Bioinspired Metal–Polyphenol Materials: Self-Healing and Beyond
Abstract
:1. Introduction
1.1. The Blue Mussel Byssus
1.2. pH-Dependent Material Behavior
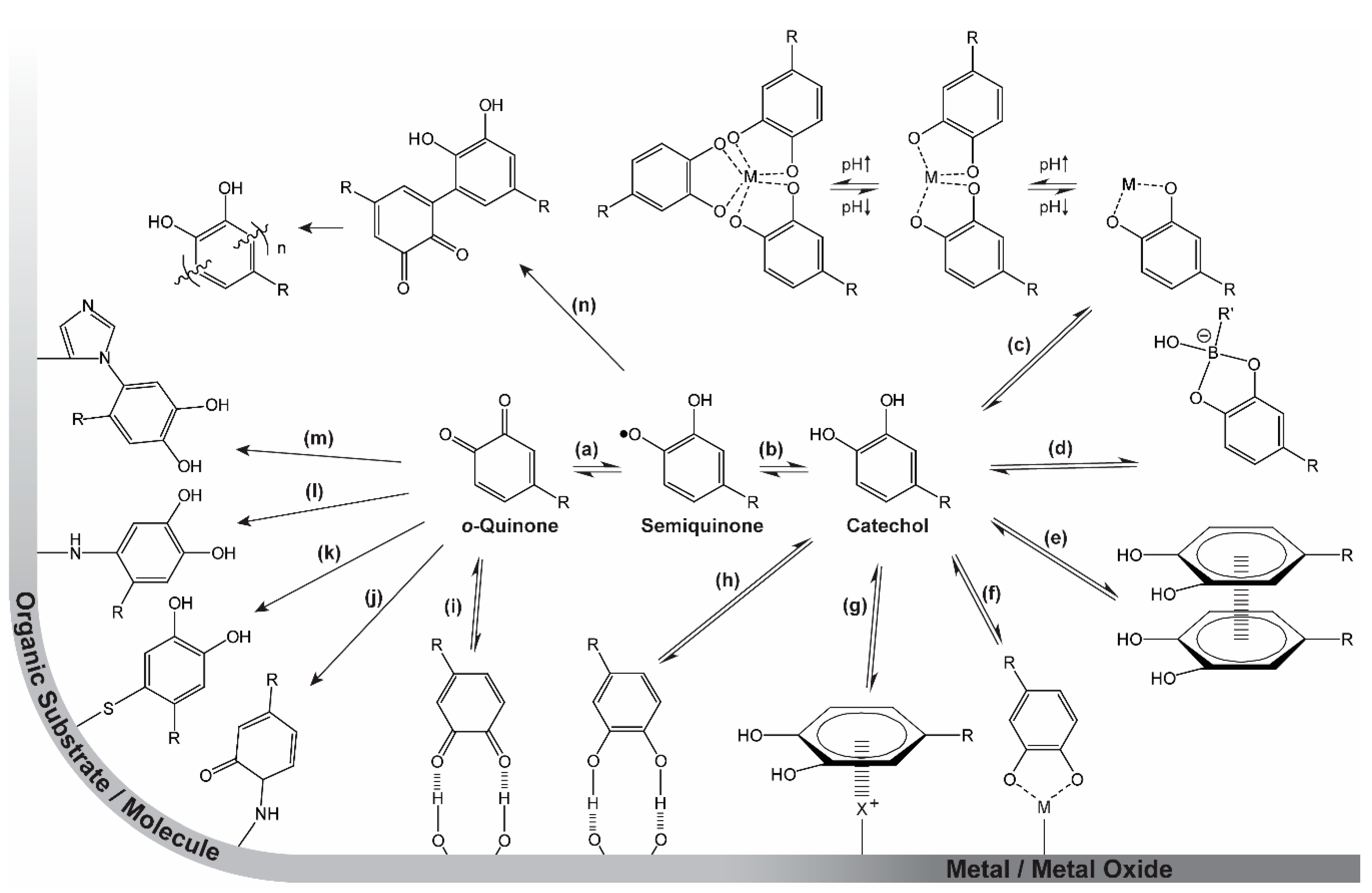
2. Catechol Chemistry
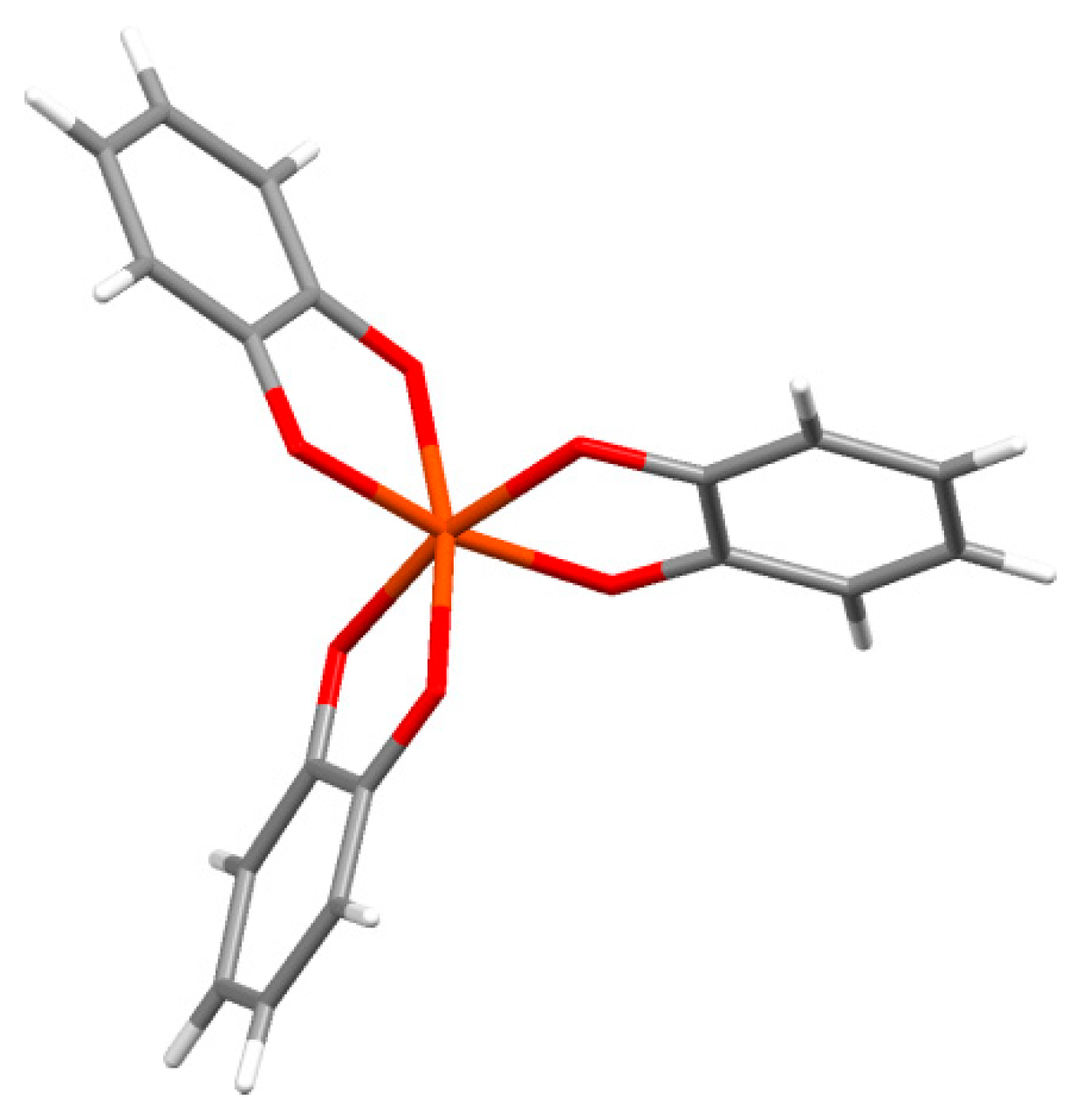
2.1. Catechol Coordination Chemistry
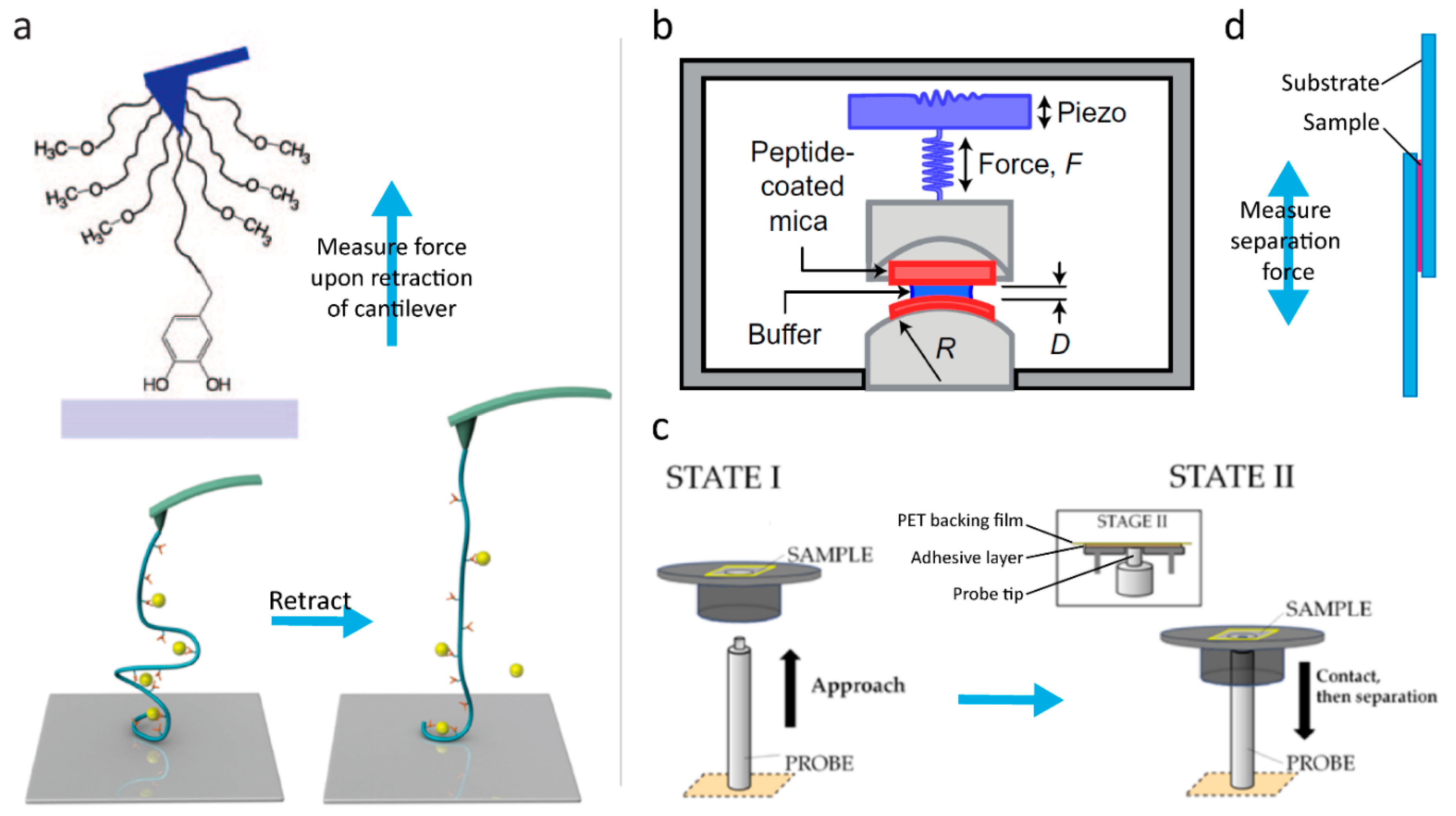
2.2. Strengths of Catechol Metal Binding
3. Self-Healing Materials Based on DOPA
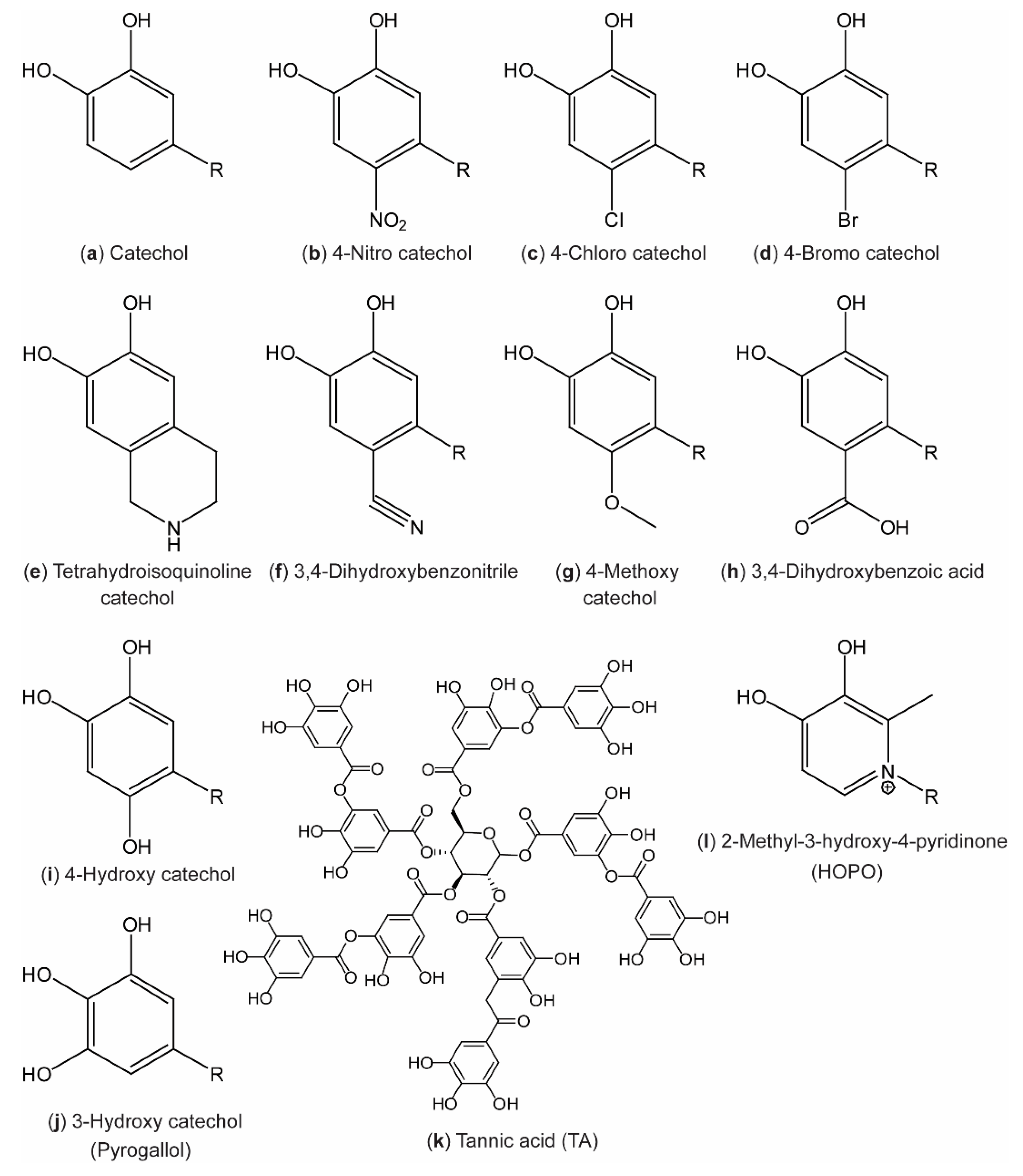
4. DOPA Analogues: Other Polyphenols
4.1. Ring-Substituted Catechols
4.2. Ring Modification: Pyridinone Catechols
5. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef]
- Waite, J.H. Nature’s underwater adhesive specialist. Int. J. Adhes. Adhes. 1987, 7, 9–14. [Google Scholar] [CrossRef]
- Waite, J.H. Adhesion in byssally attached bivalves. Biol. Rev. 1983, 58, 209–231. [Google Scholar] [CrossRef]
- Lichtenegger, H.C.; Schöberl, T.; Bartl, M.H.; Waite, H.; Stucky, G.D. High abrasion resistance with sparse mineralization: Copper biomineral in worm jaws. Science 2002, 298, 389–392. [Google Scholar] [CrossRef]
- Martinez Rodriguez, N.R.; Das, S.; Kaufman, Y.; Israelachvili, J.N.; Waite, J.H. Interfacial pH during mussel adhesive plaque formation. Biofouling 2015, 31, 221–227. [Google Scholar] [CrossRef]
- Taylor, S.W.; Chase, D.B.; Emptage, M.H.; Nelson, M.J.; Waite, J.H. Ferric ion complexes of a DOPA-containing adhesive protein from Mytilus edulis. Inorg. Chem. 1996, 35, 7572–7577. [Google Scholar] [CrossRef]
- Zeng, H.; Hwang, D.S.; Israelachvili, J.N.; Waite, J.H. Strong reversible Fe3+-mediated bridging between dopa-containing protein films in water. Proc. Natl. Acad. Sci. USA 2010, 107, 12850–12853. [Google Scholar] [CrossRef]
- Holten-Andersen, N.; Zhao, H.; Waite, J.H. Stiff coatings on compliant biofibers: The cuticle of Mytilus californianus byssal threads. Biochemistry 2009, 48, 2752–2759. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H.; Qin, X. Polyphosphoprotein from the adhesive pads of Mytilus edulis. Biochemistry 2001, 40, 2887–2893. [Google Scholar] [CrossRef]
- Krogsgaard, M.; Nue, V.; Birkedal, H. Mussel-inspired materials: Self-healing through coordination chemistry. Chem. Eur. J. 2016, 22, 844–857. [Google Scholar] [CrossRef]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-inspired adhesives and coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef]
- Ejima, H.; Richardson, J.J.; Caruso, F. Metal-phenolic networks as a versatile platform to engineer nanomaterials and biointerfaces. Nano Today 2017, 12, 136–148. [Google Scholar] [CrossRef]
- Papov, V.V.; Diamond, T.V.; Biemann, K.; Waite, J.H. Hydroxyarginine-containing polyphenolic proteins in the adhesive plaques of the marine mussel Mytilus edulis. J. Biol. Chem. 1995, 207, 20183–20192. [Google Scholar] [CrossRef]
- Burzio, L.A.; Waite, J.H. Cross-linking in adhesive quinoproteins: Studies with model decapeptides. Biochemistry 2000, 39, 11147–11153. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, H.; Messersmith, P.B.; Lee, B.P.; Dalsin, J.L. Biomimetic adhesives and coatings based on mussel adhesive proteins. In Biological Adhesives; Springer International Publishing: Cham, Switzerland, 2016; pp. 345–378. [Google Scholar]
- Rahim, M.A.; Kristufek, S.L.; Pan, S.; Richardson, J.J.; Caruso, F. Phenolic building blocks for the assembly of functional materials. Angew. Chem. Int. Ed. 2019, 58, 1904–1927. [Google Scholar] [CrossRef]
- Petrone, L.; Kumar, A.; Sutanto, C.N.; Patil, N.J.; Kannan, S.; Palaniappan, A.; Amini, S.; Zappone, B.; Verma, C.; Misere, A. Mussel adhesion is dictated by time-regulated secretion and molecular conformation of mussel adhesive proteins. Nat. Commun. 2015, 6, 8737. [Google Scholar] [CrossRef]
- Yu, J.; Wei, W.; Danner, E.; Ashley, R.K.; Israelachvili, J.N.; Waite, J.H. Mussel protein adhesion depends on interprotein thiol-mediated redox modulation. Nat. Chem. Biol. 2011, 7, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Zechel, S.; Hager, M.D.; Priemel, T.; Harrington, M.J. Healing through histidine: Bioinspired pathways to self-healing polymers via imidazole–metal coordination. Biomimetics 2019, 4, 20. [Google Scholar] [CrossRef]
- Saiz-Poseu, J.; Mancebo-Aracil, J.; Nador, F.; Busqué, F.; Ruiz-Molina, D. The chemistry behind catechol-based adhesion. Angew. Chem. Int. Ed. 2019, 58, 696–714. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired catechol chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef]
- Deming, T.J. Mussel byssus and biomolecular materials. Curr. Opin. Chem. Biol. 1999, 3, 100–106. [Google Scholar] [CrossRef]
- Yang, J.; Cohen Stuart, M.A.; Kamperman, M. Jack of all trades: Versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 2014, 43, 8271–8298. [Google Scholar] [CrossRef]
- McBride, M.B.; Wesselink, L.G. Chemisorption of catechol on gibbsite, boehmite, and noncrystalline alumina surfaces. Environ. Sci. Technol. 1988, 22, 703–708. [Google Scholar] [CrossRef]
- Das, P.; Reches, M. Revealing the role of catechol moieties in the interactions between peptides and inorganic surfaces. Nanoscale 2016, 8, 15309–15316. [Google Scholar] [CrossRef]
- Gebbie, M.A.; Wei, W.; Schrader, A.M.; Cristiani, T.R.; Dobbs, H.A.; Idso, M.; Chmelka, B.F.; Waite, J.H.; Israelachvili, J.N. Tuning underwater adhesion with cation–π interactions. Nat. Chem. 2017, 9, 473–479. [Google Scholar] [CrossRef]
- Mu, Y.; Wu, X.; Pei, D.; Wu, Z.; Zhang, C.; Zhou, D.; Wan, X. Contribution of the polarity of mussel-inspired adhesives in the realization of strong underwater bonding. ACS Biomater. Sci. Eng. 2017, 3, 3133–3140. [Google Scholar] [CrossRef]
- Avdeef, A.; Sofen, S.R.; Bregante, T.L.; Raymond, K.N. Coordination chemistry of microbial iron transport compounds. 9. Stability constants for catechol models of enterobactin. J. Am. Chem. Soc. 1978, 100, 5362–5370. [Google Scholar] [CrossRef]
- Taylor, S.W.; Luther, G.W.; Waite, J.H. Polarographic and spectrophotometric investigation of iron(III) complexation to 3,4-dihydroxyphenylalanine-containing peptides and proteins from Mytilus edulis. Inorg. Chem. 1994, 33, 5819–5824. [Google Scholar] [CrossRef]
- Sever, M.J.; Wilker, J.J. Visible absorption spectra of metal–catecholate and metal–tironate complexes. Dalton Trans. 2004, 7, 1061–1072. [Google Scholar] [CrossRef]
- Sever, M.J.; Wilker, J.J. Absorption spectroscopy and binding constants for first-row transition metal complexes of a DOPA-containing peptide. Dalton Trans. 2006, 6, 813–822. [Google Scholar] [CrossRef]
- Krogsgaard, M.; Hansen, M.R.; Birkedal, H. Metals & polymers in the mix: Fine-tuning the mechanical properties & color of self-healing mussel-inspired hydrogels. J. Mater. Chem. B 2014, 2, 8292–8297. [Google Scholar] [CrossRef]
- Li, Y.; Wen, J.; Qin, M.; Cao, Y.; Ma, H.; Wang, W. Single-molecule mechanics of catechol-iron coordination bonds. ACS Biomater. Sci. Eng. 2017, 3, 979–989. [Google Scholar] [CrossRef]
- Holten-Andersen, N.; Harrington, M.J.; Birkedal, H.; Lee, B.P.; Messersmith, P.B.; Lee, K.Y.C.; Waite, J.H. pH-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli. Proc. Natl. Acad. Sci. USA 2011, 108, 2651–2655. [Google Scholar] [CrossRef]
- Yoshino, K.; Kotaka, M.; Okamoto, M.; Kakihana, H. 11B-NMR study of the complex formation of borate with catechol and l-dopa. Bull. Chem. Soc. Jpn. 1979, 52, 3005–3009. [Google Scholar] [CrossRef]
- Babcock, L.; Pizer, R. Comments on the formation of bis(catecholato)borates. Potassium bis(4-methylcatecholato)borate(III). Inorg. Chem. 1983, 22, 174–176. [Google Scholar] [CrossRef]
- Narkar, A.R.; Barker, B.; Clisch, M.; Jiang, J.; Lee, B.P. pH responsive and oxidation resistant wet adhesive based on reversible catechol–boronate complexation. Chem. Mater. 2016, 28, 5432–5439. [Google Scholar] [CrossRef]
- Shan, M.; Gong, C.; Li, B.; Wu, G. A pH, glucose, and dopamine triple-responsive, self-healable adhesive hydrogel formed by phenylborate–catechol complexation. Polym. Chem. 2017, 8, 2997–3005. [Google Scholar] [CrossRef]
- Yu, M.; Hwang, J.; Deming, T.J. Role of l-3,4-dihydroxyphenylalanine in mussel adhesive proteins. J. Am. Chem. Soc. 1999, 121, 5825–5826. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Anderson, T.H.; Yu, J.; Estrada, A.; Hammer, M.U.; Waite, J.H.; Israelachvili, J.N. The contribution of DOPA to substrate-peptide adhesion and internal cohesion of mussel-inspired synthetic peptide films. Adv. Funct. Mater. 2010, 20, 4196–4205. [Google Scholar] [CrossRef]
- Baty, A.M.; Leavitt, P.K.; Siedlecki, C.A.; Tyler, B.J.; Suci, P.A.; Marchant, R.E.; Geesey, G.G. Adsorption of adhesive proteins from the marine mussel, Mytilus edulis, on polymer films in the hydrated state using angle dependent X-ray photoelectron spectroscopy and atomic force microscopy. Langmuir 1997, 13, 5702–5710. [Google Scholar] [CrossRef]
- Birkedal, H. Cation-π interactions: Mimicking mussel mechanics. Nat. Chem. 2017, 9, 408–409. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Butler, L.G. The specificity of proanthocyanidin-protein interactions. J. Biol. Chem. 1981, 256, 4494–4497. [Google Scholar]
- Anderson, B.F.; Buckingham, D.A.; Robertson, G.B.; Webb, J. Structure of piperidinium tris(pyrocatecholato)ferrate(II) sesquihydrate. Acta Cryst. C 1982, 38, 1927–1931. [Google Scholar] [CrossRef]
- Lee, B.P.; Dalsin, J.L.; Messersmith, P.B. Synthesis and gelation of DOPA-modified poly(ethylene glycol) hydrogels. Biomacromolecules 2002, 3, 1038–1047. [Google Scholar] [CrossRef]
- Dalsin, J.L.; Hu, B.-H.; Lee, B.P.; Messersmith, P.B. Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J. Am. Chem. Soc. 2003, 125, 4253–4258. [Google Scholar] [CrossRef]
- Krogsgaard, M.; Behrens, M.A.; Pedersen, J.S.; Birkedal, H. Self-healing mussel-inspired multi-pH-responsive hydrogels. Biomacromolecules 2013, 14, 297–301. [Google Scholar] [CrossRef]
- Holten-Andersen, N.; Jaishankar, A.; Harrington, M.; Fullenkamp, D.E.; DiMarco, G.; He, L.; McKinley, G.H.; Messersmith, P.B.; Lee, K.Y. Metal-coordination: Using one of nature’s tricks to control soft material mechanics. J. Mater. Chem. B 2014, 2, 2467–2472. [Google Scholar] [CrossRef]
- Guo, Z.; Ni, K.; Wei, D.; Ren, Y. Fe3+-induced oxidation and coordination cross-linking in catechol–chitosan hydrogels under acidic pH conditions. RSC Adv. 2015, 5, 37377–37384. [Google Scholar] [CrossRef]
- Azevedo, S.; Costa, A.M.S.; Andersen, A.; Choi, I.S.; Birkedal, H.; Mano, J.F. Bioinspired ultratough hydrogel with fast recovery, self-healing, injectability and cytocompatibility. Adv. Mater. 2017, 29, 1700759–1700765. [Google Scholar] [CrossRef]
- Lu, Q.; Oh, D.X.; Lee, Y.; Jho, Y.; Hwang, D.S.; Zeng, H. Nanomechanics of cation–π interactions in aqueous solution. Angew. Chem. Int. Ed. 2013, 52, 3944–3948. [Google Scholar] [CrossRef]
- Clancy, S.K.; Sodano, A.; Cunningham, D.J.; Huang, S.S.; Zalicki, P.J.; Shin, S.; Ahn, B.K. Marine bioinspired underwater contact adhesion. Biomacromolecules 2016, 17, 1869–1874. [Google Scholar] [CrossRef]
- Maier, G.P.; Rapp, M.V.; Waite, J.H.; Israelachvili, J.N.; Butler, A. Adaptive synergy between catechol and lysine promotes wet adhesion by surface salt displacement. Science 2015, 349, 628–632. [Google Scholar] [CrossRef]
- Wei, W.; Yu, J.; Gebbie, M.A.; Tan, Y.; Martinez Rodriguez, N.R.; Israelachvili, J.N.; Waite, J.H. Bridging adhesion of mussel-inspired peptides: Role of charge, chain length, and surface type. Langmuir 2015, 31, 1105–1112. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, X.; Li, L.; Zhang, H.; Liu, G.; Zhong, H.; Zeng, H. Probing the reversible Fe3+–DOPA-mediated bridging interaction in mussel foot protein-1. J. Phys. Chem. C 2016, 120, 21670–21677. [Google Scholar] [CrossRef]
- Yang, B.; Lim, C.; Hwang, D.S.; Cha, H.J. Switch of surface adhesion to cohesion by dopa–Fe3+ complexation, in response to microenvironment at the mussel plaque/substrate interface. Chem. Mater. 2016, 28, 7982–7989. [Google Scholar] [CrossRef]
- Byette, F.; Laventure, A.; Marcotte, I.; Pellerin, C. Metal–ligand interactions and salt bridges as sacrificial bonds in mussel byssus-derived materials. Biomacromolecules 2016, 17, 3277–3286. [Google Scholar] [CrossRef]
- Fan, C.; Fu, J.; Zhu, W.; Wang, D.-A. A mussel-inspired double-crosslinked tissue adhesive intended for internal medical use. Acta Biomater. 2016, 33, 51–63. [Google Scholar] [CrossRef]
- Chen, N.; Qin, L.; Pan, Q. Mussel-inspired healing of a strong and stiff polymer. J. Mater. Chem. A 2018, 6, 6667–6674. [Google Scholar] [CrossRef]
- Gao, Z.; Duan, L.; Yang, Y.; Hu, W.; Gao, G. Mussel-inspired tough hydrogels with self-repairing and tissue adhesion. Appl. Surf. Sci. 2018, 427, 74–82. [Google Scholar] [CrossRef]
- Alegre-Requena, J.V.; Häring, M.; Herrera, R.P.; Díaz Díaz, D. Regulatory parameters of self-healing alginate hydrogel networks prepared via mussel-inspired dynamic chemistry. New J. Chem. 2016, 40, 8493–8501. [Google Scholar] [CrossRef]
- Lee, J.; Chang, K.; Kim, S.; Gite, V.; Chung, H.; Sohn, D. Phase controllable hyaluronic acid hydrogel with iron(III) ion–catechol induced dual cross-linking by utilizing the gap of gelation kinetics. Macromolecules 2016, 49, 7450–7459. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Ding, H.; Qian, J.; Yin, J.; Wu, Z.L.; Song, Y.; Zheng, Q. Metal-coordination complexes mediated physical hydrogels with high toughness, stick-slip tearing behavior, and good processability. Macromolecules 2016, 49, 9637–9646. [Google Scholar] [CrossRef]
- Kim, C.; Ejima, H.; Yoshie, N. Non-swellable self-healing polymer with long-term stability under seawater. RSC Adv. 2017, 7, 19288–19295. [Google Scholar] [CrossRef]
- Huang, W.-C.; Ali, F.; Zhao, J.; Rhee, K.; Mou, C.; Bettinger, C.J. Ultrasound-mediated self-healing hydrogels based on tunable metal-organic bonding. Biomacromolecules 2017, 18, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Mrlík, M.; Špírek, M.; Al-Khori, J.; Ahmad, A.A.; Mosnaček, J.; AlMaadeed, M.A.; Kasák, P. Mussel-mimicking sulfobetaine-based copolymer with metal tunable gelation, self-healing and antibacterial capability. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Cholewinski, A.; Yang, F.; Zhao, B. Algae–mussel-inspired hydrogel composite glue for underwater bonding. Mater. Horiz. 2019, 6, 285–293. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Filippidi, E.; Cristiani, T.R.; Eisenbach, C.D.; Waite, J.H.; Israelachvili, J.N.; Ahn, B.K.; Valentine, M.T. Toughening elastomers using mussel-inspired iron–catechol complexes. Science 2017, 358, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ejima, H.; Yoshie, N. Seawater-assisted self-healing of catechol polymers via hydrogen bonding and coordination interactions. ACS Appl. Mater. Interfaces 2016, 8, 19047–19053. [Google Scholar] [CrossRef]
- Xia, N.N.; Xiong, X.M.; Wang, J.; Rong, M.Z.; Zhang, M.Q. A seawater triggered dynamic coordinate bond and its application for underwater self-healing and reclaiming of lipophilic polymer. Chem. Sci. 2016, 7, 2736–2742. [Google Scholar] [CrossRef]
- Qin, L.; Chen, N.; Zhou, X.; Pan, Q. A superhydrophobic aerogel with robust self-healability. J. Mater. Chem. A 2018, 6, 4424–4431. [Google Scholar] [CrossRef]
- Lee, B.P.; Narkar, A.; Wilharm, R. Effect of metal ion type on the movement of hydrogel actuator based on catechol–metal ion coordination chemistry. Sens. Actuators B Chem. 2016, 227, 248–254. [Google Scholar] [CrossRef]
- Li, Q.; Barrett, D.G.; Messersmith, P.B.; Holten-Andersen, N. Controlling hydrogel mechanics via bio-inspired polymer–nanoparticle bond dynamics. ACS Nano 2016, 10, 1317–1324. [Google Scholar] [CrossRef]
- Zhang, Q.; Nurumbetov, G.; Simula, A.; Zhu, C.; Li, M.; Wilson, P.; Kempe, K.; Yang, B.; Tao, L.; Haddleton, D.M. Synthesis of well-defined catechol polymers for surface functionalization of magnetic nanoparticles. Polym. Chem. 2016, 7, 7002–7010. [Google Scholar] [CrossRef]
- Han, L.; Yan, L.; Wang, K.; Fang, L.; Zhang, H.; Tang, Y.; Ding, Y.; Weng, L.-T.; Xu, J.; Weng, J.; et al. Tough, self-healable and tissue-adhesive hydrogel with tunable multifunctionality. NPG Asia Mater. 2017, 9. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Y.; Wang, L.; Zhang, M.; Chen, X.; Liu, M.; Fan, J.; Liu, J.; Zhou, F.; Wang, Z. Bio-inspired reversible underwater adhesive. Nat. Commun. 2017, 8, 221. [Google Scholar] [CrossRef]
- Patil, N.; Jérôme, C.; Detrembleur, C. Recent advances in the synthesis of catechol-derived (bio)polymers for applications in energy storage and environment. Prog. Polym. Sci. 2018, 82, 34–91. [Google Scholar] [CrossRef]
- Ding, X.; Vegesna, G.K.; Meng, H.; Winter, A.; Lee, B.P. Nitro-group functionalization of dopamine and its contribution to the viscoelastic properties of catechol-containing nanocomposite hydrogels. Macromol. Chem. Phys. 2015, 216, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, Z.; Cui, J.; Pastor-Pérez, L.; San Miguel, V.; Gropeanu, R.A.; Serrano, C.; del Campo, A. Bioinspired underwater bonding and debonding on demand. Angew. Chem. Int. Ed. 2012, 51, 4332–4335. [Google Scholar] [CrossRef]
- Jameson, R.F.; Wilson, M.F. Thermodynamics of the interactions of catechol with transition metals. Part III. The effect of 4-chloro- and 4-nitro-substitution on proton and metal catechol complex formation. J. Chem. Soc., Dalton Trans. 1972, 23, 2617–2621. [Google Scholar] [CrossRef]
- Cui, J.; Iturri, J.; Paez, J.; Shafiq, Z.; Serrano, C.; d’Ischia, M.; del Campo, A. Dopamine-based coatings and hydrogels: Toward substitution-related structure–property relationships. Macromol. Chem. Phys. 2014, 215, 2403–2413. [Google Scholar] [CrossRef]
- García-Fernández, L.; Cui, J.; Serrano, C.; Shafiq, Z.; Gropeanu, R.A.; Miguel, V.S.; Ramos, J.I.; Wang, M.; Auernhammer, G.K.; Ritz, S.; et al. Antibacterial strategies from the sea: Polymer-bound Cl-catechols for prevention of biofilm formation. Adv. Mater. 2013, 25, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Rote, J.C.; Malkowski, S.N.; Cochrane, C.S.; Bailey, G.E.; Brown, N.S.; Cafiero, M.; Peterson, L.W. Catechol reactivity: Synthesis of dopamine derivatives substituted at the 6-position. Synth. Commun. 2017, 47, 435–441. [Google Scholar] [CrossRef]
- Chen, N.; Pan, Q. Mussel-inspired self-healing of ultralight magnetic frameworks. ACS Sustain. Chem. Eng. 2017, 5, 7905–7911. [Google Scholar] [CrossRef]
- Zhao, P.; Wei, K.; Feng, Q.; Chen, H.; Wong, D.S.H.; Chen, X.; Wu, C.-C.; Bian, L. Mussel-mimetic hydrogels with defined cross-linkers achieved via controlled catechol dimerization exhibiting tough adhesion for wet biological tissues. Chem. Commun. 2017, 53, 12000–12003. [Google Scholar] [CrossRef] [PubMed]
- Coumes, F.; Malfait, A.; Bria, M.; Lyskawa, J.; Woisel, P.; Fournier, D. Catechol/boronic acid chemistry for the creation of block copolymers with a multi-stimuli responsive junction. Polym. Chem. 2016, 7, 4682–4692. [Google Scholar] [CrossRef]
- Coumes, F.; Woisel, P.; Fournier, D. Facile access to multistimuli-responsive self-assembled block copolymers via a catechol/boronic acid ligation. Macromolecules 2016, 49, 8925–8932. [Google Scholar] [CrossRef]
- Zhang, J.; Cheah, Y.S.; Santhanakrishnan, S.; Neoh, K.G.; Chai, C.L.L. Methoxy group substitution on catechol ring of dopamine facilitates its polymerization and formation of surface coatings. Polymer 2017, 116, 5–15. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Hlushko, R.; Hlushko, H.; Sukhishvili, S.A. A family of linear phenolic polymers with controlled hydrophobicity, adsorption and antioxidant properties. Polym. Chem. 2018, 9, 506–516. [Google Scholar] [CrossRef]
- Zhan, K.; Ejima, H.; Yoshie, N. Antioxidant and adsorption properties of bioinspired phenolic polymers: A comparative study of catechol and gallol. ACS Sustain. Chem. Eng. 2016, 4, 3857–3863. [Google Scholar] [CrossRef]
- Liu, F.; Long, Y.; Zhao, Q.; Liu, X.; Qiu, G.; Zhang, L.; Ling, Q.; Gu, H. Gallol-containing homopolymers and block copolymers: ROMP synthesis and gelation properties by metal-coordination and oxidation. Polymer 2018, 143, 212–227. [Google Scholar] [CrossRef]
- Rahim, M.A.; Björnmalm, M.; Suma, T.; Faria, M.; Ju, Y.; Kempe, K.; Müllner, M.; Ejima, H.; Stickland, A.D.; Caruso, F. Metal–phenolic supramolecular gelation. Angew. Chem. Int. Ed. 2016, 55, 13803–13807. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Peng, L.; Liu, T.; Wang, Y.; Shi, S.; Wang, H. Poly(vinyl alcohol)–tannic acid hydrogels with excellent mechanical properties and shape memory behaviors. ACS Appl. Mater. Interfaces 2016, 8, 27199–27206. [Google Scholar] [CrossRef]
- Prajatelistia, E.; Ju, S.-W.; Sanandiya, N.D.; Jun, S.H.; Ahn, J.-S.; Hwang, D.S. Tunicate-inspired gallic acid/metal ion complex for instant and efficient treatment of dentin hypersensitivity. Adv. Healthc. Mater. 2016, 5, 919–927. [Google Scholar] [CrossRef]
- Guo, J.; Tardy, B.L.; Christofferson, A.J.; Dai, Y.; Richardson, J.J.; Zhu, W.; Hu, M.; Ju, Y.; Cui, J.; Dagastine, R.R.; et al. Modular assembly of superstructures from polyphenol-functionalized building blocks. Nat. Nanotechnol. 2016, 11, 1105–1111. [Google Scholar] [CrossRef]
- Liang, H.; Li, J.; He, Y.; Xu, W.; Liu, S.; Li, Y.; Chen, Y.; Li, B. Engineering multifunctional films based on metal-phenolic networks for rational pH-responsive delivery and cell imaging. ACS Biomater. Sci. Eng. 2016, 2, 317–325. [Google Scholar] [CrossRef]
- Kim, B.J.; Han, S.; Lee, K.-B.; Choi, I.S. Biphasic supramolecular self-assembly of ferric ions and tannic acid across interfaces for nanofilm formation. Adv. Mater. 2017, 29, 1700784. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, K.; Lee, J.; Choi, J.Y.; Hong, D.; Yang, S.H.; Caruso, F.; Lee, Y.; Choi, I.S. A cytoprotective and degradable metal–polyphenol nanoshell for single-cell encapsulation. Angew. Chem. Int. Ed. 2014, 53, 12420–12425. [Google Scholar] [CrossRef]
- Yang, W.; Sousa, A.M.M.; Fan, X.; Jin, T.; Li, X.; Tomasula, P.M.; Liu, L. Electrospun ultra-fine cellulose acetate fibrous mats containing tannic acid–Fe3+ complexes. Carbohydr. Polym. 2017, 157, 1173–1179. [Google Scholar] [CrossRef]
- Oh, J.Y.; Jung, Y.; Cho, Y.S.; Choi, J.; Youk, J.H.; Fechler, N.; Yang, S.J.; Park, C.R. Metal–phenolic carbon nanocomposites for robust and flexible energy-storage devices. ChemSusChem 2017, 10, 1675–1682. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Z.-W.; Guo, H.; Yao, Z.; Ma, X.-H.; Song, X.; Feng, S.-P.; Tang, C.Y. Tannic acid/Fe3+ nanoscaffold for interfacial polymerization: Toward enhanced nanofiltration performance. Environ. Sci. Technol. 2018, 52, 9341–9349. [Google Scholar] [CrossRef]
- Shen, Y.; Du, C.; Zhou, J.; Ma, F. Application of nano FeIII–tannic acid complexes in modifying aqueous acrylic latex for controlled-release coated urea. J. Agric. Food Chem. 2017, 65, 1030–1036. [Google Scholar] [CrossRef]
- Fang, X.; Li, J.; Li, X.; Pan, S.; Sun, X.; Shen, J.; Han, W.; Wang, L.; Van der Bruggen, B. Iron–tannin-framework complex modified PES ultrafiltration membranes with enhanced filtration performance and fouling resistance. J. Colloid Interface Sci. 2017, 505, 642–652. [Google Scholar] [CrossRef]
- Bartzoka, E.D.; Lange, H.; Thiel, K.; Crestini, C. Coordination complexes and one-step assembly of lignin for versatile nanocapsule engineering. ACS Sustain. Chem. Eng. 2016, 4, 5194–5203. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, S.; Li, X.; Liu, X.; Xu, Q.; Liu, J.; Wang, Z. Tannic acid modified Fe3O4 core-shell nanoparticles for adsorption of Pb2+ and Hg2+. J. Taiwan Inst. Chem. Eng. 2017, 72, 163–170. [Google Scholar] [CrossRef]
- Çakar, S.; Güy, N.; Özacar, M.; Fındık, F. Investigation of vegetable tannins and their iron complex dyes for dye sensitized solar cell applications. Electrochim. Acta 2016, 209, 407–422. [Google Scholar] [CrossRef]
- Çakar, S.; Özacar, M. Fe–tannic acid complex dye as photo sensitizer for different morphological ZnO based DSSCs. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 163, 79–88. [Google Scholar] [CrossRef]
- Sileika, T.S.; Barrett, D.G.; Zhang, R.; Lau, K.H.A.; Messersmith, P.B. Colorless multifunctional coatings inspired by polyphenols found in tea, chocolate, and wine. Angew. Chem. Int. Ed. 2013, 52, 10766–10770. [Google Scholar] [CrossRef]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; van Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, S.; Moon, H.C.; Seo, H.; Kim, J.Y.; Hong, S.-P.; Lee, B.S.; Kang, E.; Lee, J.; Ryu, D.H.; et al. Antimicrobial spray nanocoating of supramolecular Fe(III)–tannic acid metal-organic coordination complex: Applications to shoe insoles and fruits. Sci. Rep. 2017, 7, 6980. [Google Scholar] [CrossRef]
- Park, J.H.; Hong, D.; Lee, J.; Choi, I.S. Cell-in-shell hybrids: Chemical nanoencapsulation of individual cells. Acc. Chem. Res. 2016, 49, 792–800. [Google Scholar] [CrossRef]
- Creative Commons—Attribution 4.0 International (CC BY 4.0). Available online: https://creativecommons.org/licenses/by/4.0/.
- Menyo, M.S.; Hawker, C.J.; Waite, J.H. Rate-dependent stiffness and recovery in interpenetrating network hydrogels through sacrificial metal coordination bonds. ACS Macro Lett. 2015, 4, 1200–1204. [Google Scholar] [CrossRef]
- Andersen, A.; Krogsgaard, M.; Birkedal, H. Mussel-inspired self-healing double-cross-linked hydrogels by controlled combination of metal coordination and covalent cross-linking. Biomacromolecules 2017, 19, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Grindy, S.C.; Learsch, R.; Mozhdehi, D.; Cheng, J.; Barrett, D.G.; Guan, Z.; Messersmith, P.B.; Holten-Andersen, N. Control of hierarchical polymer mechanics with bioinspired metal-coordination dynamics. Nat. Mater. 2015, 14, 1210–1216. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersen, A.; Chen, Y.; Birkedal, H. Bioinspired Metal–Polyphenol Materials: Self-Healing and Beyond. Biomimetics 2019, 4, 30. https://doi.org/10.3390/biomimetics4020030
Andersen A, Chen Y, Birkedal H. Bioinspired Metal–Polyphenol Materials: Self-Healing and Beyond. Biomimetics. 2019; 4(2):30. https://doi.org/10.3390/biomimetics4020030
Chicago/Turabian StyleAndersen, Amanda, Yaqing Chen, and Henrik Birkedal. 2019. "Bioinspired Metal–Polyphenol Materials: Self-Healing and Beyond" Biomimetics 4, no. 2: 30. https://doi.org/10.3390/biomimetics4020030
APA StyleAndersen, A., Chen, Y., & Birkedal, H. (2019). Bioinspired Metal–Polyphenol Materials: Self-Healing and Beyond. Biomimetics, 4(2), 30. https://doi.org/10.3390/biomimetics4020030




