1. Introduction
Biological surfaces—especially biomimetically interesting surfaces and (micro-) structures of animals and plants—have seen more and more scientific interest in the past few decades. Discoveries like the lotus effect [
1,
2], moisture harvesting in beetles [
3], spider silk [
4] and lizards [
5,
6], the interesting properties of butterflies’ wing scale structures [
7,
8] or the adaptive camouflage of bugs when in contact with rain [
9] all have in common they consist on biological surfaces that exhibit an interesting phenomenon that scientists try to understand and transfer onto technical surfaces. Here, a common problem arises: morphological features of the biological antetype, which are responsible for the effect of interest, are often very small (within the nano- and micrometer range). Therefore, investigation becomes a challenge as it requires various forms of sample modification in order to prepare the biological material even for common methods like scanning electron microscopy (SEM) or transmission electron microscopy (TEM). Fixation and staining are necessary to enhance sample toughness and feature contrast. Additionally, biological samples often do not (or too faintly) exhibit a desired material based feature like thermal conductivity (e.g., human skin with a conductivity value of only 1–3 W m
−1 K
−1 [
10], compared to copper with around 400 W m
−1 K
−1 [
11]). This becomes problematic when investigating phenomena like the abovementioned adaptive camouflage on flat bugs and moisture harvesting, which are suspected to be influenced by conditions that lead to condensation. Since condensation is a phenomenon that involves relative ambient values of pressure, temperature and humidity with regard to a given surface, samples that exhibit the natural surface morphology but adjustable thermal conductivity are desirable for testing the theory.
Replication of just the morphological surface features is a task that generally can be achieved by various techniques. In simple cases, the surface is scanned (e.g., by SEM or atomic force microscopy (AFM), inferometric methods, laser or mechanical scanning), and in a digitally aided abstraction process, the relevant surface features are reduced to a required minimum. Afterwards, the features can be introduced into a desired material using a fitting manufacturing process. Casting, three-dimensional (3D) printing, lithography, laser etching or micromilling in technical surfaces like polymers and metals are just a few commonly used procedures. Successful examples for this kind of processing are various [
12,
13,
14,
15], however they all share a certain loss of detail in the final product—usually due to the reduction of information in the abstraction process or technical limitations. Such replicas can be good enough (and reasonable with concern to production cost and effort), however in many cases a true 1:1 copy is needed. To achieve this, in many cases the detailed imprinting capabilities of polymers are used to generate molds of a certain surface (e.g., [
16,
17,
18]). Afterwards, these can be used for casting with a desired polymer (like resins or plastics) [
19]. Such replicas show excellent replication detail and have been successfully used in the past to create precise copies of biological samples, and ready-to-use kits have reached the market (e.g., President SEM replication kit for high-resolution SEM replicas, Coltene/Whaledent, Switzerland). The chemical and mechanical material properties in these cases obviously depend on the chosen casting material. However, after testing different methods and materials, as well as screening the literature, we found no established replication technique that satisfied our demands with regard to material properties and simplicity. Investigating any influence of condensation phenomena in the liquid handling requires such samples to be (thermally) conductive in order to be able to quickly adjust sample temperatures in an environmental testing chamber. Hence, the all-metal approach was chosen.
Here, we report a novel approach to generate all-metal replicas of biological (and technical) surfaces, in short named AMROBS. These replicas, due to their metallic nature, have the benefits of sturdiness and durability, exhibit excellent conductivity values and still copy the target surface to maximum detail.
3. Results
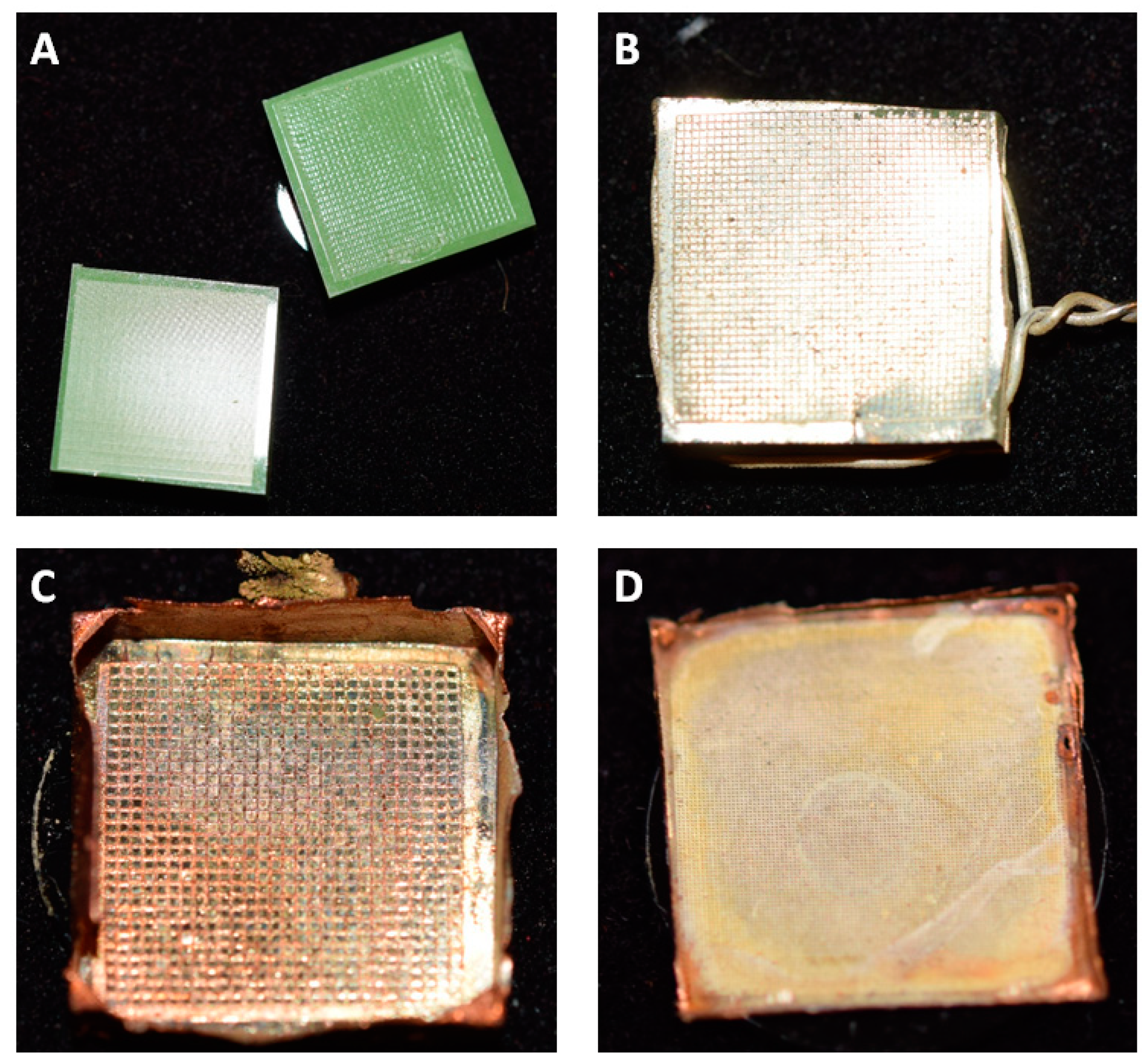
The first replicas were produced from diffraction grid foils. These foils exhibited highly regular, periodic microstructures—successful replication of their surface was the first step in evaluating the feasibility of the approach for delicate biological samples. A comparison between the original grid foil and the all-metal replica is shown in
Figure 2.
Figure 2 shows that the individual grid lines of the foil can be successfully replicated into the copper–silver surface matrix of the replicas. Individual lines were cleanly copied (
Figure 2C,D) and no apparent major defects were visible. In detail, however, the replica (
Figure 2D) revealed a slightly grainier texture in comparison to the smoothness of the original. Still, as shown in
Figure 2E, even though the surface is not as smooth as the original, the replica exhibited a rainbow like iridescence that is typical for periodically microstructured surfaces.
Next, in order to determine the copper deposition rate, we performed a series of verification trials. Standardized steel cubes and copper sheet metal squares were manufactured and galvanized according to the protocol above (galvanizing at 50 mA cm−2 and 3 V). The steel cubes (n = 6) were plated for 30 min, while the copper sheet metal samples (n = 4; 1 cm2, 1 mm thick) were plated for 2 h. Weighing the cubes after galvanization revealed the deposition rate, while the sheet metal squares were cut in half with a small diamond cutting disc (Dremel cutting disc Ø 22 mm, Dremel, Racine, WI, USA) to allow for measurement of layer thickness and the evaluation of connection quality in between the two coating layers.
As a result, we found that the steel cubes gained 0.175 ± 0.02 g after 30 min of plating, which translated into 63.8 ± 7.7 µm h
−1 of layer growth. Measurements of the cross-cut thickness of “Layer 2”, shown in
Supplementary Figure S2A,B, revealed a thickness of 129.4 ± 22.1 μm after 2 h deposition (
n = 10 for each of the four manufactured samples). This translated into a deposition rate of 64.7 ± 11.05 µm h
−1 and, therefore, confirmed the value found for the steel cubes.
Replications of biological samples were produced specimen of Texas horned lizard (
P. cornutum), as well as the dorsal surface of a flat bug (
D. lunatus). For the lizard, silicone imprints of different body parts were selected in order to replicate the various kinds of scales that these animals exhibit.
Figure 3 summarizes the exemplary achieved by the all-metal replicas technique.
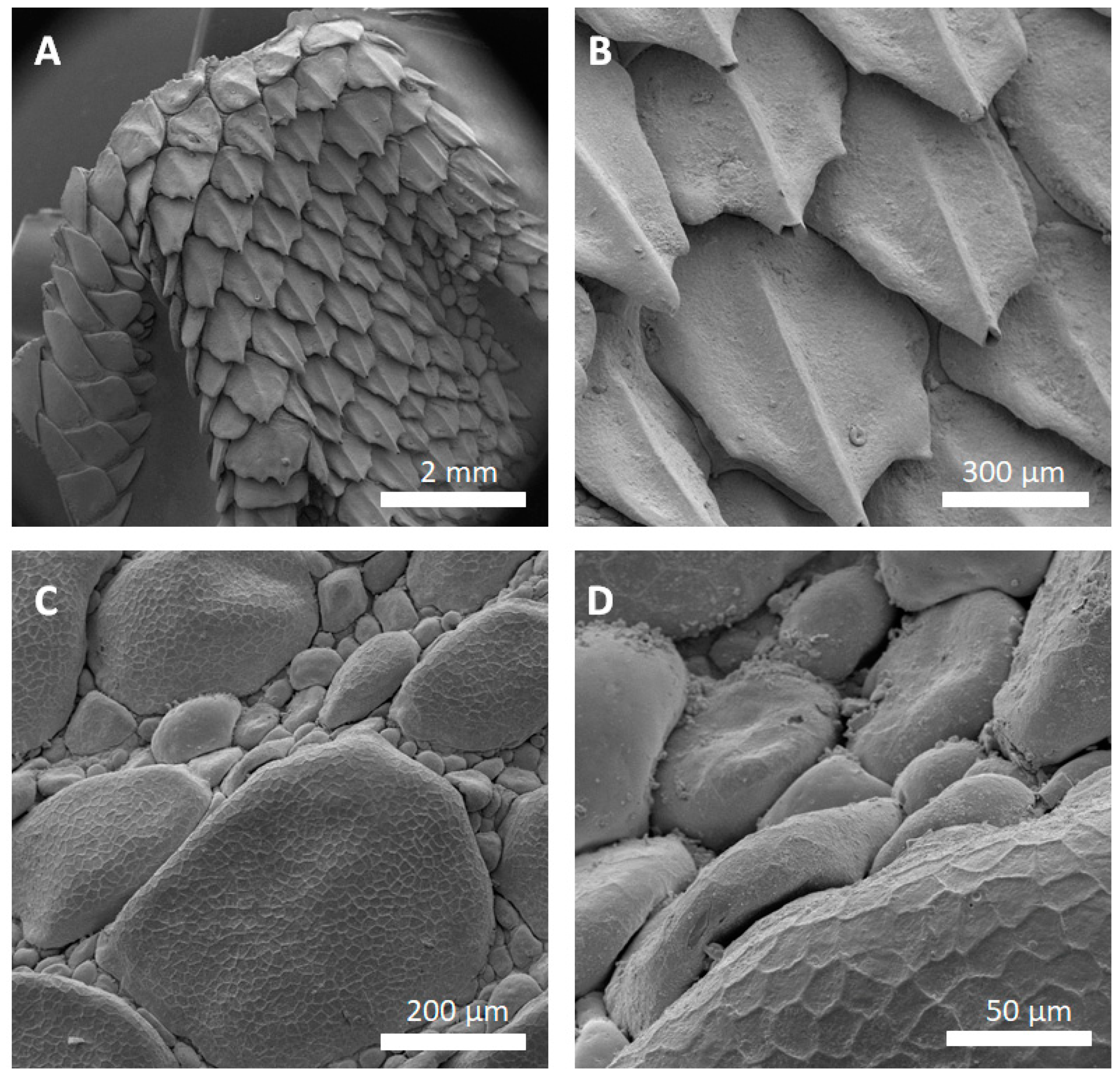
Figure 4 shows SEM images of successfully replicated skin regions of
P. cornutum. All relevant skin features of the lizard were successfully replicated into the metal and the lizards’ surface microstructures were identified in the SEM images. Noteworthy, the honeycomb-like microstructure covering the lizards’ scales (as described in [
5]), was clearly copied into the metal (
Figure 4C,D).
Figure 4A,B show that high aspect ratios and the undercuts are reproducible: the big, overlapping scales of the lizards’ foot were sharply replicated. Minor replication defects can be found in the gold-coated silver–copper replicas of the lizards’ foot (
Figure 4A,B, tips of the scales are missing).
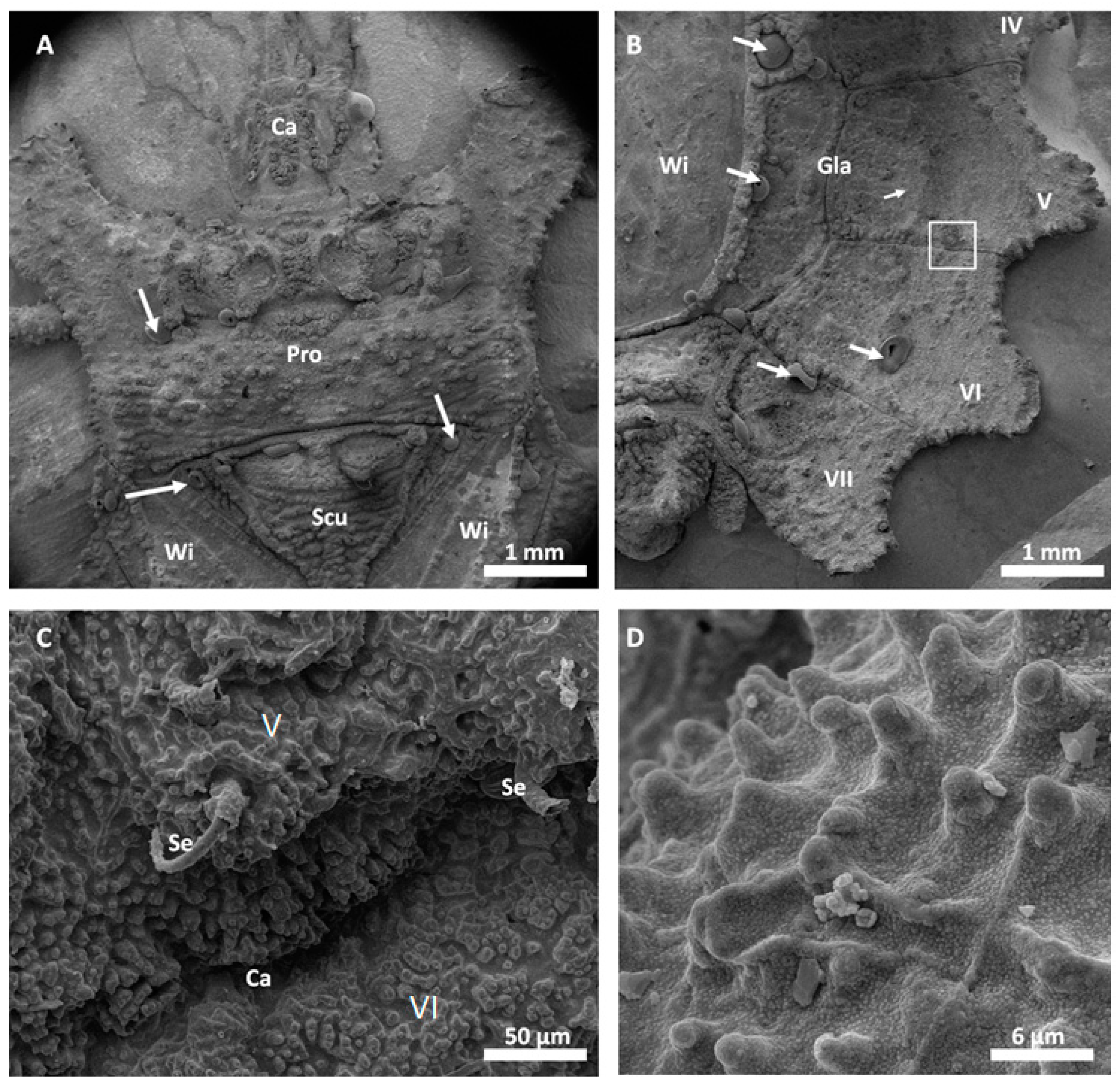
Scanning electron microscopy images of the successfully replicated dorsal surface of
D. lunatus are shown in
Figure 5 and
Figure 6. These images revealed that all relevant morphological features of the flat bugs’ surface were replicated into the metal surface. Wings, capillaries, setae and the waxy microstructure of the integument were cleanly copied and resembled the morphology of the original surfaces as shown and described in [
9]. The overall amount of surface defects in this replica was relatively low (bubbles, marked by white arrows).
In order to test the functionality of the replicated falt bug surface, the wetting behavior was tested analog to the experiments we presented in [
9]. On the surface of a real bug, water spreads fast in all directions, wetting the insect’s integument. Water transport hereby is facilitated by the surface chemistry, the microstructure and the capillary system in between the segmental plates. To account for the changed surface chemistry of the all-metal replica, we dropped small amounts of WD-40 multipurpose oil (WD-40 Company, San Diego, CA, USA) onto the replica and recorded how the oil spread.
Supplementary Video S1 shows this experiment and
Figure 7 summarizes the results. The use of WD-40 oil served no other purpose than to exemplarily show that the artificial surface of the replica is able to perform similar tasks as the natural antetype, however in a completely different chemical regime: on the strongly hydrophilic surface of the original flat bug, fluid spread with oil would never occur. Instead, a droplet of WD-40 oil would form a sphere and eventually roll off the surface. Additionally, WD-40 oil is a penetrating oil and exhibits low viscosity, which speeds up the spreading and improves the visual effect.
4. Discussion
The experiments and results reported here can be considered preliminary, and extensive testing with concern to reproducibility, functionality and actual degree of replication is planned for the future. However, the presented findings clearly suggest the overall feasibility of the AMROBS concept and the justification to continue to work in this direction. We achieved to copy delicate technical and biological surfaces into metal with a high degree of replication accuracy while allowing the replicas to offer all the benefits of the metallic material matrix: rigidity, durability and wear resistance, as well as excellent conductivity.
Replication of the technical surface (diffraction grid foil, as shown in
Figure 2) yielded excellent results and the justification to attempt replication of biological samples. Following the AMROBS approach, all-metal replicas of different surface parts of lizard (
P. cornutum) skin and flat bug (
D. lunatus) could be fabricated (as shown in
Figure 4,
Figure 5 and
Figure 6). The SEM images revealed the the described replication method to be successful: individual morphological features of the replicas’ natural counterparts were virtually copied 1:1. Features like the honeycomb-like microstructures on the scales of
P. cornutum (
Figure 4) were pronounced almost as sharply as on the natural skin, and even the fine and delicate wax microstructures of
Dysodius exhibited the typical appearance (
Figure 5C,D). In a direct comparison with the natural counterpart (
Figure 6), one can clearly see how exact the surface was replicated into all-metal. Even shapes that exhibited fine and hollow features like setae (
Figure 5C and
Figure 6B) can be reproduced by this technique. This indicates that (1) the imprinting process using dental silicone is an appropriate way to mold very fine surface structures without destroying them; and (2) the subsequent surface coatings with silver and copper produce a matrix fine enough to infill these most delicate imprints in the mold.
However, the yielded metal surfaces were not completely void of smaller defects. As depicted in
Figure 4B, as well as in
Figure 5B, small, bubble-like artefacts occurred in the replicas. These were most likely the result of small enclosures of air during imprinting with silicone (and, therefore, result in the empty pockets that were getting filled with copper in the electroplating process). More dedicated testing towards this problem has to be addressed in the future, and a possible solution might be to execute imprinting and curing in an evacuated environment. Also, small empty spots occurred in the replicas, as exemplarily shown for the tips of the lizard scales (
Figure 4B). Here, most likely deposition of the conductive material (silver) did not reach the deepest spots in the silicone mold. Consequently, no copper could be deposited there as the electroplating process filled the mold in a consecutive manner (hence, the tips of the scales were missing in the replica). To avoid future defects of this kind, it is suggested to choose the orientation of the molds in the silver precipitation bath carefully in order to avoid precipitation shadows. Also, achieving better control in the silver precipitation step will be key (i.e., controlling the temperature and, therefore, the reaction speed).
Despite the abovementioned defects, AMROBS samples will allow for carrying out a variety of desired experiments in the future. Thanks to the excellent thermal conductivity, experiments concerning wetting, evaporation and condensation will become possible: AMROBS samples allow for quick changing of sample temperature, for example, by the usage of proper sized Peltiere elements. In addition to that, the sturdy qualities of the all-metal replicas will allow for multiple usage of the same sample, a feature that not necessarily applies to the originals. Additionally, given the uniform surface chemistry, all-metal replicas can be used for individual experiments that require explicitly designed chemical surfaces. In case of
Dysodius, for example, it is feasible to coat the metal replicas with thin layers of artificially designed waxes. This could be achieved by vacuum deposition and has already been successfully shown for technical replications of self-organizing waxes, biomimetically inspired by plant surfaces (e.g., [
19]). Afterwards, the liquid–surface interaction of flat bugs, as presented in [
9], would become reproducible and adjustable. Additionally, gold used as a surface finish (in the presented work mainly to prevent further oxidation) is an excellent starter for further modifying the surface chemistry in general: the mercapto head group of thiols is known to form complexes with gold, whereas the rest of the molecule can be designed and modified to individual needs to achieve a desired surface chemistry (e.g., [
22,
23]). Combining this feature with gold-sputtered copper replicas may allow for diverse experiments that require stable surface morphology and adjustable surface chemistry, as well as excellent thermal and electrical conductivities.
Do’s and Do Nots of AMROBS
One of our main reasons for publishing the results in such an early stage is to present this novel but easy approach of replication to the broader audience. In our opinion, research with the need for conductive copies of particularly, however not only, biological samples might benefit from this knowledge with the chance of adjusting and verifying the process to individual needs. Therefore, some general rules and observations of our initial work with the AMROBS approach should be addressed.
We found that one of the most crucial steps in the whole process was the initial coverage of silicone molds with a conductive layer in order to enable electrochemical deposition in the following electroplating step. As numerous pretests showed, this layer could be achieved by different means, however the result can drastically differ. We tested different approaches to render a conductive surface, such as sputter coating or evaporation-based applications. These transfer a small layer of metal or conductive non-metal material (like carbon). In both cases, the outcome could indeed be galvanized, and all-metal replicas were generated, however for different reasons, the final result was undesirable: sputter coating formed a very good bond between the silicone and the matrix of sputtered and electroplated material. The more (micro-) structured a surface, the stronger the bonding. In many cases, this rendered detachment of the replica from the mold impossible. For evaporation-based methods (like cathodic arc evaporation of carbon), the problem was that most evaporation-based coating techniques heat up the silicone mold and cause it to expand. In this case, we could achieve a successful replication process, however we found that the surface features were changed. After coating and cooling down, the silicone shrunk and most coatings started to wrinkle and form fault lines. From our experience, these fault lines will override major parts of the original (micro-) structures.