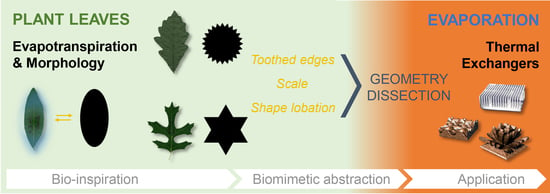
Biomimetic Groundwork for Thermal Exchange Structures Inspired by Plant Leaf Design
Abstract
1. Introduction
1.1. Thermal Design Innovation and Biomimetics
1.2. Plant Structures, Leaf Exchange, and Thermodynamics
2. Materials and Methods
- Literature review and identification of botanical case studies pointing to a relation between leaf thermal function and morphology patterns—listed as leaf role models;
- Definition of leaf morphotypes and shape features of interest involved in such relations, presumed relevant for leaf exchange and plant thermal management, based on botany and transfer physics literature;
- Identification of thermal design features and hypotheses abstracted from the leaf literature review—listed as leaf-inspired design principles;
- Biology research addressing one instance of the reviewed case studies, that of sun–shade leaf dimorphism in oak trees. The experimental approach involved shape analysis of oak leaves with single-parameter metrics. This tested the suitability of basic geometrical parameters for differentiating sun and shade leaves (Section 2.1);
- Translation of a subset of design principles into a family of two-dimensional abstract geometries, reflecting leaf “morphotypes” and some results of the shape analysis of oak leaves. Paper models were used as leaf analogs in “proof-of-concept” evaporation tests, to observe and compare the evaporative transfer of these leaf-inspired geometries (Section 2.3).
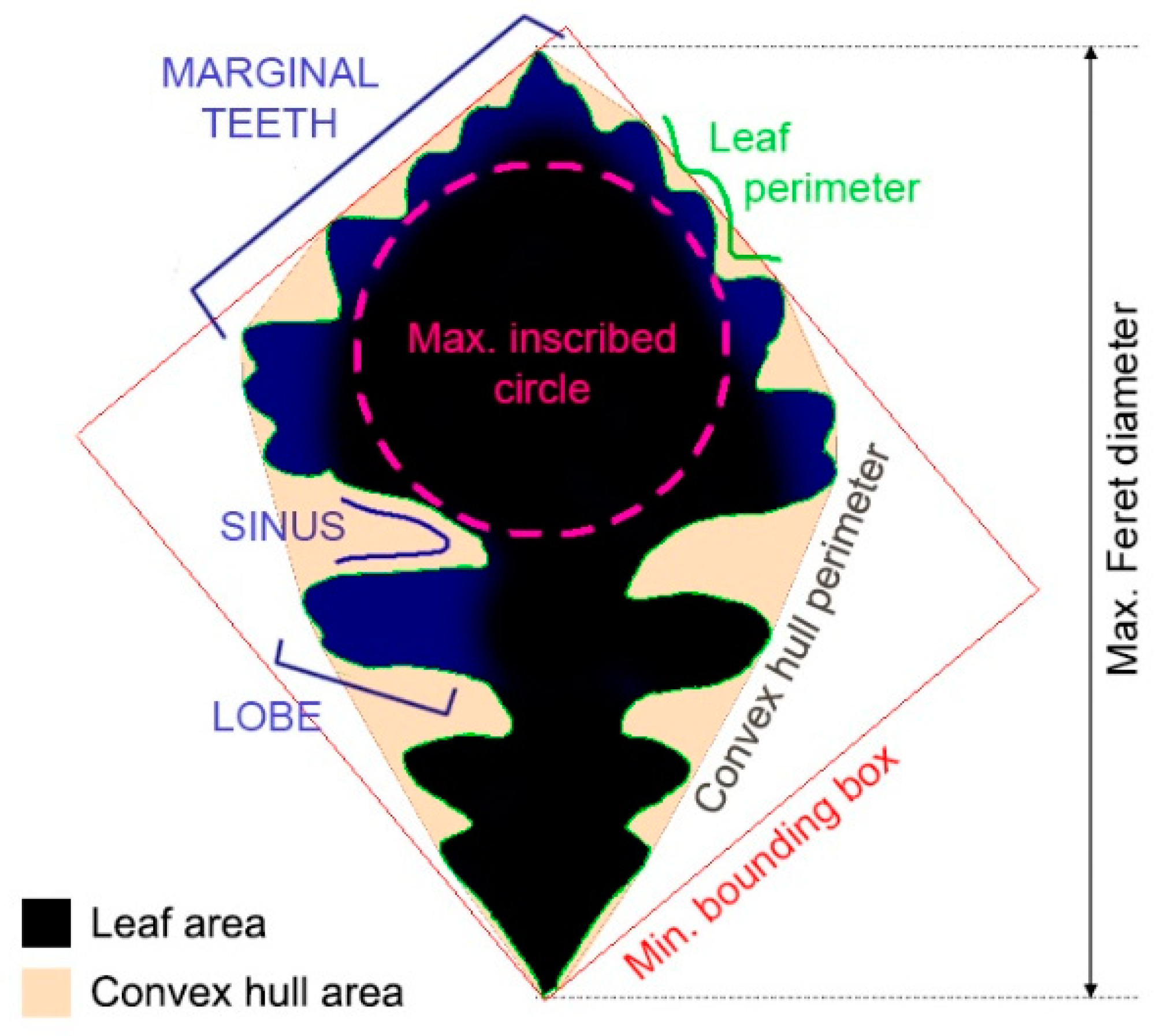
2.1. Biology Research: Shape Analysis of Oak Leaves
2.2. Biomimetic Design Principles: Abstracted Leaf-Inspired Geometries
2.3. Proof-of-Concept Evaporation Study with Bio-Inspired Paper Models
3. Results
3.1. Biology Research Findings
3.1.1. Leaf Role Models for Evaporative Thermal Design
3.1.2. Quantifying Oak Leaf Dimorphism
3.2. Leaf-Inspired Design Principles
3.2.1. Geometrical Abstraction from Leaf Role Models
3.2.2. Leaf-Inspired Morphotypical Geometries
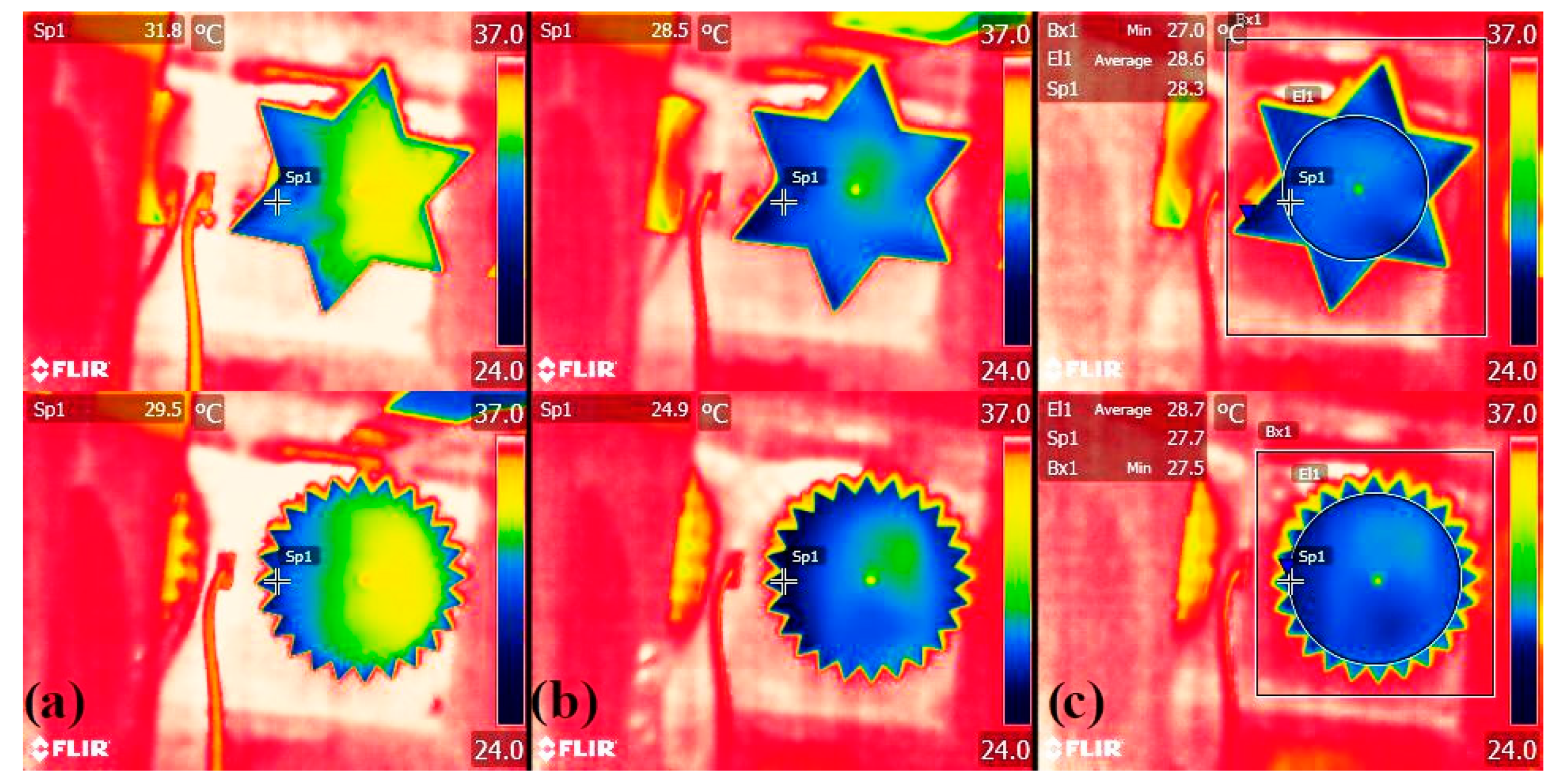
3.3. Proof-Of-Concept Evaporation Tests
4. Discussion
4.1. Mass Transfer Enhanced by Geometry Dissection
4.2. Biological and Biomimetic Significance
4.2.1. Heterophyllous Leaf Dissection
4.2.2. Sun–Shade Leaf Dimorphism
4.2.3. Marginal Teeth
4.2.4. Leaves from Temperate to Tropical Climates as Exchangers
4.2.5. The Multifactorial and Multifunctional Realm of Leaf Morphology
4.3. Applicability of Shape Parameters
4.3.1. Sun–Shade Leaf Morphology
4.3.2. Shape Parameters Relation to Evaporative Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chu, K.; Zhu, Y.; Miljkovic, N.; Nam, Y.; Enright, R.; Wang, E.N. Enhanced boiling heat transfer with copper oxide hierarchical surfaces. In Proceedings of the 2013 Transducers Eurosensors XXVII, 17th International Conference on Solid-State Sensors, Actuators and Microsystems, Barcelona, Spain, 16–20 June 2013; pp. 2272–2275, ISBN 978-1-4673-5983-2. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, G.; Chen, Q.; Zeng, M. Review of Improvements on Shell-and-Tube Heat Exchangers with Helical Baffles. Heat Transf. Eng. 2010, 31, 836–853. [Google Scholar] [CrossRef]
- Incropera, F.P. Fundamentals of Heat and Mass Transfer; John Wiley: Hoboken, NJ, USA, 2007; pp. 574–576. [Google Scholar]
- Jaluria, Y. Computational Heat Transfer; Routledge: Abingdon, UK, 2017; pp. 203–222. [Google Scholar]
- Shang, J.S. Three decades of accomplishments in computational fluid dynamics. Prog. Aerosp. Sci. 2004, 40, 173–197. [Google Scholar] [CrossRef]
- Shah, R.K.; Sekulic, D.P. Fundamentals of Heat Exchanger Design; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 78–80. [Google Scholar]
- Angilletta, M.J., Jr.; Angilletta, M.J. Thermal Adaptation: A Theoretical and Empirical Synthesis; OUP Oxford: Oxford, UK, 2009; p. 126. [Google Scholar]
- Cafsia, L. Plyskin; insulating façade panel based on Biomimicry of the polar bear skin and fur. In Proceedings of the IABSE Conference, Bath, UK, 19–20 April 2017; IABSE Symposium Report. Volume 108, pp. 58–59. [Google Scholar] [CrossRef]
- Dongjin, C. Sea Otter Fur Structure Imitated Waterproof and Warm-Keeping Fabric and Preparation Method Thereof. CN Patent CN108330703A, 27 July 2018. [Google Scholar]
- Transport of Heat and Mass in Natural Porous Materials with Graded Structure. Available online: https://www.trr141.de/index.php/research-areas-2/research-areas/ (accessed on 20 May 2019).
- Turner, J.S.; Soar, R.C. Beyond biomimicry: What termites can tell us about realizing the living building. In Proceedings of the First International Conference on Industrialized, Intelligent Construction (I3CON), Loughborough University, Loughborough, UK, 14–16 May 2008. [Google Scholar]
- Kerbel, M.; Kulyk, R. Method and Apparatus for Managing an Energy Consuming Load. U.S. Patent 8,527,109B2, 3 September 2013. [Google Scholar]
- Smith, C.A.; Bernett, A.; Hanson, E.; Garvin, C. Tapping into Nature; Terrapin Bright Green LLC: New York, NY, USA, 2015; p. 27. [Google Scholar]
- Fraunhofer, I.S.E. FracTherm® Technology in Roll-Bond Solar Absorbers; Fraunhofer Institute for Solar Energy Systems ISE Press Release: Freiburg, Germany, 2013. [Google Scholar]
- Reichert, S.; Menges, A.; Correa, D. Meteorosensitive architecture: Biomimetic building skins based on materially embedded and hygroscopically enabled responsiveness. Comput.-Aided Des. 2015, 60, 50–69. [Google Scholar] [CrossRef]
- Kim, H.; Kim, K.; Lee, S.J. Compact and Thermosensitive Nature-inspired Micropump. Sci. Rep. 2016, 6, 36085. [Google Scholar] [CrossRef] [PubMed]
- Lütz, C. Plants in Alpine Regions: Cell Physiology of Adaption and Survival Strategies; Springer Science & Business Media: Berlin, Germany, 2011; pp. 62–63. [Google Scholar]
- Gates, D.M. Biophysical Ecology; Springer Inc.: New York, NY, USA, 1980; pp. 25–28. [Google Scholar]
- Sinha, R.K. Modern Plant Physiology; CRC Press: Boca Raton, FL, USA, 2004; p. 84. [Google Scholar]
- Mahan, J.R.; Upchurch, D.R. Maintenance of constant leaf temperature by plants—I. Hypothesis-limited homeothermy. Environ. Exp. Bot. 1988, 28, 351–357. [Google Scholar] [CrossRef]
- Michaletz, S.T.; Weiser, M.D.; Zhou, J.; Kaspari, M.; Helliker, B.R.; Enquist, B.J. Plant Thermoregulation: Energetics, Trait—Environment Interactions, and Carbon Economics. Trends Ecol. Evol. 2015, 30, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.F.V.; Bogatyreva, O.A.; Bogatyrev, N.R.; Bowyer, A.; Pahl, A.-K. Biomimetics: Its practice and theory. J. R. Soc. Interface 2006, 3, 471–482. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, W.; Mou, J.; Zheng, S.; Jiang, L.; Sun, Z.; Wang, E. Research progress of biomimetic superhydrophobic surface characteristics, fabrication, and application. Adv. Mech. Eng. 2017, 9, 1687814017746859. [Google Scholar] [CrossRef]
- Miguel, S.; Hehn, A.; Bourgaud, F. Nepenthes: State of the art of an inspiring plant for biotechnologists. J. Biotechnol. 2018, 265, 109–115. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, X.; Xing, Z.; Tu, T. Exploring the underwater air-retaining ability and thermal insulating effect of terry fabrics inspired by Salvinia molesta. Text. Res. J. 2018. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Leigh, A.; Boyce, C.K.; Jones, C.S.; Niklas, K.J.; Royer, D.L.; Tsukaya, H. The evolution and functional significance of leaf shape in the angiosperms. Funct. Plant Biol. 2011, 38, 535–552. [Google Scholar] [CrossRef]
- Helms, M.; Vattam, S.S.; Goel, A.K. Biologically inspired design: Process and products. Des. Stud. 2009, 30, 606–622. [Google Scholar] [CrossRef]
- Gratani, L. Plant Phenotypic Plasticity in Response to Environmental Factors. Adv. Bot. 2014, 208747. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2016, 15, 259–263. [Google Scholar] [CrossRef]
- Wagner, T.; Lipinski, H.-G. IJBlob: An ImageJ Library for Connected Component Analysis and Shape Analysis. J. Open Res. Softw. 2013, 1, e6. [Google Scholar]
- Leigh, A.; Sevanto, S.; Close, J.D.; Nicotra, A.B. The influence of leaf size and shape on leaf thermal dynamics: Does theory hold up under natural conditions? Plant Cell Environ. 2017, 40, 237–248. [Google Scholar] [CrossRef]
- Schuepp, P. Tansley review no. 59 leaf boundary layers. New Phytol. 1993, 125, 477–507. [Google Scholar] [CrossRef]
- Gruber, P.; Rupp, A.I.K.S. Investigation of leaf shape and edge design for faster evaporation in biomimetic heat dissipation systems. In Bioinspiration, Biomimetics, and Bioreplication VIII, Proceedings of the SPIE Smart Structures and Materials + Nondestructive Evaluation and Health Monitoring, Denver, CO, USA, 5–7 March 2018; SPIE-International Society for Optics and Photonics: Bellingham, WA, USA, 2018; Volume 10593, p. 105930H. [Google Scholar]
- Royer, D.L.; Wilf, P.; Janesko, D.A.; Kowalski, E.A.; Dilcher, D.L. Correlations of climate and plant ecology to leaf size and shape: Potential proxies for the fossil record. Am. J. Bot. 2005, 92, 1141–1151. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, H.; Ahn, S. Water transport in porous hydrogel structures analogous to leaf mesophyll cells. Microfluid. Nanofluidics 2015, 18, 775–784. [Google Scholar] [CrossRef]
- Shahraeeni, E.; Lehmann, P.; Or, D. Coupling of evaporative fluxes from drying porous surfaces with air boundary layer: Characteristics of evaporation from discrete pores. Water Resour. Res. 2012, 48. [Google Scholar] [CrossRef]
- Winn, A.A. The Functional Significance and Fitness Consequences of Heterophylly. Int. J. Plant Sci. 1999, 160, S113–S121. [Google Scholar] [CrossRef] [PubMed]
- Schmerler, S.B.; Clement, W.L.; Beaulieu, J.M.; Chatelet, D.S.; Sack, L.; Donoghue, M.J.; Edwards, E.J. Evolution of leaf form correlates with tropical-temperate transitions in Viburnum (Adoxaceae). Proc. Biol. Sci. 2012, 279, 3905–3913. [Google Scholar] [CrossRef] [PubMed]
- Peppe, D.J.; Royer, D.L.; Cariglino, B.; Oliver, S.Y.; Newman, S.; Leight, E.; Enikolopov, G.; Fernandez-Burgos, M.; Herrera, F.; Adams, J.M.; et al. Sensitivity of leaf size and shape to climate: Global patterns and paleoclimatic applications. New Phytol. 2011, 190, 724–739. [Google Scholar] [CrossRef]
- Talbert, C.M.; Holch, A.E. A study of the lobing of sun and shade leaves. Ecology 1957, 38, 655–658. [Google Scholar] [CrossRef]
- Sack, L.; Melcher, P.J.; Liu, W.H.; Middleton, E.; Pardee, T. How strong is intracanopy leaf plasticity in temperate deciduous trees? Am. J. Bot. 2006, 93, 829–839. [Google Scholar] [CrossRef]
- Billings, F.H. Precursory Leaf Serrations of Ulmus. Bot. Gaz. 1905, 40, 224–225. [Google Scholar] [CrossRef][Green Version]
- Macmillan, R.J. Aspects of heterophylly in Morus alba. Ph.D. Thesis, City University of New York, New York, NY, USA, 2002. [Google Scholar]
- Lewis, M.C. Genecological differentiation of leaf morphology in Geranium sanguineum L. New Phytol. 1969, 68, 481–503. [Google Scholar] [CrossRef]
- Royer, D.L.; McElwain, J.C.; Adams, J.M.; Wilf, P. Sensitivity of leaf size and shape to climate within Acer rubrum and Quercus kelloggii. New Phytol. 2008, 179, 808–817. [Google Scholar] [CrossRef]
- Royer, D.L.; Wilf, P. Why do Toothed Leaves Correlate with Cold Climates? Gas Exchange at Leaf Margins Provides New Insights into a Classic Paleotemperature Proxy. Int. J. Plant Sci. 2006, 167, 11–18. [Google Scholar] [CrossRef]
- Vogel, S. Leaves in the lowest and highest winds: Temperature, force and shape. New Phytol. 2009, 183, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S. ‘Sun Leaves’ and ‘Shade Leaves’: Differences in Convective Heat Dissipation. Ecology 1968, 49, 1203–1204. [Google Scholar] [CrossRef]
- Zwieniecki, M.A.; Boyce, C.K.; Holbrook, N.M. Hydraulic limitations imposed by crown placement determine final size and shape of Quercus rubra L. leaves. Plant Cell Environ. 2005, 27, 357–365. [Google Scholar] [CrossRef]
- Jebb, M.H. A revision of the genus Trevesia (Araliaceae). Glasra 1998, 3, 85–113. [Google Scholar]
- Gray, E.; Gray, R.E. Leaf Lobation Patterns in Mulberry. Castanea 1987, 52, 216–224. [Google Scholar]
- Gottschlich, D.E.; Smith, A.P. Convective heat transfer characteristics of toothed leaves. Oecologia 1982, 53, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Bergles, A.E. Some Perspectives on Enhanced Heat Transfer—Second-Generation Heat Transfer Technology. J. Heat Transf. 1988, 110, 1082–1096. [Google Scholar] [CrossRef]
- Llano-Sánchez, L.E.; Domínguez-Cajeli, D.M.; Ruiz-Cárdenas, L.C. Thermal transfer analysis of tubes with extended surface with fractal design. Fac. Ing. 2018, 27, 31–37. [Google Scholar] [CrossRef]
- Kuddusi, L.; Eğrican, N. A critical review of constructal theory. Energy Convers. Manag. 2008, 49, 1283–1294. [Google Scholar] [CrossRef]
- Xu, P.; Yu, B.; Yun, M.; Zou, M. Heat conduction in fractal tree-like branched networks. Int. J. Heat Mass Transf. 2006, 49, 3746–3751. [Google Scholar] [CrossRef]
- Huang, Z.; Hwang, Y.; Aute, V.; Radermacher, R. Review of Fractal Heat Exchangers. In Proceedings of the 16th International Refrigeration and Air Conditioning Conference, Purdue, IN, USA, 11–14 July 2016. [Google Scholar]
- Herr, M. Design Criteria for Low-Noise Trailing-Edges. In Proceedings of the 13th AIAA/CEAS Aeroacoustics Conference, Rome, Italy, 21–23 May 2007; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2007. [Google Scholar] [CrossRef]
- Wells, C.L.; Pigliucci, M. Adaptive phenotypic plasticity: The case of heterophylly in aquatic plants. Perspect. Plant Ecol. Evol. Syst. 2000, 3, 1–18. [Google Scholar] [CrossRef]
- Rice, S.K.; Schuepp, P.H. On the ecological and evolutionary significance of branch and leaf morphology in aquatic Sphagnum (Sphagnaceae). Am. J. Bot. 1995, 82, 833–846. [Google Scholar] [CrossRef]
- Potter, K.; Davidowitz, G.; Woods, H.A. Insect eggs protected from high temperatures by limited homeothermy of plant leaves. J. Exp. Biol. 2009, 212, 3448–3454. [Google Scholar] [CrossRef] [PubMed]
- Slot, M.; Krause, G.H.; Krause, B.; Hernández, G.G.; Winter, K. Photosynthetic heat tolerance of shade and sun leaves of three tropical tree species. Photosynth. Res. 2018, 141, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Terashima, I.; Miyazawa, S.-I.; Hanba, Y.T. Why are sun leaves thicker than shade leaves?—Consideration based on analyses of CO2 diffusion in the leaf. J. Plant Res. 2001, 114, 93–105. [Google Scholar] [CrossRef]
- Schymanski, S.J.; Or, D.; Zwieniecki, M. Stomatal Control and Leaf Thermal and Hydraulic Capacitances under Rapid Environmental Fluctuations. PLoS ONE 2013, 8, e54231. [Google Scholar] [CrossRef]
- Givnish, T.J.; Kriebel, R. Causes of ecological gradients in leaf margin entirety: Evaluating the roles of biomechanics, hydraulics, vein geometry, and bud packing. Am. J. Bot. 2017, 104, 354–366. [Google Scholar] [CrossRef]
- Royer, D.L. Climate reconstruction from leaf size and shape: New developments and challenges. Paleontol. Soc. Pap. 2012, 18, 195–212. [Google Scholar] [CrossRef][Green Version]
- Seddon, G. Xerophytes, xeromorphs and sclerophylls: The history of some concepts in ecology. Biol. J. Linn. Soc. 1974, 6, 65–87. [Google Scholar] [CrossRef]
- Wickens, G.E. Ecophysiology of Economic Plants in Arid and Semi-Arid Lands; Springer Science & Business Media: Berlin, Germany, 2013; pp. 155–160. [Google Scholar]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef]
- Parkhurst, D.F.; Loucks, O. Optimal leaf size in relation to environment. J. Ecol. 1972, 60, 505–537. [Google Scholar] [CrossRef]
- Bradshaw, A.D. Evolutionary Significance of Phenotypic Plasticity in Plants. Adv. Genet. 1965, 13, 115–155. [Google Scholar]
- Chitwood, D.H.; Rundell, S.M.; Li, D.Y.; Woodford, Q.L.; Yu, T.T.; Lopez, J.R.; Greenblatt, D.; Kang, J.; Londo, J.P. Climate and Developmental Plasticity: Interannual Variability in Grapevine Leaf Morphology. Plant Physiol. 2016, 170, 1480–1491. [Google Scholar] [CrossRef]
- Vaz Monteiro, M.; Blanuša, T.; Verhoef, A.; Hadley, P.; Cameron, R.W. Relative importance of transpiration rate and leaf morphological traits for the regulation of leaf temperature. Aust. J. Bot. 2016, 64, 32–44. [Google Scholar] [CrossRef]
- Grace, J.; Wilson, J. The Boundary Layer over a Populus Leaf. J. Exp. Bot. 1976, 27, 231–241. [Google Scholar] [CrossRef]
- Yamazaki, K. Gone with the wind: Trembling leaves may deter herbivory. Biol. J. Linn. Soc. 2011, 104, 738–747. [Google Scholar] [CrossRef]
- Collatz, G.J.; Ball, J.T.; Grivet, C.; Berry, J.A. Physiological and environmental regulation of stomatal conductance, photosynthesis and transpiration: A model that includes a laminar boundary layer. Agric. Meteorol. 1991, 54, 107–136. [Google Scholar] [CrossRef]
- Rohsenow, W.M.; Hartnett, J.P.; Ganic, E.N. Handbook of Heat Transfer Applications; McGraw-Hill Book Co: New York, NY, USA, 1985; pp. 1–7. [Google Scholar]
- McLellan, T.; Endler, J.A. The Relative Success of Some Methods for Measuring and Describing the Shape of Complex Objects. Syst. Biol. 1998, 47, 264–281. [Google Scholar] [CrossRef]
- Biot, E.; Cortizo, M.; Burguet, J.; Kiss, A.; Oughou, M.; Maugarny-Calès, A.; Gonçalves, B.; Adroher, B.; Andrey, P.; Boudaoud, A.; et al. Multiscale quantification of morphodynamics: MorphoLeaf software for 2D shape analysis. Development 2016, 143, 3417–3428. [Google Scholar] [CrossRef]
- Parkhurst, D.F.; Duncan, P.R.; Gates, D.M.; Kreith, F. Wind-tunnel modelling of convection of heat between air and broad leaves of plants. Agric. Meteorol. 1968, 5, 33–47. [Google Scholar] [CrossRef]
- Lusk, C.H.; Clearwater, M.J.; Laughlin, D.C.; Harrison, S.P.; Prentice, I.C.; Nordenstahl, M.; Smith, B. Frost and leaf-size gradients in forests: Global patterns and experimental evidence. New Phytol. 2018, 219, 565–573. [Google Scholar] [CrossRef] [PubMed]
| Leaf Shape Variation | Plant Case Studies, Reported Observations | References |
|---|---|---|
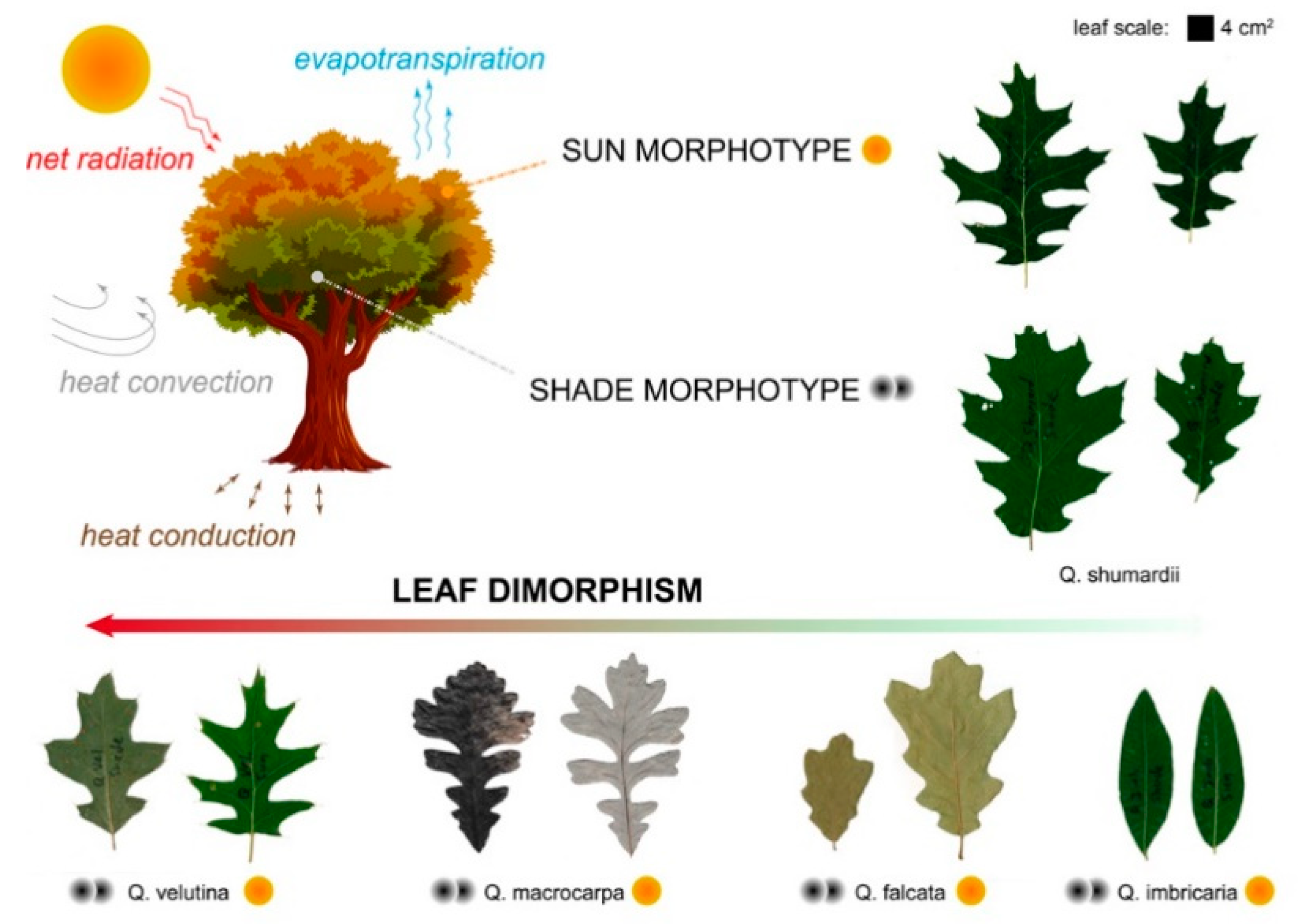
| Temporal variation: SEASONAL • summer vs. winter heterophylly | Southern coastal violet (Viola septemloba): Developed more lobed leaves in summer, which maintained lower leaf temperatures | [38] |
| Spatial variation: GLOBAL • geographical trends • plant local adaptation | Geranium sanguineum: Leaves develop more elongated lobes in drier, continental habitats. | [45] |
| Viburnum (Adoxaceae) clade: Leaves in warmer climates are more elongated and entire, overall. | [39] | |
| LEAF • developing leaves • margin transpiration | Maple (Acer genus): Greater transpiration at the margins of young leaves; leaves grown in colder environments become more dissected, develop more numerous and larger marginal teeth. | [46,47] |
| Elm (Ulmus genus): Leaf tissues with evaporative role develop prematurely at the margins of young leaves. | [43] | |
| PLANT CANOPY • sun vs. shade leaf dimorphism | Oak (Quercus genus): Sun leaves have deeper lobes and greater transpiring capacity; transpiring sun leaves reach colder temperatures than shade leaves; sun leaf models in low wind convect heat better, more independently of orientation thanks to sinuses. | [40,48,49,50] |
| Combined variation temporal and spatial: PLANT LIFETIME • heteroblasty • young vs. mature plants | Snowflake Aralia (Trevesia palmata): Sun leaves are more dissected; young plants have palmately lobed leaves, mature plants have pseudo-compound leaves. | [51] |
| Mulberry (Morus genus): Lobes develop preferentially on leaves’ outer side; many-lobed leaves retain lower temperatures; young plants have more lobed leaves. | [44,52] |
| Shape Parameter | Leaf Type | Mean | SD | Dif. | t ratio | Std Err Dif. | df | p | 95% CL Dif. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||||
| Perimeter (cm) | SUN | 59 | 24 | 14 | 4.72 | 3.06 | 182.81 | <0.0001 | 8 | 20 |
| SHADE | 45 | 19 | ||||||||
| LDI (normalized perimeter) | SUN | 89 | 47 | 26 | 4.30 | 6.02 | 184.07 | <0.0001 | 14 | 38 |
| SHADE | 63 | 38 | ||||||||
| Convexity | SUN | 0.55 | 0.15 | −0.08 | −3.75 | 0.02 | 203.46 | 0.0002 | −0.13 | −0.04 |
| SHADE | 0.64 | 0.17 | ||||||||
| Roundness | SUN | 0.60 | 0.08 | −0.04 | −3.28 | 0.01 | 186.64 | 0.001 | −0.07 | −0.02 |
| SHADE | 0.64 | 0.10 | ||||||||
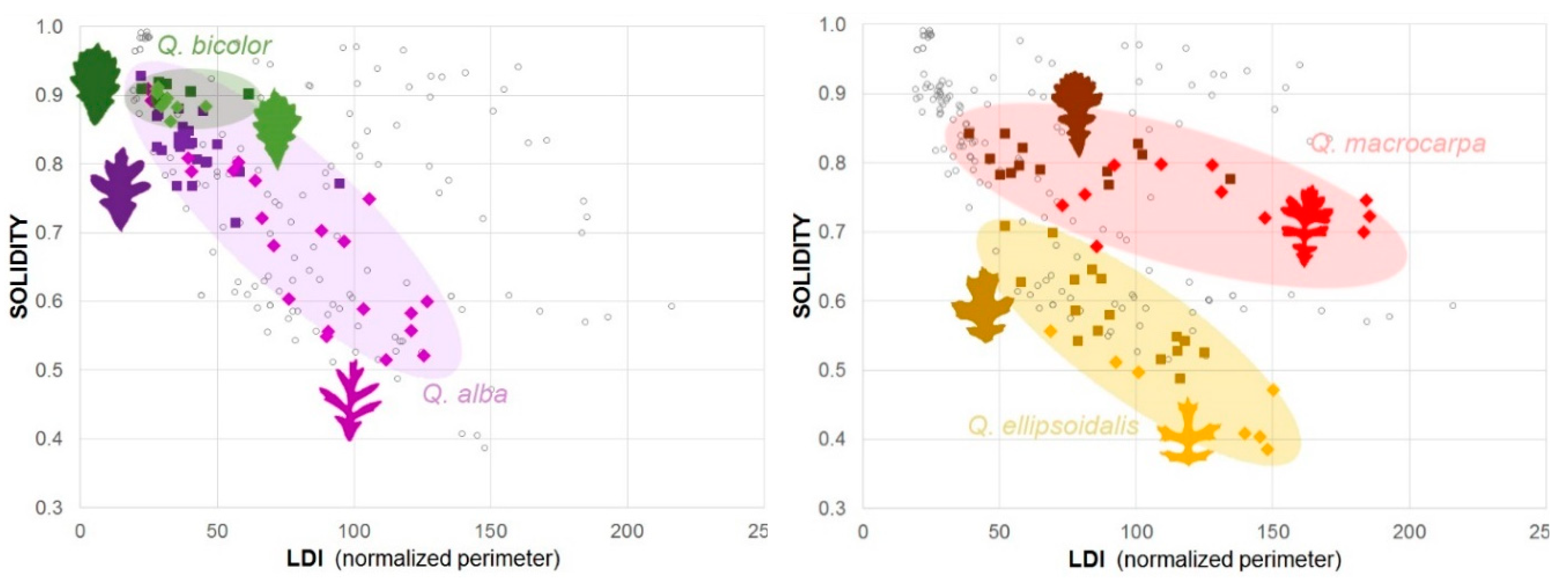
| Oak Species (Sample Size) | Parameter | Leaf Type | Mean | SD | Dif. | t Ratio | Std Err Dif. | df | p | 95% CL Dif. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||||||
| Q. alba2 | ||||||||||||
| Nsun = 21 |  | LDI | SUN | 81 | 33 | 38 | 4.89 | 7.71 | 27.14 | <0.0001 | 22 | 54 |
| SHADE | 43 | 15 | ||||||||||
| Nsha = 26 |  | Solidity | SUN | 0.68 | 0.12 | −0.13 | −4.53 | 0.03 | 28.45 | <0.0001 | −0.19 | −0.07 |
| SHADE | 0.82 | 0.06 | ||||||||||
| Q. macrocarpa2 | ||||||||||||
| Nsun = 11 |  | LDI | SUN | 127 | 43 | 55 | 3.61 | 15.19 | 16.80 | 0.0022 | 23 | 87 |
| SHADE | 72 | 28 | ||||||||||
| Nsha = 13 |  | Solidity | SUN | 0.75 | 0.04 | −0.06 | −4.04 | 0.01 | 16.02 | 0.0009 | −0.09 | −0.02 |
| SHADE | 0.80 | 0.02 | ||||||||||
| Q. ellipsoidalis2 | ||||||||||||
| Nsun = 7 |  | LDI | SUN | 121 | 33 | 29 | 2.16 | 13.65 | 8.59 | 0.0609 | −2 | 61 |
| SHADE | 91 | 23 | ||||||||||
| Nsha = 16 |  | Solidity | SUN | 0.46 | 0.06 | −0.12 | −4.17 | 0.03 | 11.79 | 0.0014 | −0.19 | −0.06 |
| SHADE | 0.58 | 0.07 | ||||||||||
| BIOLOGY Plant Role Model | ABSTRACTION Shape Features, Transfer Hypotheses | APPLICATION Transfer Regime |
|---|---|---|
| Viola septemloba Geranium sanguineum | • Elliptic lobation • Lobe elongation Elliptic lobes in two-dimensional exchangers enhance convection. Elliptic lobes of higher aspect ratio (elongated) enhance convection. | SENSIBLE HEAT convective cooling |
| Viburnum genus | • Leaf blade elongation Shapes of high aspect ratio enhance convection. | |
| Acer rubrum | • Toothed edges • Teeth proportions and shape Toothed edges, especially with proportionally large teeth, enhance vapor dissipation. | LATENT HEAT evaporation |
| Ulmus genus | • Hierarchically toothed edges Hierarchical teeth enhance vapor dissipation. | |
| Quercus genus | • Sinus profile in lobed shapes Sinuses of lobed shapes enhance orientation-independence of transfer in free convection, and inclination-independence under strong airflows. | HEAT and MASS TRANSFER evaporative cooling |
| Trevesia palmata | • Compoundness • Fenestration A large surface dissected into semi-distinct, space-filling parts enhances transfer. | |
| Morus genus | • Circular lobation • Convex teeth A hierarchical design of major obovate lobes and marginal curved teeth enhances transfer. |
| Geometry (i.d. and Visual) | Abstract Design Features | Relative 1 Perimeter | LDI | Relative 1 Max. Inscribed Circle Diameter | Relative 1 Leff 2 (Effective Dimension) | Protrusion to Core Ratio | Convexity | Solidity | |
|---|---|---|---|---|---|---|---|---|---|
| C |  | circular, control | 1 | 13 | 1 | 1 | 0 | 1 | 1 |
| E |  | ellipse, aspect ratio and elongation | 1.1 | 15 | 0.70 | 0.70 2 | 0 | 1 | 1 |
| L |  | “lobes”, few and large protrusions | 1.5 | 28 | 0.78 | 0.79 | 0.4 | 0.87 | 0.67 |
| T |  | marginal teeth, many small protrusions | 1.8 | 41 | 0.9 | 0.82 | 0.2 | 0.61 | 0.83 |
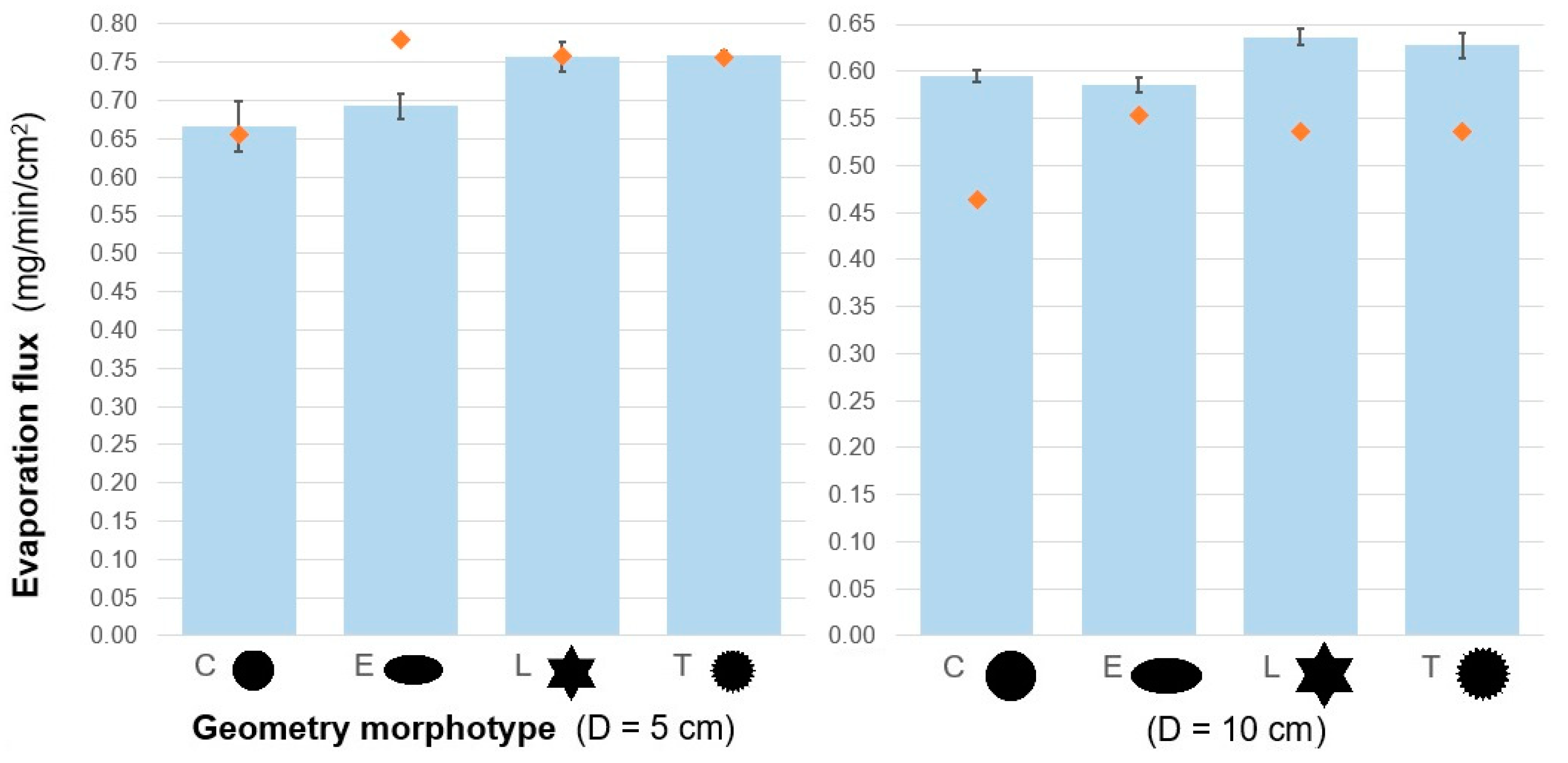
| Characteristic Dimension | Shape | Evap. Flux Mean 1 (mg/min/cm2) | SD | Std Err | Relative2 Enhancement | df | F Ratio | p | |
|---|---|---|---|---|---|---|---|---|---|
| D = 5 cm | C |  | 0.67 | 0.03 | 0.01 | - | 3 | 19.08 | <0.0001 |
| E |  | 0.69 | 0.02 | 0.01 | +4% | ||||
| L |  | 0.76 | 0.02 | 0.01 | +14% | ||||
| T |  | 0.76 | 0.01 | 0.01 | +14% | ||||
| D = 10 cm | C |  | 0.59 | 0.01 | 0.005 | - | 3 | 28.89 | <0.0001 |
| E |  | 0.59 | 0.01 | 0.005 | −1% | ||||
| L |  | 0.64 | 0.01 | 0.005 | +7% | ||||
| T |  | 0.63 | 0.01 | 0.005 | +6% | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rupp, A.I.K.S.; Gruber, P. Biomimetic Groundwork for Thermal Exchange Structures Inspired by Plant Leaf Design. Biomimetics 2019, 4, 75. https://doi.org/10.3390/biomimetics4040075
Rupp AIKS, Gruber P. Biomimetic Groundwork for Thermal Exchange Structures Inspired by Plant Leaf Design. Biomimetics. 2019; 4(4):75. https://doi.org/10.3390/biomimetics4040075
Chicago/Turabian StyleRupp, Ariana I. K. S., and Petra Gruber. 2019. "Biomimetic Groundwork for Thermal Exchange Structures Inspired by Plant Leaf Design" Biomimetics 4, no. 4: 75. https://doi.org/10.3390/biomimetics4040075
APA StyleRupp, A. I. K. S., & Gruber, P. (2019). Biomimetic Groundwork for Thermal Exchange Structures Inspired by Plant Leaf Design. Biomimetics, 4(4), 75. https://doi.org/10.3390/biomimetics4040075