: A systematic review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1358–1368. [Google Scholar] [CrossRef]
- Celik, B.N.; Mutluay, M.S.; Arikan, V.; Sari, S. The evaluation of MTA and Biodentine as a pulpotomy materials for carious exposures in primary teeth. Clin. Oral Investig. 2019, 23, 661–666. [Google Scholar] [CrossRef]
- Burger, E.H.; Klein-Nulend, J.; Van Der Plas, A.; Nijweide, P.J. Function of osteocytes in bone—Their role in mechanotransduction. J. Nutr. 1995, 125 (Suppl. 7), 2020S–2023S. [Google Scholar] [CrossRef]
- Thi, M.M.; Suadicani, S.O.; Schaffler, M.B.; Weinbaum, S.; Spray, D.C. Mechanosensory responses of osteocytes to physiological forces occur along processes and not cell body and require αVβ3 integrin. Proc. Natl. Acad. Sci. USA 2013, 110, 21012–21017. [Google Scholar] [CrossRef]
- Lloyd, S.A.; Loiselle, A.E.; Zhang, Y.; Donahue, H.J. Shifting paradigms on the role of connexin43 in the skeletal response to mechanical load. J. Bone Miner. Res. 2014, 29, 275–286. [Google Scholar] [CrossRef]
- Cambra-Moo, O.; Nacarino Meneses, C.; Rodríguez Barbero, M.Á.; García Gil, O.; Rascón Pérez, J.; Rello-Varona, S.; D’Angelo, M.; Campo Martín, M.; González Martín, A. An approach to the histomorphological and histochemical variations of the humerus cortical bone through human ontogeny. J. Anat. 2014, 224, 634–646. [Google Scholar] [CrossRef]
- Katsamenis, O.L.; Chong, H.M.; Andriotis, O.G.; Thurner, P.J. Load-bearing in cortical bone microstructure: Selective stiffening and heterogeneous strain distribution at the lamellar level. J. Mech. Behav. Biomed. Mater. 2013, 17, 152–165. [Google Scholar] [CrossRef]
- Beddoe, A.; Darley, P.; Spiers, F. Measurements of trabecular bone structure in man (for radionuclide dosimetry. Phys. Med. Biol. 1976, 21, 589. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, E.; Grogan, J.; Niebur, G.; McNamara, L.; McHugh, P. Computational modelling of the mechanics of trabecular bone and marrow using fluid structure interaction techniques. Ann. Biomed. Eng. 2013, 41, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.K.; Gautieri, A.; Chang, S.-W.; Buehler, M.J. Molecular mechanics of mineralized collagen fibrils in bone. Nat. Commun. 2013, 4, 1724. [Google Scholar] [CrossRef] [PubMed]
- Itälä, A.; Koort, J.; Ylänen, H.O.; Hupa, M.; Aro, H.T. Biologic significance of surface microroughing in bone incorporation of porous bioactive glass implants. J. Biomed. Mater. Res. Part A 2003, 67A, 496–503. [Google Scholar] [CrossRef]
- Launey, M.E.; Buehler, M.J.; Ritchie, R.O. On the Mechanistic Origins of Toughness in Bone. Annu. Rev. Mater. Res. 2010, 40, 25–53. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46 (Suppl. 21), 92–102. [Google Scholar] [CrossRef]
- Misch, C.E.; Qu, Z.; Bidez, M.W. Mechanical properties of trabecular bone in the human mandible: Implications for dental implant treatment planning and surgical placement. J. Oral Maxillofac. Surg. 1999, 57, 700–706. [Google Scholar] [CrossRef]
- Zhao, W.; Li, X.; Liu, X.; Zhang, N.; Wen, X. Effects of substrate stiffness on adipogenic and osteogenic differentiation of human mesenchymal stem cells. Mater. Sci. Eng. C 2014, 40, 316–323. [Google Scholar] [CrossRef]
- Gittens, R.A.; Olivares-Navarrete, R.; Cheng, A.; Anderson, D.M.; McLachlan, T.; Stephan, I.; Geis-Gerstorfer, J.; Sandhage, K.H.; Fedorov, A.G.; Rupp, F.; et al. The roles of titanium surface micro/nanotopography and wettability on the differential response of human osteoblast lineage cells. Acta Biomater. 2013, 9, 6268–6277. [Google Scholar] [CrossRef]
- Pamula, E.; Filová, E.; Bačáková, L.; Lisá, V.; Adamczyk, D. Resorbable polymeric scaffolds for bone tissue engineering: The influence of their microstructure on the growth of human osteoblast-like MG 63 cells. J. Biomed. Mater. Res. Part A 2009, 89A, 432–443. [Google Scholar] [CrossRef]
- Ghayor, C.; Weber, F.E. Osteoconductive Microarchitecture of Bone Substitutes for Bone Regeneration Revisited. Front. Physiol. 2018, 9, 960. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Sheikh, Z.; Abdallah, M.N.; Hanafi, A.A.; Misbahuddin, S.; Rashid, H.; Glogauer, M. Mechanisms of in Vivo Degradation and Resorption of Calcium Phosphate Based Biomaterials. Materials 2015, 8, 7913–7925. [Google Scholar] [CrossRef]
- Thomas, M.V.; Puleo, D.A. Infection, inflammation, and bone regeneration: A paradoxical relationship. J. Dent. Res. 2011, 90, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Nuss, K.M.; von Rechenberg, B. Biocompatibility issues with modern implants in bone-a review for clinical orthopedics. Open Orthop. J. 2008, 2, 66. [Google Scholar] [CrossRef] [PubMed]
- Gristina, A. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Chris Arts, J.J.; Schmidmaier, G.; Larsson, S. What should be the characteristics of the ideal bone graft substitute? Injury 2011, 42 (Suppl. 2), S1–S2. [Google Scholar] [CrossRef]
- Juodzbalys, G. Regenerative bone potential after sinus floor elevation using various bone graft materials: A systematic review. Quintessence Int. 2019, 50, 548–558. [Google Scholar]
- Jo, S.H.; Kim, Y.K.; Choi, Y.H. Histological Evaluation of the Healing Process of Various Bone Graft Materials after Engraftment into the Human Body. Materials 2018, 11, 714. [Google Scholar] [CrossRef]
- Kunert-Keil, C.; Botzenhart, U.; Gedrange, T.; Gredes, T. Interrelationship between bone substitution materials and skeletal muscle tissue. Ann. Anat. 2015, 199, 73–78. [Google Scholar] [CrossRef]
- Wang, X.; Shao, Z.; Zhang, H.Z.; Zhu, F.; Shen, H.; Shang, Z.J. Experimental study on ectopic prefabrication of vascularized mandible graft with autogenous ribs. Zhonghua Kou Qiang Yi Xue Za Zhi 2012, 47, 544–546. [Google Scholar] [PubMed]
- Huang, R.-L.; Kobayashi, E.; Liu, K.; Li, Q. Bone Graft Prefabrication Following the In Vivo Bioreactor Principle. EBioMedicine 2016, 12, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Blume, O.; Donkiewicz, P.; Back, M.; Born, T. Bilateral maxillary augmentation using CAD/CAM manufactured allogenic bone blocks for restoration of congenitally missing teeth: A case report. J. Esthet. Restor. Dent. Off. Publ. Am. Acad. Esthet. Dent. 2019, 31, 171–178. [Google Scholar] [CrossRef]
- Yamada, M.; Egusa, H. Current bone substitutes for implant dentistry. J Prosthodont Res 2018, 62, 152–161. [Google Scholar] [CrossRef]
- Peker, E.; Karaca, I.R.; Yildirim, B. Experimental Evaluation of the Effectiveness of Demineralized Bone Matrix and Collagenated Heterologous Bone Grafts Used Alone or in Combination with Platelet-Rich Fibrin on Bone Healing in Sinus Floor Augmentation. Int. J. Oral Maxillofac. Implant. 2016, 31, e24–e31. [Google Scholar] [CrossRef] [PubMed]
- Lambert, F.; Lecloux, G.; Leonard, A.; Sourice, S.; Layrolle, P.; Rompen, E. Bone regeneration using porous titanium particles versus bovine hydroxyapatite: A sinus lift study in rabbits. Clin. Implant Dent. Relat. Res. 2013, 15, 412–426. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.; Spiller, K.; Bernhard, J.; Vunjak-Novakovic, G. Biomimetic approaches for bone tissue engineering. Tissue Eng. Part B Rev. 2017, 23, 480–493. [Google Scholar] [CrossRef]
- Paduano, F.; Marrelli, M.; Alom, N.; Amer, M.; White, L.J.; Shakesheff, K.M.; Tatullo, M. Decellularized bone extracellular matrix and human dental pulp stem cells as a construct for bone regeneration. J. Biomater. Sci. Polym. Ed. 2017, 28, 730–748. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Patil, S.; Gao, Y.-G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Li, J. Natural protein bioinspired materials for regeneration of hard tissues. J. Mater. Chem. B 2020, 8, 2199–2215. [Google Scholar] [CrossRef] [PubMed]
- Dobretsov, S.; Abed, R.M.; Teplitski, M. Mini-review: Inhibition of biofouling by marine microorganisms. Biofouling 2013, 29, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Rodriguez, P.; López-Álvarez, M.; Serra, J.; González, P.; Landín, M. Current stage of marine ceramic grafts for 3D bone tissue regeneration. Mar. Drugs 2019, 17, 471. [Google Scholar] [CrossRef] [PubMed]
- Green, D.W.; Lai, W.F.; Jung, H.S. Evolving marine biomimetics for regenerative dentistry. Mar. Drugs 2014, 12, 2877–2912. [Google Scholar] [CrossRef] [PubMed]
- Coringa, R.; de Sousa, E.M.; Botelho, J.N.; Diniz, R.S.; de Sá, J.C.; da Cruz, M.C.F.N.; Paschoal, M.A.B.; Gonçalves, L.M. Bone substitute made from a Brazilian oyster shell functions as a fast stimulator for bone-forming cells in an animal model. PLoS ONE 2018, 13, e0198697. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Binnewerg, B.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; Bechmann, N.; et al. Naturally Prefabricated Marine Biomaterials: Isolation and Applications of Flat Chitinous 3D Scaffolds from Ianthella labyrinthus (Demospongiae: Verongiida). Int. J. Mol. Sci. 2019, 20, 5105. [Google Scholar] [CrossRef]
- Wong, S.H.M.; Lim, S.S.; Tiong, T.J.; Show, P.L.; Zaid, H.F.M.; Loh, H.S. Preliminary In Vitro Evaluation of Chitosan-Graphene Oxide Scaffolds on Osteoblastic Adhesion, Proliferation, and Early Differentiation. Int. J. Mol. Sci. 2020, 21, 5202. [Google Scholar] [CrossRef]
- Moutinho, I.; da Costa Oliveira, I.; Santos, M.C.; Vasconcelos, M.; Portela, A. Different Chitosan-Based Biomaterials and their Biomedical Applications. Eur. J. Med. Res. Clin. Trials 2019, 1, 1–12. [Google Scholar]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. A Publ. Assoc. Bone Jt. Surg. ®| Corr® 2002, 395, 81–98. [Google Scholar] [CrossRef]
- Miron, R.J.; Sculean, A.; Shuang, Y.; Bosshardt, D.D.; Gruber, R.; Buser, D.; Chandad, F.; Zhang, Y. Osteoinductive potential of a novel biphasic calcium phosphate bone graft in comparison with autographs, xenografts, and DFDBA. Clin. Oral Implant. Res. 2016, 27, 668–675. [Google Scholar] [CrossRef]
- Nevins, M.; Nevins, M.L.; Schupbach, P.; Kim, S.W.; Lin, Z.; Kim, D.M. A prospective, randomized controlled preclinical trial to evaluate different formulations of biphasic calcium phosphate in combination with a hydroxyapatite collagen membrane to reconstruct deficient alveolar ridges. J. Oral Implantol. 2013, 39, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.G.; de Ruiter, A.P.; van Hout, W.; van Miegem, V.; Gawlitta, D.; Groot, F.B.; Meijer, G.J.; Rosenberg, A.; Koole, R. Microstructured β-Tricalcium Phosphate Putty Versus Autologous Bone for Repair of Alveolar Clefts in a Goat Model. Cleft Palate Craniofac J. 2017, 54, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Marinucci, L.; Balloni, S.; Becchetti, E.; Bistoni, G.; Calvi, E.M.; Lumare, E.; Ederli, F.; Locci, P. Effects of Hydroxyapatite and Biostite® on Osteogenic Induction of hMSC. Ann. Biomed. Eng. 2010, 38, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, A.; Mascitti, M.; Orsini, G.; Memè, L.; Rocchetti, R.; Tiriduzzi, P.; Sampalmieri, F.; Putignano, A.; Procaccini, M.; Lo Muzio, L.; et al. Osteopontin, osteocalcin and OB-cadherin expression in Synthetic nanohydroxyapatite vs. bovine hydroxyapatite cultured Osteoblastic-like cells. J. Biol. Regul. Homeost Agents 2014, 28, 523–529. [Google Scholar]
- Dorozhkin, S.V. Calcium Orthophosphate-Based Bioceramics. Materials 2013, 6, 3840–3942. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Hatton, J.; Davis, G.R.; Mourad, A.-H.I.; Cherupurakal, N.; Hill, R.G.; Mohsin, S. Fabrication of porous bone scaffolds using alginate and bioactive glass. J. Funct. Biomater. 2019, 10, 15. [Google Scholar] [CrossRef]
- Migliaresi, C.; Motta, A. Scaffolds for Tissue Engineering: Biological Design, Materials, and Fabrication; CRC Press: Singapore, 2014. [Google Scholar]
- Peroglio, M.; Gremillard, L.; Chevalier, J.; Chazeau, L.; Gauthier, C.; Hamaide, T. Toughening of bio-ceramics scaffolds by polymer coating. J. Eur. Ceram. Soc. 2007, 27, 2679–2685. [Google Scholar] [CrossRef]
- Zhang, W.; Liao, S.; Cui, F. Hierarchical self-assembly of nano-fibrils in mineralized collagen. Chem. Mater. 2003, 15, 3221–3226. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, L.; Cui, Y.; Song, T.X.; Qiu, Z.Y.; Wang, X.M.; Tan, B.S. Clinical evaluations of mineralized collagen in the extraction sites preservation. Regen. Biomater. 2016, 3, 41–48. [Google Scholar] [CrossRef][Green Version]
- Rampichová, M.; Chvojka, J.; Jenčová, V.; Kubíková, T.; Tonar, Z.; Erben, J.; Buzgo, M.; Daňková, J.; Litvinec, A.; Vocetková, K.; et al. The combination of nanofibrous and microfibrous materials for enhancement of cell infiltration and in vivo bone tissue formation. Biomed. Mater. 2018, 13, 025004. [Google Scholar] [CrossRef] [PubMed]
- McCullen, S.D.; Zhu, Y.; Bernacki, S.H.; Narayan, R.J.; Pourdeyhimi, B.; Gorga, R.E.; Loboa, E.G. Electrospun composite poly(L-lactic acid)/tricalcium phosphate scaffolds induce proliferation and osteogenic differentiation of human adipose-derived stem cells. Biomed. Mater. 2009, 4, 035002. [Google Scholar] [CrossRef] [PubMed]
- Puwanun, S.; Delaine-Smith, R.M.; Colley, H.E.; Yates, J.M.; MacNeil, S.; Reilly, G.C. A simple rocker-induced mechanical stimulus upregulates mineralization by human osteoprogenitor cells in fibrous scaffolds. J. Tissue Eng. Regen. Med. 2018, 12, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Topsakal, A.; Uzun, M.; Ugar, G.; Ozcan, A.; Altun, E.; Oktar, F.N.; Ikram, F.; Ozkan, O.; Turkoglu Sasmazel, H.; Gunduz, O. Development of Amoxicillin-Loaded Electrospun Polyurethane/Chitosan/$\beta$ -Tricalcium Phosphate Scaffold for Bone Tissue Regeneration. IEEE Trans. Nanobioscience 2018, 17, 321–328. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Lu, H.; Zhuang, Z.; Wang, X.P.; Fang, Q.F. Nano-hydroxyapatite/poly(l-lactic acid) composite synthesized by a modified in situ precipitation: Preparation and properties. J. Mater. Sci. Mater. Med. 2010, 21, 3077–3083. [Google Scholar] [CrossRef]
- Chen, Z.; Song, Y.; Zhang, J.; Liu, W.; Cui, J.; Li, H.; Chen, F. Laminated electrospun nHA/PHB-composite scaffolds mimicking bone extracellular matrix for bone tissue engineering. Mater. Sci. Eng. C 2017, 72, 341–351. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Shi, R.; Gong, M.; Chi, C.; Huang, Y.; Li, W.; Li, G.; Ye, J.; Liao, M.; Zhang, L.; Tian, W. Nano twin-fiber membrane with osteogenic and antibacterial dual functions as artificial periosteum for long bone repairing. J. Biomed. Nanotechnol. 2019, 15, 272–287. [Google Scholar] [CrossRef]
- Sharif, F.; Tabassum, S.; Mustafa, W.; Asif, A.; Zarif, F.; Tariq, M.; Siddiqui, S.A.; Gilani, M.A.; Ur Rehman, I.; MacNeil, S. Bioresorbable antibacterial PCL-PLA-nHA composite membranes for oral and maxillofacial defects. Polym. Compos. 2019, 40, 1564–1575. [Google Scholar] [CrossRef]
- Corbella, S.; Taschieri, S.; Weinstein, R.; Del Fabbro, M. Histomorphometric outcomes after lateral sinus floor elevation procedure: A systematic review of the literature and meta-analysis. Clin. Oral Implant. Res. 2016, 27, 1106–1122. [Google Scholar] [CrossRef]
- Starch-Jensen, T.; Mordenfeld, A.; Becktor, J.P.; Jensen, S.S. Maxillary Sinus Floor Augmentation With Synthetic Bone Substitutes Compared With Other Grafting Materials: A Systematic Review and Meta-analysis. Implant Dent. 2018, 27, 363–374. [Google Scholar] [CrossRef] [PubMed]
- La Monaca, G.; Iezzi, G.; Cristalli, M.P.; Pranno, N.; Sfasciotti, G.L.; Vozza, I. Comparative Histological and Histomorphometric Results of Six Biomaterials Used in Two-Stage Maxillary Sinus Augmentation Model after 6-Month Healing. Biomed Res. Int. 2018, 2018, 9430989. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; Padial-Molina, M.; Lopez-Chaichio, L.; Gutiérrez-Garrido, L.; Martín-Morales, N.; O’Valle, F. Algae-derived hydroxyapatite behavior as bone biomaterial in comparison with anorganic bovine bone: A split-mouth clinical, radiological, and histologic randomized study in humans. Clin. Oral Implant. Res. 2020, 31, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Shin, H.K.; Yun, J.H.; Cho, K.S. Randomized Clinical Trial of Maxillary Sinus Grafting using Deproteinized Porcine and Bovine Bone Mineral. Clin. Implant Dent. Relat. Res. 2017, 19, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Jelusic, D.; Zirk, M.L.; Fienitz, T.; Plancak, D.; Puhar, I.; Rothamel, D. Monophasic s-TCP vs. biphasic HA/s-TCP in two-stage sinus floor augmentation procedures—A prospective randomized clinical trial. Clin. Oral Implant. Res. 2017, 28, e175–e183. [Google Scholar] [CrossRef]
- Pang, K.M.; Lee, J.K.; Choi, S.H.; Kim, Y.K.; Kim, B.J.; Lee, J.H. Maxillary Sinus Augmentation With Calcium Phosphate Double-Coated Anorganic Bovine Bone: Comparative Multicenter Randomized Clinical Trial With Histological and Radiographic Evaluation. Implant Dent. 2019, 28, 39–45. [Google Scholar] [CrossRef]
- Stacchi, C.; Lombardi, T.; Oreglia, F.; Alberghini Maltoni, A.; Traini, T. Histologic and Histomorphometric Comparison between Sintered Nanohydroxyapatite and Anorganic Bovine Xenograft in Maxillary Sinus Grafting: A Split-Mouth Randomized Controlled Clinical Trial. Biomed Res. Int. 2017, 2017, 9489825. [Google Scholar] [CrossRef]
- Palumbo, C.; Baldini, A.; Cavani, F.; Sena, P.; Benincasa, M.; Ferretti, M.; Zaffe, D. Immunocytochemical and structural comparative study of committed versus multipotent stem cells cultured with different biomaterials. Micron 2013, 47, 1–9. [Google Scholar] [CrossRef]
- Hendrijantini, N.; Kusumaningsih, T.; Rostiny, R.; Mulawardhana, P.; Danudiningrat, C.P.; Rantam, F.A. A potential therapy of human umbilical cord mesenchymal stem cells for bone regeneration on osteoporotic mandibular bone. Eur. J. Dent. 2018, 12, 358–362. [Google Scholar] [CrossRef]
- Gjerde, C.; Mustafa, K.; Hellem, S.; Rojewski, M.; Gjengedal, H.; Yassin, M.A.; Feng, X.; Skaale, S.; Berge, T.; Rosen, A. Cell therapy induced regeneration of severely atrophied mandibular bone in a clinical trial. Stem Cell Res. Ther. 2018, 9, 213. [Google Scholar] [CrossRef]
- Peel Kim, H.; Ji, Y.-h.; Chul Rhee, S.; Sang Dhong, E.; Ha Park, S.; Yoon, E.-S. Enhancement of bone regeneration using osteogenic-induced adipose-derived stem cells combined with demineralized bone matrix in a rat critically-sized calvarial defect model. Curr. Stem Cell Res. Ther. 2012, 7, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Chamieh, F.; Collignon, A.-M.; Coyac, B.R.; Lesieur, J.; Ribes, S.; Sadoine, J.; Llorens, A.; Nicoletti, A.; Letourneur, D.; Colombier, M.-L.; et al. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci. Rep. 2016, 6, 38814. [Google Scholar] [CrossRef] [PubMed]
- D’Aquino, R.; De Rosa, A.; Lanza, V.; Tirino, V.; Laino, L.; Graziano, A.; Desiderio, V.; Laino, G.; Papaccio, G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur. Cell Mater. 2009, 18, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Chamila Prageeth Pandula, P.; Samaranayake, L.; Jin, L.; Zhang, C. Periodontal ligament stem cells: An update and perspectives. J. Investig. Clin. Dent. 2014, 5, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Ito, K.; Nakamura, S.; Ueda, M.; Nagasaka, T. Promising cell-based therapy for bone regeneration using stem cells from deciduous teeth, dental pulp, and bone marrow. Cell Transplant. 2011, 20, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, T.; Kunimatsu, R.; Nakajima, K.; Abe, T.; Yamada, S.; Rikitake, K.; Tanimoto, K. Stem cell-derived conditioned media from human exfoliated deciduous teeth promote bone regeneration. Oral Dis. 2020, 26, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, W.; Watanabe, J.; Toyama, N.; Osugi, M.; Sakaguchi, K.; Hibi, H. Clinical Study of Bone Regeneration by Conditioned Medium From Mesenchymal Stem Cells After Maxillary Sinus Floor Elevation. Implant Dent. 2017, 26, 607–612. [Google Scholar] [CrossRef]
- Tsukamoto, J.; Naruse, K.; Nagai, Y.; Kan, S.; Nakamura, N.; Hata, M.; Omi, M.; Hayashi, T.; Kawai, T.; Matsubara, T. Efficacy of a self-assembling peptide hydrogel, SPG-178-gel, for bone regeneration and three-dimensional osteogenic induction of dental pulp stem cells. Tissue Eng. Part A 2017, 23, 1394–1402. [Google Scholar] [CrossRef]
- Yu, B.-H.; Zhou, Q.; Wang, Z.-L. Periodontal ligament versus bone marrow mesenchymal stem cells in combination with Bio-Oss scaffolds for ectopic and in situ bone formation: A comparative study in the rat. J. Biomater. Appl. 2014, 29, 243–253. [Google Scholar] [CrossRef]
- Korn, P.; Hauptstock, M.; Range, U.; Kunert-Keil, C.; Pradel, W.; Lauer, G.; Schulz, M.C. Application of tissue-engineered bone grafts for alveolar cleft osteoplasty in a rodent model. Clin. Oral Investig. 2017, 21, 2521–2534. [Google Scholar] [CrossRef]
- Martin-del-Campo, M.; Rosales-Ibañez, R.; Alvarado, K.; Sampedro, J.G.; Garcia-Sepulveda, C.A.; Deb, S.; San Román, J.; Rojo, L. Strontium folate loaded biohybrid scaffolds seeded with dental pulp stem cells induce in vivo bone regeneration in critical sized defects. Biomater. Sci. 2016, 4, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmady, H.H.; Abd Elazeem, A.F.; Bellah Ahmed, N.E.-m.; Shawkat, W.M.; Elmasry, M.; Abdelrahman, M.A.; Abderazik, M.A. Combining autologous bone marrow mononuclear cells seeded on collagen sponge with Nano Hydroxyapatite, and platelet-rich fibrin: Reporting a novel strategy for alveolar cleft bone regeneration. J. Cranio-Maxillofac. Surg. 2018, 46, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The roles of signaling pathways in bone repair and regeneration. J. Cell. Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Q.; Wang, Z. A comparative study of the effect of Bio-Oss® in combination with concentrated growth factors or bone marrow-derived mesenchymal stem cells in canine sinus grafting. J. Oral Pathol. Med. 2017, 46, 528–536. [Google Scholar] [CrossRef]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef]
- Wozney, J.M. The bone morphogenetic protein family and osteogenesis. Mol. Reprod. Dev. 1992, 32, 160–167. [Google Scholar] [CrossRef]
- Khojasteh, A.; Behnia, H.; Naghdi, N.; Esmaeelinejad, M.; Alikhassy, Z.; Stevens, M. Effects of different growth factors and carriers on bone regeneration: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e405–e423. [Google Scholar] [CrossRef]
- Sudheesh Kumar, P.T.; Hashimi, S.; Saifzadeh, S.; Ivanovski, S.; Vaquette, C. Additively manufactured biphasic construct loaded with BMP-2 for vertical bone regeneration: A pilot study in rabbit. Mater. Sci. Eng. C Mater Biol. Appl. 2018, 92, 554–564. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, K.-H.; Kim, S.; Lee, Y.-M.; Seol, Y.-J. BMP-2 gene delivery-based bone regeneration in dentistry. Pharmaceutics 2019, 11, 393. [Google Scholar] [CrossRef]
- Fliefel, R.; Kühnisch, J.; Ehrenfeld, M.; Otto, S. Gene Therapy for Bone Defects in Oral and Maxillofacial Surgery: A Systematic Review and Meta-Analysis of Animal Studies. Stem Cells Dev. 2016, 26, 215–230. [Google Scholar] [CrossRef]
- Keramaris, N.; Calori, G.; Nikolaou, V.; Schemitsch, E.; Giannoudis, P. Fracture vascularity and bone healing: A systematic review of the role of VEGF. Injury 2008, 39, S45–S57. [Google Scholar] [CrossRef]
- Patel, Z.S.; Young, S.; Tabata, Y.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008, 43, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Cho, T.H.; Han, J.J.; Kim, I.S.; Park, Y.; Hwang, S.J. Comparative study of BMP-2 alone and combined with VEGF carried by hydrogel for maxillary alveolar bone regeneration. Tissue Eng. Regen. Med. 2016, 13, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Beenken, A.; Mohammadi, M. The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009, 8, 235–253. [Google Scholar] [CrossRef]
- Reible, B.; Schmidmaier, G.; Moghaddam, A.; Westhauser, F. Insulin-Like Growth Factor-1 as a Possible Alternative to Bone Morphogenetic Protein-7 to Induce Osteogenic Differentiation of Human Mesenchymal Stem Cells in Vitro. Int. J. Mol. Sci. 2018, 19, 1674. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. PDGF in bone formation and regeneration: New insights into a novel mechanism involving MSCs. J. Orthop. Res. 2011, 29, 1795–1803. [Google Scholar] [CrossRef]
- Li, F.; Yu, F.; Liao, X.; Wu, C.; Wang, Y.; Li, C.; Lou, F.; Li, B.; Yin, B.; Wang, C.; et al. Efficacy of Recombinant Human BMP2 and PDGF-BB in Orofacial Bone Regeneration: A Systematic Review and Meta-analysis. Sci. Rep. 2019, 9, 8073. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Ghazizadeh Ahsaie, M.; Rezai Rad, M.; Baghani, M.t.; Motamedian, S.R.; Khojasteh, A. Application of selected scaffolds for bone tissue engineering: A systematic review. Oral Maxillofac. Surg. 2017, 21, 109–129. [Google Scholar] [CrossRef]
- Stumbras, A.; Januzis, G.; Kubilius, R.; Gervickas, A.; Juodzbalys, G. Randomized clinical trial of bone healing after alveolar ridge preservation using xenografts and allografts vs. plasma rich in growth factors. J. Oral Implantol. 2020. [Google Scholar] [CrossRef]
- Talaat, W.M.; Ghoneim, M.M.; Salah, O.; Adly, O.A. Autologous Bone Marrow Concentrates and Concentrated Growth Factors Accelerate Bone Regeneration After Enucleation of Mandibular Pathologic Lesions. J. Craniofacial Surg. 2018, 29, 992–997. [Google Scholar] [CrossRef]
- Yu, T.-T.; Liu, J.; Yin, J.-J.; Xu, X.-N.; Yan, S.-J.; Lan, J. Effects of concentrated growth factors on relieving postoperative reaction of guided bone regeneration in the esthetic zone. Hua Xi Kou Qiang Yi Xue Za Zhi 2019, 37, 398–402. [Google Scholar] [PubMed]
- Feinberg, S.E.; Aghaloo, T.L.; Cunningham, L.L. Role of tissue engineering in oral and maxillofacial reconstruction: Findings of the 2005 AAOMS Research Summit. J. Oral Maxillofac. Surg. 2005, 63, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011. [Google Scholar] [CrossRef]
- Kato, H.; Marcelo, C.L.; Washington, J.B.; Bingham, E.L.; Feinberg, S.E. Fabrication of large size ex vivo-produced oral mucosal equivalents for clinical application. Tissue Eng. Part C Methods 2015, 21, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, T.; Nakamura, T.; Yamamoto, T.; Kinoshita, S.; Kanamura, N. Autologous transplantation of oral mucosal epithelial cell sheets cultured on an amniotic membrane substrate for intraoral mucosal defects. PLoS ONE 2015, 10, e0125391. [Google Scholar] [CrossRef] [PubMed]
- Köseoğlu, S.; Duran, İ.; Sağlam, M.; Bozkurt, S.B.; Kırtıloğlu, O.S.; Hakkı, S.S. Efficacy of collagen membrane seeded with autologous gingival fibroblasts in gingival recession treatment: A randomized, controlled pilot study. J. Periodontol. 2013, 84, 1416–1424. [Google Scholar] [CrossRef]
- Fischer, K.R.; Testori, T.; Wachtel, H.; Mühlemann, S.; Happe, A.; Del Fabbro, M. Soft tissue augmentation applying a collagenated porcine dermal matrix during second stage surgery: A prospective multicenter case series. Clin. Implant Dent. Relat. Res. 2019, 21, 923–930. [Google Scholar] [CrossRef] [PubMed]
- De Santis, D.; Gelpi, F.; Castellani, R.; Palumbo, C.; Ferretti, M.; Zanotti, G.; Zotti, F.; Montagna, L.; Luciano, U.; Marconcini, S.; et al. Bi-layered collagen nano-structured membrane prototype collagen matrix CM-10826 for oral soft tissue regeneration: An in vivo ultrastructural study on 13 patients. J. Biol. Regul. Homeost Agents 2019, 33 (Suppl. 1), 29–41. [Google Scholar]
- Echazú, M.I.A.; Tuttolomondo, M.V.; Foglia, M.L.; Mebert, A.M.; Alvarez, G.S.; Desimone, M.F. Advances in collagen, chitosan and silica biomaterials for oral tissue regeneration: From basics to clinical trials. J. Mater. Chem. B 2016, 4, 6913–6929. [Google Scholar] [CrossRef]
- Toledano, M.; Toledano-Osorio, M.; Carrasco-Carmona, Á.; Vallecillo, C.; Lynch, C.D.; Osorio, M.T.; Osorio, R. State of the Art on Biomaterials for Soft Tissue Augmentation in the Oral Cavity. Part I: Natural Polymers-Based Biomaterials. Polymers 2020, 12, 1850. [Google Scholar] [CrossRef]
- Toledano, M.; Toledano-Osorio, M.; Osorio, R.; Carrasco-Carmona, Á.; Gutiérrez-Pérez, J.-L.; Gutiérrez-Corrales, A.; Serrera-Figallo, M.-A.; Lynch, C.D.; Torres-Lagares, D. Doxycycline and Zinc Loaded Silica-Nanofibrous Polymers as Biomaterials for Bone Regeneration. Polymers 2020, 12, 1201. [Google Scholar] [CrossRef]
- Blackwood, K.A.; McKean, R.; Canton, I.; Freeman, C.O.; Franklin, K.L.; Cole, D.; Brook, I.; Farthing, P.; Rimmer, S.; Haycock, J.W. Development of biodegradable electrospun scaffolds for dermal replacement. Biomaterials 2008, 29, 3091–3104. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Tobita, M.; Mizuno, H. Periodontal disease and periodontal tissue regeneration. Curr. Stem Cell Res. 2010, 5, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Villar, C.C.; Cochran, D.L. Regeneration of periodontal tissues: Guided tissue regeneration. Dent Clin. N. Am. 2010, 54, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Sculean, A. Does periodontal tissue regeneration really work? Periodontol 2000 2009, 51, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.; Bostanci, N.; Remboutsika, E.; De Bari, C.; Mitsiadis, T.A. Future dentistry: Cell therapy meets tooth and periodontal repair and regeneration. J. Cell Mol. Med. 2011, 15, 1054–1065. [Google Scholar] [CrossRef]
- Trubiani, O.; Orsini, G.; Zini, N.; Di Iorio, D.; Piccirilli, M.; Piattelli, A.; Caputi, S. Regenerative potential of human periodontal ligament derived stem cells on three-dimensional biomaterials: A morphological report. J. Biomed Mater Res. A 2008, 87, 986–993. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef]
- Qin, Y.; Guan, J.; Zhang, C. Mesenchymal stem cells: Mechanisms and role in bone regeneration. Postgrad Med. J. 2014, 90, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, N.; Kawaguchi, H.; Hirachi, A.; Takeda, K.; Mizuno, N.; Nishimura, M.; Koike, C.; Tsuji, K.; Iba, H.; Kato, Y.; et al. Behavior of transplanted bone marrow-derived mesenchymal stem cells in periodontal defects. J. Periodontol. 2006, 77, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Tobita, M.; Mizuno, H. Adipose-derived stem cells and periodontal tissue engineering. Int. J. Oral Maxillofac. Implant. 2013, 28, e487–e493. [Google Scholar] [CrossRef] [PubMed]
- Benatti, B.B.; Silverio, K.G.; Casati, M.Z.; Sallum, E.A.; Nociti, F.H., Jr. Physiological features of periodontal regeneration and approaches for periodontal tissue engineering utilizing periodontal ligament cells. J. Biosci. Bioeng. 2007, 103, 1–6. [Google Scholar] [CrossRef]
- Spagnuolo, G.; Codispoti, B.; Marrelli, M.; Rengo, C.; Rengo, S.; Tatullo, M. Commitment of Oral-Derived Stem Cells in Dental and Maxillofacial Applications. Dent. J. 2018, 6, 13. [Google Scholar] [CrossRef]
- Trubiani, O.; Marconi, G.D.; Pierdomenico, S.D.; Piattelli, A.; Diomede, F.; Pizzicannella, J. Human Oral Stem Cells, Biomaterials and Extracellular Vesicles: A Promising Tool in Bone Tissue Repair. Int. J. Mol. Sci. 2019, 20, 9. [Google Scholar] [CrossRef]
- Duan, X.; Tu, Q.; Zhang, J.; Ye, J.; Sommer, C.; Mostoslavsky, G.; Kaplan, D.; Yang, P.; Chen, J. Application of induced pluripotent stem (iPS) cells in periodontal tissue regeneration. J. Cell Physiol. 2011, 226, 150–157. [Google Scholar] [CrossRef]
- Csete, M. Translational prospects for human induced pluripotent stem cells. Regen. Med. 2010, 5, 509–519. [Google Scholar] [CrossRef]
- Wada, N.; Menicanin, D.; Shi, S.; Bartold, P.M.; Gronthos, S. Immunomodulatory properties of human periodontal ligament stem cells. J. Cell Physiol. 2009, 219, 667–676. [Google Scholar] [CrossRef]
- Zhang, J.; An, Y.; Gao, L.N.; Zhang, Y.J.; Jin, Y.; Chen, F.M. The effect of aging on the pluripotential capacity and regenerative potential of human periodontal ligament stem cells. Biomaterials 2012, 33, 6974–6986. [Google Scholar] [CrossRef]
- Park, J.C.; Kim, J.M.; Jung, I.H.; Kim, J.C.; Choi, S.H.; Cho, K.S.; Kim, C.S. Isolation and characterization of human periodontal ligament (PDL) stem cells (PDLSCs) from the inflamed PDL tissue: In vitro and in vivo evaluations. J. Clin. Periodontol. 2011, 38, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, S.; Song, Y.; Tang, L.; Ma, D.; Liu, B.; Jin, Y. The biological effect of dentin noncollagenous proteins (DNCPs) on the human periodontal ligament stem cells (HPDLSCs) in vitro and in vivo. Tissue Eng. Part A 2008, 14, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, K.H.; Seo, B.M.; Koo, K.T.; Kim, T.I.; Seol, Y.J.; Ku, Y.; Rhyu, I.C.; Chung, C.P.; Lee, Y.M. Alveolar bone regeneration by transplantation of periodontal ligament stem cells and bone marrow stem cells in a canine peri-implant defect model: A pilot study. J. Periodontol. 2009, 80, 1815–1823. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Ding, G.; Fang, D.; Zhang, C.; Bartold, P.M.; Gronthos, S.; Shi, S.; Wang, S. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells 2008, 26, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.M.; Anusaksathien, O.; Webb, S.A.; Rutherford, R.B.; Giannobile, W.V. Gene therapy of bone morphogenetic protein for periodontal tissue engineering. J. Periodontol. 2003, 74, 202–213. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, K.H.; Gwak, E.H.; Rhee, S.H.; Lee, J.C.; Shin, S.Y.; Koo, K.T.; Lee, Y.M.; Seol, Y.J. Ex vivo bone morphogenetic protein 2 gene delivery using periodontal ligament stem cells for enhanced re-osseointegration in the regenerative treatment of peri-implantitis. J. Biomed. Mater. Res. A 2015, 103, 38–47. [Google Scholar] [CrossRef]
- Park, J.C.; Lee, S.M.; Kim, J.C.; Yun, J.H.; Cho, K.S.; Im, G.I.; Kim, B.S.; Kim, C.S. Effect of humoral factors from hPDLSCs on the biologic activity of hABCs. Oral Dis. 2012, 18, 537–547. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Nikou, G.; Ivanovic, A.; Chapple, I.L.; Stavropoulos, A. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontology 2000 2015, 68, 182–216. [Google Scholar] [CrossRef]
- Seciu, A.-M.; Craciunescu, O.; Stanciuc, A.-M.; Zarnescu, O. Tailored Biomaterials for Therapeutic Strategies Applied in Periodontal Tissue Engineering. Stem Cells Dev. 2019, 28, 963–973. [Google Scholar] [CrossRef]
- Tsumanuma, Y.; Iwata, T.; Washio, K.; Yoshida, T.; Yamada, A.; Takagi, R.; Ohno, T.; Lin, K.; Yamato, M.; Ishikawa, I.; et al. Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials 2011, 32, 5819–5825. [Google Scholar] [CrossRef]
- Yu, Y.; Mu, J.; Fan, Z.; Lei, G.; Yan, M.; Wang, S.; Tang, C.; Wang, Z.; Yu, J.; Zhang, G. Insulin-like growth factor 1 enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells via ERK and JNK MAPK pathways. Histochem. Cell Biol. 2012, 137, 513–525. [Google Scholar] [CrossRef]
- McAllister, B.S. Stem cell-containing allograft matrix enhances periodontal regeneration: Case presentations. Int. J. Periodontics Restor. Dent. 2011, 31, 149–155. [Google Scholar]
- Feng, F.; Akiyama, K.; Liu, Y.; Yamaza, T.; Wang, T.M.; Chen, J.H.; Wang, B.B.; Huang, G.T.; Wang, S.; Shi, S. Utility of PDL progenitors for in vivo tissue regeneration: A report of 3 cases. Oral Dis 2010, 16, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Gao, L.N.; Tian, B.M.; Zhang, X.Y.; Zhang, Y.J.; Dong, G.Y.; Lu, H.; Chu, Q.; Xu, J.; Yu, Y.; et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: A randomized clinical trial. Stem Cell Res. Ther. 2016, 7, 33. [Google Scholar] [CrossRef]
- Lieberman, J.R.; Daluiski, A.; Einhorn, T.A. The role of growth factors in the repair of bone. Biology and clinical applications. J. Bone Jt. Surg Am. 2002, 84, 1032–1044. [Google Scholar] [CrossRef]
- Strauss, F.J.; Nasirzade, J.; Kargarpoor, Z.; Stahli, A.; Gruber, R. Effect of platelet-rich fibrin on cell proliferation, migration, differentiation, inflammation, and osteoclastogenesis: A systematic review of in vitro studies. Clin. Oral Investig. 2020, 24, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Dragonas, P.; Schiavo, J.H.; Avila-Ortiz, G.; Palaiologou, A.; Katsaros, T. Plasma rich in growth factors (PRGF) in intraoral bone grafting procedures: A systematic review. J. Cranio-Maxillo-Facial Surg. 2019, 47, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Duan, J.; Zhang, Y.; Chu, Y.; Sun, C. The effect of concentrated growth factors in the treatment of periodontal intrabony defects. Future Sci. Oa 2016, 2, FS136. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, E.A.; Abo Shady, T.E. Clinical and radiographic evaluation of nanocrystalline hydroxyapatite with or without platelet-rich fibrin membrane in the treatment of periodontal intrabony defects. J. Indian Soc. Periodontol. 2015, 19, 61–65. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, N.D.; Jain, A. Platelet rich fibrin combined with decalcified freeze-dried bone allograft for the treatment of human intrabony periodontal defects: A randomized split mouth clinical trail. Acta Odontol. Scand. 2016, 74, 36–43. [Google Scholar] [CrossRef]
- Biswas, S.; Sambashivaiah, S.; Kulal, R.; Bilichodmath, S.; Kurtzman, G.M. Comparative Evaluation of Bioactive Glass (Putty) and Platelet Rich Fibrin in Treating Furcation Defects. J. Oral Implantol. 2016, 42, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, A.R.; Nagpal, K.; Karvekar, S.; Patnaik, K.; Naik, S.B.; Guruprasad, C.N. Platelet-rich fibrin with 1% metformin for the treatment of intrabony defects in chronic periodontitis: A randomized controlled clinical trial. J. Periodontol. 2015, 86, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Kornsuthisopon, C.; Pirarat, N.; Osathanon, T.; Kalpravidh, C. Autologous platelet-rich fibrin stimulates canine periodontal regeneration. Sci. Rep. 2020, 10, 1850. [Google Scholar] [CrossRef]
- Fang, D.; Hu, S.; Liu, Y.; Quan, V.-H.; Seuntjens, J.; Tran, S.D. Identification of the active components in Bone Marrow Soup: A mitigator against irradiation-injury to salivary glands. Sci. Rep. 2015, 5, 16017. [Google Scholar] [CrossRef]
- Kang, W.; Liang, Q.; Du, L.; Shang, L.; Wang, T.; Ge, S. Sequential application of bFGF and BMP-2 facilitates osteogenic differentiation of human periodontal ligament stem cells. J. Periodontal. Res. 2019, 54, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yu, F.; Xu, X.; Li, C.; Huang, D.; Zhou, X.; Ye, L.; Zheng, L. Evaluation of Recombinant Human FGF-2 and PDGF-BB in Periodontal Regeneration: A Systematic Review and Meta-Analysis. Sci. Rep. 2017, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.P.; Vaughn, O.L.; Anderson, P.A. Systematic Review and Meta-Analysis of Recombinant Human Bone Morphogenetic Protein-2 in Localized Alveolar Ridge and Maxillary Sinus Augmentation. J. Oral Maxillofac. Surg 2016, 74, 928–939. [Google Scholar] [CrossRef]
- Gestrelius, S.; Lyngstadaas, S.P.; Hammarström, L. Emdogain—Periodontal regeneration based on biomimicry. Clin. Oral Investig. 2000, 4, 120–125. [Google Scholar] [CrossRef]
- Tokiyasu, Y.; Takata, T.; Saygin, E.; Somerman, M. Enamel Factors Regulate Expression of Genes Associated With Cementoblasts. J. Periodontol. 2000, 71, 1829–1839. [Google Scholar] [CrossRef]
- Wang, S.S.; Rausch-fan, X.; Andrukov, O.; Lin, Y.; Lin, L.S.; Shi, B. The effect of emdogain on proliferation and differentiation of co-cultured osteoblasts and endothelial cells. China J. Oral Maxillofac. Surg. 2019, 17, 32–39. [Google Scholar]
- Talebi Ardakani, M.R.; Meimandi, M.; Shaker, R.; Golmohammadi, S. The Effect of Platelet-Rich Fibrin (PRF), Plasma Rich in Growth Factors (PRGF), and Enamel Matrix Proteins (Emdogain) on Migration of Human Gingival Fibroblasts. J. Dent. (Shiraz.) 2019, 20, 232–239. [Google Scholar]
- Takeuchi, T.; Masuno, K.; Kato, H.; Taguchi, Y.; Umeda, M.; Okusa, N.; Tanaka, A.; Tominaga, K. A Human Amelogenin-Derived Oligopeptide Enhances Osteogenic Differentiation of Human Periodontal Ligament Stem Cells. J. Hard Tissue Biol. 2019, 28, 251–258. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Papanikolaou, N.; Coulthard, P.; Worthington, H.V. Enamel matrix derivative (Emdogain(R)) for periodontal tissue regeneration in intrabony defects. Cochrane Database Syst. Rev. 2009, 2009, CD003875. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, A.; Attin, T. Efficacy of enamel matrix derivatives (Emdogain®) in treatment of replanted teeth—A systematic review based on animal studies. Dent. Traumatol. 2008, 24, 498–502. [Google Scholar] [CrossRef]
- Mohamed, R.N.; Basha, S.; Al-Thomali, Y.; Tawfik Enan, E. Enamel matrix derivative (Emdogain) in treatment of replanted teeth—A systematic review. Acta Odontol. Scand. 2019, 77, 168–172. [Google Scholar] [CrossRef]
- Lekovic, V.; Milinkovic, I.; Aleksic, Z.; Jankovic, S.; Stankovic, P.; Kenney, E.B.; Camargo, P.M. Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects. J. Periodontal. Res. 2012, 47, 409–417. [Google Scholar] [CrossRef]
- Lindhe, J. Clinical periodontology and implant dentistry; Lang, N.P., Karring, T., Eds.; Blackwell Munksgaard: Copenhagen, Denmark, 2003; Volume 4, pp. 650–754. [Google Scholar]
- Nyman, S. Bone regeneration using the principle of guided tissue regeneration. J. Clin. Periodontol. 1991, 18, 494–498. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.M.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Goker, F.; Larsson, L.; Del Fabbro, M.; Asa’ad, F. Gene Delivery Therapeutics in the Treatment of Periodontitis and Peri-Implantitis: A State of the Art Review. Int. J. Mol. Sci. 2019, 20, 20. [Google Scholar] [CrossRef]
- Zhang, N.; Nichols, H.L.; Tylor, S.; Wen, X. Fabrication of nanocrystalline hydroxyapatite doped degradable composite hollow fiber for guided and biomimetic bone tissue engineering. Mater. Sci. Eng. C 2007, 27, 599–606. [Google Scholar] [CrossRef]
- Jia, J.; Liu, G.; Guo, Z.-X.; Yu, J.; Duan, Y. Preparation and characterization of soluble eggshell membrane protein/PLGA electrospun nanofibers for guided tissue regeneration membrane. J. Nanomater. 2012, 2012, 282736. [Google Scholar] [CrossRef]
- Gredes, T.; Kubasiewicz-Ross, P.; Gedrange, T.; Dominiak, M.; Kunert-Keil, C. Comparison of surface modified zirconia implants with commercially available zirconium and titanium implants: A histological study in pigs. Implant Dent. 2014, 23, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, M.; Lauret, J.; Saadoun, A. Mastication forces and implant-bearing surface. Pract. Periodontics Aesthetic Dent. Ppad 1994, 6, 37. [Google Scholar]
- Murphy, K.G.; Polack, M.A.; Arzadon, J.M.; Hickerson, R.D.; Scheyer, E.T. A Report of Three Cases from an Ongoing Prospective Clinical Study on a Novel Pink Biomimetic Implant System. Compend. Contin. Educ. Dent. 2016, 37, S1–S12. [Google Scholar] [PubMed]
- Sicilia, A.; Cuesta, S.; Coma, G.; Arregui, I.; Guisasola, C.; Ruiz, E.; Maestro, A. Titanium allergy in dental implant patients: A clinical study on 1500 consecutive patients. Clin. Oral Implant. Res. 2008, 19, 823–835. [Google Scholar] [CrossRef]
- Barwacz, C.A.; Brogden, K.A.; Stanford, C.M.; Dawson, D.V.; Recker, E.N.; Blanchette, D. Comparison of pro-inflammatory cytokines and bone metabolism mediators around titanium and zirconia dental implant abutments following a minimum of 6 months of clinical function. Clin. Oral Implant. Res. 2015, 26, e35–e41. [Google Scholar] [CrossRef]
- Houshmand, A.; Donkiewicz, P.; Smeets, R.; Jung, O.; Barbeck, M. Incidental finding of a degrading zirconia dental implant 29 months after implantation: Histological and histomorphometrical analysis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2919–2923. [Google Scholar] [CrossRef]
- Shiu, H.T.; Goss, B.; Lutton, C.; Crawford, R.; Xiao, Y. Formation of blood clot on biomaterial implants influences bone healing. Tissue Eng. Part B Rev. 2014, 20, 697–712. [Google Scholar] [CrossRef]
- Ananth, H.; Kundapur, V.; Mohammed, H.S.; Anand, M.; Amarnath, G.S.; Mankar, S. A Review on Biomaterials in Dental Implantology. Int. J. Biomed. Sci. 2015, 11, 113–120. [Google Scholar]
- Kundu, R.; Rathee, M. Effect of platelet-rich-plasma (PRP) and implant surface topography on implant stability and bone. J. Clin. Diagn. Res. JCDR 2014, 8, ZC26. [Google Scholar] [CrossRef]
- Strauss, F.J.; Stähli, A.; Gruber, R. The use of platelet-rich fibrin to enhance the outcomes of implant therapy: A systematic review. Clin. Oral Implant. Res. 2018, 29, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Ge, S. Application of Antimicrobial Nanoparticles in Dentistry. Molecules 2019, 24, 15. [Google Scholar] [CrossRef] [PubMed]
- Kasraei, S.; Sami, L.; Hendi, S.; Alikhani, M.Y.; Rezaei-Soufi, L.; Khamverdi, Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor. Dent. Endod. 2014, 39, 109–114. [Google Scholar] [CrossRef]
- Garner, S.J.; Nobbs, A.H.; McNally, L.M.; Barbour, M.E. An antifungal coating for dental silicones composed of chlorhexidine nanoparticles. J. Dent. 2015, 43, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jo, J.K.; Kim, D.A.; Patel, K.D.; Kim, H.W.; Lee, H.H. Nano-graphene oxide incorporated into PMMA resin to prevent microbial adhesion. Dent. Mater. 2018, 34, e63–e72. [Google Scholar] [CrossRef] [PubMed]
- Allan, I.; Newman, H.; Wilson, M. Antibacterial activity of particulate bioglass against supra- and subgingival bacteria. Biomaterials 2001, 22, 1683–1687. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Weir, M.D.; Zhang, K.; Zhou, Y.; Xu, H.H.K.; Reynolds, M.A. Novel multifunctional dental bonding agent for Class-V restorations to inhibit periodontal biofilms. RSC Adv. 2017, 7, 29004–29014. [Google Scholar] [CrossRef]
- Wang, L.; Melo, M.A.; Weir, M.D.; Xie, X.; Reynolds, M.A.; Xu, H.H. Novel bioactive nanocomposite for Class-V restorations to inhibit periodontitis-related pathogens. Dent. Mater. 2016, 32, e351–e361. [Google Scholar] [CrossRef]
- Wang, L.; Xie, X.; Imazato, S.; Weir, M.D.; Reynolds, M.A.; Xu, H.H.K. A protein-repellent and antibacterial nanocomposite for Class-V restorations to inhibit periodontitis-related pathogens. Mater Sci. Eng. C Mater Biol. Appl. 2016, 67, 702–710. [Google Scholar] [CrossRef]
- do Nascimento, C.; Paulo, D.F.; Pita, M.S.; Pedrazzi, V.; de Albuquerque Junior, R.F. Microbial diversity of the supra- and subgingival biofilm of healthy individuals after brushing with chlorhexidine- or silver-coated toothbrush bristles. Can. J. Microbiol. 2015, 61, 112–123. [Google Scholar] [CrossRef]
- Mackevica, A.; Olsson, M.E.; Hansen, S.F. The release of silver nanoparticles from commercial toothbrushes. J. Hazard. Mater. 2017, 322 Pt A, 270–275. [Google Scholar] [CrossRef]
- Fernandes, J.S.; Gentile, P.; Pires, R.A.; Reis, R.L.; Hatton, P.V. Multifunctional bioactive glass and glass-ceramic biomaterials with antibacterial properties for repair and regeneration of bone tissue. Acta Biomater. 2017, 59, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xu, J.; Yu, L.; Fang, X.-Y.; Khor, K. Spark plasma sintering of sol-gel derived 45S5 Bioglass (R)-ceramics: Mechanical properties and biocompatibility evaluation. Mater. Sci. Eng. C 2012, 32, 494–502. [Google Scholar] [CrossRef]
- Daguano, J.K.; Strecker, K.; Ziemath, E.C.; Rogero, S.O.; Fernandes, M.H.; Santos, C. Effect of partial crystallization on the mechanical properties and cytotoxicity of bioactive glass from the 3CaO.P(2)O(5)-SiO(2)-MgO system. J. Mech. Behav. Biomed. Mater. 2012, 14, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.S.; Gentile, P.; Moorehead, R.; Crawford, A.; Miller, C.A.; Pires, R.A.; Hatton, P.V.; Reis, R.L. Design and Properties of Novel Substituted Borosilicate Bioactive Glasses and Their Glass-Ceramic Derivatives. Cryst. Growth Des. 2016, 16, 3731–3740. [Google Scholar] [CrossRef]
- Freeman, C.O.; Brook, I.M.; Johnson, A.; Hatton, P.V.; Hill, R.G.; Stanton, K.T. Crystallization modifies osteoconductivity in an apatite-mullite glass-ceramic. J. Mater Sci. Mater. Med. 2003, 14, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Kanchanarat, N.; Miller, C.; Hatton, P.; James, P.; Reaney, I. Early Stages of Crystallization in Canasite-Based Glass Ceramics. J. Am. Ceram. Soc. 2005, 88, 3198–3204. [Google Scholar] [CrossRef]
- Wallace, K.E.; Hill, R.G.; Pembroke, J.T.; Brown, C.J.; Hatton, P.V. Influence of sodium oxide content on bioactive glass properties. J. Mater Sci. Mater. Med. 1999, 10, 697–701. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 1998, 81, 1705–1728. [Google Scholar] [CrossRef]
- Rahaman, M.N. Bioactive ceramics and glasses for tissue engineering. In Tissue Engineering Using Ceramics and Polymers; Woodhead Publishing: Cambridge, UK, 2014; pp. 67–114. [Google Scholar]
- Ahmed, A.A.; Ali, A.A.; Mahmoud, D.; El-Fiqi, A. Preparation and Characterization of Antibacterial P2O5–CaO–Na2O–Ag2O Glasses. J. Biomed. Mater. Res. Part A 2011, 98, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Bellantone, M.; Williams, H.D.; Hench, L.L. Broad-spectrum bactericidal activity of Ag(2)O-doped bioactive glass. Antimicrob. Agents Chemother. 2002, 46, 1940–1945. [Google Scholar] [CrossRef]
- Goh, Y.F.; Alshemary, A.Z.; Akram, M.; Abdul Kadir, M.; Hussain, R. In vitro Characterization of Antibacterial Bioactive Glass Containing Ceria. Ceram. Int. 2013, 40, 729–737. [Google Scholar] [CrossRef]
- Mulligan, A.M.; Wilson, M.; Knowles, J.C. The effect of increasing copper content in phosphate-based glasses on biofilms of Streptococcus sanguis. Biomaterials 2003, 24, 1797–1807. [Google Scholar] [CrossRef]
- Domingues, E.P.; Ribeiro, R.F.; Horta, M.C.R.; Manzi, F.R.; Cosso, M.G.; Zenobio, E.G. Vertical augmentation of the posterior atrophic mandible by interpositional grafts in a split-mouth design: A human tomography evaluation pilot study. Clin. Oral Implant. Res. 2017, 28, e193–e200. [Google Scholar] [CrossRef]
- Paladini, F.; Pollini, M.; Sannino, A.; Ambrosio, L. Metal-Based Antibacterial Substrates for Biomedical Applications. Biomacromolecules 2015, 16, 1873–1885. [Google Scholar] [CrossRef]
- Simchi, A.; Tamjid, E.; Pishbin, F.; Boccaccini, A.R. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomedicine 2011, 7, 22–39. [Google Scholar] [CrossRef]
- Vassena, C.; Fenu, S.; Giuliani, F.; Fantetti, L.; Roncucci, G.; Simonutti, G.; Romanò, C.L.; De Francesco, R.; Drago, L. Photodynamic antibacterial and antibiofilm activity of RLP068/Cl against Staphylococcus aureus and Pseudomonas aeruginosa forming biofilms on prosthetic material. Int. J. Antimicrob. Agents 2014, 44, 47–55. [Google Scholar] [CrossRef]
- Mishnaevsky, L.; Levashov, E.; Valiev, R.Z.; Segurado, J.; Sabirov, I.; Enikeev, N.; Prokoshkin, S.; Solov’yov, A.V.; Korotitskiy, A.; Gutmanas, E.; et al. Nanostructured titanium-based materials for medical implants: Modeling and development. Mater. Sci. Eng. R Rep. 2014, 81, 1–19. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.; Asokamani, R.; Gogia, A. Ti based biomaterials, the ultimate choice for orthopaedic implants A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- D’Almeida, M.; Attik, N.; Amalric, J.; Brunon, C.; Renaud, F.; Abouelleil, H.; Toury, B.; Grosgogeat, B. Chitosan coating as an antibacterial surface for biomedical applications. PLoS ONE 2017, 12, e0189537. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, S.; Venkatesan, J.; Kim, S.K.; Bumgardner, J.D.; Jayakumar, R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93 Pt B, 1338–1353. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. In vivo evaluation of chitosan-PVP-titanium dioxide nanocomposite as wound dressing material. Carbohydr. Polym. 2013, 95, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Khor, E.; Lim, L.Y. Implantable applications of chitin and chitosan. Biomaterials 2003, 24, 2339–2349. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- Behera, S.S.; Das, U.; Kumar, A.; Bissoyi, A.; Singh, A.K. Chitosan/TiO(2) composite membrane improves proliferation and survival of L929 fibroblast cells: Application in wound dressing and skin regeneration. Int. J. Biol. Macromol. 2017, 98, 329–340. [Google Scholar] [CrossRef]
- Singla, A.K.; Chawla, M. Chitosan: Some pharmaceutical and biological aspects—An update. J. Pharm. Pharm. 2001, 53, 1047–1067. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential--a critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef]
- Kumirska, J.; Weinhold, M.; Thöming, J.; Stepnowski, P. Biomedical Activity of Chitin/Chitosan Based Materials—Influence of Physicochemical Properties Apart from Molecular Weight and Degree of N-Acetylation. Polymers 2011, 3, 1875–1901. [Google Scholar] [CrossRef]
- Wenling, C.; Duohui, J.; Jiamou, L.; Yandao, G.; Nanming, Z.; Xiufang, Z. Effects of the degree of deacetylation on the physicochemical properties and Schwann cell affinity of chitosan films. J. Biomater. Appl. 2005, 20, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Tchemtchoua, V.T.; Atanasova, G.; Aqil, A.; Filée, P.; Garbacki, N.; Vanhooteghem, O.; Deroanne, C.; Noël, A.; Jérome, C.; Nusgens, B.; et al. Development of a chitosan nanofibrillar scaffold for skin repair and regeneration. Biomacromolecules 2011, 12, 3194–3204. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.M.; Toury, B.; D’Almeida, M.; Attik, G.N.; Ferrand, A.; Renoud, P.; Grosgogeat, B. Acidic pH resistance of grafted chitosan on dental implant. Odontology 2015, 103, 210–217. [Google Scholar] [CrossRef]
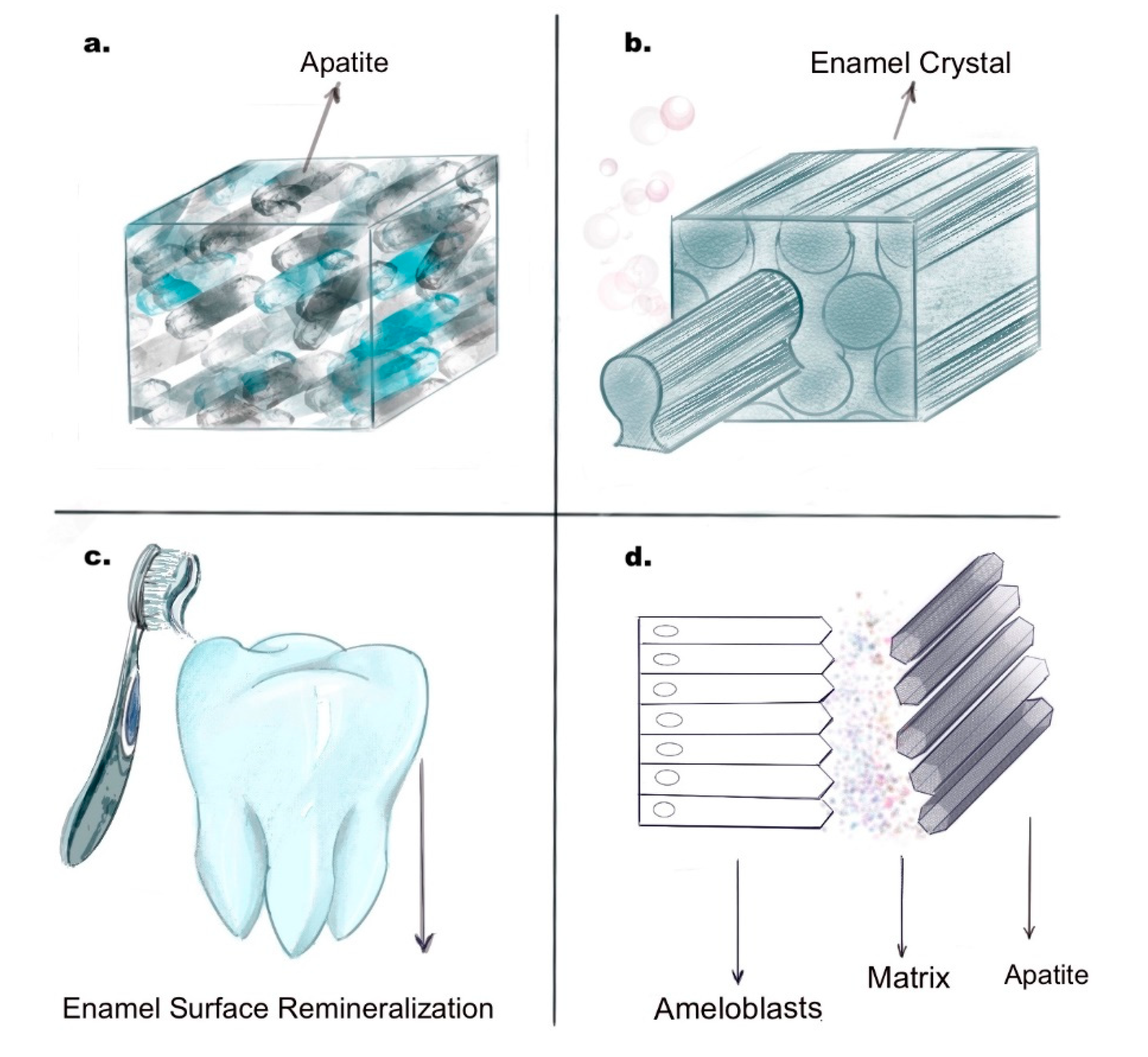
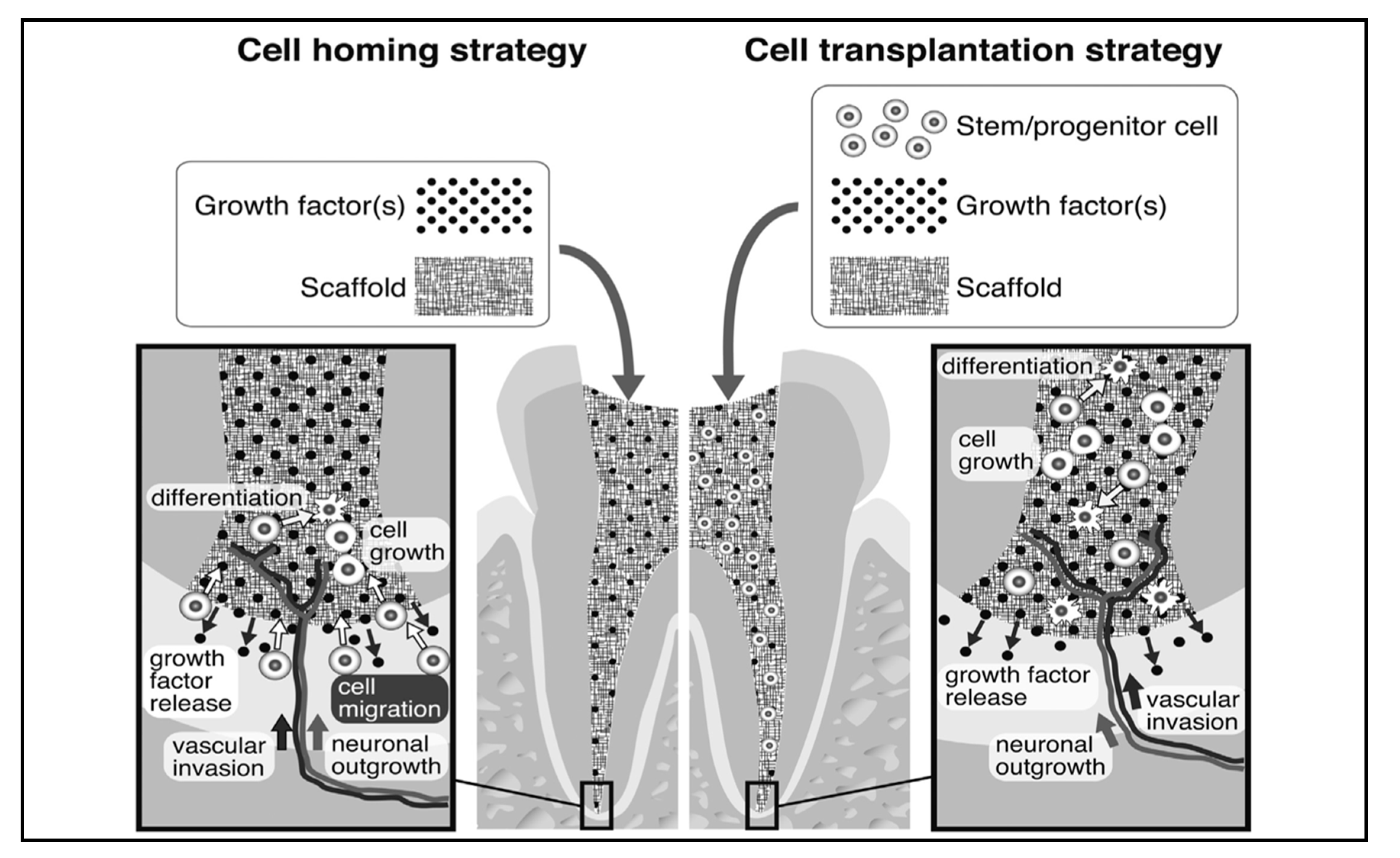
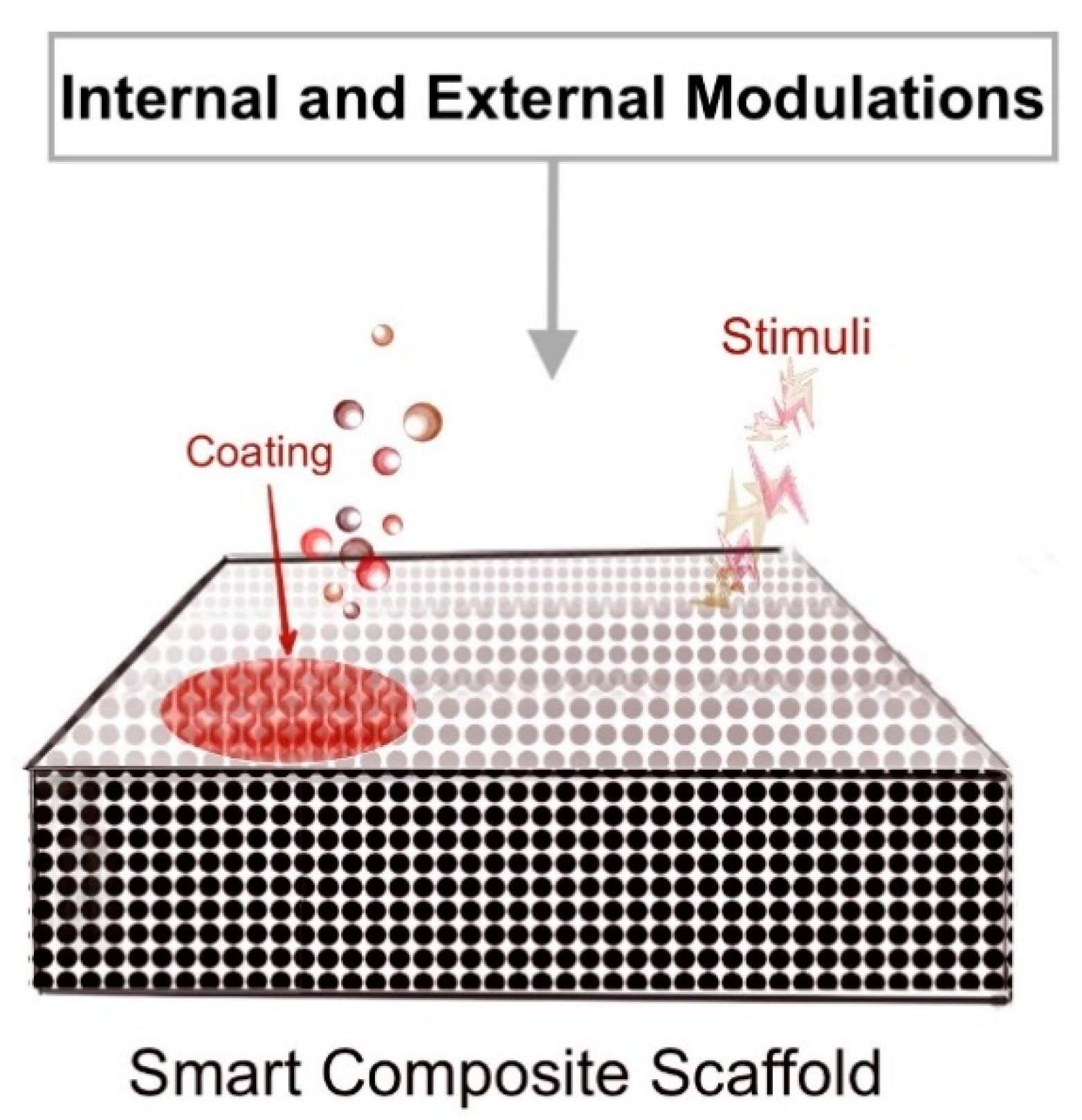
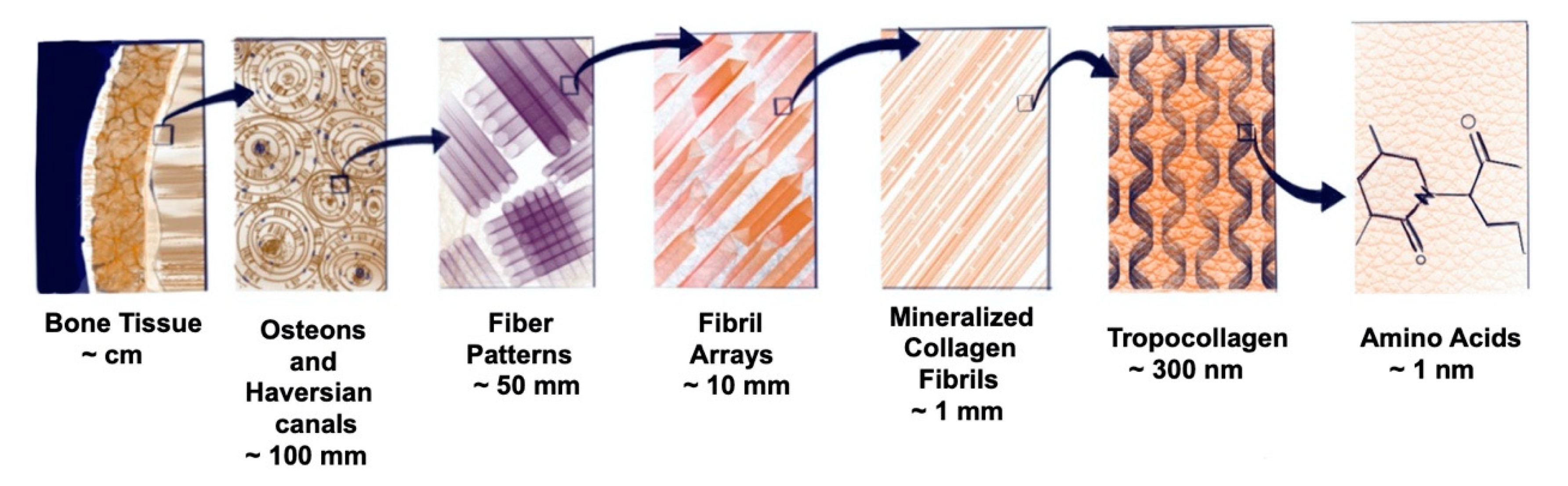
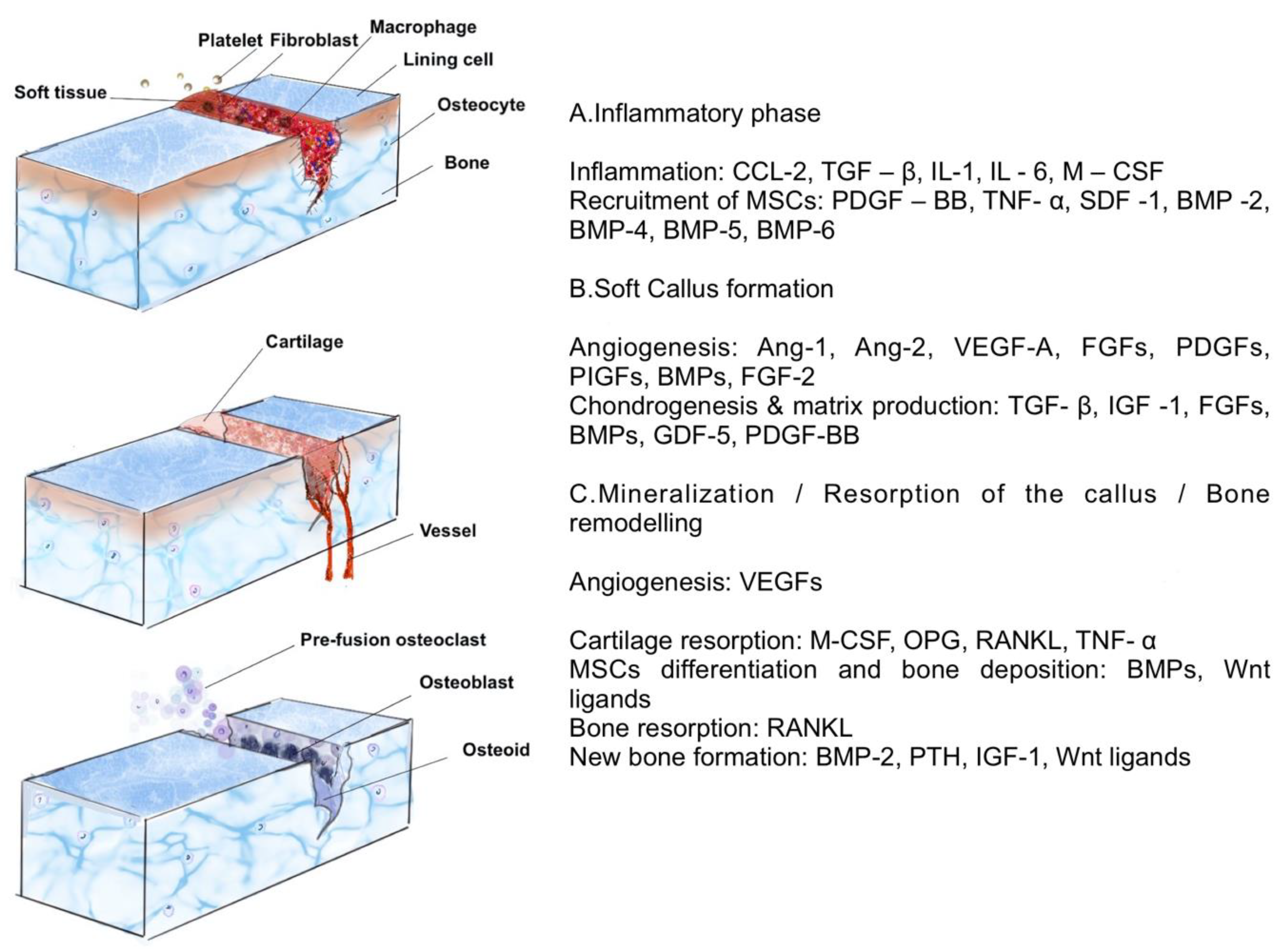
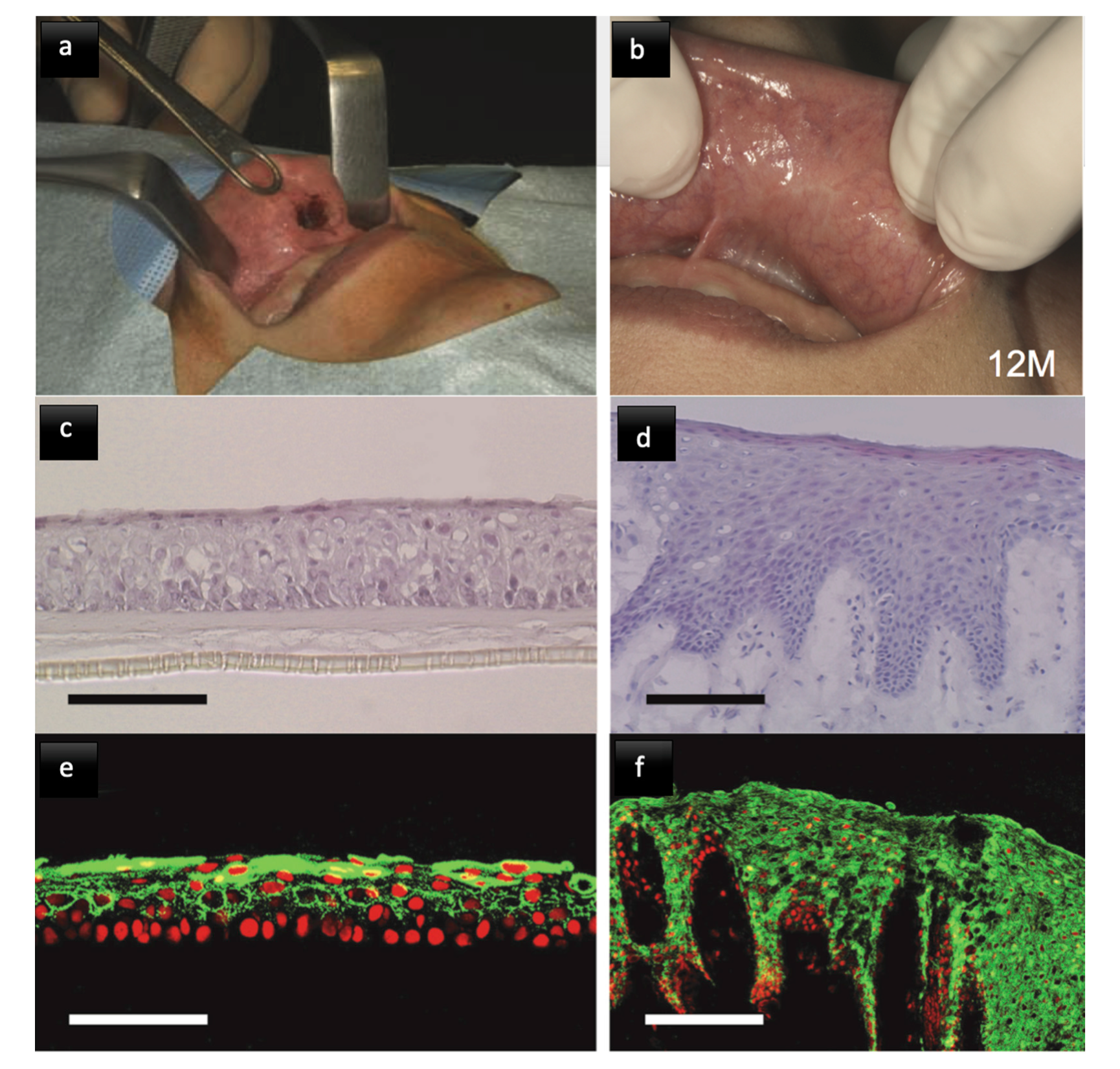
| Material | Modifications and Use | Ref. |
|---|---|---|
| Bioactive glass (BAG) | Modified with zinc, copper, fluoride, and PAA (polyacrylic acid), used as adhesive agents in dentin resin bonding interfaces, and used in dentin hypersensitivity treatment. | [27,28,29] |
| Calcium silicates | Di/tricalcium silicates and Mineral trioxide aggregate (MTA); bioactivity by alkalisation of hydroxyl groups from the CaOH phase, leading to an increase in pH, decreased activity of MMP, activated mineral precipitation, and antimicrobial activity. | [30] |
| Calcium orthophosphates | Modifications based on different concentrations of Ca–P, dicalcium phosphate anhydrous (DCPA), dicalcium phosphate dihydrate (DCPD), and tetra calcium phosphate (TTCP) as resin adhesives for dentin remineralisation and improved dentin resin interfaces. | [31] |
| ZnO particles | Zinc oxide and Zn-loaded polymeric nanomaterials additives in resin, protective action in collagen degradation, and initiation of precipitation of poorly crystallised apatite crystals. | [32,33] |
| Biomimetic Analogues | Modifications and Use | Ref. |
|---|---|---|
| Polyacrylic acid9 (PAA9) | Calcium-binding molecule analogous to dentin matrix protein 1(DMP 1) stabilises and controls dimensions of calcium carbonate and calcium phosphate phases. | [34,35] |
| Polyvinyl phosphonic acid (PVA) | Functions analogous to collagen-binding matrix phosphoproteins like DMP1 and dentin phosphoproteins. | [35,36] |
| Sodium trimetaphosphate (STMP) | It is a phosphophoryn analogue, binds to collagen fibrils, creates negatively charged sites to receive nanoprecursors, and initiates nucleation of apatite crystals. | [34,37] |
| Polyaspartic acid (PAS) | Is an analogue for calcium utilisation, released from hardened calcium silicate cements or calcium phosphate mineralising solutions, and assists in controlling the size of ACP nano precursors and their movement into the collagen fibrils. | [38,39] |
| Biomaterial | Indication/Mechanism/Results | Ref. |
|---|---|---|
| Calcium hydroxide (CaOH2) | It is a gold standard, with high pH inducing necrosis and mineralisation, good antibacterial properties, and formation of heterogeneous dentin bridge with tunnel defects; it increases recruitment, migration, proliferation, and mineralisation of DPSCs and periodontal ligament stem cells (PDLSCs) through the expression of STRO-1 and CD146 markers; and calcium increases the synthesis of biomolecules such as fibronectin and bone morphogenetic proteins (BMPs) and causes precipitation mineralisation. | [43,44,45,46,47] |
| Mineral trioxide aggregate (MTA)/Calcium silicates/modifications | It has an antibacterial effect by releasing calcium hydroxide, a superior sealing ability, low solubility, higher strength, and more stability than CaOH; it works well in a moist environment; it forms thicker dentin bridges; it has less inflammatory response, hyperaemia and lower pulp tissue necrosis; modifications include calcium chloride additions, leads to lower setting time, and more biocompatibility; light cured, resin-modified calcium-silicate-based MTA provides immediate polymerization, material preservation, and superior physical properties; and it induces generation of proangiogenic factors like IL-8 and IL-beta (interleukins). | [45,48,49,50,51,52] |
| Bioactive glasses | It is a mixture of silica, sodium, and phosphorous oxides with the ability to bond to bone by controlled release of ions forming apatite crystals repairing hard tissues; it mimics the natural apatite structure; studies show dentin bridge formation on pulp capping, no necrosis of pulp tissue, and mild inflammatory response; it can form different qualities of reparative dentin with varying porosities and mechanical properties; and it is noncytotoxic and improves cell metabolic activities on in vitro testing. | [53,54] |
| BiodentineTM | Induces differentiation of DPSCs by MAPK (mitogen-activated protein kinase) and calcium calmodulin-dependent protein kinase II (CaMKII) pathways; faster mineralisation of pulp tissue due to the release of transforming growth factor (TGF- Beta 1). | [55,56] |
| CEM (Calcium enriched mixture) | It has dentinogenic, cementogenic, and osteogenic properties; they increase the expression of fibroblast growth factor 4 (FGF-4) and bone morphogenetic protein 2 (BMP-2), which favours remineralisation and regeneration. | [57] |
| Glass ionomers and adhesive resins | It has a proliferative effect on pulp tissue comparable to CaOH when used as a lining material for dentin pulp regeneration, no noticeable antibacterial effect, more inflammatory response seen on pulp, upregulation of fibroblasts and endothelial cells, and an inhibitory effect on Hohl cells; in mechanically injured pulp tissue HEMA (hydroxyethyl methacrylate), it induces secretion of proangiogenic factors like vascular endothelial growth factors (VEGF) and decreases expression of FGF-2; and concerns remain regarding the efficiency and quality of tertiary dentin formation after pulp injury. | [58,59,60] |
| Enamel matrix derivatives (EMD) | It is shown to be more effective than CaOH and MTA in differentiation and proliferation of human tooth germ stem cells; it is highly biocompatible and has known chemotactic effect and angiogenic effects; studies indicate their use for periodontal regeneration; and it is inversely shown to cause more inflammation on pulp tissue with little or less hard tissue formation when compared with CaOH application. | [61,62,63] |
| Cells | Indications/Mechanism/Result | Positive Markers | Negative Markers | Ref. |
|---|---|---|---|---|
| Stem cells from apical papilla (SCAP) | Present next to immature tooth root apex; remains active even in cases of pulp infections or necrosis due to collateral blood supply; has the potential to differentiate into odontoblast like cells; and shows increased telomerase activity, higher resistance to infection, faster multiplication, and migratory efficiency within root canals | CD49d, CD51/61, CD56, CD73, CD90, CD105, CD106, CD146, CD166 | CD14, CD18, CD34, CD45, CD117, CD150 | [65,66,67,68] |
| Dental pulp stem cells (DPSC) | Derived usually from human third molar pulp tissue; has high proliferation and colony-forming ability as dense calcified structures; can differentiate into osteoblasts, odontoblasts, adipocytes, and chondrocytes; and can be used as stem cells in neural disorders due to their ability to induce axonal guidance and differentiate into functional neural cells. | CD9, CD10, CD13, CD29, CD44, CD49d, CD59, CD73, CD90, CD105, CD106, CD146, CD166 | CD14, CD31, CD34, CD45, CD117, CD133 | [69,70,71,72,73,74,75,76,77] |
| Stem cells from human exfoliated deciduous teeth (SHEDs) | SHED can form bone, dentin, and differentiate into other nondental mesenchymal cell derivatives; it has a higher proliferation rate that DPSC and bone marrow-derived MSCs, faster population doubling, and osteoinductive properties; and transplantation has shown the architecture and cellularity of tissue formed by SHED to resemble dental pulp closely. | CD13, CD44, CD73, CD90, CD105, CD146 | CD14, CD19, CD34, CD43, CD45 | [78,79,80,81,82] |
| Dental follicle stem cells (DFSC) | It has ectomesenchyme-derived connective tissue surrounding enamel and dental papilla; contains progenitors for cementoblasts, osteoblasts, and PDL; and exhibits the ability to differentiate into PDL fibroblasts, to secrete collagen, and to consequently interact with fibres in the bone and cementum surface, and DFSC from human third molars in vitro shows rapid growth and expresses stem cell markers, including Nestin and Notch 1. | CD9, CD10, CD13, CD29, CD44, CD49d, CD59, CD73, CD90, CD105, CD106, CD166 | CD31, CD34, CD45, CD133 | [75,83,84,85,86,87] |
| Scaffold | Indications/Mechanism/Results | Ref. |
|---|---|---|
| Intracanal blood clot | It induces apical bleeding, leading to delivery of SCAP to root canal space; it is an autologous scaffold with growth factors, is clinically efficient, and is economical; and it can be an unstable and unreliable movement of stem cells within the canal space after revascularization. | [88,89,90,91] |
| Platelet-rich plasma (PRP) | Autologous injectable scaffold: it can be delivered via collagen sponges; platelet elevation results in increased production and secretion of growth factors PDGF, TGF-b, Insulin-like growth factor (IGF), epidermal growth factor (EGF), and epithelial cell growth factor (ECGF), leading to improved angiogenesis and cell proliferation | [88,92] |
| Alginate | Natural polysaccharides from cell walls and seaweeds: stem cells can be incorporated during scaffold processing; it supports 3-D printing in combination with proteins like DMPs; it includes easy diffusion of nutrients and waste debris due to porous structure; it is highly biocompatible, has low immune reactions, is economic, and is easy to fabricate; and has low mechanical strength of the scaffold when used alone. | [93,94] |
| Hyaluronic acid (HA)and derivatives | Glycosaminoglycans which mimic ECM components: it interacts with stem cell receptors and drives them towards the area of regeneration; it is shown to have a role in dentin matrix and pulp tissue development; it exhibits good biocompatibility, biodegradability, and bioactivity; HA derivatives induce proangiogenic factors release; it improves stem cell mineralisation and odontogenic differentiation; it has low mechanical strength and needs combination with growth factors to improve regenerative potential; and it may cause hypersensitivity reactions. | [90,94,95,96,97] |
| Chitosan derivatives | Linear amino polysaccharide mimics ECM structure and composition: it is easy to fabricate, is highly porous, and allows easy migration of cells and growth factors; when fabricated as nanoparticles, it improved properties due to increased surface area, has better mechanical strength, and is resistant to enzymatic degradation; it allows the controlled release of growth factors and improves stem cell or SCAP adhesion, viability, and differentiation; and it is highly biocompatible, has controlled biodegradation, and has low cytotoxicity with antibacterial properties. | [98,99,100] |
| Gelatin | Consists of proteins from hydrolysis of hard and soft tissue-derived collagen; they are biocompatible and biodegradable, elicits no immune responses, and is cost-efficient; they can be modified with RBDs (receptor binding motifs), which promotes cell attachment and allows chemical modifications to improve the scaffold’s physiochemical properties; it is used as a drug delivery medium or in 2D and 3D cultures; and the use of FGF-2 with gelatin shows the formation of osteo-dentin-like calcified tissue for dentin pulp complex regeneration. | [101,102] |
| Cellulose | Naturally occurring scaffold obtained from green plants and algae: they are not biodegradable due to the absence of cellulase enzymes in humans; they possess high tensile strength, high crystallinity, fine fibrous structure, and good formability and is biocompatible; they have higher chances of immune response; and they are used mostly in target-specific drug delivery or growth factor release in dental tissue engineering. | [103,104] |
| Collagen | It is a natural biomaterial, is easily adapted to root canal morphology, and mimics ECM; the most used is type I, suitable for DPSCs proliferation and mineralisation; it is biocompatible, provides bioactivity by facilitating adhesion and attachment of stem cells, and induces signalling pathways that promote differentiation; the highly porous structure allows easy cell seeding for site-specific delivery; and commercially available SynnOss (bovine type 1 collagen) in conjunction with revascularization forms mineralised cementum-like tissues. | [105,106,107,108,109] |
| Self-assembling peptide hydrogels -Puramatrix | Synthetic, biocompatible, biodegradable, nontoxic, 3D matrix gel available as a liquid phase, which solidifies when in contact with a physiologic salt environment: in vitro studies show pure matrix support DPSC cell proliferation and viability when evaluated over three weeks within tooth slices; puramatrix showed better in vitro results in terms of cell viability and odontogenic differentiation when used with a co-culture of DPSC/HUVEC (human umbilical vein endothelial cells). | [110,111,112,113] |
| Poly L- Lactic acid (PLLA) nanofibrous microspheres | Injectable scaffold with integrated BMP-2, when combined with polylactic acid (PLA) and polyglycolic acid (PGA), significantly improving the properties and half-life of the PLLA and prolonged BMP-2 release: it is easily adapts to root canal shape and is biodegradable; it can incorporate drugs/growth factors and is conductive for cells, including DPSC and SHED; it has favourable viscosity and porosity; it does not elicit any adverse immune response; it is cheap and reproducible; the regenerated dentin structure may be disorganized and may not replicate the natural tooth architecture; and degradation metabolites might cause unfavourable conditions for surrounding cells but can be excreted to urine without complications. | [114,115,116] |
| Poly (lactide-co gylcolide)-polyethylene glycol (PLGA-PE) NP | It has better conductivity for dental pulp fibroblasts proliferation; it is clinically biodegradable, has fast setting, has low toxicity, has good biocompatibility, and has low immunogenicity; but, it lacks intrinsic signalling abilities and is more expensive than other synthetic scaffolds. | [106,117] |
| Type of Graft | Action | Advantage | Disadvantage |
|---|---|---|---|
| Autograft | Osteogenic Osteoinductive Osteoconductive | Histocompatible Negligible immunogenicity Ideal physical and mechanical properties | Donor site injury Scarring Longer recovery time Limited size |
| Ectopic prefabrication | Osteogenic Osteoinductive Osteoconductive | Histocompatible Negligible immunogenicity Ideal physical and mechanical properties No shape or volume limitation | Donor site injury Scarring Longer recovery time |
| Allografts | Osteogenic Osteoconductive | Histocompatible Ideal physical and mechanical properties. | Immune reaction Transmission of infection |
| Xenografts | Osteogenic Osteoconductive | Histocompatible Ideal physical and mechanical properties. | Immune reaction Transmission of infection |
| Ref. | Type of Study | Type of Graft | Method of Evaluation—In Vitro/In Vivo | Sample Size | Conclusions |
|---|---|---|---|---|---|
| [193] | Randomized clinical trial (NCT03496688) | MCBA FDBA ABB EB HA-TCP-30/70 BC | Histological and histomorphometric analysis | 6 patients | All materials showed good biocompatibility and Osseo conductivity with FDBA as the best material, but only one patient per sample was used, so a larger sample size is required. |
| [194] | Randomized split-mouth study (NCT03682315) | ACB + ABB ACB + BP | Radiographic analysis, mRNA analysis, histopathological analysis, Immunohistochemistry, TEM | 8 patients | Biphasic psychogenic biomaterial (BP) induced a higher radiographical vertical resorption and graft collapse in comparison with the combination with an organic bovine bone (ABB). |
| [195] | Randomized clinical trial | DPBM vs. DBBM | CT and trephine biopsy histology | 11 participants for PPA, 12 ITT | Porcine bone (DPBM) showed comparable results with the widely used bovine bone (DBBM). A larger sample size and more extended studies are still required. |
| [196] | Randomized clinical trial | MBS BBS | Histological Histomorphometric CBCT | 60 patients | BBS remains more stable in terms of volume maintenance and radiological graft homogeneity after a healing period of 6 months. |
| [197] | Randomized clinical trial | Calcium phosphate crystal double- coated bovine bone and an organic bovine bone | Histological Histomorphometric radiographic | 33 patients | Both materials showed comparable histomorphometric and radiographic results. |
| [198] | Randomized split-mouth study (NCT03077867) | NHA ABB | Histomorphometric | 28 patients | After six months of healing, no statistically significant difference was present in histomorphometric outcomes between the NHA and ABB groups. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upadhyay, A.; Pillai, S.; Khayambashi, P.; Sabri, H.; Lee, K.T.; Tarar, M.; Zhou, S.; Harb, I.; Tran, S.D. Biomimetic Aspects of Oral and Dentofacial Regeneration. Biomimetics 2020, 5, 51. https://doi.org/10.3390/biomimetics5040051
Upadhyay A, Pillai S, Khayambashi P, Sabri H, Lee KT, Tarar M, Zhou S, Harb I, Tran SD. Biomimetic Aspects of Oral and Dentofacial Regeneration. Biomimetics. 2020; 5(4):51. https://doi.org/10.3390/biomimetics5040051
Chicago/Turabian StyleUpadhyay, Akshaya, Sangeeth Pillai, Parisa Khayambashi, Hisham Sabri, Kyungjun T. Lee, Maryam Tarar, Stephanie Zhou, Ingrid Harb, and Simon D. Tran. 2020. "Biomimetic Aspects of Oral and Dentofacial Regeneration" Biomimetics 5, no. 4: 51. https://doi.org/10.3390/biomimetics5040051
APA StyleUpadhyay, A., Pillai, S., Khayambashi, P., Sabri, H., Lee, K. T., Tarar, M., Zhou, S., Harb, I., & Tran, S. D. (2020). Biomimetic Aspects of Oral and Dentofacial Regeneration. Biomimetics, 5(4), 51. https://doi.org/10.3390/biomimetics5040051







