Towards a Dynamic Model of the Kangaroo Knee for Clinical Insights into Human Knee Pathology and Treatment: Establishing a Static Biomechanical Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection
2.2. Specimen Imaging
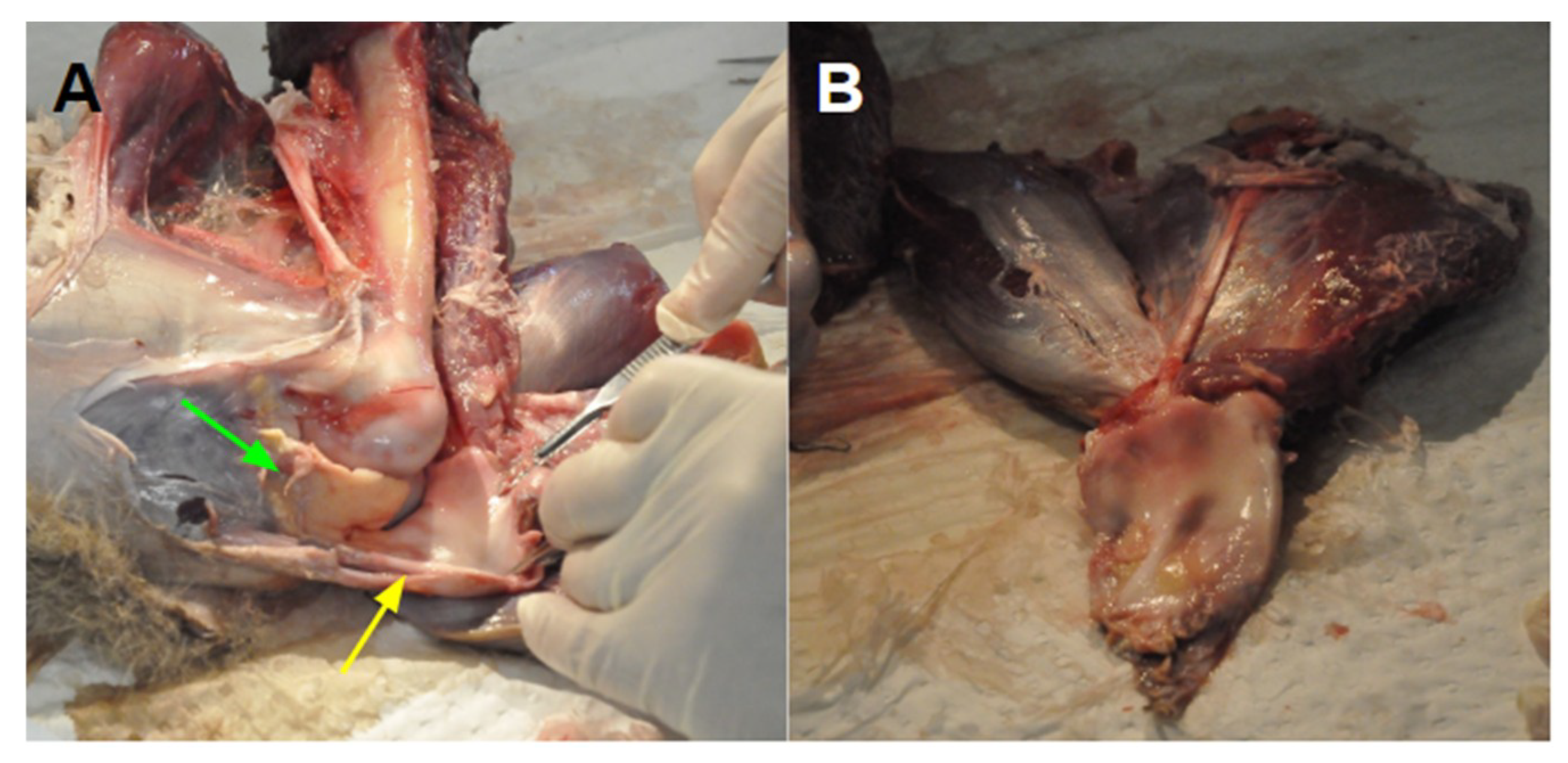
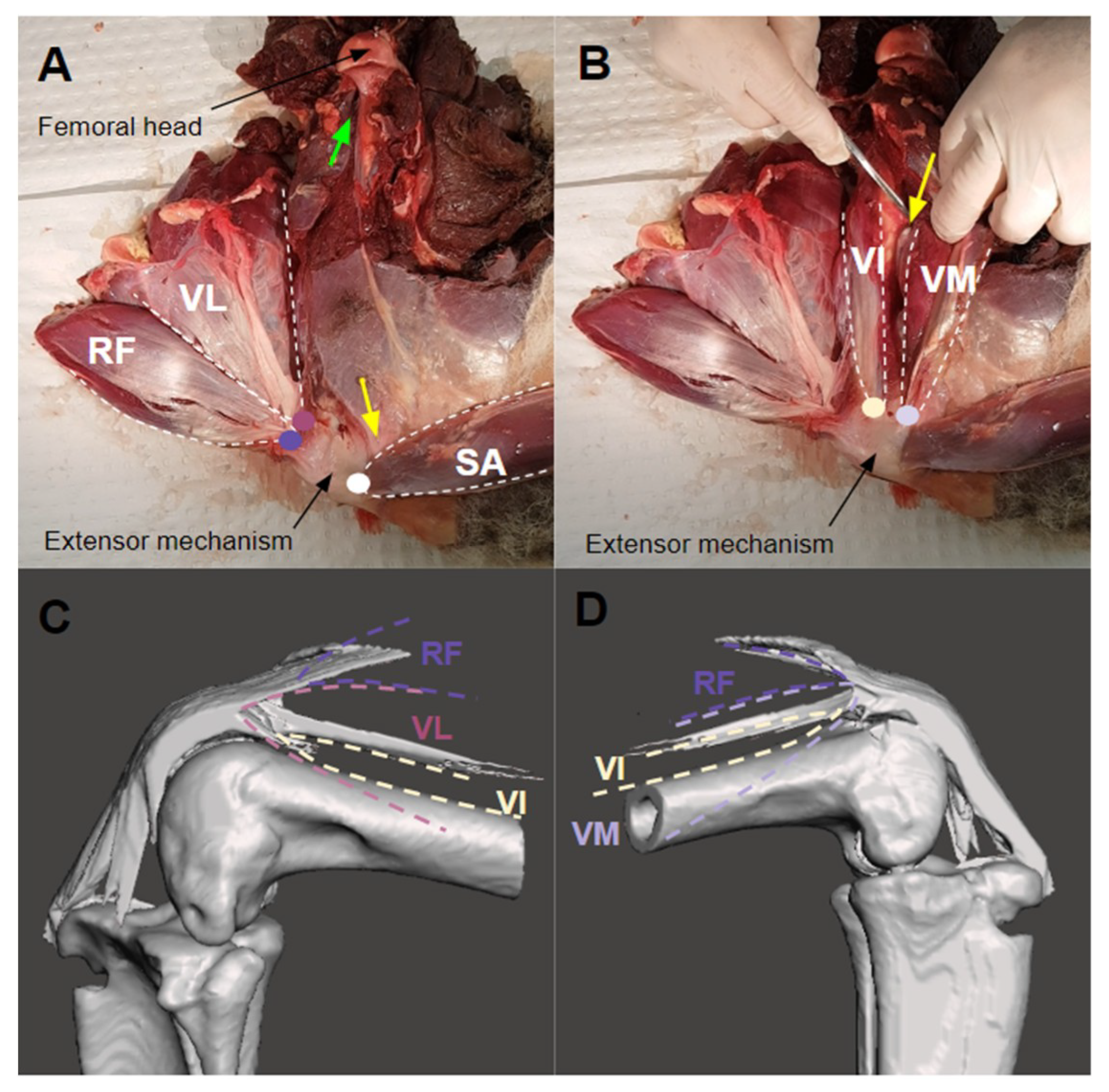
2.3. Specimen Dissection
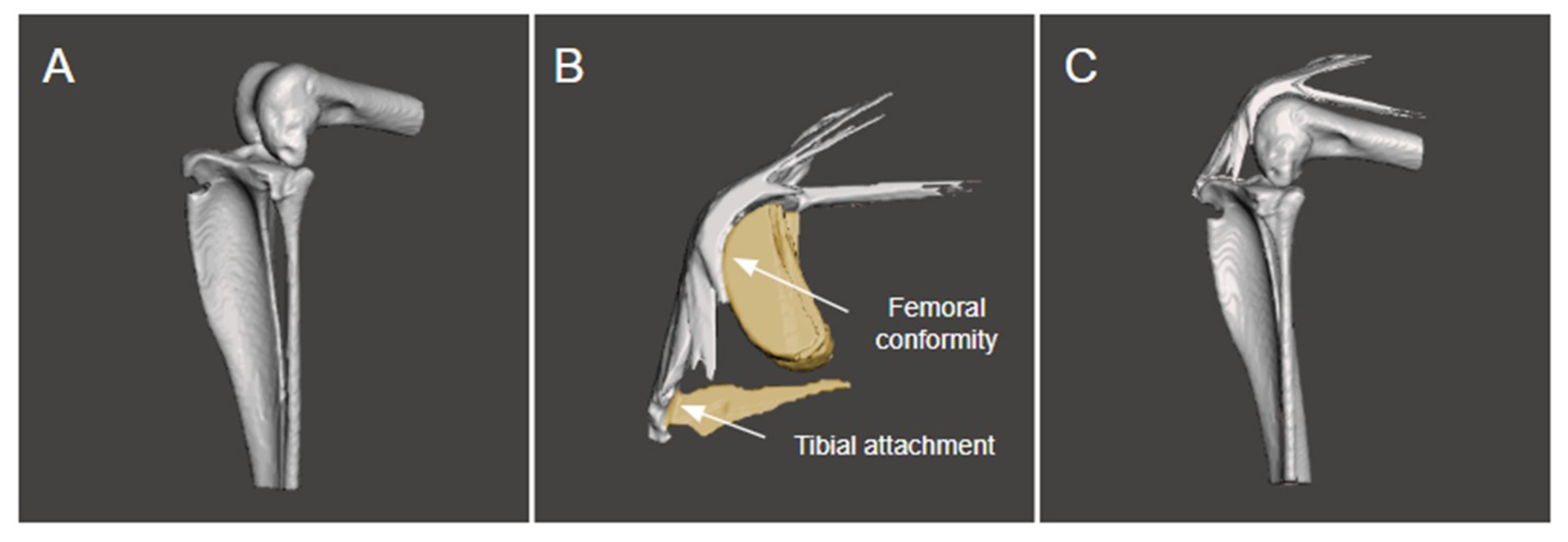
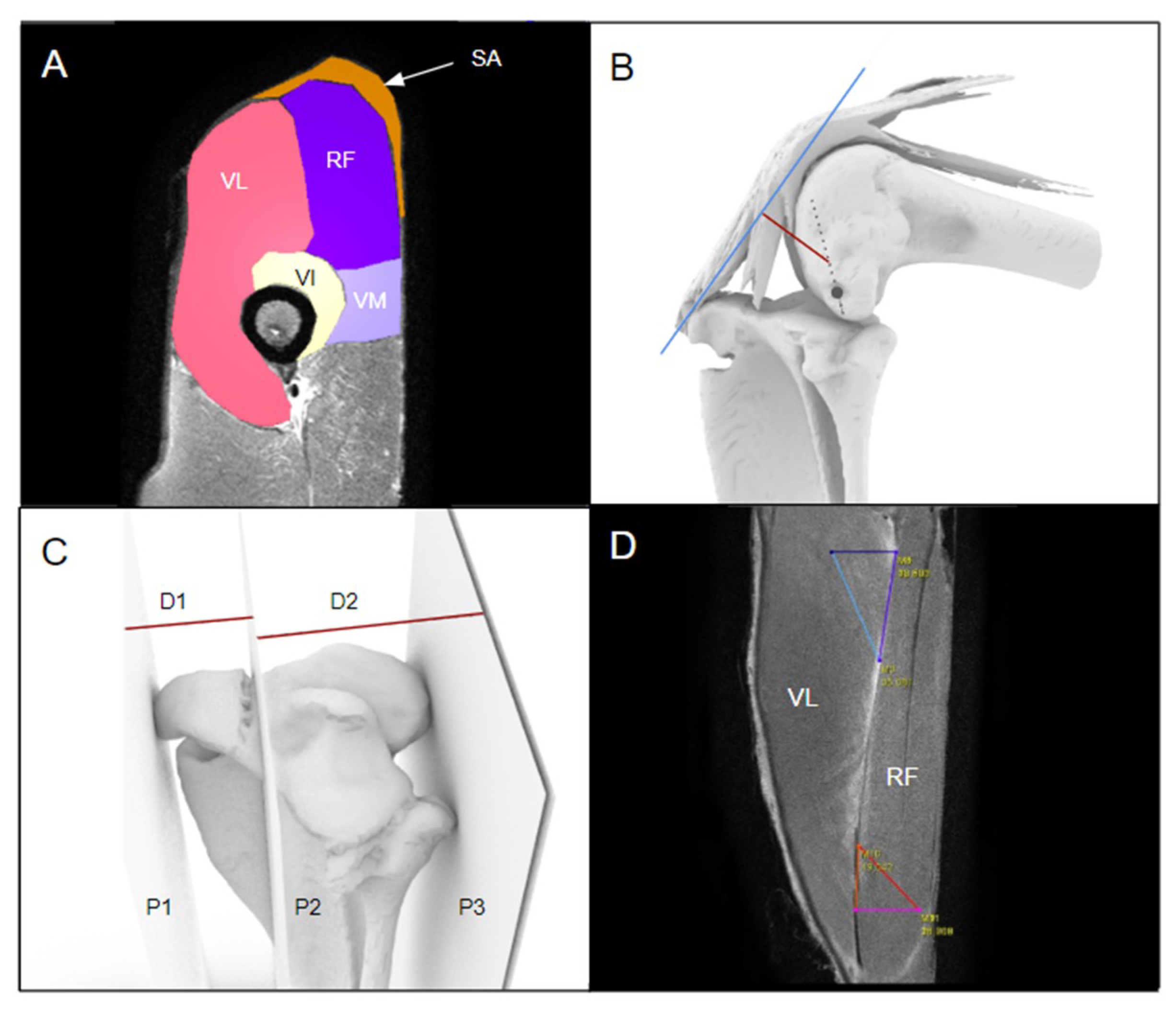
2.4. Modelling the Bone and Extensor Mechanism
2.5. Static Measurements
3. Results
3.1. The Extensor Mechanism
3.2. Static Measurements of the Kangaroo Stifle
3.3. Muscle Attachment and Insertion Points
4. Discussion
4.1. Structural Characteristics
4.2. Functional Characteristics
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hart, H.F.; Filbay, S.R.; Coburn, S.; Charlton, J.M.; Sritharan, P.; Crossley, K.M. Is quality of life reduced in people with patellofemoral osteoarthritis and does it improve with treatment? A systematic review, meta-analysis and regression. Disabil. Rehabil. 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Coburn, S.L.; Barton, C.J.; Filbay, S.R.; Hart, H.F.; Rathleff, M.S.; Crossley, K.M. Quality of life in individuals with patellofemoral pain: A systematic review including meta-analysis. Phys. Ther. Sport 2018, 33, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Wyndow, N.; Collins, N.; Vicenzino, B.; Tucker, K.; Crossley, K. Is There a Biomechanical Link Between Patellofemoral Pain and Osteoarthritis? A Narrative Review. Sports Med. 2016, 46, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Fulkerson, J.P. Diagnosis and treatment of patients with patellofemoral pain. Am. J. Sports Med. 2002, 30, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Farrokhi, S.; Keyak, J.H.; Powers, C.M. Individuals with patellofemoral pain exhibit greater patellofemoral joint stress: A finite element analysis study. Osteoarthr. Cartil. 2011, 19, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Saranathan, A.; Kirkpatrick, M.S.; Mani, S.; Smith, L.G.; Cosgarea, A.J.; Tan, J.S.; Elias, J.J. The effect of tibial tuberosity realignment procedures on the patellofemoral pressure distribution. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Günal, I.; Karatosun, V. Patellectomy: An overview with reconstructive procedures. Clin. Orthop. Relat. Res. 2001, 74–78. [Google Scholar] [CrossRef]
- Sutton, F.S., Jr.; Thompson, C.H.; Lipke, J.; Kettelkamp, D.B. The effect of patellectomy on knee function. J. Bone Joint Surg. Am. 1976, 58, 537–540. [Google Scholar] [CrossRef]
- Wendt, B.Y.P.P.; Johnson, R.P. A Study of Quadriceps Excursion, Torque, and the Effect of Patellectomy on Cadaver Knees. J. Bone Joint Surg. Am. Vol. 1985, 67, 726–732. [Google Scholar] [CrossRef]
- Asopa, V.; Willis-Owen, C.; Keene, G. Patellectomy for osteoarthritis: A new tension preserving surgical technique to reconstruct the extensor mechanism with retrospective review of long-term follow-up. J. Orthop. Surg. Res. 2015, 10, 107. [Google Scholar] [CrossRef]
- Samuels, M.E.; Regnault, S.; Hutchinson, J.R. Evolution of the patellar sesamoid bone in mammals. PeerJ 2017, 5, e3103. [Google Scholar] [CrossRef]
- Reese, S.; Pfuderer, U.R.; Bragulla, H.; Loeffler, K.; Budras, K.D. Topography, structure and function of the patella and the patelloid in marsupials. Anat. Histol. Embryol. 2001, 30, 289–294. [Google Scholar] [CrossRef]
- Holladay, S.D.; Smith, B.J.; Smallwood, J.E.; Hudson, L.C. Absence of an osseous patella and other observations in Macropodidae stifle. Anat. Rec. 1990, 226, 112–114. [Google Scholar] [CrossRef]
- Hutchinson, J.R. Biomechanical modeling and sensitivity analysis of bipedal running ability. I. Extant taxa. J. Morphol. 2004, 262, 421–440. [Google Scholar] [CrossRef]
- Miller, A.C.; Cake, M.A.; Warburton, N.M. The fibular meniscus of the kangaroo as an adaptation against external tibial rotation during saltatorial locomotion. J. Anat. 2017, 231, 931–938. [Google Scholar] [CrossRef]
- Johnson, W.L.; Jindrich, D.L.; Roy, R.R.; Reggie Edgerton, V. A three-dimensional model of the rat hindlimb: Musculoskeletal geometry and muscle moment arms. J. Biomech. 2008, 41, 610–619. [Google Scholar] [CrossRef]
- Young, F.; Rode, C.; Hunt, A.; Quinn, R. Analyzing Moment Arm Profiles in a Full-Muscle Rat Hindlimb Model. Biomimetics 2019, 4, 10. [Google Scholar] [CrossRef]
- Hopwood, P.R.; Butterfield, R.M. The musculature of the proximal pelvic limb of the Eastern Grey Kangaroo Macropus major (Shaw) Macropus giganteus (Zimm). J. Anat. 1976, 121, 259–277. [Google Scholar]
- Kanehisa, H.; Ikegawa, S.; Tsunoda, N.; Fukunaga, T. Strength and cross-sectional area of knee extensor muscles in children. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 68, 402–405. [Google Scholar] [CrossRef]
- Miller, J.A.; Gross, M.M. Locomotor advantages of Neandertal skeletal morphology at the knee and ankle. J. Biomech. 1998, 31, 355–361. [Google Scholar] [CrossRef]
- Lee, D.; Li, Z.; Sohail, Q.Z.; Jackson, K.; Fiume, E.; Agur, A. A three-dimensional approach to pennation angle estimation for human skeletal muscle. Comput. Methods Biomech. Biomed. Engin. 2015, 18, 1474–1484. [Google Scholar] [CrossRef]
- Krevolin, J.L.; Pandy, M.G.; Pearce, J.C. Moment arm of the patellar tendon in the human knee. J. Biomech. 2004, 37, 785–788. [Google Scholar] [CrossRef]
- O’Brien, T.D.; Reeves, N.D.; Baltzopoulos, V.; Jones, D.A.; Maganaris, C.N. Moment arms of the knee extensor mechanism in children and adults. J. Anat. 2009, 215, 198–205. [Google Scholar] [CrossRef]
- Trinkaus, E. The Shanidar Neandertals; Academic Press: Cambridge, MA, USA, 2014; ISBN 9781483276472. [Google Scholar]
- Kear, B.P.; Lee, M.S.Y.; Gerdtz, W.R.; Flannery, T.F. Evolution of Hind Limb Proportions in Kangaroos (Marsupialia: Macropodoidea). In Mammalian Evolutionary Morphology; Vertebrate Paleobiology and Paleoanthropology Series; Springer: Dordrecht, The Netherlands, 2008; pp. 25–35. ISBN 9781402069963. [Google Scholar]
- Pietak, A.; Ma, S.; Beck, C.W.; Stringer, M.D. Fundamental ratios and logarithmic periodicity in human limb bones. J. Anat. 2013, 222, 526–537. [Google Scholar] [CrossRef]
- Liao, T.-C.; Keyak, J.H.; Powers, C.M. Runners with Patellofemoral Pain Exhibit Greater Peak Patella Cartilage Stress Compared with Pain-Free Runners. J. Appl. Biomech. 2018, 34, 298–305. [Google Scholar] [CrossRef]
- Besier, T.F.; Pal, S.; Draper, C.E.; Fredericson, M.; Gold, G.E.; Delp, S.L.; Beaupré, G.S. The Role of Cartilage Stress in Patellofemoral Pain. Med. Sci. Sports Exerc. 2015, 47, 2416–2422. [Google Scholar] [CrossRef]
- Pal, S.; Besier, T.F.; Gold, G.E.; Fredericson, M.; Delp, S.L.; Beaupre, G.S. Patellofemoral cartilage stresses are most sensitive to variations in vastus medialis muscle forces. Comput. Methods Biomech. Biomed. Eng. 2018, 206–216. [Google Scholar] [CrossRef]
- Smith, T.O.; Nichols, R.; Harle, D.; Donell, S.T. Do the vastus medialis obliquus and vastus medialis longus really exist? A systematic review. Clin. Anat. 2009, 22, 183–199. [Google Scholar] [CrossRef]
- Glenn, L.L.; Samojla, B.G. A critical reexamination of the morphology, neurovasculature, and fiber architecture of knee extensor muscles in animal models and humans. Biol. Res. Nurs. 2002, 4, 128–141. [Google Scholar] [CrossRef]
- Purevsuren, T.; Elias, J.J.; Kim, K.; Kim, Y.H. Dynamic simulation of tibial tuberosity realignment: Model evaluation. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 1606–1610. [Google Scholar] [CrossRef][Green Version]
- Liao, T.-C.; Yang, N.; Ho, K.-Y.; Farrokhi, S.; Powers, C.M. Femur Rotation Increases Patella Cartilage Stress in Females with Patellofemoral Pain. Med. Sci. Sports Exerc. 2015, 47, 1775–1780. [Google Scholar] [CrossRef]
- Liao, T.-C.; Powers, C.M. Tibiofemoral kinematics in the transverse and frontal planes influence the location and magnitude of peak patella cartilage stress: An investigation of runners with and without patellofemoral pain. Clin. Biomech. 2019, 62, 72–78. [Google Scholar] [CrossRef]

| TL (mm) | FL (mm) | Flexion Angle (deg) | QCA (cm2) | QCA.FL (cm2·m) | Moment Arm (mm) | TT Projection Index | TP:TT RATIO | Pennation Angle (deg) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| VL | RF | |||||||||
| Limb 1 | 395.5 | 196.1 | 78.2 | 77.1 | 15.1 | 29.1 | 15.3 | 1.4 | 32.1 | 45.8 |
| Limb 2 | 392.9 | 189.9 | 70.0 | 81.6 | 15.4 | 28.6 | 18.2 | 1.9 | 19.0 | 20.8 |
| Limb 3 | 418.9 | 205.2 | 68.4 | 101.7 | 20.9 | 28.5 | 17.8 | 1.7 | 24.8 | 29.3 |
| Limb 4 | 405.3 | 209.2 | 88.6 | 86.1 | 18.0 | 32.2 | 18.0 | 1.8 | 29.9 | 27.9 |
| Median | 400.4 | 200.7 | 74.1 | 83.9 | 16.7 | 28.9 | 17.9 | 1.8 | 27.4 | 28.6 |
| Range | 26.0 | 20.1 | 20.2 | 24.6 | 5.8 | 3.8 | 2.9 | 0.5 | 13.1 | 25.0 |
| Muscle | Origin | Insertion into Extensor Mechanism | Femoral Attachment |
|---|---|---|---|
| SA | Tuber coxae [18] | Most superficial insertion | None |
| RF | Lateral spine of the ilium cranial to the acetabulum [18] | Inferior to SA insertion | None |
| VL | Greater trochanter | Lateral insertion, inferior to RF | Greater trochanter only |
| VI | Greater trochanter | Inferior to VL and VM insertion | Continuous along the lateral aspect of femoral shaft |
| VM | Lesser trochanter | Medial insertion, inferior to RF | None, attached to medial intermuscular septum with VI |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, M.; Scholes, C.J.; Zhong, E.; Kohan, L. Towards a Dynamic Model of the Kangaroo Knee for Clinical Insights into Human Knee Pathology and Treatment: Establishing a Static Biomechanical Profile. Biomimetics 2019, 4, 52. https://doi.org/10.3390/biomimetics4030052
Fatima M, Scholes CJ, Zhong E, Kohan L. Towards a Dynamic Model of the Kangaroo Knee for Clinical Insights into Human Knee Pathology and Treatment: Establishing a Static Biomechanical Profile. Biomimetics. 2019; 4(3):52. https://doi.org/10.3390/biomimetics4030052
Chicago/Turabian StyleFatima, Manaal, Corey J. Scholes, Emily Zhong, and Lawrence Kohan. 2019. "Towards a Dynamic Model of the Kangaroo Knee for Clinical Insights into Human Knee Pathology and Treatment: Establishing a Static Biomechanical Profile" Biomimetics 4, no. 3: 52. https://doi.org/10.3390/biomimetics4030052
APA StyleFatima, M., Scholes, C. J., Zhong, E., & Kohan, L. (2019). Towards a Dynamic Model of the Kangaroo Knee for Clinical Insights into Human Knee Pathology and Treatment: Establishing a Static Biomechanical Profile. Biomimetics, 4(3), 52. https://doi.org/10.3390/biomimetics4030052





