Biomimetic Aspects of Restorative Dentistry Biomaterials
Abstract
1. Introduction
2. Dental Hard Tissues
2.1. Enamel
2.2. Dentin–Pulp Complex
2.3. Cementum
3. Biomimetic Approaches for Dental Restorative Biomaterials
3.1. Biomimetic Mechanical Perspective of Restorative Materials
3.2. Aesthetics Perspective of Restorative Materials
3.3. Biocompatibility of Dental Restorative Materials
3.4. Biomimetic Mineralization of Enamel and Dentin: A Current Approach in Restorative Dentistry
4. Biomimetic Endodontics and Regenerative Aspects
4.1. Endodontic Irrigants
4.2. Intra-canal Medicaments
4.3. Biomimetic Endodontic Cements
4.3.1. Calcium Hydroxide
4.3.2. Triple Antibiotic Paste
4.3.3. Bioceramics
5. Biomimetic Tissue-Engineering Aspects
5.1. Desired Properties for Biomimetic Tissue-Engineering Scaffolds
5.2. Materials for Biomimetic Scaffold Fabrication
5.2.1. Polymers
5.2.2. Bioceramics
5.2.3. Metals
5.3. Methods of Processing 3D Biomimetic Scaffolds
5.4. Dental Stem Cells Therapy for Biomimetic Tissue Regeneration
5.5. Biological Cell Signaling Growth Factors for Biomimetic Tissue Engineering
5.5.1. Bone Morphogenetic Proteins
5.5.2. Vascular Endothelial Growth Factor
5.5.3. Platelet-Derived Growth Factor
5.5.4. Fibroblast Growth Factor
5.5.5. Transforming Growth Factor
6. Conclusions and Future Trends
Funding
Acknowledgments
Conflicts of Interest
References
- Harkness, J.M. An idea man (the life of Otto Herbert Schmitt). IEEE Eng. Med. Biol. Mag. 2004, 23, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B. Biomimetics. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2009, 367, 1443–1444. [Google Scholar] [CrossRef] [PubMed]
- Kottoor, J. Biomimetic endodontics: Barriers and strategies. Health Sci. 2013, 2, 7–12. [Google Scholar]
- Sharma, V.; Srinivasan, A.; Nikolajeff, F.; Kumar, S. Biomineralization process in hard tissues: The interaction complexity within protein and inorganic counterparts. Acta Biomater. 2020. [Google Scholar] [CrossRef] [PubMed]
- Cleymand, F.; Rousseau, M.; Mano, J.F. Introducing biomimetic approaches to materials development and product design for engineering students. Bioinspired Biomim. Nanobiomaterials 2015, 4, 207–212. [Google Scholar] [CrossRef]
- Santulli, C.; Langella, C. Introducing students to bio-inspiration and biomimetic design: A workshop experience. Int. J. Technol. Des. Educ. 2011, 21, 471–485. [Google Scholar] [CrossRef]
- Ullah, R.; Zafar, M.S. Oral and dental delivery of fluoride: A review. Fluoride 2015, 48, 195–204. [Google Scholar]
- Zafar, M.S.; Ahmed, N. Therapeutic roles of fluoride released from restorative dental materials. Fluoride 2015, 48, 184–194. [Google Scholar]
- Akyuz, S.; Yarat, A.; Alturfan, E.E.; Kaya, S. Fluoride in Saliva and Its Impact on Health; Preedy, V.R., Ed.; Fluorine Chemistry Group, Royal Society of Chemistry: Cambridge, UK, 2015; pp. 173–185. [Google Scholar]
- Donnermeyer, D.; Bürklein, S.; Dammaschke, T.; Schäfer, E. Endodontic sealers based on calcium silicates: A systematic review. Odontology 2019, 107, 1–16. [Google Scholar] [CrossRef]
- Sanz, J.L.; Rodríguez-Lozano, F.J.; Llena, C.; Sauro, S.; Forner, L. Bioactivity of bioceramic materials used in the dentin-pulp complex therapy: A systematic review. Materials 2019, 12, 1015. [Google Scholar] [CrossRef]
- Bazos, P.; Magne, P. Bio-emulation: Biomimetically emulating nature utilizing a histo-anatomic approach; structural analysis. Eur. J. Esthet. Dent. 2011, 6, 8–19. [Google Scholar] [PubMed]
- Tirlet, G.; Crescenzo, H.; Crescenzo, D.; Bazos, P. Ceramic adhesive restorations and biomimetic dentistry: Tissue preservation and adhesion. Int. J. Esthet. Dent. 2014, 9, 354–369. [Google Scholar]
- Yu, X.; Wang, L.; Jiang, X.; Rowe, D.; Wei, M. Biomimetic CaP coating incorporated with parathyroid hormone improves the osseointegration of titanium implant. J. Mater. Sci. Mater. Med. 2012, 23, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Barrere, F.; van der Valk, C.; Meijer, G.; Dalmeijer, R.; de Groot, K.; Layrolle, P. Osteointegration of biomimetic apatite coating applied onto dense and porous metal implants in femurs of goats. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 67, 655–665. [Google Scholar] [CrossRef]
- Zafar, M.S.; Fareed, M.A.; Riaz, S.; Latif, M.; Habib, S.R.; Khurshid, Z. Customized therapeutic surface coatings for dental implants. Coatings 2020, 10, 568. [Google Scholar] [CrossRef]
- Galler, K.M.; D’Souza, R.N.; Hartgerink, J.D. Biomaterials and their potential applications for dental tissue engineering. J. Mater. Chem. 2010, 20, 8730–8746. [Google Scholar] [CrossRef]
- Zafar, M.; Khurshid, Z.; Almas, K. Oral tissue engineering progress and challenges. Tissue Eng. Regen. Med. 2015, 12, 387–397. [Google Scholar] [CrossRef]
- Bottino, M.C.; Kamocki, K.; Yassen, G.H.; Platt, J.A.; Vail, M.M.; Ehrlich, Y.; Spolnik, K.J.; Gregory, R.L. Bioactive nanofibrous scaffolds for regenerative endodontics. J. Dent. Res. 2013, 92, 963–969. [Google Scholar] [CrossRef]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative endodontics: A review of current status and a call for action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef]
- Yang, J.; Yuan, G.; Chen, Z. Pulp regeneration: Current approaches and future challenges. Front. Physiol. 2016, 7, 58. [Google Scholar] [CrossRef]
- Boushell, L.W.; Sturdevant, J.R. Sturdevant’s Art and Science of Operative Dentistry. In 1—Clinical Significance of Dental Anatomy, Histology, Physiology, and Occlusion; Ritter, A.V., Boushell, L.W., Walter, R., Eds.; Elsevier: St. Louis, MO, USA, 2019; pp. 1–39. [Google Scholar]
- Nanci, A. Ten Cate’s Oral Histology: Development, Structure, and Function; Mosby: St. Louis, MO, USA, 2012; p. 411. [Google Scholar]
- Ruan, Q.; Moradian-Oldak, J. Amelogenin and enamel biomimetics. J. Mater. Chem. B 2015, 3, 3112–3129. [Google Scholar] [CrossRef]
- Perdigão, J.; Walter, R.; Miguez, P.A.; Swift, E.J. Sturdevant’s Art and Science of Operative Dentistry. In 5–Fundamental Concepts of Enamel and Dentin Adhesion; Ritter, A.V., Boushell, L.W., Walter, R., Eds.; Elsevier: St. Louis, MO, USA, 2019; pp. 136–169. [Google Scholar]
- Fincham, A.; Moradian-Oldak, J.; Simmer, J. The structural biology of the developing dental enamel matrix. J. Struct. Biol. 1999, 126, 270–299. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Akamine, A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J. Endod. 2005, 31, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Berkovitz, B.K.; Holland, G.R.; Moxham, B.J. Oral Anatomy, Histology and Embryology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Slavkin, H.C. Biomimetics: Replacing body parts is no longer science fiction. J. Am. Dent. Assoc. 1996, 127, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Mann, S. The biomimetics of enamel: A paradigm for organized biomaterials synthesis. Ciba Found. Symp. 1997, 205, 261–274. [Google Scholar]
- Magne, P.; Belser, U. Understanding the Intact Tooth and the Biomimetic Principle. Bonded Porcelain Restorations in the Anterior Dentition A Biomimetic Approach; Quintessence Publishing CO: Chicago, IL, USA, 2002; pp. 23–55. [Google Scholar]
- Magne, P. Composite resins and bonded porcelain: The post amalgam era. CDA J. 2006, 34, 135–147. [Google Scholar]
- Morin, D.; DeLong, R.; Douglas, W. Clinical science cusp reinforcement by the acid-etch technique. J. Dent. Res. 1984, 63, 1075–1078. [Google Scholar] [CrossRef]
- Ngo, H. Biological Properties of Glass-Ionomers. In An Atlas of Glass-Ionomer Cements a Clinician’s Guide, 3rd ed.; Martin Dunitz: London, UK, 2002; pp. 43–55. [Google Scholar]
- Scribante, A.; Bollardi, M.; Chiesa, M.; Poggio, C.; Colombo, M. Flexural properties and elastic modulus of different esthetic restorative materials: Evaluation after exposure to acidic drink. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- Wang, Y.; Darvell, B. Effect of elastic modulus mismatch on failure behaviour of glass ionomer cement under Hertzian indentation. Dent. Mater. 2012, 28, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Boaro, L.C.C.; Gonçalves, F.; Guimarães, T.C.; Ferracane, J.L.; Versluis, A.; Braga, R.R. Polymerization stress, shrinkage and elastic modulus of current low-shrinkage restorative composites. Dent. Mater. 2010, 26, 1144–1150. [Google Scholar] [CrossRef]
- Junior, R.; Adalberto, S.; Zanchi, C.H.; Carvalho, R.V.D.; Demarco, F.F. Flexural strength and modulus of elasticity of different types of resin-based composites. Braz. Oral Res. 2007, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Yap, A.; Tsai, K.; Yap, F. Elastic modulus of resin based dental restorative materials: A microindentation approach. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 72, 246–253. [Google Scholar] [CrossRef]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials 2003, 24, 655–665. [Google Scholar] [CrossRef]
- Trindade, F.Z.; Valandro, L.F.; de Jager, N.; Bottino, M.A.; Kleverlaan, C.J. Elastic properties of lithium disilicate versus feldspathic inlays: Effect on the bonding by 3D finite element analysis. J. Prosthodont. 2018, 27, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Al-Samadani, K.H. Surface hardness of dental composite resin restorations in response to preventive agents. J. Contemp. Dent. Pract. 2016, 17, 978–984. [Google Scholar] [CrossRef]
- Abed, Y.A.; Sabry, H.A.; Alrobeigy, N.A. Degree of conversion and surface hardness of bulk-fill composite versus incremental-fill composite. Tanta Dent. J. 2015, 12, 71–80. [Google Scholar] [CrossRef]
- Jafari, K.; Hekmatfar, S.; Badakhsh, S. The effect of mouthwashes on surface hardness of dental ceramics. J. Dent. Biomater. 2014, 1, 23–26. [Google Scholar]
- Davallo, A.; Tavangar, M.; Darabi, F.; Pourhabibi, Z.; Alamouti, N. The surface hardness value of a light cured hybrid composite resin after 12 hours immersion in three alcohol-free mouthwashes. J. Dentomaxillofac. Radiol. Pathol. Surg. 2013, 2, 1–6. [Google Scholar] [CrossRef][Green Version]
- Bala, O.; Arisu, D.H.; Yikilgan, I.; Arslan, S.; Gullu, A. Evaluation of surface roughness and hardness of different glass ionomer cements. Eur. J. Dent. 2012, 6, 079–086. [Google Scholar] [CrossRef]
- Keogh, P.; Ray, N.J.; Lynch, C.D.; Burke, F.M.; Hannigan, A. Surface microhardness of a resin composite exposed to a “first-generation” LED curing lamp, in vitro. Eur. J. Prosthodont. Restor. Dent. 2004, 12, 177–180. [Google Scholar]
- Nakayama, W.T.; Hall, D.R.; Grenoble, D.E.; Katz, J.L. Elastic properties of dental resin restorative materials. J. Dent. Res. 1974, 53, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.W.; Rizkalla, A.S. Characterization of experimental composite biomaterials. J. Biomed. Mater. Res. 1996, 33, 89–100. [Google Scholar] [CrossRef]
- Mahoney, E.; Holt, A.; Swain, M.; Kilpatrick, N. The hardness and modulus of elasticity of primary molar teeth:an ultra-micro-indentation study. J. Dent. 2000, 28, 589–594. [Google Scholar] [CrossRef]
- Zafar, M.S. A Comparison of dental restorative materials and mineralized dental tissues for surface nanomechanical properties. Life Sci. J. 2014, 11, 19–24. [Google Scholar]
- Naghipur, S.; Pesun, I.; Nowakowski, A.; Kim, A. Twelve-year survival of 2-surface composite resin and amalgam premolar restorations placed by dental students. J. Prosthet. Dent. 2016, 116, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Opdam, N.J.M.; Bronkhorst, E.M.; Roeters, J.M.; Loomans, B.A.C. A retrospective clinical study on longevity of posterior composite and amalgam restorations. Dent. Mater. 2007, 23, 2–8. [Google Scholar] [CrossRef]
- Magne, P.; Douglas, W.H. Rationalization of esthetic restorative dentistry based on biomimetics. J. Esthet. Restor. Dent. 1999, 11, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Naasan, M.A.; Watson, T.F. Conventional glass ionomers as posterior restorations. A status report for the American Journal of Dentistry. Am. J. Dent. 1998, 11, 36–45. [Google Scholar] [PubMed]
- Ilie, N.; Hickel, R.; Valceanu, A.S.; Huth, K.C. Fracture toughness of dental restorative materials. Clin. Oral Investig. 2012, 16, 489–498. [Google Scholar] [CrossRef]
- Zafar, M.S. Effects of surface pre-reacted glass particles on fluoride release of dental restorative materials. World Appl. Sci. J. 2013, 28, 457–462. [Google Scholar]
- Glasspoole, E.A.; Erickson, R.L.; Davidson, C.L. Effect of surface treatments on the bond strength of glass ionomers to enamel. Dent. Mater. 2002, 18, 454–462. [Google Scholar] [CrossRef]
- Mount, G.J. Glass-ionomer cements—Past, present and future. Oper. Dent. 1994, 19, 82–90. [Google Scholar] [PubMed]
- Wiegand, A.; Buchalla, W.; Attin, T. Review on fluoride-releasing restorative materials—Fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent. Mater. 2007, 23, 343–362. [Google Scholar] [CrossRef]
- Pereira, L.C.G.; Nunes, M.C.P.; Dibb, R.G.P.; Powers, J.M.; Roulet, J.; de Lima Navarro, M.F. Mechanical properties and bond strength of glass-ionomer cements. J. Adhes. Dent. 2002, 4, 73–80. [Google Scholar]
- Zimehl, R.; Hannig, M. Non metallic restorative materials based on glass ionomer cements—Recent trends and developments. Colloids Surf. Physicochem. Eng. Asp. 2000, 163, 55–62. [Google Scholar] [CrossRef]
- Bonifácio, C.; Kleverlaan, C.J.; Raggio, D.P.; Werner, A.; de Carvalho, R.; van Amerongen, W. Physical-mechanical properties of glass ionomer cements indicated for atraumatic restorative treatment. Aust. Dent. J. 2009, 54, 233–237. [Google Scholar] [CrossRef]
- McCabe, J.F.; Walls, A. Applied Dental Materials; John Wiley and Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Svanberg, M.; Mjör, I.; Ørstavik, D. Mutans streptococci in plaque from margins of amalgam, composite, and glass-ionomer restorations. J. Dent. Res. 1990, 69, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Bahir, R.; Matalon, S.; Domb, A.J.; Weiss, E.I. Streptococcus mutans biofilm changes surface-topography of resin composites. Dent. Mater. 2008, 24, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Pedrini, D.; Gaetti-Jardim, E.J.; Vasconcelos, A.C.D. Retention of oral microorganisms on conventional and resin-modified glass-ionomer cements. Pesqui. Odontológica Bras. 2001, 15, 196–200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baran, G.; Boberick, K.; McCool, J. Fatigue of restorative materials. Critical reviews in oral biology and medicine: An official publication of the American association of oral biologists. Crit. Rev. Oral Biol. Med. 2001, 12, 350–360. [Google Scholar] [CrossRef]
- Kumar, N.; Zafar, M.S.; Dahri, W.M.; Khan, M.A.; Khurshid, Z.; Najeeb, S. Effects of deformation rate variation on biaxial flexural properties of dental resin composites. J. Taibah Univ. Med Sci. 2018, 13, 319–326. [Google Scholar] [CrossRef]
- Sabbagh, J.; Vreven, J.; Leloup, G. Dynamic and static moduli of elasticity of resin-based materials. Dent. Mater. 2002, 18, 64–71. [Google Scholar] [CrossRef]
- Satish, G.; Nainan, M.T. Invitro evaluation of flexural strength and flexural modulus of elasticity of different composite restoratives. J. Conserv. Dent. 2006, 9, 140. [Google Scholar] [CrossRef]
- Masouras, K.; Silikas, N.; Watts, D.C. Correlation of filler content and elastic properties of resin-composites. Dent. Mater. 2008, 24, 932–939. [Google Scholar] [CrossRef]
- Leprince, J.G.; Palin, W.M.; Vanacker, J.; Sabbagh, J.; Devaux, J.; Leloup, G. Physico-mechanical characteristics of commercially available bulk-fill composites. J. Dent. 2014, 42, 993–1000. [Google Scholar] [CrossRef]
- Gomes, T.; Carvalho, E.M.; Costa, J.F.; Grande, R.H.M.; Bauer, J. Effect of manipulation surface on the mechanical properties and fluoride release of resin-modified GIC. Rev. Port. Estomatol. Med. Dentária Cir. Maxilofac. 2016, 57, 132–137. [Google Scholar] [CrossRef]
- Helvatjoglu-Antoniades, M.; Papadogiannis, Y.; Lakes, R.; Palaghias, G.; Papadogiannis, D. The effect of temperature on viscoelastic properties of glass ionomer cements and compomers. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 460–467. [Google Scholar] [CrossRef]
- Zoergiebel, J.; Ilie, N. Evaluation of a conventional glass ionomer cement with new zinc formulation: Effect of coating, aging and storage agents. Clin. Oral Investig. 2013, 17, 619–626. [Google Scholar] [CrossRef]
- Ilie, N.; Hickel, R. Mechanical behavior of glass ionomer cements as a function of loading condition and mixing procedure. Dent. Mater. J. 2007, 26, 526–533. [Google Scholar] [CrossRef][Green Version]
- Magni, E.; Ferrari, M.; Hickel, R.; Ilie, N. Evaluation of the mechanical properties of dental adhesives and glass-ionomer cements. Clin. Oral Investig. 2010, 14, 79–87. [Google Scholar] [CrossRef]
- Isgrò, G.; Rodi, D.; Sachs, A.; Hashimoto, M. Modulus of elasticity of two ceramic materials and stress-inducing mechanical deformation following fabrication techniques and adhesive cementation procedures of a dental ceramic. Int. J. Biomater. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Rizkalla, A.S.; Jones, D.W. Mechanical properties of commercial high strength ceramic core materials. Dent. Mater. 2004, 20, 207–212. [Google Scholar] [CrossRef]
- Elsaka, S.E.; Elnaghy, A.M. Mechanical properties of zirconia reinforced lithium silicate glass-ceramic. Dent. Mater. 2016, 32, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Bosquiroli, V.; Brandt, W.C.; Boaro, L.C.; Silva, I.D.; Sinhoreti, M.A. Influence of the composition of different resin composites in the Knoop hardness and bond strength between tooth/restoration. Appl. Adhes. Sci. 2014, 2, 14. [Google Scholar] [CrossRef]
- Stockton, L.; Williams, P.; Attallah, C. The effect of prolonged packing on the surface hardness of posterior composites. Oper. Dent. 2002, 27, 266–270. [Google Scholar]
- Galvao, M.R.; Caldas, S.G.; Bagnato, V.S.; de Souza Rastelli, A.N.; de Andrade, M.F. Evaluation of degree of conversion and hardness of dental composites photo-activated with different light guide tips. Eur. J. Dent. 2013, 7, 86–93. [Google Scholar]
- Ali, S.; Sangi, L.; Kumar, N.; Kumar, B.; Khurshid, Z.; Zafar, M.S. Evaluating antibacterial and surface mechanical properties of chitosan modified dental resin composites. Technol. Health Care 2019, 28, 1–9. [Google Scholar] [CrossRef]
- Brito, C.R.; Velasco, L.G.; Bonini, G.A.; Imparato, J.C.P.; Raggio, D.P. Glass ionomer cement hardness after different materials for surface protection. J. Biomed. Mater. Res. Part A 2010, 93, 243–246. [Google Scholar] [CrossRef]
- Buldur, M.; Karaarslan, E.S. Microhardness of glass carbomer and high-viscous glass Ionomer cement in different thickness and thermo-light curing durations after thermocycling aging. BMC Oral Health 2019, 19, 273. [Google Scholar] [CrossRef]
- Silva, R.; Zuanon, A.C.C.; Esberard, R.; Candido, M.; Machado, J. In vitro microhardness of glass ionomer cements. J. Mater. Sci. Mater. Med. 2007, 18, 139–142. [Google Scholar] [CrossRef]
- Žmak, I.; Ćorić, D.; Mandic, V.; Ćurković, L. Hardness and indentation fracture toughness of slip cast alumina and alumina-zirconia ceramics. Materials 2020, 13, 122. [Google Scholar]
- Alamoush, R.A.; Silikas, N.; Salim, N.A.; Al-Nasrawi, S.; Satterthwaite, J.D. Effect of the composition of CAD/CAM composite blocks on mechanical properties. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Seghi, R.; Denry, I.; Rosenstiel, S. Relative fracture toughness and hardness of new dental ceramics. J. Prosthet. Dent. 1995, 74, 145–150. [Google Scholar] [CrossRef]
- Burke, F.; Lucarotti, P. The ultimate guide to restoration longevity in England and Wales. Part 3: Glass ionomer restorations—Time to next intervention and to extraction of the restored tooth. Br. Dent. J. 2018, 224, 865–874. [Google Scholar] [CrossRef]
- Layton, D.; Walton, T. An up to 16-year prospective study of 304 porcelain veneers. Int. J. Prosthodont. 2007, 20, 389. [Google Scholar] [PubMed]
- Fradeani, M.; Redemagni, M.; Corrado, M. Porcelain laminate veneers: 6-to 12-year clinical evaluation—A retrospective study. Int. J. Periodontics Restor. Dent. 2005, 25, 9–17. [Google Scholar]
- Stoll, R.; Cappel, I.; Jablonski-Momeni, A.; Pieper, K.; Stachniss, V. Survival of inlays and partial crowns made of IPS empress after a 10-year observation period and in relation to various treatment parameters. Oper. Dent. 2007, 32, 556–563. [Google Scholar] [CrossRef]
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef]
- Gaengler, P.; Hoyer, I.; Montag, R. Clinical evaluation of posterior composite restorations: The 10-year report. J. Adhes. Dent. 2001, 3, 185–194. [Google Scholar]
- Wilder Jr, A.D.; May Jr, K.N.; Bayne, S.C.; Taylor, D.F.; Leinfelder, K.F. Seventeen-year clinical study of ultraviolet-cured posterior composite Class I and II restorations. J. Esthet. Restor. Dent. 1999, 11, 135–142. [Google Scholar] [CrossRef]
- Piotrowski, B.T.; Gillette, W.B.; Hancock, E.B. Examining the prevalence and characteristics of abfractionlike cervical lesions in a population of U.S. veterans. J. Am. Dent. Assoc. 2001, 132, 1694–1701. [Google Scholar] [CrossRef] [PubMed]
- Beier, U.S.; Kapferer, I.; Burtscher, D.; Dumfahrt, H. Clinical performance of porcelain laminate veneers for up to 20 years. Int. J. Prosthodont. 2012, 25, 79–85. [Google Scholar] [PubMed]
- D′Arcangelo, C.; De Angelis, F.; Vadini, M.; D′Amario, M. Clinical evaluation on porcelain laminate veneers bonded with light-cured composite: Results up to 7 years. Clin. Oral Investig. 2012, 16, 1071–1079. [Google Scholar] [CrossRef]
- Edelhoff, D.; Sorensen, J.A. Tooth structure removal associated with various preparation designs for anterior teeth. J. Prosthet. Dent. 2002, 87, 503–509. [Google Scholar] [CrossRef]
- Goldberg, M. In vitro and in vivo studies on the toxicity of dental resin components: A review. Clin. Oral Investig. 2008, 12, 1–8. [Google Scholar] [CrossRef]
- Gao, W.; Smales, R. Fluoride release/uptake of conventional and resin-modified glass ionomers, and compomers. J. Dent. 2001, 29, 301–306. [Google Scholar] [CrossRef]
- Stanley, H.R. Pulp capping: Conserving the dental pulp—Can it be done? Is it worth it? Oral Surg. Oral Med. Oral Pathol. 1989, 68, 628–639. [Google Scholar] [CrossRef]
- Matinlinna, J.; Vallittu, P. Bonding of resin composites to etchable ceramic surfaces–an insight review of the chemical aspects on surface conditioning. J. Oral Rehabil. 2007, 34, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.; Matinlinna, J. Insights on porcelain as a dental material. Part II: Chemical surface treatments. Silicon 2011, 3, 117–123. [Google Scholar] [CrossRef]
- Roulet, J.; Söderholm, K.; Longmate, J. Effects of treatment and storage conditions on ceramic/composite bond strength. J. Dent. Res. 1995, 74, 381–387. [Google Scholar] [CrossRef]
- Featherstone, J.D. Dental caries: A dynamic disease process. Aust. Dent. J. 2008, 53, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sun, Z.; Moradian-Oldak, J. Effect of fluoride on the morphology of calcium phosphate crystals grown on acid-etched human enamel. Caries Res. 2009, 43, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Yun, S.; Fang, J.; Chen, H. Chemical regeneration of human tooth enamel under near-physiological conditions. Chem. Commun. 2009, 39, 5892–5894. [Google Scholar] [CrossRef]
- Fowler, C.E.; Li, M.; Mann, S.; Margolis, H.C. Influence of surfactantassembly on the formation of calcium phosphate materials—A model for dental enamel formation. J. Mater. Chem. 2005, 15, 3317–3325. [Google Scholar] [CrossRef]
- Ye, W.; Wang, X. Ribbon-like and rod-like hydroxyapatite crystals deposited on titanium surface with electrochemical method. Mater. Lett. 2007, 61, 4062–4065. [Google Scholar] [CrossRef]
- Chen, H.; Tang, Z.; Liu, J.; Sun, K.; Chang, S.; Peters, M.C.; Mansfield, J.F.; Czajka-Jakubowska, A.; Clarkson, B.H. Acellular synthesis of a human enamel-like microstructure. Adv. Mater. 2006, 18, 1846–1851. [Google Scholar] [CrossRef]
- Yamagishi, K.; Onuma, K.; Suzuki, T.; Okada, F.; Tagami, J.; Otsuki, M.; Senawangse, P. A synthetic enamel for rapid tooth repair. Nature 2005, 433, 819. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.Y.; Mei, M.L.; Li, Q.; Lo, E.C.M.; Chu, C.H. Methods for biomimetic mineralisation of human enamel: A systematic review. Materials 2015, 8, 2873–2886. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Habelitz, S.; Kinney, J.H.; Marshall, S.J.; Marshall, G.W.J. Biomechanical perspective on the remineralization of dentin. Caries Res. 2009, 43, 70–77. [Google Scholar] [CrossRef]
- Cao, C.Y.; Mei, M.L.; Li, Q.; Lo, E.C.M.; Chu, C.H. Methods for biomimetic remineralization of human dentine: A systematic review. Int. J. Mol. Sci. 2015, 16, 4615–4627. [Google Scholar] [CrossRef]
- Tabassum, S.; Khan, F.R. Failure of endodontic treatment: The usual suspects. Eur. J. Dent. 2016, 10, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Jafarzadeh, H.; Shalavi, S.; Yaripour, S.; Sharifi, F.; Kinoshita, J.I. A review on triple antibiotic paste as a suitable material used in regenerative endodontics. Iran. Endod. J. 2018, 13, 1–6. [Google Scholar]
- Diogenes, A.; Henry, M.A.; Teixeira, F.B.; Hargreaves, K.M. An update on clinical regenerative endodontics. Endod. Top. 2013, 28, 2–23. [Google Scholar] [CrossRef]
- Kandaswamy, D.; Venkateshbabu, N. Root canal irrigants. J. Conserv. Dent. 2010, 13, 256–264. [Google Scholar] [CrossRef]
- Dioguardi, M.; Di Gioia, G.; Illuzzi, G.; Laneve, E.; Cocco, A.; Troiano, G. Endodontic irrigants: Different methods to improve efficacy and related problems. Eur. J. Dent. 2018, 12, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Scott II, M.B.; Zilinski, G.S.; Kirkpatrick, T.C.; Himel, V.T.; Sabey, K.A.; Lallier, T.E. The effects of irrigants on the survival of human stem cells of the apical papilla, including Endocyn. J. Endod. 2018, 44, 263–268. [Google Scholar] [CrossRef]
- Senia, E.S.; Marshall, F.J.; Rosen, S. The solvent action of sodium hypochlorite on pulp tissue of extracted teeth. Oral Surg. Oral Med. Oral Pathol. 1971, 31, 96–103. [Google Scholar] [CrossRef]
- Yu, C.; Abbott, P.V. An overview of the dental pulp: Its functions and responses to injury. Aust. Dent. J. 2007, 52, S4–S6. [Google Scholar] [CrossRef]
- Trevino, E.G.; Patwardhan, A.N.; Henry, M.A.; Perry, G.; Dybdal-Hargreaves, N.; Hargreaves, K.M.; Diogenes, A. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J. Endod. 2011, 37, 1109–1115. [Google Scholar] [CrossRef]
- Martin, D.E.; de Almeida, J.F.A.; Henry, M.A.; Khaing, Z.Z.; Schmidt, C.E.; Teixeira, F.B.; Diogenes, A. Concentration-dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. J. Endod. 2014, 40, 51–55. [Google Scholar] [CrossRef]
- Galler, K.M.; Buchalla, W.; Hiller, K.; Federlin, M.; Eidt, A.; Schiefersteiner, M.; Schmalz, G. Influence of root canal disinfectants on growth factor release from dentin. J. Endod. 2015, 41, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Widbiller, M.; Althumairy, R.I.; Diogenes, A. Direct and indirect effect of chlorhexidine on survival of stem cells from the apical papilla and its neutralization. J. Endod. 2019, 45, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Jain, A. Overview on the current antibiotic containing agents used in endodontics. N. Am. J. Med. Sci. 2014, 6, 351–358. [Google Scholar] [PubMed]
- Hegde, J. Endodontics: Prep. Manual for Undergraduates; Elsevier India: Chennai, India, 2009. [Google Scholar]
- Althumairy, R.I.; Teixeira, F.B.; Diogenes, A. Effect of dentin conditioning with intracanal medicaments on survival of stem cells of apical papilla. J. Endod. 2014, 40, 521–525. [Google Scholar] [CrossRef]
- Madarati, A.A.; Zafar, M.S.; Sammani, A.M.; Mandorah, A.O.; Bani-Younes, H.A. Preference and usage of intracanal medications during endodontic treatment. Saudi Med. J. 2017, 38, 755–763. [Google Scholar] [CrossRef]
- Rizvi, A.; Zafar, M.S.; Farid, W.M.; Gazal, G. Assessment of antimicrobial efficacy of MTAD, sodium hypochlorite, EDTA and chlorhexidine for endodontic applications: An in vitro study. Middle E J. Sci. Res. 2014, 21, 353–357. [Google Scholar]
- Byström, A.; Sundqvist, G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Eur. J. Oral Sci. 1981, 89, 321–328. [Google Scholar] [CrossRef]
- Ruparel, N.B.; Teixeira, F.B.; Ferraz, C.C.; Diogenes, A. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J. Endod. 2012, 38, 1372–1375. [Google Scholar] [CrossRef]
- Alghilan, M.; Windsor, L.J.; Palasuk, J.; Yassen, G.H. Attachment and proliferation of dental pulp stem cells on dentine treated with different regenerative endodontic protocols. Int. Endod. J. 2017, 50, 667–675. [Google Scholar] [CrossRef]
- Ardeshna, S.; Qualtrough, A.; Worthington, H. An in vitro comparison of pH changes in root dentine following canal dressing with calcium hydroxide points and a conventional calcium hydroxide paste. Int. Endod. J. 2002, 35, 239–244. [Google Scholar] [CrossRef]
- Kaval, M.E.; Güneri, P.; Çalışkan, M.K. Regenerative endodontic treatment of perforated internal root resorption: A case report. Int. Endod. J. 2018, 51, 128–137. [Google Scholar] [CrossRef]
- Pacios, M.G.; de la Casa, M.L.; de los Ángeles Bulacio, M.; López, M.E. Influence of different vehicles on the pH of calcium hydroxide pastes. J. Oral Sci. 2004, 46, 107–111. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Dummer, P.M.H. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int. Endod. J. 2011, 44, 697–730. [Google Scholar] [CrossRef]
- Siqueira Jr, J.; Lopes, H. Mechanisms of antimicrobial activity of calcium hydroxide: A critical review. Int. Endod. J. 1999, 32, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Neelakantan, P. Antiseptics and antibiotics used in regenerative endodontics. Int. J. Pharm. Clin. Res. 2013, 5, 141–144. [Google Scholar]
- Bakkal, M.; Kaya, M.S. Success rate of using metronidazole paste in pulpal treatment of infected deciduous molars: 6–30 months follow up. J. Dent. Spec. 2018, 6, 115–120. [Google Scholar] [CrossRef]
- Lin, J.; Zeng, Q.; Wei, X.; Zhao, W.; Cui, M.; Gu, J.; Lu, J.; Yang, M.; Ling, J. Regenerative endodontics versus apexification in immature permanent teeth with apical periodontitis: A prospective randomized controlled study. J. Endod. 2017, 43, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Kahler, S.L.; Kahler, B. Alkaline material effects on roots of teeth. Materials 2017, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
- Nerness, A.; Ehrlich, Y.; Spolnik, K.; Platt, J.; Yassen, G. Effect of triple antibiotic paste with or without ethylenediaminetetraacetic acid on surface loss and surface roughness of radicular dentine. Odontology 2016, 104, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Banchs, F.; Trope, M. Revascularization of immature permanent teeth with apical periodontitis: New treatment protocol? J. Endod. 2004, 30, 196–200. [Google Scholar] [CrossRef]
- Rödig, T.; Vogel, S.; Zapf, A.; Hülsmann, M. Efficacy of different irrigants in the removal of calcium hydroxide from root canals. Int. Endod. J. 2010, 43, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Margelos, J.; Eliades, G.; Verdelis, C.; Palaghias, G. Interaction of calcium hydroxide with zinc oxide-eugenol type sealers: A potential clinical problem. J. Endod. 1997, 23, 43–48. [Google Scholar] [CrossRef]
- Adefurin, A.; Sammons, H.; Jacqz-Aigrain, E.; Choonara, I. Ciprofloxacin safety in paediatrics: A systematic review. Arch. Dis. Child. 2011, 96, 874–880. [Google Scholar] [CrossRef]
- Ramu, C.; Padmanabhan, T. Indications of antibiotic prophylaxis in dental practice—A review. Asian Pac. J. Trop. Biomed. 2012, 2, 749–754. [Google Scholar] [CrossRef]
- Kubin, R. Safety and efficacy of ciprofloxacin in paediatric patients. Infection 1993, 21, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Windley III, W.; Teixeira, F.; Levin, L.; Sigurdsson, A.; Trope, M. Disinfection of immature teeth with a triple antibiotic paste. J. Endod. 2005, 31, 439–443. [Google Scholar] [CrossRef]
- Krittika, R.; CRRI. Cytotoxicity of root canal disinfecting agents against stem cells of apical papilla—A review. J. Pharm. Sci. Res. 2017, 9, 2052–2055. [Google Scholar]
- Peng, C.; Zhao, Y.; Wang, W.; Yang, Y.; Qin, M.; Ge, L. Histologic findings of a human immature revascularized/regenerated tooth with symptomatic irreversible pulpitis. J. Endod. 2017, 43, 905–909. [Google Scholar] [CrossRef]
- Niwa, T.; Yamakoshi, Y.; Yamazaki, H.; Karakida, T.; Chiba, R.; Hu, J.C.; Nagano, T.; Yamamoto, R.; Simmer, J.P.; Margolis, H.C. The dynamics of TGF-β in dental pulp, odontoblasts and dentin. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Ivica, A.; Zehnder, M.; Mateos, J.M.; Ghayor, C.; Weber, F.E. Biomimetic conditioning of human dentin using citric acid. J. Endod. 2019, 45, 45–50. [Google Scholar] [CrossRef]
- Alyas, S.M.; Fischer, B.I.; Ehrlich, Y.; Spolnik, K.; Gregory, R.L.; Yassen, G.H. Direct and indirect antibacterial effects of various concentrations of triple antibiotic pastes loaded in a methylcellulose system. J. Oral Sci. 2016, 58, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Labban, N.; Yassen, G.H.; Windsor, L.J.; Platt, J.A. The direct cytotoxic effects of medicaments used in endodontic regeneration on human dental pulp cells. Dent. Traumatol. 2014, 30, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Latham, J.; Fong, H.; Jewett, A.; Johnson, J.D.; Paranjpe, A. Disinfection efficacy of current regenerative endodontic protocols in simulated necrotic immature permanent teeth. J. Endod. 2016, 42, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- American Association of Endodontists AAE Clinical Considerations for a Regenerative Procedure. Available online: http://www.aae.org/specialty/wp-content/uploads/sites/2/2017/06/currentregenerativeendodonticconsiderations.pdf (accessed on 27 June 2020).
- Brave, A.D. A review of bioceramic technology in endodontics. CE Article 2012, 4, 6–12. [Google Scholar]
- Koch, K.; Brave, D. A new day has dawned: The increased use of bioceramics in endodontics. Dent. April 2009, 10, 39–43. [Google Scholar]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive glass applications in dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Montesin, F.E.; Brady, K.; Sweeney, R.; Curtis, R.V.; Ford, T.R.P. The constitution of mineral trioxide aggregate. Dent. Mater. 2005, 21, 297–303. [Google Scholar] [CrossRef]
- Torabinejad, M.; Chivian, N. Clinical applications of mineral trioxide aggregate. J. Endod. 1999, 25, 197–205. [Google Scholar] [CrossRef]
- Perez, A.; Spears, R.; Gutmann, J.; Opperman, L. Osteoblasts and MG-63 osteosarcoma cells behave differently when in contact with ProRootTM MTA and white MTA. Int. Endod. J. 2003, 36, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Mante, F.K.; Romanow, W.J.; Kim, S. Chemical analysis of powder and set forms of Portland cement, gray ProRoot MTA, white ProRoot MTA, and gray MTA-Angelus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, 809–815. [Google Scholar] [CrossRef]
- Kogan, P.; He, J.; Glickman, G.N.; Watanabe, I. The effects of various additives on setting properties of MTA. J. Endod. 2006, 32, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.R. Considerations in the selection of a root-end filling material. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1999, 87, 398–404. [Google Scholar] [CrossRef]
- Bodrumlu, E. Biocompatibility of retrograde root filling materials: A review. Aust. Endod. J. 2008, 34, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Filho, J.E.; Rodrigues, G.; Watanabe, S.; Bernabé, P.F.E.; Lodi, C.S.; Gomes, A.C.; Faria, M.D.; dos Santos, A.D.; Moraes, J.C.S. Evaluation of the tissue reaction to fast endodontic cement (CER) and Angelus MTA. J. Endod. 2009, 35, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Modification of mineral trioxide aggregate. Physical and mechanical properties. Int. Endod. J. 2008, 41, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Shahabi, S.; Jafarzadeh, T.; Amini, S.; Kheirieh, S. The properties of a new endodontic material. J. Endod. 2008, 34, 990–993. [Google Scholar] [CrossRef]
- Laurent, P.; Camps, J.; De Méo, M.; Déjou, J.; About, I. Induction of specific cell responses to a Ca3SiO5-based posterior restorative material. Dent. Mater. 2008, 24, 1486–1494. [Google Scholar] [CrossRef]
- Koh, E.T.; McDonald, F.; Pitt Ford, T.R.; Torabinejad, M. Cellular response to mineral trioxide aggregate. J. Endod. 1998, 24, 543–547. [Google Scholar] [CrossRef]
- Vajrabhaya, L.; Korsuwannawong, S.; Jantarat, J.; Korre, S. Biocompatibility of furcal perforation repair material using cell culture technique: Ketac molar versus ProRoot MTA. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, e48–e50. [Google Scholar] [CrossRef]
- Takita, T.; Hayashi, M.; Takeichi, O.; Ogiso, B.; Suzuki, N.; Otsuka, K.; Ito, K. Effect of mineral trioxide aggregate on proliferation of cultured human dental pulp cells. Int. Endod. J. 2006, 39, 415–422. [Google Scholar] [CrossRef]
- Laurent, P.; Camps, J.; About, I. BiodentineTM induces TGF-B1 release from human pulp cells and early dental pulp mineralization. Int. Endod. J. 2012, 45, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, T.; Gençoğlu, N.; Firat, I.; Perk, C.; Guzel, O. Histologic study of furcation perforations treated with MTA or Super EBA in dogs’ teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 100, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Al-Daafas, A.; Al-Nazhan, S. Histological evaluation of contaminated furcal perforation in dogs’ teeth repaired by MTA with or without internal matrix. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 103, e92. [Google Scholar] [CrossRef]
- Chong, B.; Pitt Ford, T.; Hudson, M. A prospective clinical study of mineral Trioxide aggregate and IRM when used as root—End filling materials in endodontic surgery. Int. Endod. J. 2003, 36, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Bezgin, T.; Yilmaz, A.D.; Celik, B.N.; Kolsuz, M.E.; Sonmez, H. Efficacy of platelet-rich plasma as a scaffold in regenerative endodontic treatment. J. Endod. 2015, 41, 36–44. [Google Scholar] [CrossRef]
- Mullaguri, H.; Suresh, N.; Surendran, S.; Velmurugan, N.; Chitra, S. Role of pH changes on transforming growth factor-β1 release and on the fibrin architecture of platelet-rich Fibrin when layered with biodentine, glass ionomer cement, and intermediate restorative material. J. Endod. 2016, 42, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Vallés, M.; Roig, M.; Duran-Sindreu, F.; Martínez, S.; Mercadé, M. Color stability of teeth restored with biodentine: A 6-month in vitro study. J. Endod. 2015, 41, 1157–1160. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Nör, J. Buonocore memorial lecture: Tooth regeneration in operative dentistry. Oper. Dent. 2006, 31, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Ikada, Y. Challanges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef]
- Kemppainen, J.M.; Hollister, S.J. Tailoring the mechanical properties of 3D-designed poly (glycerol sebacate) scaffolds for cartilage applications. J. Biomed. Mater. Res. Part A 2010, 94, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Bowlin, G. Enhanced porosity without compromising structural integrity: The nemesis of electrospun scaffolding. J. Tissue Sci. Eng. 2011, 2, 103e. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Kadler, K. Matrix loading: Assembly of extracellular matrix collagen fibrils during embryogenesis. Birth Defects Res. Part C Embryo Today Rev. 2004, 72, 1–11. [Google Scholar] [CrossRef]
- Elsdale, T.; Bard, J. Collagen substrata for studies on cell behavior. J. Cell Biol. 1972, 54, 626–637. [Google Scholar] [CrossRef]
- Kadler, K.E.; Holmes, D.F.; Trotter, J.A.; Chapman, J.A. Collagen fibril formation. Biochem. J. 1996, 316, 1–11. [Google Scholar] [CrossRef]
- Yu, J.; Xia, H.; Ni, Q. A three-dimensional porous hydroxyapatite nanocomposite scaffold with shape memory effect for bone tissue engineering. J. Mater. Sci. 2018, 53, 4734–4744. [Google Scholar] [CrossRef]
- Jaschouz, S.; Mehl, A. Reproducibility of habitual intercuspation in vivo. J. Dent. 2014, 42, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Bouët, G.; Marchat, D.; Cruel, M.; Malaval, L.; Vico, L. In vitro three-dimensional bone tissue models: From cells to controlled and dynamic environment. Tissue Eng. Part B Rev. 2015, 21, 133–156. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.; Schuetz, M.A.; Hutmacher, D.W. Bone regeneration based on tissue engineering conceptions—A 21st century perspective. Bone Res. 2013, 1, 216. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, S.; Laurencin, C.; Deng, M. Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; Volume 17. [Google Scholar]
- Qasim, S.S.B.; Zafar, M.S.; Niazi, F.H.; Alshahwan, M.; KS, H.A.; Daood, U. Functionally graded biomimetic biomaterials in dentistry: An evidence-based update. J. Biomater. Sci. Polym. Ed. 2020, 31, 1–20. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Husain, S.; Alotaibi, H.; Rehman, R.; Zafar, M.S.; Farooq, I.; Khan, A.S. Chapter 18—Novel Techniques of Scaffold Fabrication for Bioactive Glasses. In Biomedical Therapeutic and Clinical Applications of Bioactive Glasses, 1st ed.; Elsevier: Lodon, UK, 2019; pp. 497–519. [Google Scholar]
- Fu, Q.; Rahaman, M.N.; Fu, H.; Liu, X. Silicate, borosilicate, and borate bioactive glass scaffolds with controllable degradation rate for bone tissue engineering applications. I. Preparation and in vitro degradation. J. Biomed. Mater. Res. Part A 2010, 95, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Garcia-Fuentes, M. Bioactive Scaffolds for The Controlled Formation of Complex Skeletal Tissues. Regenerative Medicine and Tissue Engineering: Cells and Biomaterials; Eberli, D., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 393–432. [Google Scholar]
- Ge, Z.; Jin, Z.; Cao, T. Manufacture of degradable polymeric scaffolds for bone regeneration. Biomed. Mater. 2008, 3, 022001. [Google Scholar] [CrossRef] [PubMed]
- Nigam, R.; Mahanta, B. An overview of various biomimetic scaffolds: Challenges and applications in tissue engineering. J. Tissue Sci. Eng. 2014, 5, 1. [Google Scholar]
- Ige, O.O.; Umoru, L.E.; Aribo, S. Natural products: A minefield of biomaterials. ISRN Mater. Sci. 2012, 2012. [Google Scholar] [CrossRef]
- Schmidt, C.E.; Baier, J.M. Acellular vascular tissues: Natural biomaterials for tissue repair and tissue engineering. Biomaterials 2000, 21, 2215–2231. [Google Scholar] [CrossRef]
- Rios, H.F.; Lin, Z.; Oh, B.; Park, C.H.; Giannobile, W.V. Cell- and gene-based therapeutic strategies for periodontal regenerative medicine. J. Periodontol. 2011, 82, 1223–1237. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, M.; Liu, N.; Lv, X.; Zhang, J.; Chen, F.; Chen, Y. The combined use of cell sheet fragments of periodontal ligament stem cells and platelet-rich fibrin granules for avulsed tooth reimplantation. Biomaterials 2013, 34, 5506–5520. [Google Scholar] [CrossRef]
- Femminella, B.; Iaconi, M.C.; Di Tullio, M.; Romano, L.; Sinjari, B.; D’Arcangelo, C.; De Ninis, P.; Paolantonio, M. Clinical comparison of platelet-rich fibrin and a gelatin sponge in the management of palatal wounds after epithelialized free gingival graft harvest: A randomized clinical trial. J. Periodontol. 2016, 87, 103–113. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-rich fibrin and soft tissue wound healing: A systematic review. Tissue Eng. Part B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Khurshid, Z.; Agwan, M.A.S.; Ansari, S.A.; Zafar, M.S.; Matinlinna, J.P. Regenerative potential of platelet rich fibrin (PRF) for curing intrabony periodontal defects: A systematic review of clinical studies. Tissue Eng. Regen. Med. 2017, 14, 735–742. [Google Scholar] [CrossRef]
- Ajwani, H.; Shetty, S.; Gopalakrishnan, D.; Kathariya, R.; Kulloli, A.; Dolas, R.S.; Pradeep, A.R. Comparative evaluation of platelet-rich fibrin biomaterial and open flap debridement in the treatment of two and three wall intrabony defects. J. Int. Oral Health. 2015, 7, 32–37. [Google Scholar] [PubMed]
- Agarwal, A.; Gupta, N.D.; Jain, A. Platelet rich fibrin combined with decalcified freeze-dried bone allograft for the treatment of human intrabony periodontal defects: A randomized split mouth clinical trail. Acta Odontol. Scand. 2016, 74, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Bains, V.K.; Gupta, V.; Jhingran, R.; Singh, G.P. Evaluation of intrabony defects treated with platelet-rich fibrin or autogenous bone graft: A comparative analysis. Eur. J. Dent. 2015, 9, 100–108. [Google Scholar] [CrossRef]
- Shah, M.; Patel, J.; Dave, D.; Shah, S. Comparative evaluation of platelet-rich fibrin with demineralized freeze-dried bone allograft in periodontal infrabony defects: A randomized controlled clinical study. J. Indian. Soc. Periodontol. 2015, 19, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Bansal, C.; Bharti, V. Evaluation of efficacy of autologous platelet-rich fibrin with demineralized-freeze dried bone allograft in the treatment of periodontal intrabony defects. J. Indian. Soc. Periodontol. 2013, 17, 361–366. [Google Scholar] [PubMed]
- Cortese, A.; Pantaleo, G.; Borri, A.; Caggiano, M.; Amato, M. Platelet-rich fibrin (PRF) in implant dentistry in combination with new bone regenerative technique in elderly patients. Int. J. Surg. Case Rep. 2016, 28, 52–56. [Google Scholar] [CrossRef]
- Yeo, I.; Oh, J.; Jeong, L.; Lee, T.S.; Lee, S.J.; Park, W.H.; Min, B. Collagen-based biomimetic nanofibrous scaffolds: Preparation and characterization of collagen. Biomacromolecules 2008, 9, 1106–1116. [Google Scholar] [CrossRef]
- Asti, A.; Gioglio, L. Natural and synthetic biodegradable polymers: Different scaffolds for cell expansion and tissue formation. Int. J. Artif. Organs 2014, 37, 187–205. [Google Scholar]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Iafiscol, M.; Quirici, N.; Foltran, I.; Rimondini, L. Electrospun collagen mimicking the reconstituted extracellular matrix improves osteoblastic differentiation onto titanium surfaces. J. Nanosci. Nanotechnol. 2013, 13, 4720–4726. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Cheng, X.R.; Wang, J.W.; Xu, D.X.; Wang, G. Preparation and evaluation of porous chitosan/collagen scaffolds for periodontal tissue engineering. J. Bioact. Compat. Polym. 2006, 21, 207–220. [Google Scholar] [CrossRef]
- Lotfi, G.; Shokrgozar, M.A.; Mofid, R.; Abbas, F.M.; Ghanavati, F.; Baghban, A.A.; Yavari, S.K.; Pajoumshariati, S. Biological evaluation (in vitro and in vivo) of Bilayered collagenous coated (nano electrospun and solid wall) Chitosan membrane for periodontal guided bone regeneration. Ann. Biomed. Eng. 2016, 44, 2132–2144. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.R.; Lee, D.H.; Chung, P.; Yang, H. Distinct differentiation properties of human dental pulp cells on collagen, gelatin, and chitosan scaffolds. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, e94–e100. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Li, X.; Wen, P.; Zhang, Y.; Long, Y.; Wang, X.; Guo, Y.; Xing, F.; Gao, J. Preparation of aligned porous gelatin scaffolds by unidirectional freeze-drying method. Acta Biomater. 2010, 6, 1167–1177. [Google Scholar] [CrossRef]
- Peter, M.; Binulal, N.S.; Nair, S.V.; Selvamurugan, N.; Tamura, H.; Jayakumar, R. Novel biodegradable chitosan-gelatin/nano-bioactive glass ceramic composite scaffolds for alveolar bone tissue engineering. Chem. Eng. J. 2010, 158, 353–361. [Google Scholar] [CrossRef]
- Wang, J.; Yang, C.X.; Wan, Y.Z.; Luo, H.L.; He, F.; Dai, K.R.; Huang, Y. Laser patterning of bacterial cellulose hydrogel and its modification with gelatin and hydroxyapatite for bone tissue engineering. Soft Mater. 2013, 11, 173–180. [Google Scholar]
- Hunter, K.T.; Ma, T. In vitro evaluation of hydroxyapatite-chitosan-gelatin composite membrane in guided tissue regeneration. J. Biomed. Mater. Res. Part A 2013, 101, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhang, Z.; Zhou, Y.; Liu, Y.; Wang, Z.; Tong, H.; Shen, X.; Wang, Y. Surface functionalization of Titanium with Chitosan/Gelatin via electrophoretic deposition: Characterization and cell behavior. Biomacromolecules 2010, 11, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Konovalova, M.V.; Markov, P.A.; Durnev, E.A.; Kurek, D.V.; Popov, S.V.; Varlamov, V.P. Preparation and biocompatibility evaluation of pectin and chitosan cryogels for biomedical application. J. Biomed. Mater. Res. Part A 2017, 105, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.F.; Otte, A.D.; Gregory, R.L.; Pinal, R.; Ferreira-Zandona, A.; Bottino, M.C. Physicomechanical and antibacterial properties of experimental resin-based dental sealants modified with nylon-6 and chitosan nanofibers. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 103, 1560–1568. [Google Scholar] [CrossRef]
- Ali, S.; Sangi, L.; Kumar, N. Exploring antibacterial activity and hydrolytic stability of resin dental composite restorative materials containing chitosan. Technol. Health Care 2017, 25, 11–18. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Huang, Q.; Ghaffar, A.; Abid, M.A.; Zafar, M.S.; Khurshid, Z.; Latif, M. A Smart drug Delivery system based on biodegradable Chitosan/Poly (allylamine hydrochloride) blend films. Pharmaceutics 2020, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhai, J.; Cheng, S.; Wang, Y.; Yang, L.; Liu, H.; Ran, R. The application of chitosan and its derivatives as nanosized carriers for the delivery of chemical drugs and genes or proteins. Curr. Drug Targets 2015, 17. [Google Scholar] [CrossRef]
- Luo, Y.; Teng, Z.; Li, Y.; Wang, Q. Solid lipid nanoparticles for oral drug delivery: Chitosan coating improves stability, controlled delivery, mucoadhesion and cellular uptake. Carbohydr. Polym. 2015, 122, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Strandman, S.; Zhu, J.X.X.; Barralet, J.; Cerruti, M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-based solutions for tissue engineering and regenerative medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan biomaterials for current and potential dental applications. Materials 2017, 10, 602. [Google Scholar] [CrossRef]
- Qasim, S.B.; Najeeb, S.; Delaine-Smith, R.M.; Rawlinson, A.; Rehman, I.U. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent. Mater. 2017, 33, 71–83. [Google Scholar] [CrossRef]
- La Noce, M.; Paino, F.; Spina, A.; Naddeo, P.; Montella, R.; Desiderio, V.; De Rosa, A.; Papaccio, G.; Tirino, V.; Laino, L. Dental pulp stem cells: State of the art and suggestions for a true translation of research into therapy. J. Dent. 2014, 42, 761–768. [Google Scholar] [CrossRef]
- Chen, H.; Fan, M. Chitosan/carboxymethyl cellulose polyelectrolyte complex scaffolds for pulp cells regeneration. J. Bioact. Compat. Polym. 2007, 22, 475–491. [Google Scholar] [CrossRef]
- Liao, F.; Chen, Y.; Li, Z.; Wang, Y.; Shi, B.; Gong, Z.; Cheng, X. A novel bioactive three-dimensional β-tricalcium phosphate/chitosan scaffold for periodontal tissue engineering. J. Mater. Sci. Mater. Med. 2010, 21, 489–496. [Google Scholar] [CrossRef]
- Ravindran, S.; Song, Y.; George, A. Development of three-dimensional biomimetic scaffold to study epithelial-mesenchymal interactions. Tissue Eng. Part A 2010, 16, 327–342. [Google Scholar] [CrossRef]
- Chavanne, P.; Stevanovic, S.; Wüthrich, A.; Braissant, O.; Pieles, U.; Gruner, P.; Schumacher, R. 3D printed chitosan/hydroxyapatite scaffolds for potential use in regenera-tive medicine. Biomed. Tech. 2013, 58, 1. [Google Scholar]
- Liu, H.; Peng, H.; Wu, Y.; Zhang, C.; Cai, Y.; Xu, G.; Li, Q.; Chen, X.; Ji, J.; Zhang, Y. The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs. Biomaterials 2013, 34, 4404–4417. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, H.; Liu, X.; Mao, J.; Gu, S.; Xu, W. Electrospinning of carboxyethyl chitosan/poly (vinyl alcohol)/silk fibroin nanoparticles for wound dressings. Int. J. Biol. Macromol. 2013, 53, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, S.; Lai, G. Preparation and characterization of biomimetic silk fibroin/chitosan composite nanofibers by electrospinning for osteoblasts culture. Nanoscale Res. Lett. 2012, 7. [Google Scholar] [CrossRef]
- Deng, J.; She, R.; Huang, W.; Dong, Z.; Mo, G.; Liu, B. A silk fibroin/chitosan scaffold in combination with bone marrow-derived mesenchymal stem cells to repair cartilage defects in the rabbit knee. J. Mater. Sci. Mater. Med. 2013, 24, 2037–2046. [Google Scholar] [CrossRef]
- Yang, X.; Han, G.; Pang, X.; Fan, M. Chitosan/collagen scaffold containing bone morphogenetic protein-7 DNA supports dental pulp stem cell differentiation in vitro and in vivo. J. Biomed. Mater. Res. Part A 2012, 00A, 1–8. [Google Scholar] [CrossRef]
- Li, W.; Long, Y.; Liu, Y.; Long, K.; Liu, S.; Wang, Z.; Wang, Y.; Ren, L. Fabrication and characterization of chitosan–collagen crosslinked membranes for corneal tissue engineering. J. Biomater. Sci. Polym. Ed. 2014, 25, 1962–1972. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, R.; Ke, Q.; Morsi, Y.; Zhang, K.; Mo, X. Electrospun collagen–chitosan–TPU nanofibrous scaffolds for tissue engineered tubular grafts. Colloids Surf. B Biointerfaces 2011, 82, 307–315. [Google Scholar] [CrossRef]
- Soran, Z.; Aydin, R.S.; Gumusderelioglu, M. Chitosan scaffolds with BMP-6 loaded alginate microspheres for periodontal tissue engineering. J. Microencapsul. 2012, 29, 770–780. [Google Scholar] [CrossRef]
- Jeong, S.I.; Krebs, M.D.; Bonino, C.A.; Samorezov, J.E.; Khan, S.A.; Alsberg, E. Electrospun Chitosan-alginate nanofibers with in situ Polyelectrolyte complexation for use as tissue engineering scaffolds. Tissue Eng. Part A 2011, 17, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Miranda, D.G.; Malmonge, S.M.; Campos, D.M.; Attik, N.G.; Grosgogeat, B.; Gritsch, K. A chitosan-hyaluronic acid hydrogel scaffold for periodontal tissue engineering. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2015, 104, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, N.; Hanna, C.; Nair, S.V.; Nair, L.S. Enzymatically cross-linked alginic-hyaluronic acid composite hydrogels as cell delivery vehicles. Int. J. Biol. Macromol. 2013, 55, 289–294. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Gwon, K.; Kim, E.; Tae, G. Heparin-hyaluronic acid hydrogel in support of cellular activities of 3D encapsulated adipose derived stem cells. Acta Biomater. 2017, 49, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Ehrbar, M.; Djonov, V.G.; Schnell, C.; Tschanz, S.A.; Martiny-Baron, G.; Schenk, U.; Wood, J.; Burri, P.H.; Hubbell, J.A.; Zisch, A.H. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ. Res. 2004, 94, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Zarkoob, S.; Eby, R.K.; Reneker, D.H.; Hudson, S.D.; Ertley, D.; Adams, W.W. Structure and morphology of electrospun silk nanofibers. Polymer 2004, 45, 3973–3977. [Google Scholar] [CrossRef]
- Hardy, J.G.; Römer, L.M.; Scheibel, T.R. Polymeric materials based on silk proteins. Polymer 2008, 49, 4309–4327. [Google Scholar] [CrossRef]
- Shimura, K.; Kikuchi, A.; Ohtomo, K.; Katagata, Y.; Hyodo, A. Studies on silk fibroin of Bombyx mori. I. Fractionation of fibroin prepared from the posterior silk gland. J. Biochem. 1976, 80, 693–702. [Google Scholar] [CrossRef]
- Zafar, M.S.; Belton, D.J.; Hanby, B.; Kaplan, D.L.; Perry, C.C. Functional material features of Bombyx Mori silk light vs. heavy chain proteins. Biomacromolecules 2015, 16, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, W.; Xiang, R.; Zhuge, L.; Gao, W.; Wang, W. Formation of silk fibroin nanoparticles in water-miscible organic solvent and their characterization. J. Nanoparticle Res. 2007, 9, 885–900. [Google Scholar] [CrossRef]
- Kim, K.; Jeong, L.; Park, H.; Shin, S.; Park, W.; Lee, S.; Kim, T.; Park, Y.; Seol, Y.; Lee, Y.; et al. Biological efficacy of silk fibroin nanofiber membranes for guided bone regeneration. J. Biotechnol. 2005, 120, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, O.; Knight, D.P.; Vollrath, F.; Vadgama, P. Spider and mulberry silkworm silks as compatible biomaterials. Compos. Part B Eng. 2007, 38, 324–337. [Google Scholar] [CrossRef]
- Gosline, J.; Guerette, P.; Ortlepp, C.; Savage, K. The mechanical design of spider silks: From fibroin sequence to mechanical function. J. Exp. Biol. 1999, 202, 3295–3303. [Google Scholar] [PubMed]
- Sheu, H.; Phyu, K.W.; Jean, Y.; Chiang, Y.; Tso, I.; Wu, H.; Yang, J.; Ferng, S. Lattice deformation and thermal stability of crystals in spider silk. Int. J. Biol. Macromol. 2004, 34, 267–273. [Google Scholar] [CrossRef]
- Lammel, A.S.; Hu, X.; Park, S.; Kaplan, D.L.; Scheibel, T.R. Controlling silk fibroin particle features for drug delivery. Biomaterials 2010, 31, 4583–4591. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kapoor, S.; Kundu, S.C. Silk fibroin/polyacrylamide semi-interpenetrating network hydrogels for controlled drug release. Biomaterials 2009, 30, 2826–2836. [Google Scholar] [CrossRef]
- Wenk, E.; Wandrey, A.J.; Merkle, H.P.; Meinel, L. Silk fibroin spheres as a platform for controlled drug delivery. J. Control. Release 2008, 132, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, U.; Kim, H.S.; Li, C.; Wada, M.; Leisk, G.G.; Kaplan, D.L. Bone tissue engineering with premineralized silk scaffolds. Bone 2008, 42, 1226–1234. [Google Scholar] [CrossRef]
- Soffer, L.; Wang, X.; Mang, X.; Kluge, J.; Dorfmann, L.; Kaplan, D.L.; Leisk, G. Silk-based electrospun tubular scaffolds for tissue-engineered vascular grafts. J. Biomater. Sci. Polym. Ed. 2008, 19, 653–664. [Google Scholar] [CrossRef]
- Wang, Y.; Rudym, D.D.; Walsh, A.; Abrahamsen, L.; Kim, H.; Kim, H.S.; Kirker-Head, C.; Kaplan, D.L. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials 2008, 29, 3415–3428. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, W.; Asrican, R.; Kim, H.; Kaplan, D.L.; Yelick, P.C. Accurately shaped tooth bud cell-derived mineralized tissue formation on silk scaffolds. Tissue Eng. Part A 2008, 14, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Jindal, S.K.; Kiamehr, M.; Sun, W.; Yang, X.B. Silk Biomaterials for Tissue Engineering and Regenerative Medicine. In 15—Silk Scaffolds for Dental Tissue Engineering; Kundu, S.C., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 403–428. [Google Scholar]
- Zafar, M.S.; Al-Samadani, K.H. Potential use of natural silk for bio-dental applications. J. Taibah Univ. Med. Sci. 2014, 9, 171–177. [Google Scholar] [CrossRef]
- Kweon, H.; Lee, S.; Hahn, B.; Lee, Y.; Kim, S. Hydroxyapatite and silk combination-coated dental implants result in superior bone formation in the peri-implant area compared with hydroxyapatite and collagen combination-coated implants. J. Oral Maxillofac. Surg. 2014, 72, 1928–1936. [Google Scholar] [CrossRef]
- Zafar, M.S. Developing Silica Based Nanocomposite Materials for Dental Applications Using Bombyx Mori Silk Proteins. Ph.D. Thesis, Nottingham Trent University, Nottingham, UK, October 2011. [Google Scholar]
- Rodroiguez-Lozano, F.J.; Garcia-Bernal, D.; Aznar-Cervantes, S.; Ros-Roca, M.; AlguerÃ, M.C.; Atucha, N.M.; Lozano-GarcÃa, A.A.; Moraleda, J.M.; Cenis, J.L. Effects of composite films of silk fibroin and graphene oxide on the proliferation, cell viability and mesenchymal phenotype of periodontal ligament stem cells. J. Mater. Sci. Mater. Med. 2014, 25, 2731–2741. [Google Scholar] [CrossRef]
- Cai, Z.; Mo, X.; Zhang, K.; Fan, L.; Yin, A.; He, C.; Wang, H. Fabrication of chitosan/silk fibroin composite nanofibers for wound-dressing applications. Int. J. Mol. Sci. 2010, 11, 3529–3539. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Scheibel, T.R. Composite materials based on silk proteins. Prog. Polym. Sci. 2010, 35, 1093–1115. [Google Scholar] [CrossRef]
- Liu, X.; Holzwarth, J.M.; Ma, P.X. Functionalized Synthetic Biodegradable polymer scaffolds for tissue engineering. Macromol. Biosci. 2012, 12, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials: A review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011. [Google Scholar] [CrossRef]
- Matassi, F.; Nistri, L.; Chicon Paez, D.; Innocenti, M. New biomaterials for bone regeneration. Clin. Cases Miner. Bone Metab. 2011, 8, 21–24. [Google Scholar]
- Erol-Taygun, M.; Zheng, K.; Boccaccini, A.R. Nanoscale bioactive glasses in medical applications. Int. J. Appl. Glass Sci. 2013, 4, 136–148. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, L.; Liu, J.; Weir, M.D.; Zhou, X.; Xu, H.H. Bone tissue engineering via nanostructured calcium phosphate biomaterials and stem cells. Bone Res. 2014, 2, 14017. [Google Scholar] [CrossRef]
- Grigolato, R.; Pizzi, N.; Brotto, M.C.; Corrocher, G.; Desando, G.; Grigolo, B. Magnesium-enriched hydroxyapatite as bone filler in an ameloblastoma mandibular defect. Int. J. Clin. Exp. Med. 2015, 8, 281–288. [Google Scholar]
- Chou, D.; Wells, D.; Hong, D.; Lee, B.; Kuhn, H.; Kumta, P.N. Novel processing of iron-manganese alloy-based biomaterials by inkjet 3-D printing. Acta Biomater. 2013, 9, 8593–8603. [Google Scholar] [CrossRef]
- Zheng, Y.; Gu, X.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Zhuang, H.; Han, Y.; Feng, A. Preparation, mechanical properties and in vitro biodegradation of porous magnesium scaffolds. Mater. Sci. Eng. C 2008, 28, 1462–1466. [Google Scholar] [CrossRef]
- Vorndran, E.; Wunder, K.; Moseke, C.; Biermann, I.; Müller, F.A.; Zorn, K.; Gbureck, U. Hydraulic setting Mg3 (PO4) 2 powders for 3D printing technology. Adv. Appl. Ceram. 2011, 110, 476–481. [Google Scholar] [CrossRef]
- Gong, T.; Xie, J.; Liao, J.; Zhang, T.; Lin, S.; Lin, Y. Nanomaterials and bone regeneration. Bone Res. 2015, 3, 15029. [Google Scholar] [CrossRef] [PubMed]
- Marrella, A.; Tedeschi, G.; Giannoni, P.; Lagazzo, A.; Sbrana, F.; Barberis, F.; Quarto, R.; Puglisi, F.; Scaglione, S. “Green-reduced” graphene oxide induces in vitro an enhanced biomimetic mineralization of polycaprolactone electrospun meshes. Mater. Sci. Eng. C 2018, 93, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Zan, X.; Sitasuwan, P.; Feng, S.; Wang, Q. Effect of roughness on in situ biomineralized CaP-collagen coating on the osteogenesis of mesenchymal stem cells. Langmuir 2016, 32, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.L. Modifications of dental implant surfaces at the micro- and nano-level for enhanced osseointegration. Materials 2020, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Lim, C.H.Y.; Shi, H.; Tang, L.A.; Wang, Y.; Lim, C.T.; Loh, K.P. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 2011, 5, 7334–7341. [Google Scholar] [CrossRef]
- Cheng, X.; Wan, Q.; Pei, X. Graphene family materials in bone tissue regeneration: Perspectives and challenges. Nanoscale Res. Lett. 2018, 13, 289. [Google Scholar] [CrossRef] [PubMed]
- Prasadh, S.; Suresh, S.; Wong, R. Osteogenic potential of graphene in bone tissue engineering scaffolds. Materials 2018, 11, 1430. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef]
- Raslan, A.; del Burgo, L.S.; Ciriza, J.; Pedraz, J.L. Graphene oxide and reduced graphene oxide-based scaffolds in regenerative medicine. Int. J. Pharm. 2020, 119226. [Google Scholar] [CrossRef]
- Foroutan, T.; Nazemi, N.; Tavana, M.; Kassaee, M.Z.; Motamedi, E.; Sonieshargh, S.; Zare Zardini, H. Suspended graphene oxide nanoparticle for accelerated multilayer osteoblast attachment. J. Biomed. Mater. Res. Part. A 2018, 106, 293–303. [Google Scholar] [CrossRef]
- Pinto, A.M.; Goncalves, I.C.; Magalhaes, F.D. Graphene-based materials biocompatibility: A review. Colloids Surf. B Biointerfaces 2013, 111, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Kalisz, M.; Grobelny, M.; Mazur, M.; Zdrojek, M.; Wojcieszak, D.; Świniarski, M.; Judek, J.; Kaczmarek, D. Comparison of mechanical and corrosion properties of graphene monolayer on Ti–Al–V and nanometric Nb2O5 layer on Ti–Al–V alloy for dental implants applications. Thin Solid Film. 2015, 589, 356–363. [Google Scholar] [CrossRef]
- Su, J.; Du, Z.; Xiao, L.; Wei, F.; Yang, Y.; Li, M.; Qiu, Y.; Liu, J.; Chen, J.; Xiao, Y. Graphene oxide coated titanium surfaces with osteoimmunomodulatory role to enhance osteogenesis. Mater. Sci. Eng. C 2020, 110983. [Google Scholar] [CrossRef] [PubMed]
- Radunovic, M.; de Colli, M.; de Marco, P.; di Nisio, C.; Fontana, A.; Piattelli, A.; Cataldi, A.; Zara, S. Graphene oxide enrichment of collagen membranes improves DPSCs differentiation and controls inflammation occurrence. J. Biomed. Mater. Res. Part A 2017, 105, 2312–2320. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 013001. [Google Scholar] [CrossRef]
- Ventola, C.L. Medical applications for 3D Printing: Current and projected uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar]
- Jeong, C.G.; Atala, A. Engineering Mineralized and Load Bearing Tissues. In 3D Printing and Biofabrication for Load Bearing Tissue Engineering; Springer: Berlin, Germany, 2015; pp. 3–14. [Google Scholar]
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of electrospun nanofibers for biomedical and dental applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mauck, R.L.; Tuan, R.S. Electrospun nanofibrous scaffolds: Production, characterization, and applications for tissue engineering and drug delivery. J. Biomed. Nanotechnol. 2005, 1, 259–275. [Google Scholar] [CrossRef]
- Cho, M.K.; Singu, B.S.; Na, Y.H.; Yoon, K.R. Fabrication and characterization of double-network agarose/polyacrylamide nanofibers by electrospinning. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Thavornyutikarn, B.; Chantarapanich, N.; Sitthiseripratip, K.; Thouas, G.A.; Chen, Q. Bone tissue engineering scaffolding: Computer-aided scaffolding techniques. Prog. Biomater. 2014, 3, 61–102. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.O.; Liu, X.H.; Smith, L.A.; Ma, P.X. Nanostructured polymer scaffolds for tissue engineering and regenerative medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 226–236. [Google Scholar] [CrossRef]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef]
- Maspero, F.; Ruffieux, K.; Müller, B.; Wintermantel, E. Resorbable defect analog PLGA scaffolds using CO2 as solvent: Structural characterization. J. Biomed. Mater. Res. 2002, 62, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.; Mather, M.L.; Howard, D.; Wang, W.; White, L.J.; Crowe, J.A.; Morgan, S.P.; Chandra, A.; Williams, D.J.; Howdle, S.M. Control of pore size and structure of tissue engineering scaffolds produced by supercritical fluid processing. Eur. Cell Mater. 2007, 14, 64–77. [Google Scholar] [CrossRef]
- Galler, K.M.; Cavender, A.; Yuwono, V.; Dong, H.; Shi, S.; Schmalz, G.; Hartgerink, J.D.; D’Souza, R.N. Self-assembling peptide amphiphile nanofibers as a scaffold for dental stem cells. Tissue Eng. Part A 2008, 14, 2051–2058. [Google Scholar] [CrossRef]
- Yang, Z.; Jin, F.; Zhang, X.; Ma, D.; Han, C.; Huo, N.; Wang, Y.; Zhang, Y.; Lin, Z.; Jin, Y. Tissue engineering of cementum/periodontal-ligament complex using a novel three-dimensional pellet cultivation system for human periodontal ligament stem cells. Tissue Eng. Part C Methods 2009, 15, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Su, P.; Chen, X.; Meng, Y.; Yu, W.; Xiang, A.P.; Wang, Y. Biocompatibility and osteogenesis of biomimetic Bioglass-Collagen-Phosphatidylserine composite scaffolds for bone tissue engineering. Biomaterials 2011, 32, 1051–1058. [Google Scholar] [CrossRef]
- Bajaj, P.; Schweller, R.M.; Khademhosseini, A.; West, J.L.; Bashir, R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014, 16, 247–276. [Google Scholar] [CrossRef]
- Giannitelli, S.; Basoli, F.; Mozetic, P.; Piva, P.; Bartuli, F.; Luciani, F.; Arcuri, C.; Trombetta, M.; Rainer, A.; Licoccia, S. Graded porous polyurethane foam: A potential scaffold for oro-maxillary bone regeneration. Mater. Sci. Eng. C 2015, 51, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Annabi, N.; Khademhosseini, A.; Dehghani, F. Fabrication of porous chitosan scaffolds for soft tissue engineering using dense gas Co2. Acta Biomater. 2011, 7, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of scaffolds for bone-tissue regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Chen, X. Biofabrication of tissue scaffolds. In Advances in biomaterials science and biomedical applications; InTech: Rijeka, Croatia, 2013; pp. 315–328. [Google Scholar]
- Badami, A.S.; Kreke, M.R.; Thompson, M.S.; Riffle, J.S.; Goldstein, A.S. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly (lactic acid) substrates. Biomaterials 2006, 27, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Oh, S.H.; Liu, J.; Soker, S.; Atala, A.; Yoo, J.J. The use of thermal treatments to enhance the mechanical properties of electrospun poly (É›-caprolactone) scaffolds. Biomaterials 2008, 29, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Su, K.; Varshney, R.R.; Wang, Y.; Wang, D. Sintered microsphere scaffolds for controlled release and tissue engineering. Pharm. Res. 2011, 28, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, X.; Ren, L.; Wang, C.; Wang, D. Porous poly (lactic-co-glycolide) microsphere sintered scaffolds for tissue repair applications. Mater. Sci. Eng. C 2009, 29, 2502–2507. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Castro, N.J.; O’brien, J.; Zhang, L.G. Integrating biologically inspired nanomaterials and table-top stereolithography for 3D printed biomimetic osteochondral scaffolds. Nanoscale 2015, 7, 14010–14022. [Google Scholar] [CrossRef]
- Timashev, P.; Kuznetsova, D.; Koroleva, A.; Prodanets, N.; Deiwick, A.; Piskun, Y.; Bardakova, K.; Dzhoyashvili, N.; Kostjuk, S.; Zagaynova, E. Novel biodegradable star-shaped polylactide scaffolds for bone regeneration fabricated by two-photon polymerization. Nanomedicine 2016, 11, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Yan, Y.; Wang, S.; Zhang, R.; Zhang, C. Fabrication of porous scaffolds for bone tissue engineering via low-temperature deposition. Scripta. Materialia 2002, 46, 771–776. [Google Scholar] [CrossRef]
- Chou, Y.; Lee, D.; Chang, T.; Hsu, Y.; Yu, Y.; Liu, S.; Ueng, S.W. Development of a three-dimensional (3D) printed biodegradable cage to convert morselized corticocancellous bone chips into a structured cortical bone graft. Int. J. Mol. Sci. 2016, 17, 595. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef]
- Rasperini, G.; Pilipchuk, S.; Flanagan, C.; Park, C.; Pagni, G.; Hollister, S.; Giannobile, W. 3D-printed bioresorbable scaffold for periodontal repair. J. Dent. Res. 2015, 94, 153S–157S. [Google Scholar] [CrossRef]
- Roskies, M.; Jordan, J.O.; Fang, D.; Abdallah, M.; Hier, M.P.; Mlynarek, A.; Tamimi, F.; Tran, S.D. Improving PEEK bioactivity for craniofacial reconstruction using a 3D printed scaffold embedded with mesenchymal stem cells. J. Biomater. Appl. 2016, 31, 132–139. [Google Scholar] [CrossRef]
- Duan, B.; Cheung, W.L.; Wang, M. Optimized fabrication of Ca-P/PHBV nanocomposite scaffolds via selective laser sintering for bone tissue engineering. Biofabrication 2011, 3, 015001. [Google Scholar] [CrossRef]
- Barron, J.A.; Krizman, D.B.; Ringeisen, B.R. Laser printing of single cells: Statistical analysis, cell viability, and stress. Ann. Biomed. Eng. 2005, 33, 121–130. [Google Scholar] [CrossRef]
- Jakab, K.; Norotte, C.; Marga, F.; Murphy, K.; Vunjak-Novakovic, G.; Forgacs, G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication 2010, 2, 022001. [Google Scholar] [CrossRef]
- Yen, A.H.; Sharpe, P.T. Stem cells and tooth tissue engineering. Cell Tissue Res. 2008, 331, 359–372. [Google Scholar] [CrossRef]
- Rodriguez-Lozano, F.; Bueno, C.; Insausti, C.L.; Meseguer, L.; Ramirez, M.; Blanquer, M.; Marin, N.; MartÃnez, S.; Moraleda, J.M. Mesenchymal stem cells derived from dental tissues. Int. Endod. J. 2011, 44, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lozano, F.J.; Insausti, C.L.; Iniesta, F.; Blanquer, M.; Ramirez, M.D.; Meseguer, L.; Meseguer-Henarejos, A.B.; Marin, N.; Martinez, S.; Moraleda, J.M. Mesenchymal dental stem cells in regenerative dentistry. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e1062–e1067. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.; Huss, R. Adult stem cell lines in regenerative medicine and reconstructive surgery. J. Surg. Res. 2005, 124, 201–208. [Google Scholar] [CrossRef]
- Chagastelles, P.C.; Nardi, N.B. Biology of stem cells: An overview. Kidney Int. Suppl. 2011, 1, 63–67. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Lindroos, B.; Mäenpää, K.; Ylikomi, T.; Oja, H.; Suuronen, R.; Miettinen, S. Characterisation of human dental stem cells and buccal mucosa fibroblasts. Biochem. Biophys. Res. Commun. 2008, 368, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Walboomers, X.F.; Van Kuppevelt, T.H.; Daamen, W.F.; Van Damme, P.A.; Bian, Z.; Jansen, J.A. In vivo evaluation of human dental pulp stem cells differentiated towards multiple lineages. J. Tissue Eng. Regen. Med. 2008, 2, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.J.; Yamaza, T.; Song, Y.; Fouad, A.F.; Romberg, E.E.; Shi, S.; Tuan, R.S.; Huang, G.T. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen. Med. 2010, 5, 617–631. [Google Scholar] [CrossRef]
- Karaöz, E.; Doğan, B.N.; Aksoy, A.; Gacar, G.; Akyüz, S.; Ayhan, S.; Genç, Z.S.; Yürüker, S.; Duruksu, G.; Demircan, P.C. Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem. Cell Biol. 2010, 133, 95. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Zhu, X.; Zhang, C.; Jin, L. Characterization of dental pulp stem cells isolated from canine premolars. J. Endod. 2011, 37, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.; Sonoyama, W.; Yamaza, T.; Coppe, C.; Kikuiri, T.; Akiyama, K.; Lee, J.; Shi, S. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008, 14, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Alipour, R.; Sadeghi, F.; Hashemi-Beni, B.; Zarkesh-Esfahani, S.H.; Heydari, F.; Mousavi, S.B.; Adib, M.; Narimani, M.; Esmaeili, N. Phenotypic characterizations and comparison of adult dental stem cells with adipose-derived stem cells. Int. J. Prev. Med. 2010, 1, 164–171. [Google Scholar]
- Nourbakhsh, N.; Soleimani, M.; Taghipour, Z.; Karbalaie, K.; Mousavi, S.B.; Talebi, A.; Nadali, F.; Tanhaei, S.; Kiyani, G.A.; Nematollahi, M.; et al. Induced in vitro differentiation of neural-like cells from human exfoliated deciduous teeth-derived stem cells. Int. J. Dev. Biol. 2011, 55, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.M.; Dong, Z.; Kaneko, T.; Zhang, Z.; Miyazawa, M.; Shi, S.; Smith, A.J.; Nör, J.E. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J. Endod. 2008, 34, 962–969. [Google Scholar] [CrossRef]
- Gay, I.C.; Chen, S.; MacDougall, M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod. Craniofacial Res. 2007, 10, 149–160. [Google Scholar] [CrossRef]
- Shi, S.; Bartold, P.; Miura, M.; Seo, B.; Robey, P.; Gronthos, S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod. Craniofacial Res. 2005, 8, 191–199. [Google Scholar] [CrossRef]
- Park, J.C.; Kim, J.M.; Jung, I.; Kim, J.C.; Choi, S.H.; Cho, K.S.; Kim, C.S. Isolation and characterization of human periodontal ligament (PDL) stem cells (PDLSCs) from the inflamed PDL tissue: In vitro and in vivo evaluations. J. Clin. Periodontol. 2011, 38, 721–731. [Google Scholar] [CrossRef]
- Kémoun, P.; Laurencin-Dalicieux, S.; Rue, J.; Farges, J.; Gennero, I.; Conte-Auriol, F.; Briand-Mesange, F.; Gadelorge, M.; Arzate, H.; Narayanan, A.S. Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res. 2007, 329, 283–294. [Google Scholar] [CrossRef]
- Völlner, F.; Ernst, W.; Driemel, O.; Morsczeck, C. A two-step strategy for neuronal differentiation in vitro of human dental follicle cells. Differentiation 2009, 77, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Morsczeck, C.; Götz, W.; Schierholz, J.; Zeilhofer, F.; Kühn, U.; Möhl, C.; Sippel, C.; Hoffmann, K. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef]
- Shi, S.; Sonoyama, W.; Yamaza, T.; Wang, S. Mesenchymal Stem Cell-Mediated Function Tooth Regeneration. U.S. Patent No. 10,232,080, 19 March 2019. [Google Scholar]
- Garcia-Godoy, F.; Murray, P.E. Status and potential commercial impact of stem cell-based treatments on dental and craniofacial regeneration. Stem Cells Dev. 2006, 15, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Horch, R. New developments and trends in tissue engineering: An update. J. Tissue Sci. Eng. 2012, 3. [Google Scholar] [CrossRef]
- Witkowska-Zimny, M. Dental tissue as a source of stem cells: Perspectives for teeth regeneration. J. Bioeng. Biomed. Sci 2011, 2, 006. [Google Scholar] [CrossRef]
- Galler, K.M.; D’Souza, R.N. Tissue engineering approaches for regenerative dentistry. Regen. Med. 2011, 6, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Versiani, M.A.; Basrani, B.; Sousa-Neto, M.D. The Root Canal Anatomy in Permanent Dentition; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Casagrande, L.; Cordeiro, M.M.; Nör, S.A.; Nör, J.E. Dental pulp stem cells in regenerative dentistry. Odontology 2011, 99, 1–7. [Google Scholar] [CrossRef]
- Gathani, K.M.; Raghavendra, S.S. Scaffolds in regenerative endodontics: A review. Dent. Res. J. 2016, 13, 379–386. [Google Scholar]
- Aguilar, L.M.C.; Silva, S.M.; Moulton, S.E. Growth factor delivery: Defining the next generation platforms for tissue engineering. J. Control. Release 2019, 306, 40–58. [Google Scholar] [CrossRef]
- Patil, A.S.; Merchant, Y.; Nagarajan, P. Tissue engineering of craniofacial tissues—A review. J. Regen Med. Tissue Eng. 2013, 2, 6. [Google Scholar] [CrossRef]
- Nakashima, M. The application of bone morphogenetic proteins to dental tissue engineering. Nat. Biotechnol. 2003, 21, 1025. [Google Scholar] [CrossRef]
- Bansal, R.; Bansal, R. Regenerative endodontics: A state of the art. Indian J. Dent. Res. 2011, 22, 122. [Google Scholar] [CrossRef]
- Kirker-Head, C.A. Potential applications and delivery strategies for bone morphogenetic proteins. Adv. Drug Deliv. Rev. 2000, 43, 65–92. [Google Scholar] [CrossRef]
- Chiu, H.; Chiang, C.; Tu, H.; Wikesjö, U.M.; Susin, C.; Fu, E. Effects of bone morphogenetic protein-6 on periodontal wound healing/regeneration in supraalveolar periodontal defects in dogs. J. Clin. Periodontol. 2013, 40, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Nguyen, T.B.L.; Min, Y.; Lee, B. In vitro and in vivo studies of BMP-2-loaded PCL–gelatin–BCP electrospun scaffolds. Tissue Eng. Part A 2014, 20, 3279–3289. [Google Scholar] [CrossRef]
- Francis, C.S.; Mobin, S.S.; Lypka, M.A.; Rommer, E.; Yen, S.; Urata, M.M.; Hammoudeh, J.A. rhBMP-2 with a demineralized bone matrix scaffold versus autologous iliac crest bone graft for alveolar cleft reconstruction. Plast. Reconstr. Surg. 2013, 131, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Mariner, P.D.; Wudel, J.M.; Miller, D.E.; Genova, E.E.; Streubel, S.O.; Anseth, K.S. Synthetic hydrogel scaffold is an effective vehicle for delivery of INFUSE (rhBMP2) to critical-sized calvaria bone defects in rats. J. Orthop. Res. 2013, 31, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gluhak-Heinrich, J.; Martinez, M.; Li, T.; Wu, Y.; Chuang, H.H.; Chen, L.; Dong, J.; Gay, I.; MacDougall, M. Bone morphogenetic protein 2 mediates dentin sialophosphoprotein expression and odontoblast differentiation via NF-Y signaling. J. Biol. Chem. 2008, 283, 19359–19370. [Google Scholar] [CrossRef] [PubMed]
- Baum, B.J.; Kok, M.; Tran, S.D.; Yamano, S. The impact of gene therapy on dentistry: A revisiting after six years. J. Am. Dent. Assoc. 2002, 133, 35–44. [Google Scholar] [CrossRef]
- Franceschi, R. Biological approaches to bone regeneration by gene therapy. J. Dent. Res. 2005, 84, 1093–1103. [Google Scholar] [CrossRef]
- Cho, T.; Gerstenfeld, L.C.; Einhorn, T.A. Differential temporal expression of members of the transforming growth factor β superfamily during murine fracture healing. J. Bone Mineral. Res. 2002, 17, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.A.; Barbato, P.R.; Guimarães Bahia Reis, S.C.; de Morais Freitas, C.H.S.; Antunes, J.L.F. Tooth loss in Brazil: Analysis of the 2010 Brazilian oral health survey. Rev. Saude Publica 2013, 47, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Yadlapati, M.; Biguetti, C.; Cavalla, F.; Nieves, F.; Bessey, C.; Bohluli, P.; Garlet, G.P.; Letra, A.; Fakhouri, W.D.; Silva, R.M. Characterization of a vascular endothelial growth factor-loaded bioresorbable delivery system for pulp regeneration. J. Endod. 2017, 43, 77–83. [Google Scholar] [CrossRef]
- Peng, H.; Wright, V.; Usas, A.; Gearhart, B.; Shen, H.C.; Cummins, J.; Huard, J. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein. J. Clin. Investig. 2002, 110, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Farrokhi, M.; Peykanpour, F. Vascular endothelial growth factor-loaded bioresorbable delivery system for pulp regeneration. J. Endod. 2017, 43, 1414. [Google Scholar] [CrossRef]
- Aksel, H.; Huang, G.T. Combined effects of vascular endothelial growth factor and bone morphogenetic protein 2 on odonto/osteogenic differentiation of human dental pulp stem cells in vitro. J. Endod. 2017, 43, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Dissanayaka, W.L.; Zhang, C. Dental pulp stem cells overexpressing stromal-derived factor-1α and vascular endothelial growth factor in dental pulp regeneration. Clin. Oral Investig. 2019, 23, 2497–2509. [Google Scholar] [CrossRef] [PubMed]
- D’Alimonte, I.; Nargi, E.; Mastrangelo, F.; Falco, G.; Lanuti, P.; Marchisio, M.; Miscia, S.; Robuffo, I.; Capogreco, M.; Buccella, S.; et al. Vascular endothelial growth factor enhances in vitro proliferation and osteogenic differentiation of human dental pulp stem cells. J. Biol. Regul. Homeost. Agents 2011, 25, 57–69. [Google Scholar]
- Howell, T.H.; Fiorellini, J.P.; Paquette, D.W.; Offenbacher, S.; Giannobile, W.V.; Lynch, S.E. A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J. Periodontol. 1997, 68, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, R.B.; Niekrash, C.E.; Kennedy, J.E.; Charette, M.F. Platelet-derived and insulin-like growth-factors stimulate regeneration of periodontal attachment in monkeys. J. Periodont. Res. 1992, 27, 285–290. [Google Scholar] [CrossRef]
- Gamal, A.Y.; Abdel Ghaffar, K.A.; Alghezwy, O.A. Crevicular fluid growth factors release profile following the use of platelet-rich fibrin and plasma rich growth factors in treating periodontal intrabony defects: A randomized clinical trial. J. Periodontol. 2016, 87, 654–662. [Google Scholar] [CrossRef]
- Nevins, M.; Giannobile, W.V.; McGuire, M.K.; Kao, R.T.; Mellonig, J.T.; Hinrichs, J.E.; McAllister, B.S.; Murphy, K.S.; McClain, P.K.; Nevins, M.L.; et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: Results of a large multicenter randomized controlled trial. J. Periodontol. 2005, 76, 2205–2215. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, Y.M.; Lee, J.Y.; Seol, Y.J.; Chung, C.P.; Lee, S.J. Controlled release of platelet-derived growth factor-BB from chondroitin sulfate-chitosan sponge for guided bone regeneration. J. Control. Release 2000, 67, 385–394. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Pradeep, A. Gene therapy in periodontics: A review and future implications. J. Contemp. Dent. Pr. 2006, 7, 83–91. [Google Scholar] [CrossRef]
- Sood, S.; Gupta, S.; Mahendra, A. Gene therapy with growth factors for periodontal tissue engineering—A review. Med. Oral Patol. Oral Cirugía Bucal 2012, 12, e301–e310. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. Fibroblast growth factors. Genome Biol. 2001, 2. [Google Scholar] [CrossRef]
- Feldman, D.S.; Osborne, S. Fibrin as a tissue adhesive and scaffold with an angiogenic agent (FGF-1) to enhance burn graft healing in vivo and clinically. J. Funct. Biomater. 2018, 9, 68. [Google Scholar] [CrossRef]
- Nakahara, T.; Nakamura, T.; Kobayashi, E.; Inoue, M.; Shigeno, K.; Tabata, Y.; Eto, K.; Shimizu, Y. Novel approach to regeneration of periodontal tissues based on in situ tissue engineering: Effects of controlled release of basic fibroblast growth factor from a sandwich membrane. Tissue Eng. 2003, 9, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Shim, J.; Jang, J.; Kim, S.; Cho, D. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1286–1297. [Google Scholar] [CrossRef]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [PubMed]
- Isogai, Z.; Ono, R.N.; Ushiro, S.; Keene, D.R.; Chen, Y.; Mazzieri, R.; Charbonneau, N.L.; Reinhardt, D.P.; Rifkin, D.B.; Sakai, L.Y. Latent transforming growth factor β-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J. Biol. Chem. 2003, 278, 2750–2757. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.L.; Flavell, R.A. Transforming growth factor-β regulation of immune responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef]
- Tachi, K.; Takami, M.; Sato, H.; Mochizuki, A.; Zhao, B.; Miyamoto, Y.; Tsukasaki, H.; Inoue, T.; Shintani, S.; Koike, T. Enhancement of bone morphogenetic protein-2-induced ectopic bone formation by transforming growth factor-β1. Tissue Eng. Part A 2011, 17, 597–606. [Google Scholar] [CrossRef]
- Liu, Y.; de Groot, K.; Hunziker, E.B. Osteoinductive implants: The mise-en-scene for drug-bearing biomimetic coatings. Ann. Biomed. Eng. 2004, 32, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huse, R.O.; de Groot, K.; Buser, D.; Hunziker, E.B. Delivery mode and efficacy of BMP-2 in association with implants. J. Dent. Res. 2007, 86, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, G.; de Groot, K. Biomimetic coatings for bone tissue engineering of critical-sized defects. J. R. Soc. Interface 2010, 7, S631–S647. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Zhou, J.; Solomon, C.; Zheng, Y.; Suzuki, T.; Chen, M.; Song, S.; Jiang, N.; Cho, S.; Mao, J.J. Effects of growth factors on dental stem/progenitor cells. Dent. Clin. N. Am. 2012, 56, 563–575. [Google Scholar] [CrossRef]
- Liu, Y.; de Groot, K.; Hunziker, E.B. BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model. Bone 2005, 36, 745–757. [Google Scholar] [CrossRef]
- Neel, E.; Chrzanowski, W.; Salih, V.; Kim, H.W.; Knowles, J. Tissue engineering in dentistry. J. Dent. 2014, 42, 915–928. [Google Scholar] [CrossRef]
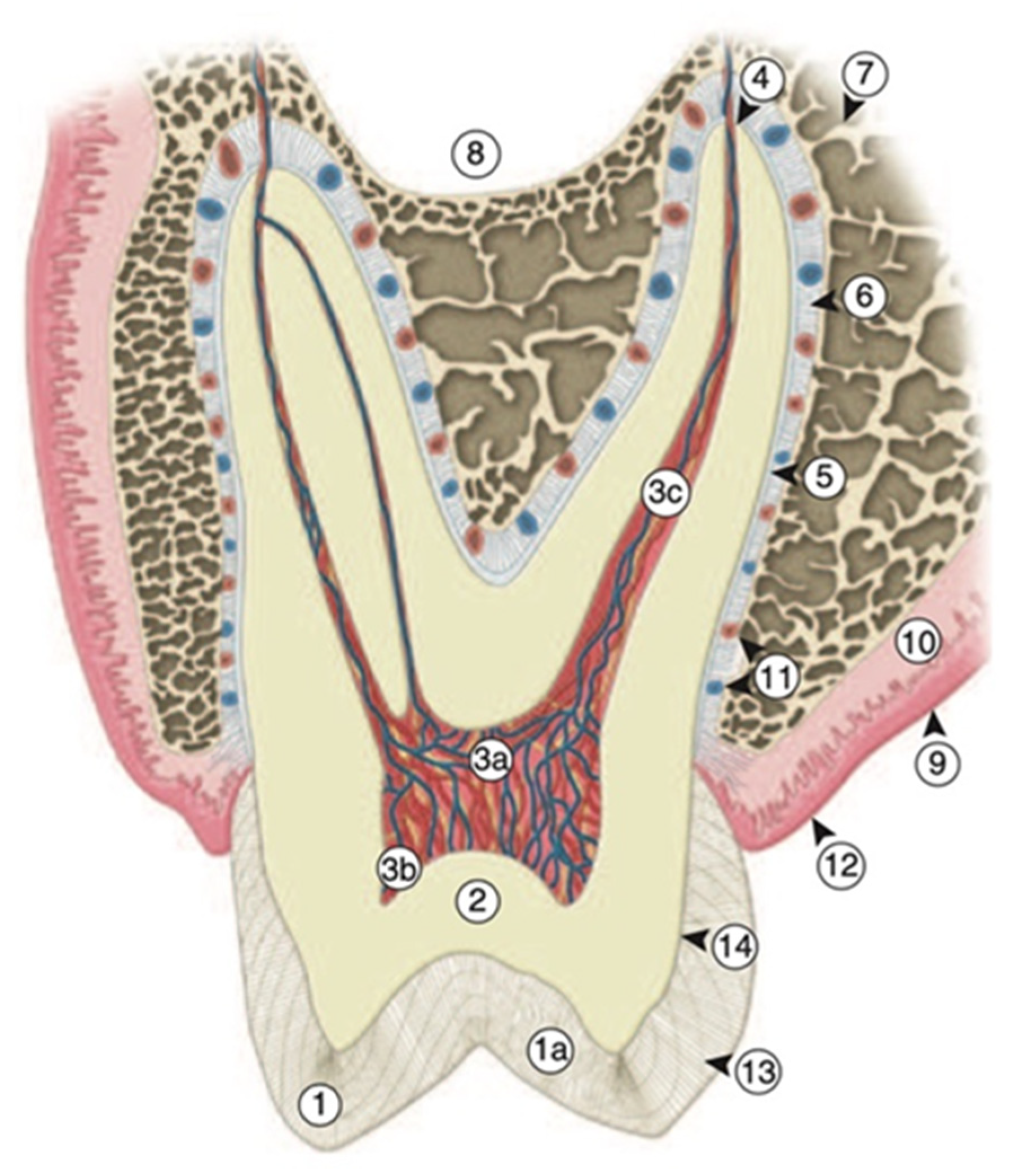
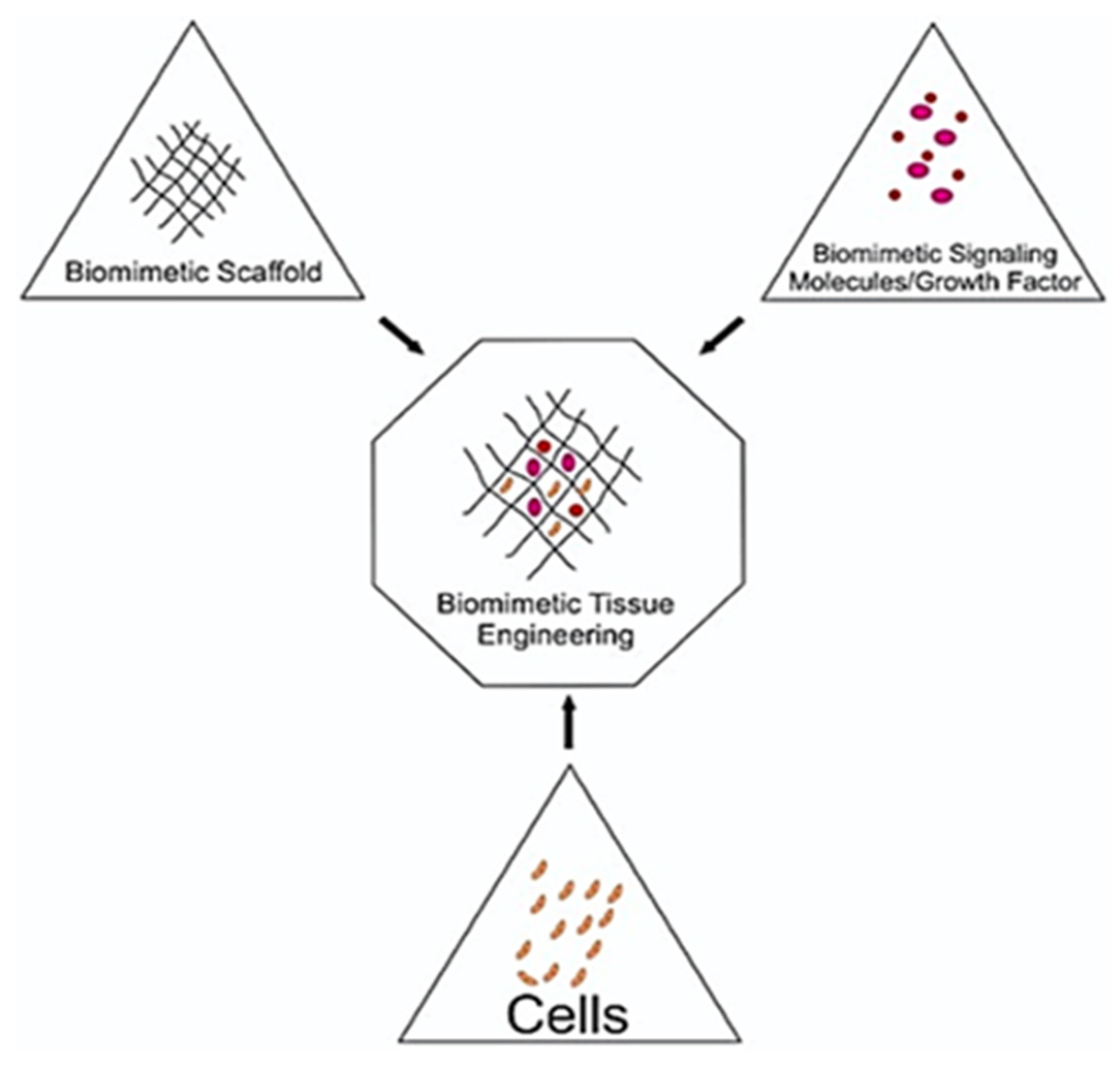

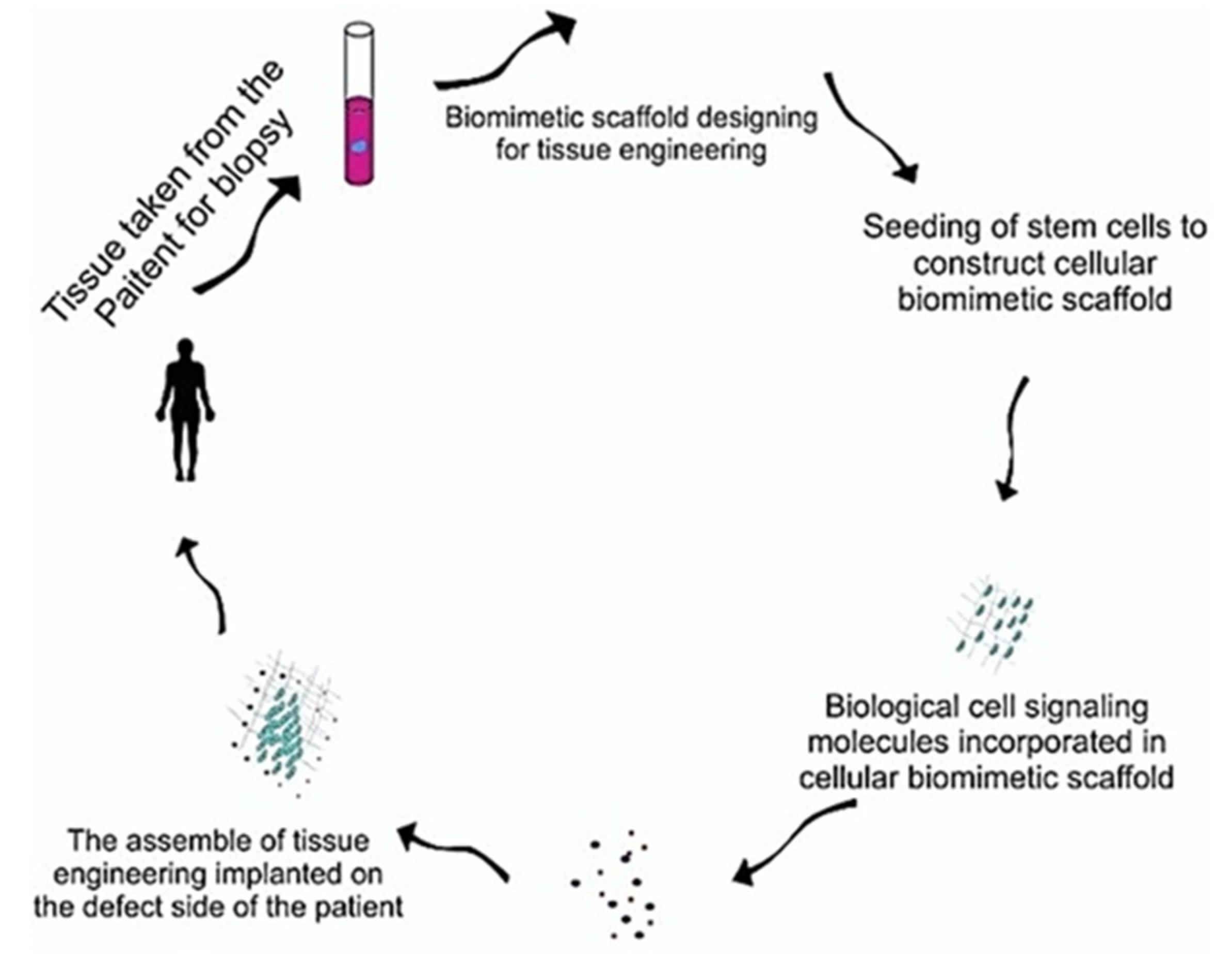
| Formative (Developmental) | Odontoblasts Produce Primary and Secondary Dentin |
|---|---|
| Nutritive | Supply of nutrition to odontoblasts processes through the blood supply (mineral ions, proteins, and water to dentin) |
| Sensory (protective) | Pulpal nerve fibers mediate the sensation of pain (motor and sensory nerve fibers initiate reflexes) |
| Defensive (reparative) | The DPC: Response to pathologic challenges |
| Restorative Materials | Test | Elastic Modulus (GPa) | References |
|---|---|---|---|
| Tooth Hard Tissues | |||
| Tooth enamel | Nanoindentation | 72.0–125.0 | [51] |
| 80.9 ± 6.6 | [50] | ||
| Tooth dentin | Nanoindentation | 14.0–38.0 | [51] |
| 20.5 ± 2.0 | [50] | ||
| Resin-Based Dental Composites (RDCs) | |||
| Z100 (Micro-hybrid RDC), (3M ESPE, USA) | Three-point bending | 18.3 ± 1.2 | [69] |
| 11.3 ± 0.5 | [39] | ||
| Z250 (Micro-hybrid RDC), 3M ESPE, USA | Three-point bending | 16.7 ± 0.8 | [69] |
| 6.9 ± 0.6 | [39] | ||
| Flitek Supreme (Nanofilled RDC), 3M ESPE, USA | Three-point bending | 13.7 ± 0.6 | [69] |
| 9.4 ± 0.7 | [35] | ||
| Tetric Ceram (Hybrid RDC), Vivedent Schaan Liechtenstein | Three-point bending | 6.9 ± 0.5 | [70] |
| 9.4 ± 0.9 | [71] | ||
| Clearfil PhotoPost (Hybrid RDC) Kuraray, Osaka, Japan | Three-point bending | 18.0 ± 1.2 | [70] |
| Point 4 (Flowable RDC), Kerr, Orange CA, USA | Compression | 3.5 ± 0.87 | [72] |
| Grandio (Hybrid Paste RDC), Voco (Cuxhaven, Germany) | Three-point bending | 15.3 | [73] |
| Glass–Ionomer Cements (GIC) | |||
| Riva Light (RMGIC) SDI, Victoria, Australia | Three-point bending | 2.1 ± 0.4 | [74] |
| Aqua ionofil U (conventional GIC, Voco, Cuxhaven Germany | Dynamic mechanical analysis (DMA) | 1.8 ± 0.01 | [75] |
| Fuji II LC (FL) (RMGIC)G. C. Belgium N.V. | DMA | 5.8 ± 0.02 | [75] |
| Riva Self-Cure (Glass–ionomer cement) SDI Limited, Victoria, Australia | Three-point bending | 6.3 ± 1.3 | [76] |
| Fujji IX, GC, Australlia | Three-point bending | 7.8 | [77] |
| Ionofil Molar Glass–Ionomer Cement, Voco, Cuxhaven, Germany | Indentation | 12.3 ± 2.1 | [78] |
| Dental Ceramics | |||
| e.max Press (Lithium-disilicate-based glass-ceramic). Ivoclar Vivadent, Schaan, Liechtenstein) | Ultrasonic pulse-echo method | 82.3 | [41] |
| PM9 Vita (Feldspathic-based ceramic), (VITA Zahnfabrik, Bad Säckingen, Germany) | Ultrasonic pulse-echo method | 44.4 | [41] |
| IPS e.max® Press glass-ceramic material Ivoclar Vivadent AG, Schaan, Liechtenstein | Deflection | 95.0 | [79] |
| Vita In-Ceram alumina core. Vita Zahnfabrik | Three-point bending | 271.3 | [80] |
| #Vita Suprinity (Zirconia reinforced lithium silicate glass-ceramic) and Vita Zahnfabrick, Bad Säckingen, Germany | Three-point bending | 70.4 ± 1.9 | [81] |
| IPS e.max (Lithium disilicate glass-ceramic) Ivoclar Vivadent, Schaan, Lichtenstein | Three-point bending | 60.6 ± 1.6 | [81] |
| Restorative Materials | Test | Surface Hardness (SH) | References |
|---|---|---|---|
| Tooth Hard Tissues | |||
| Tooth enamel | Nanoindentation | 2.2–7.2 GPa | [51] |
| 4.9 ± 0.4 GPa | [50] | ||
| Tooth dentin | Nanoindentation | 0.7–0.9 GPa | [51] |
| 0.9 ± 0.1 GPa | [50] | ||
| Resin-Based Dental Composites (RDCs) | |||
| X-tra fil (bulk-fill) Micro-hybrid RBC, Voco, Guxhaven, Germany | Vickers | 75.8 ± 7.0 VHN | [43] |
| 70.9 VHN | [73] | ||
| QuiXfil (bulk-fill) Micro-hybrid RBC, Densply, Konstanz, Germany | Vickers | 64.1 ± 6.2 VHN | [43] |
| Grandio Nano-hybrid RBC, Voco, Guxhaven, Germany | Vickers | 120.8 VHN | [73] |
| 92.6 ± 6.1 VHN | [43] | ||
| Z100 Micro-hybrid RBC, 3 M ESPE, USA | Knoop | 74.1 ± 9.0 KHN | [82] |
| 120.8 ± 15.1 KHN | [83] | ||
| Filtek Supreme Nanofilled RBC, 3 M ESPE, USA | Knoop | 58.4 ± 3.6 KHN | [82] |
| 42.8 ± 6.2 KHN | [82] | ||
| Filtek Z250, Micro-hybrid RBC, 3M ESPE, USA | Vickers | 72.0 VHN | [84] |
| 82.0 ± 4.0 VHN | [85] | ||
| Glass–Ionomer Cements (GICs) | |||
| Fuji IX, GIC, GC Corporation, Tokyo, Japan | Vickers | 26.4 ± 4.4 VHN | [46] |
| Knoop | 68.7 ± 10.9 KHN | [63] | |
| Ionofil Molar Glass–Ionomer Cement, Voco, Cuxhaven, Germany | Vickers | 74.3 ± 6.7 VHN | [46,85] |
| 57.4 ± 15.2 VHN | [78] | ||
| KetacTM Molar Easy Mix, GIC, 3M-ESPE, Saint Paul, USA | Knoop | 77.5 ± 37.7 VHN | [86] |
| Equia Forte Glass–Ionomer Cement GC, Tokyo, Japan | Vickers | 120.1 ± 10 VHN | [87] |
| Vitromolar GIC, DFL Ind’ustria e Com’ercio Ltd.a (RJ, Brazil) | Vickers | 40.9 ± 4.3 VHN | [88] |
| 40.6 ± 0.8 VHN | [88] | ||
| Dental Ceramics | |||
| Alumina Ceramic (Fabricated using slip casting technique) | Vickers | 1679 HV | [89] |
| Alumina/Zirconia Ceramics, (Fabricated using the slip casting technique) | 1447 HV | [89] | |
| Duceram love Dental Ceramics, (Degu Dent GmbH, Denstply, Germany) | 6.1 ± 0.7 GPa | [44] | |
| IPS e.max ceram Dental Ceramics, Ivoclar- Vivadent AG, Germany | 6.1 ± 0.3 GPa | [44] | |
| Feldspathic ceramic Block, Vita Zahnfabrik, Germany | 502.4 ± 2.3 kg/mm2 | [90] | |
| Vita VMK 68 Leucite Dental Ceramics, Vita Zahnfabrik, Germany | 6.9 ± 0.1 GPa | [91] | |
| Irrigant | Main Outcome | Reference |
|---|---|---|
| CHX (2%) | No survival of SCAPs Toxicity to SCAPs | [127] [130] |
| NaOCl (6%) | Survival of SCAPs (combined to the 17% EDTA) Reduced survival of SCAPs | [127] [128] |
| NaOCl (1.5%) | Survival of SCAPs | [128] |
| EDTA (17%) | Survival of SCAPs | [127,128] |
| Technique | Materials Processed | Advantages | Disadvantages |
|---|---|---|---|
| Thermally induced phase separation (TIPS) [319,320,321] | poly(L-lactic acid)-based scaffolds | Processing flexibility. Produce 3D scaffolds Enhanced osteogenic differentiation. Induction of defect healing. | Uncontrolled pore distribution and size. Limited to a few polymers. Lack of control over 3D shapes |
| Supercritical fluid-gassing [322,323] | poly(DL-lactic acid-coglycolic acid) (PLGA), Poly (DL-lactic acid) (PDLLA) | Preparation of an exact porous copy. Organic solvent is not required. | Decrease pore size. Fragile scaffold. Form nonporous layer. Take hours to complete. |
| Self-assembling [324,325] | Hydrogel scaffold and peptide-amphiphile (PA) | To engineer soft and hard mineralized matrices for dental/pulp tissue regeneration. | Inability to controlled macro-sized pores. Limited formation of mechanically stable 3D geometry. |
| Emulsion freeze-drying method/ lyophilization [289,326,327] | Natural and synthetic polymers | Highly porous scaffolds. Large surface areas. Superior mechanical properties. High temperatures can be avoided. Good biocompatibility. Extensive osteoconductivity. | Inadequate control of scaffold pore size, network and architecture. Lengthy procedures. High consumption of energy. Use of cytotoxic solvents. Formation of irregular, small size pores (15–35 μm). |
| Gas-foaming process [319,328,329] | PLGA | Highly porous. Organic, cytotoxic solvents are not required. Inert gas-foaming agents. | Technique cannot be used for hydrophilic and glassy polymers. Use of excessive heat. Close, non-interconnected pore structures. |
| Solvent-casting and particulate-leaching [319,327,330] | PGA | Most common and easy method. Sustainable equipment costs Pore size and porosity can be controlled. High porosity and interconnected pores. Capable of healing critical bone defects in rat femoral medial epicondyles. | Harmful residual solvent. Decrease in the activity of bio-inductive molecules. Impossibility of adding pharmacological agents. Process can only form simple shape scaffolds |
| Technique | Materials Processed | Advantages | Disadvantages |
|---|---|---|---|
| Electrospinning [331,332,333] | Natural polymers: collagen, silk fibroin, and fibrinogen, chitosan, gelatin Synthetic polymers: PGA, PLLA, PLGA, and PCL) | Accurate porosity and morphology. Fibers within nanometer range. Strong ability to induce osteogenic differentiation. Small pore size mimics the ECM, density and high surface area. | Require organic solvents. Difficult to create large 3D scaffold clinically. |
| Microsphere sintering [334,335,336] | Synthetic polymers: PLGA | Improved cellular attachment and proliferation. Excellent mechanical properties. | CO2 was used that creates a closed-pore structure. |
| Technique | Materials Processed | Advantages | Disadvantages |
|---|---|---|---|
| Stereolithography [330,337,338] | Synthetic polymers: PEG, PEGDA, PPF, PCL, PDLLA | Highly accurate scaffold Greatly improved adhesion, proliferation, and osteochondral differentiation. Easy removal of photopolymer by heating. | Skin irritation and cytotoxicity Photo-polymerization of materials. Expensive materials and equipment |
| Fused deposition modelling (FDM) [330,339,340] | Synthetic polymers: PCL, PLGA, PC, PPSF, PEI, PVA, ABSP400 | High porosity and controlled pore size with a complete interconnectivity Good mechanical strength No need for toxic solvent. Flexibility in material processing. Controlled porosity and size of pores. | High processing temperature. Limited material range. Inconsistent pores. Application to biodegradable polymers may be limited. |
| Selective laser sintering (SLS) [330,341,342,343,344] | Synthetic polymers: PEEK, PCL, poly(lactic acid) Ceramics: HA, TCP | High compressive strengths. Solvent-free. Complex structure. Can control pores size and porosity. | Needs powder materials that should withstand laser heat. During sintering process materials should resist shrinkage of the scaffold Pre- and post-heating treatments of the powdered material. Thermally stable polymers can be used. Limited/small pore size |
| Three-dimensional bioprinting [330,345,346] | Ceramics Polymers Hydrogel Metals | Easy process. High porosity with a controllable pore size and complete interconnectivity. Enhance cell attachment and regeneration. Capable of creating customized scaffolds that precisely fit the patient’s need. | Lack of mechanical strength. Lack of integrity. Use of toxic organic solvent. |
| Cell Type | Multi-Potentially | Source | Biomimetic Applications | CD * Antigen Expression | References |
|---|---|---|---|---|---|
| Dental pulp stem cells (DPSC) | Adipogenic Chondrogenic Myogenic Neurogenic Osteogenic Odontoblast | Pulp of natal, supernumerary, and impacted third molars. Inflamed pulp. Cryopreserved healthy molars and premolars. Diseased but vital teeth. | Regenerative endodontics Bone regeneration | Positive: CD9, CD10, CD13, CD29, CD44, CD49d, CD59, CD73, CD90, CD105, CD106, CD146, CD166 Negative: CD14, CD31, CD34, CD45, CD117, CD133 | [352,353,354,355,356,357] |
| Stem cells from human exfoliated deciduous teeth (SHED) | Adipogenic Chondrogenic Dentinogenic Myogenic Neurogenic Osteo-inductive Odontoblast | Remnant pulp of exfoliated deciduous teeth. | Regenerative endodontics and bone regeneration | Positive: CD13, CD44, CD73, CD90, CD105, CD146 Negative: CD14, CD19, CD34, CD43, CD45 | [358,359,360,361] |
| Periodontal ligament stem cells (PDLSC) | Adipogenic Chondrogenic Myogenic Neurogenic Osteogenic Cementogenic | Periodontal ligament (PDL) of healthy permanent teeth. Inflamed regenerating PDL from intrabony defects | Periodontal regeneration Bone regeneration | Positive: CD9, CD10, CD13, CD29, CD44, CD49d, CD59, CD73, CD90, CD105 CD106, CD146, CD166 Negative: CD31, CD34, CD45 | [353,362,363,364] |
| Dental follicle stem cells (DFSC) | Osteogenic, adipogenic, and periodontium-like tissues differentiation capacity Osteoblasts, Chondrocytes, Adipocytes | Normal human impacted third molars | Periodontal Regeneration Bone regeneration | Positive: CD9, CD10, CD13, CD29, CD44, CD49d, CD59, CD73, CD90, CD105, CD106, CD166 Negative: CD31, CD34, CD45, CD133 | [353,365,366,367] |
| Stem cells from apical papilla (SCAP) | Adipogenic Chondrogenic Dentinogenic Myogenic Neurogenic Odontoblast Cementoblast-like cells | Immature roots of normal human impacted third molars | Regenerative endodontics Bone regeneration | Positive: CD49d, CD51/61, CD56, CD73, CD90, CD105, CD106, CD146, CD166 Negative: CD14, CD18, CD34, CD45, | [352,358,368] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic Aspects of Restorative Dentistry Biomaterials. Biomimetics 2020, 5, 34. https://doi.org/10.3390/biomimetics5030034
Zafar MS, Amin F, Fareed MA, Ghabbani H, Riaz S, Khurshid Z, Kumar N. Biomimetic Aspects of Restorative Dentistry Biomaterials. Biomimetics. 2020; 5(3):34. https://doi.org/10.3390/biomimetics5030034
Chicago/Turabian StyleZafar, Muhammad Sohail, Faiza Amin, Muhmmad Amber Fareed, Hani Ghabbani, Samiya Riaz, Zohaib Khurshid, and Naresh Kumar. 2020. "Biomimetic Aspects of Restorative Dentistry Biomaterials" Biomimetics 5, no. 3: 34. https://doi.org/10.3390/biomimetics5030034
APA StyleZafar, M. S., Amin, F., Fareed, M. A., Ghabbani, H., Riaz, S., Khurshid, Z., & Kumar, N. (2020). Biomimetic Aspects of Restorative Dentistry Biomaterials. Biomimetics, 5(3), 34. https://doi.org/10.3390/biomimetics5030034









