Abstract
Catechols offer diverse properties and are used in biology to perform various functions that range from adhesion (e.g., mussel proteins) to neurotransmission (e.g., dopamine), and mimicking the capabilities of biological catechols have yielded important new materials (e.g., polydopamine). It is well known that catechols are also redox-active and we have observed that biomimetic catechol-modified chitosan films are redox-active and possess interesting molecular electronic properties. In particular, these films can accept, store and donate electrons, and thus offer redox-capacitor capabilities. We are enlisting these capabilities to bridge communication between biology and electronics. Specifically, we are investigating an interactive redox-probing approach to access redox-based chemical information and convert this information into an electrical modality that facilitates analysis by methods from signal processing. In this review, we describe the broad vision and then cite recent examples in which the catechol–chitosan redox-capacitor can assist in accessing and understanding chemical information. Further, this redox-capacitor can be coupled with synthetic biology to enhance the power of chemical information processing. Potentially, the progress with this biomimetic catechol–chitosan film may even help in understanding how biology uses the redox properties of catechols for redox signaling.
1. Introduction
1.1. Background: Redox-Active Catecholic Materials
Biology uses materials to perform diverse and important functions, and our understanding of these materials enables biomimetic efforts. For instance, studies of the adhesive properties of mussel proteins identified catecholic residues as critical for both surface binding (adhesion) and protein crosslinking (cohesion), and this knowledge enabled biomimetic materials with broad applications (e.g., polydopamine) [1,2,3,4,5]. Catecholic residues are also redox-active, which means that catechol residues can be switched between oxidized (quinone) and reduced (hydroquinone) states by exchanging electrons with diffusible mediators [6,7,8]. There are several studies to show that the redox activities of catecholic residues can be relevant to some biological functions such as redox buffering [9,10], antioxidant protection [11], metal chelation [12], redox signaling [13] and electron transport [6,14,15]. Although some attempts have been made to study the redox activities of catecholic residues [7,16,17,18], the mechanistic understanding is still lacking because there are few suitable techniques. We are investigating the redox activities of biomimetic catecholic materials and their biological relevance using a recently developed electrochemical reverse engineering method. We believe these redox-active catecholic materials may offer unique technological opportunities for processing chemical information. Thus, our approach may not be truly biomimetic since we are not guided by an understanding of how biology uses melanin’s redox properties to perform functions. We have rather observed intriguing redox properties of biomimetic catecholic materials and we are pursuing technological applications that may or may not be related to their biological function.
1.2. Vision: A New Paradigm for Accessing Chemical Information
Over the last 50 years, advances in information processing transformed our lives by changing the way we access, analyze, and transmit information. Each new device seems to be incrementally cheaper, smaller, faster, more powerful, and easier to use than its predecessor. However, this trend does not extend to instruments that acquire and process chemically-related information. For instance, when we need to access critical chemical information in real time, we still rely on dogs to sniff-out this information. Why have the advances in information processing not been extended to the acquisition and processing of chemically-based information? We believe one issue is that the current paradigm of chemical information is too limited, viewing chemical information through the lens of analytical chemistry and characterizing chemical information in terms of chemical composition and concentration [19]. In essence, this paradigm specifies instrument-intensive approaches (e.g., high performance liquid chromatography–mass spectrometry (HPLC–MS)) to access chemical information and incremental advances are generally accompanied by increasing costs and complexity [20,21,22].
We suggest an alternative paradigm for chemical information processing: one that accesses the power of information processing by searching “chemical space” for global signatures. We envision that this search will be rapid, cheap and convenient, but will lack the granular details of chemical composition and concentration that are targets of conventional analytical chemistry and omics approaches [22]. Thus, we envision a signal processing paradigm that is complementary to (not a replacement for) the current paradigm that focuses on composition and concentration [23,24,25,26].
As illustrated in Figure 1a, our signal processing approach is approximately analogous to sonar. Sonar uses a transmitted signal (pressure wave) to propagate through a medium in search of physical information of nearby objects. Interaction with such objects generates a reflected wave that contains information of the objects (e.g., their presence, size, shape and motion).
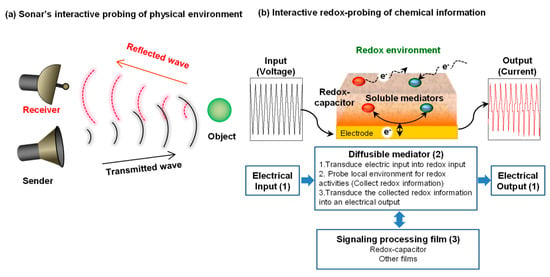
Figure 1.
Interactive probing of a local environment. (a) Sonar analogy. (b) Interactive electrochemical probing consists of: (1) complex inputs/outputs to tailor the interactive probing; (2) diffusible mediators (electron shuttles) to transmit redox signals that can probe information; and (3) signaling processing film to facilitate information processing.
Figure 1b illustrates our approach to interactively probe for chemical information in a local environment. There are three key features of our interactive electrochemical approach. First is the use of diffusible redox-active mediators (electron shuttles) that serve to transmit redox “signals” that can propagate through the local environment in search of redox-based chemical information. Electron transfer interactions between the mediators and the local environment (i.e., redox reactions) will be detected when the mediator returns to the electrode and engages in electrochemical reactions that serve to transduce the redox information into an electrical output. The second feature illustrated in Figure 1b is use of complex electrical inputs that allows redox-probing to be tailored in search of specific types of information. As will be discussed, the resulting electrical outputs contain information of the mediators’ redox interactions [27,28] and can be analyzed using approaches adapted from signal processing [29,30]. The third feature illustrated in Figure 1b is the use of thin hydrogel film coatings that are used to facilitate signal processing. A catechol-based redox-capacitor is one such film that has proven to be especially useful for processing redox-based chemical information [31,32].
In this review, we will focus on the fabrication and properties of the catechol-based redox-capacitor and we will cite several examples illustrating the value of this capacitor for processing redox-based chemical information.
2. Fabrication of Catechol-Based Redox-Capacitor
Figure 2a illustrates that the catechol-based redox-capacitor film is fabricated in two steps from catechols and the aminopolysaccharide chitosan [33,34]. The first step is the electrodeposition of a thin chitosan hydrogel film at an electrode surface. This electrodeposition step enlists chitosan’s pH-responsive self-assembling properties [35] and uses cathodic electrolytic reactions to generate the localized high pH that induces chitosan’s neutralization and gel formation [36,37]. In the second step, the chitosan-coated electrode is immersed in a catechol-containing solution and the catechol is then oxidized to generate a reactive o-quinone that grafts to chitosan through non-enzymatic reactions. Biologically, enzymes (e.g., tyrosinase) catalyze catechol oxidation [1,38,39] while Figure 2a shows that catechols can also be electrochemically oxidized by biasing the electrode to serve as an anode. Importantly, the chitosan film is a hydrogel that allows diffusion of the catechol reactant and o-quinone product. It is also important that the o-quinone product is reactive and quickly grafts to chitosan’s primary amines. These quinone grafting reactions are complex and incompletely characterized and likely involve Michael-type adduct and Schiff base chemistries [22,40,41], as suggested in Figure 2b.
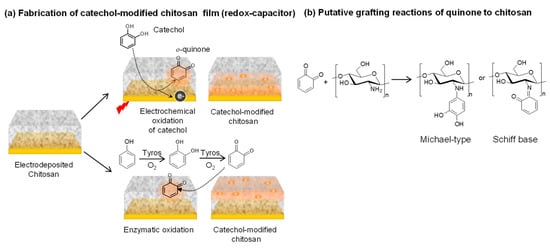
Figure 2.
Fabrication of catechol-based redox-capacitor films. (a) Catechol is electrochemically oxidized or enzymatically oxidized by tyrosinase (Tyros) and the diffusible oxidation product (o-quinone) grafts to the nucleophilic amines of the aminopolysachharide chitosan. (b) Quinone grafting likely involves Michael-type and Schiff base adduct formation.
3. The Catechol–Chitosan Film Can Accept, Store and Donate Electrons
Early studies with melanin suggested that it possesses conducting and semiconductor properties [42,43] and the obvious question was: Are the catechol–chitosan films conducting? Our experimental results indicated that these films are not conducting: electrons do not flow in response to an applied voltage and there does not appear to be direct exchange of electrons with the underlying electrode. This is not surprising given that the films are relatively thick (~1 μm when wet) and the catechols may be too far from the electrode to directly exchange electrons.
The next question was: Are the catechol–chitosan films redox-active? Specifically, can the grafted moieties be switched between oxidized states and reduced states? One challenge to assessing the redox switching capabilities of a non-conducting film is what mechanism can be used to transfer electrons from the electrode to the grafted moieties of the film. As illustrated in Figure 3a, we decided to test whether diffusible mediators (i.e., electron shuttles) could be used to engage the catechol–chitosan film in redox-cycling reactions. We found that one mediator, Ru(NH3)6Cl3 (Ru3+), could engage in reductive redox-cycling to transfer electrons from the electrode to the film to convert oxidized moieties (Q) to reduced moieties (QH2) and thereby charge the film with electrons. A second mediator, ferrocene dimethanol (Fc), could engage in oxidative redox-cycling to transfer electrons from the film to the electrode to convert the film’s QH2 to Q and thereby discharge the film.
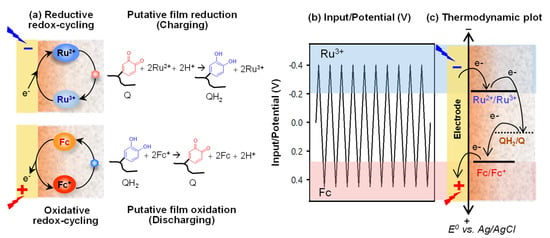
Figure 3.
Proposed redox-cycling mechanism of redox-capacitor. (a) The reductive redox-cycling reaction of Ru3+ can reduce the quinone moieties (charge, QH2) and the oxidative redox-cycling reaction of ferrocene dimethanol (Fc) can oxidize the catechol moieties (discharge, Q). (b) Electrical input potential. (c) Thermodynamics requires electrons to be transferred from more reducing (more negative) potentials to more oxidizing (more positive) potentials.
Importantly, we observed that we could immerse the film-coated electrode into a solution containing both mediators and sequentially engage it in reductive and oxidative redox-cycling reactions if we imposed cyclic voltage inputs as illustrated in Figure 3b. It is also important to note that the “flow” of electrons is constrained by thermodynamics, as illustrated in Figure 3c. The results from these initial studies demonstrated that the catechol–chitosan films are redox-active and can be switched between reduced or oxidized states by exchanging electrons with mediators.
The observation that the catechol–chitosan films are redox-active but non-conducting leads to two interesting concepts. First, the fact that catechol–chitosan films can accept, store and donate electrons essentially means that they are redox-capacitors. As will be discussed, we use these redox-capacitor properties for information processing in aqueous environments. Second, electrons “flow” through the catechol–chitosan films via distinct electron transfer steps (with intermediates) and not as a “sea” of electrons (as in electron currents in wires). This mechanism is consistent with biological electron transfer reactions (e.g., in the respiratory chain) [44,45] in which electron transfer occurs through distinct stable intermediates. Interestingly, quinones, which are the putative redox-active moieties in our capacitor film, are also important redox-active intermediates in the biological electron transfer chains of both respiration (ubiquinone) [14,15] and photosynthesis (plastoquinone) [13].
4. Molecular Electronic Properties of the Catechol–Chitosan Redox-Capacitor
As a result of its redox activities, the catechol–chitosan redox-capacitor offers interesting molecular electronic properties and we highlight four such properties: amplification, partial rectification, gating, and steady response [31,46,47,48]. The first three properties are illustrated by the results in Figure 4a. In this experiment, the electrode coated with the catechol–chitosan film was immersed in a solution containing both mediators (Fc and Ru3+) and a cyclic voltage input was imposed as illustrated by the left plot in Figure 4a. The middle plot of Figure 4a shows the current output response for these studies. The rightmost plot in Figure 4a shows a conventional cyclic voltammogram (CV) which is an alternative representation of the input–output data in which time is not explicitly shown. One control in Figure 4a is incubation of a catechol–chitosan film in the buffer solution without mediators. The output currents for this control show no discernible peaks, which is expected because the catechol–chitosan films are non-conducting. A second control is an electrode coated with a chitosan film (without catechol modification) and immersed in a solution containing both mediators. Results for this control show small output peak currents for Fc and Ru3+: these mediators can diffuse through the chitosan film and undergo electron exchange with the underlying electrode. When the electrode coated with the catechol–chitosan film was tested in solutions containing Fc and Ru3+, the output peak currents for Fc-oxidation and Ru3+-reduction were considerably amplified. Amplification of the mediator currents is consistent with the redox-cycling mechanisms of Figure 3a.
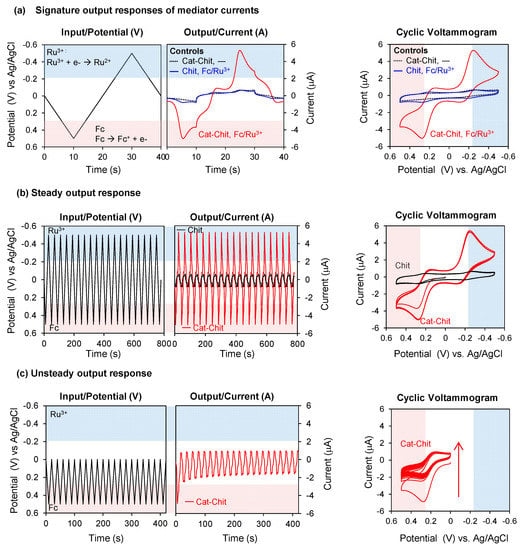
Figure 4.
Molecular electronic properties of redox-capacitor. (a) Input–output curves and cyclic voltammogram show that the redox-cycling reaction of redox-capacitor with mediators results in output currents that are amplified, partially rectified and gated. (b) The output current of mediator is steady over time under unperturbed environment. (c) The output current becomes unsteady in the limited range of input potential. Cat-Chit: Catechol-modified chitosan film; Fc: Ferrocene dimethanol. Reproduced from [31] by permission of The Royal Society of Chemistry, 2014.
Interestingly, the amplification observed in Figure 4a occurs primarily in one direction for each mediator. The Fc-oxidation current is greatly amplified while the Fc-reduction current is not amplified. The Ru3+-reduction current is greatly amplified but the Ru3+-oxidation current is not amplified. This partial rectification of the mediator currents is consistent with the thermodynamic plot in Figure 3c, which indicates that Fc can engage in oxidative redox-cycling but not reductive redox-cycling. Similarly, Ru3+ can engage in reductive but not oxidative redox-cycling.
To understand the gating property, it is useful to recognize that the currents observed in Figure 4a do not result from direct electron transfer between the film and electrode, but rather are the result of electron transfer between the mediators and electrode. Amplification of these peak currents occurs because the mediators redox-cycle with the film and shuttle electrons between the film and electrode. One requirement for redox-cycling is illustrated by the thermodynamic plot in Figure 3c: a reductive redox-cycling mediator (e.g., Ru3+) must have a redox potential (E0) that is more reducing than that of the film, and an oxidative redox-cycling mediator (e.g., Fc) must have a E0 that is more oxidizing than that of the film. A second requirement for redox-cycling is illustrated by the input voltage curve of Figure 3b: reductive redox-cycling can only occur if the imposed voltage is more reducing than the E0 of the reducing mediator, and oxidative redox-cycling can only occur if the imposed voltage is more oxidizing than the E0 of the oxidizing mediator (see below for details). In brief, the mediators’ E0 plays a critical role in determining if the film can be charged or discharged with electrons, and mediators with differing values can be used to shift the voltage that must be imposed at the electrode to initiate the redox-cycling reactions (i.e., E0 of the mediator serves as a gate).
The ability of the catechol–chitosan film to generate steady output responses is illustrated by the experiment in Figure 4b in which an oscillating voltage input was imposed over several hours in the presence of both mediators. The output response (or CV representation) for the electrode coated with the catechol–chitosan film shows that the output currents for both Fc-oxidation and Ru3+-reduction were amplified (compared to a control chitosan film) and these amplifications were nearly constant (i.e., steady) over time. From a chemical standpoint, the steady output current responses of Figure 4b indicate that the catechol–chitosan film can be repeatedly switched between oxidized and reduced states. From a signal processing standpoint, steady oscillating inputs and outputs (i.e., sine waves) are integral to the coding and decoding of information.
In contrast to the case of steady input–output, Figure 4c shows an example of unsteady response characteristics. In this unsteady case, the left plot of Figure 4c shows a more limited potential range was imposed (+0.5~0 V) to provide the oxidative voltages required to oxidize Fc but to provide reducing voltages that are insufficient to reduce Ru3+. Figure 4c shows the current output response for this unsteady case has no Ru3+-reduction peaks and the amplified currents of Fc-oxidation decrease progressively over time. Presumably, this decay in Fc-oxidation currents occurs because the catechol–chitosan film is progressively depleted of electrons during the repetitive Fc-redox-cycling reaction, but this film cannot be replenished with electrons because the imposed voltage is never sufficiently reductive for Ru3+-reductive redox-cycling. This unsteady output current response also illustrates the gating function of Ru3+: since the imposed voltage remains too oxidative (relative to the E0 for Ru3+), then reductive redox-cycling mechanism cannot be engaged to recharge the film with electrons. Under this condition, no Ru3+-reduction currents are observed and the progressive discharging of the film leads to a progressive decay in Fc-oxidation currents.
The results of Figure 4 illustrate four important molecular electronic properties of the catechol–chitosan redox-capacitor that we use for chemical information processing. One additional feature of this redox-capacitor is illustrated in Table 1. Specifically, the catechol–chitosan redox-capacitor has been observed to accept electrons from a broad range of reductants and donate electrons to various oxidants. This broad ability to exchange electrons with oxidants and reductants indicates that this redox-capacitor has a somewhat generic ability to access redox information (i.e., various different types of mediators can be used). From a chemical standpoint, the broad ability of the catechol–chitosan capacitor to exchange electrons means that it possesses redox catalytic properties and is capable of transferring electrons from reductants to oxidants in response to thermodynamic driving forces. However, we should note that not all redox-active chemicals can exchange electrons with the catechol–chitosan redox-capacitor. Thus, while thermodynamic plots may suggest what reactions are possible, some reactions do not occur within relevant timescales because redox-reactions can have significant kinetic barriers.

Table 1.
Tested redox chemicals that can redox-interact with catechol–chitosan films.
5. Examples of Chemical Information Processing Using Catechol-Based Redox-Capacitor
As suggested in Figure 1b, we believe that interactive redox-probing can provide access to chemical information, and we are especially focused on accessing chemical information relevant to redox biology. It is well-known that biology uses redox reactions for energy harvesting [56,57] (e.g., electron transfer in the respiratory chain), biosynthesis (e.g., nicotinamide adenine dinucleotide phosphate (NADPH) serves as a diffusible reductant) [10,58,59] and immune response (i.e., the oxidative burst) [60,61,62]. Redox is also emerging as an important signaling modality in biology with the use of diffusible extracellular signaling molecules (e.g., H2O2) [63,64,65] and redox-based receptor mechanisms (e.g., cysteine-based sulfur switches) [66,67,68]. In addition, redox is recognized as important in biological homeostasis with suggestions that oxidative stress is essentially redox dysregulation. Potentially, probing a local biological environment may reveal information of the redox activities and the redox context [69,70,71].
To illustrate the potential of redox-probing to access complex biological information, we cite recent studies on the development of a blood test for oxidative stress [55]. Specifically, as illustrated in Figure 5, an iridium (Ir) salt was added to serum to serve as an oxidant to detect reducing activities. Subsequent measurements of the amount of Ir reduced could be used to determine an “Ir-reducing capacity”: the lower the Ir-reducing capacity, the greater the oxidative stress. We observed that this measure of oxidative stress could correlate to clinical indicators of schizophrenia and thus this serum assay may aid in the diagnosis and assessment of symptom severity. The important point of this example is that an information processing approach was shown to rapidly access chemical information from serum and this information appears to have considerable clinical utility. Traditional approaches to develop serum tests for schizophrenia generally use panels of analytes, have high costs, and have been unsuccessful in the clinic. The Ir-mediated signal processing approach is essentially a reverse engineering approach in which the Ir mediator is used to probe the serum sample for redox-based information of oxidative stress. This approach does not rely on knowledge of underlying biological mechanisms but probes serum for redox information in a somewhat unbiased way in search of global signatures of relevant information.
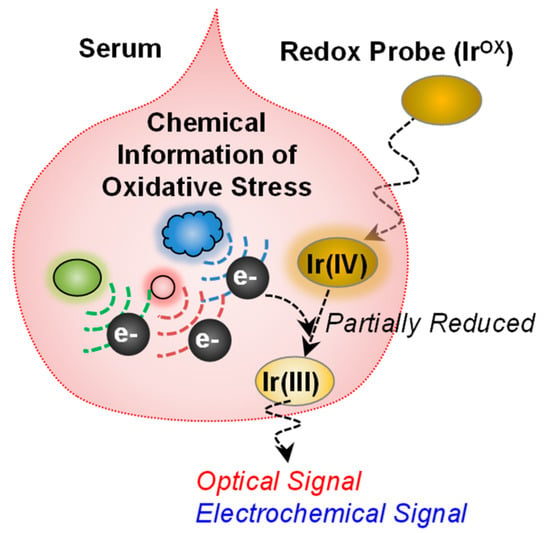
Figure 5.
Redox-probing to access chemical information of oxidative stress. The redox mediator (K2IrCl6, IrOX) can probe chemical information relevant to oxidative stress in blood. The information can be transmitted into optical and electrochemical modalities. Adapted with permission from [55]. Copyright (2017) American Chemical Society.
5.1. Interactive Redox-Probing of Biothiols
Thiols are important moieties in biology because the thiol of glutathione is important for antioxidant protection [72,73,74,75,76] and the thiols of the cysteine residues of proteins are able to serve as redox-responsive crosslinks [74]. Thiols also possess unusual chemical properties and tend to self-assemble onto gold surfaces through gold-thiol interactions rather than transferring their electrons through redox-reactions [77,78]. These chemical properties have made it difficult to detect thiols by electrochemical methods. For instance, the self-assembly of thiols tends to “foul” gold electrodes with insulating monolayer regions (i.e., patches on the gold surface) [79,80,81].
We examined whether a redox-probing approach could be used to detect the presence of the biothiol glutathione [27]. In initial studies, we immersed a gold electrode in solutions containing both the Fc and Ru3+ mediators and observed that the addition of small amounts of glutathione to this solution resulted in an attenuation of the Fc-oxidation currents. This attenuation is consistent with the self-assembly of the biothiol and a blocking of some of the electrode area to limit Fc-oxidation. When the gold electrode was coated with the catechol–chitosan redox-capacitor film and oscillating voltage inputs were imposed, as illustrated in Figure 6a, the Fc and Ru3+ currents were both amplified and the glutathione-induced signal attenuation was more easily detected. Presumably, glutathione can diffuse through the catechol–chitosan and self-assemble onto the underlying electrode as illustrated in Figure 6b. Importantly, Figure 6c shows that quantitative analysis of this signal attenuation could be correlated to glutathione concentration and a linear correlation extended over five orders of magnitude in concentration.
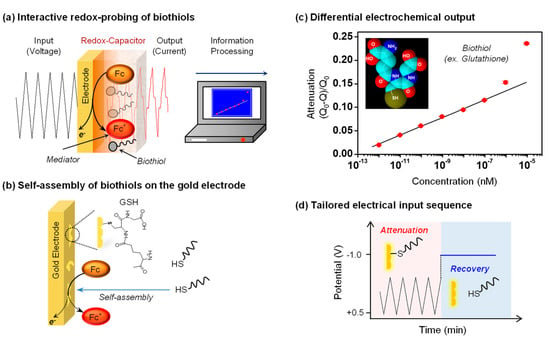
Figure 6.
Interactive redox-probing of biothiols. (a) The electrochemical information processing approach allows the acquisition of chemical information of biothiols. (b) Thiols can self-assemble on gold and potentially attenuate electrochemical signals. (c) The quantitative analysis of signal attenuation could be correlated to glutathione (GSH) concentration. Q: Ferrocene dimethanol (Fc)-oxidative charge with biothiol; Q0: Fc-oxidative charge without biothiol. (d) Tailored electrical input sequence to test the hypothesis that GSH self-assembly on gold attenuates Fc-oxidation. Adapted with permission from [27]. Copyright (2016) American Chemical Society.
This is an unusual example in the sense that we are using an electrochemical approach to detect the presence glutathione but the method is not based on a redox-reaction between glutathione and either mediator, or between glutathione and the redox-capacitor. Detection is rather believed to result because of the unique chemical capability of thiols to self-assemble onto gold and attenuate the electrode’s ability to oxidize Fc. To provide confirmatory evidence for this mechanism, we tested whether a strong reducing potential that is known to disassemble thiols from gold could be used to reverse the attenuation as illustrated in Figure 6d. We observed that indeed the use of such a reducing potential reversed the attenuated Fc currents and this sequence of attenuation and reversal could be repeated multiple times.
This work illustrates two important points. First, the catechol–chitosan redox-capacitor generates amplified signals that facilitate detection. Second, complex electrical inputs can be imposed to probe for specific information: we used oscillating inputs to generate steady outputs that facilitated quantification of attenuation and we used step changes to reducing potentials to test for biothiol disassembly and a recovery of the Fc-oxidation current. This latter point illustrates that complex electrical input signals can be designed to test specific chemical hypotheses.
5.2. Detection of a Redox-Active Bacterial Metabolite
Biology often uses diffusible redox-active metabolites to perform functions: immune cells generate reactive oxygen species to defend against pathogen attack (e.g., the oxidative burst) and H2O2 is emerging as a diffusible signaling molecule [82,83,84]. Attention has also been focused on other redox-active metabolites such as phenazines, which are among the most-studied redox-active bacterial metabolites [85,86,87]. These metabolites are believed to: (1) allow the producing bacteria to mediate signaling among cells (i.e., quorum sensing) [88]; (2) transfer electrons outside the cell for redox-balancing [89]; and (3) maintain redox homeostasis of multicellular biofilms [90]. One of the phenazines, pyocyanin (PYO), is also a virulence factor for the opportunistic pathogen Pseudomonas aeruginosa [85,91]. Importantly, P. aeruginosa is emerging as one of the most significant pathogens of nosocomial (hospital acquired) infections, especially for burn patients [92,93,94]. A rapid detection of this pathogen could be integral to successfully identifying and treating infections in this vulnerable patient population.
Figure 7a shows that P. aeruginosa secretes the redox-active metabolite PYO that can diffuse through the redox-capacitor film [50]. The diffused PYO can be electrochemically reduced at the electrode when the cathodic potential is applied and then the reduced PYO can diffuse back into the film and donate its electrons to the redox-capacitor film. Thus, PYO can undergo reductive redox-cycling with the redox capacitor film and yield amplified output currents that could facilitate detection of P. aeruginosa.
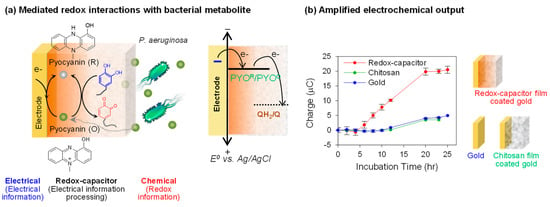
Figure 7.
Electrochemical detection of the redox-active bacterial metabolite pyocyanin (PYO). (a) Schematic shows that the redox-capacitor can amplify the reduction current of PYO due to the reductive redox-cycling reaction. QH2/Q: Catechol(QH2) moieties/quinone (Q) moieties of catechol–chitosan film. (b) Compared with bare gold and chitosan-coated electrode, the redox-capacitor can sensitively detect the PYO production by bacteria. Adapted with permission from [50]. Copyright (2013) American Chemical Society.
To illustrate the sensitive detection of PYO production, we immersed an electrode coated with the catechol–chitosan redox-capacitor into a growing bacterial culture and intermittently performed in situ electrochemical measurement (using a chronocoulometric technique). Figure 7b compares that the charge transfer (a measure of the number of electrons transferred across the electrode) for PYO-reduction against two controls, a bare gold electrode and an electrode coated with an unmodified chitosan film. Both controls show very small charge transfer for PYO-reduction compared to the amplified output generated by the electrode coated by the catechol–chitosan redox-capacitor film.
In summary, the results in Figure 7 demonstrate that the redox-active metabolite (PYO) can undergo redox interactions with the electrode and the capacitor film, and these interactions lead to amplified electrical output signals. In essence, these redox interactions serve to convert chemical information of the bacterial generation of PYO into an electrical output. Potentially, this amplified detection might allow the early detection of infections by the opportunistic pathogen, P. aeruginosa. In addition, this measurement may be useful for studying the spatiotemporal dynamics of PYO generation in complex systems (e.g., bacterial biofilms).
5.3. A Global Analysis of Redox Context
Figure 1b suggests that redox-probing and signal processing can provide a new paradigm for accessing redox-based chemical information. Our initial effort to measure global redox information is illustrated in Figure 8a in which we immersed an electrode coated with a catechol–chitosan redox-capacitor into a complex bacterial culture and imposed cyclic potential inputs [95]. As noted, these electrical inputs are transduced into redox “transmissions” by the mediators that diffuse through the film into the local environment to probe for redox information (i.e., to assess the redox context). For the example in Figure 8, we added two redox-active biological mediators: the bacterial phenazine pyocyanin that undergoes reductive redox-cycling for film-charging, and the plant phenolic acetosyringone (AS) that can undergo oxidative redox-cycling for film discharging. As an aside, it is useful to note that both molecules are believed to perform signaling functions in biology: PYO for bacterial quorum sensing and AS for a plant innate immune response [96,97,98]. In this example, the catechol–chitosan redox-capacitor films serve to manipulate the redox signals in ways that facilitate interpretation (e.g., the capacitor amplifies and partially rectifies the currents).
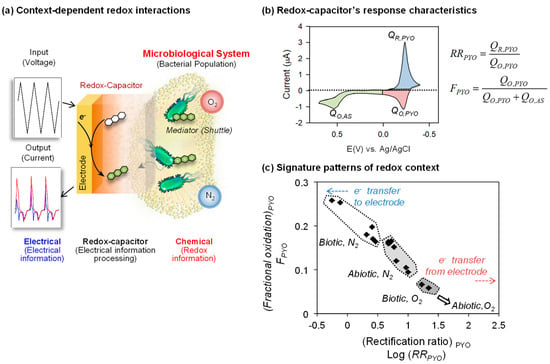
Figure 8.
Global analysis of redox context. (a) Schematic illustrates that cyclic electrical inputs–outputs are transferred via mediators to the aqueous/biological system, while the catechol–chitosan interface “processes” this information. (b) Parameters calculated from cyclic voltammograms that correlate data based on rectification of pyocyanin (PYO) currents and the fraction of total electrochemical oxidation that is attributed to PYO. QR,PYO: PYO-reductive charge; QO,PYO: PYO-oxidative charge; QO,AS: Acetosyrigone (AS)-oxidative charge. (c) Two parameters (rectification ratio for pyocyanin (RRPYO) and fraction of electrochemical oxidation occurring in the pyocyanin region (FPYO)) show the correlation for the four experimental contexts. Adapted with permission from [95]. Copyright (2013) American Chemical Society.
To evaluate the signal processing approach, we exposed this capacitor-coated electrode to different redox contexts based on whether the experimental system did or did not have a living population of Escherichia coli (biotic or abiotic) or whether there was or was not oxygen present (aerobic or anaerobic). Figure 8b shows a typical CV output response for this experiment and illustrates the signal analysis approach for analyzing the signal. As illustrated in Figure 8b, the CV signal was divided into three regions which were operationally assigned to the specific chemical processes of PYO-reduction, PYO-oxidation and AS-oxidation (note these assignments are important for signal processing but are approximations of the underlying chemistries) [53]. The currents () in these regions were integrated with respect to time () to determine the charge transfer () in these three regions. These values were then used to generate either a rectification ratio for pyocyanin (RRPYO) or the fraction of electrochemical oxidation occurring in the pyocyanin region (FPYO). Using these analytical values, the individual CVs for the four different redox contexts were compared as illustrated in Figure 8c. The correlation plot of Figure 8c shows the ability to discern these four redox contexts (see original paper [95] for details).
In summary, the results in Figure 8 illustrate that redox-probing and signal processing can provide global signatures capable of discerning difference in redox context. Potentially, this analysis could provide a new approach to extract redox-based chemical information from systems that are not well understood and are difficult to probe by conventional methods (e.g., the microbiome [99]).
5.4. Coupling Redox-Probing with Synthetic Biology to Access Biochemical Signals
As observed in previous example, some biologically important chemicals are redox-active (e.g., signaling molecules PYO and AS). In these cases, electrochemical methods can be used for direct detection. However, not all molecules are redox-active and in these cases, electrochemistry cannot be directly employed for detection. An emerging approach is to enlist synthetic biology (synbio) to create engineered cells that can recognize a specific chemical and transduce this recognition event into a redox-based signal. For instance, an important bacterial quorum sensing molecule, autoinducer-2 (AI-2), is not redox-active and a synbio reporter cell has been constructed to recognize AI-2 and convert this chemical information into a redox signal that can be electrochemically detected [23,54]. Figure 9a illustrates that this E. coli reporter cell transduces the AI-2 molecular input into the expression of the enzyme β-galactosidase (β-gal) that can convert a redox-inactive substrate (p-aminophenly β-d-galactopyranoside (PAPG)) into a redox-active product (p-aminophenol (PAP)).
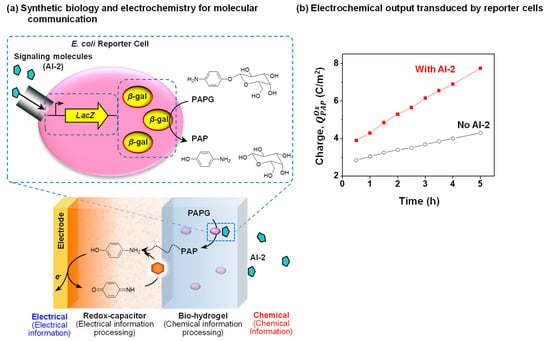
Figure 9.
Coupling redox-probing with synthetic biology to access biochemical signals. (a) Schematic shows the Escherichia coli reporter cell created to detect autoinducer-2 (AI-2) and transduce this information from the redox-inactive substrate p-aminophenly β-d-galactopyranoside (PAPG) into a redox-active intermediate that is electrochemically detected. The redox-capacitor converts p-aminophenol (PAP) into an amplified electrochemical output. (b) The electrochemical output (oxidative charge, QOx) of the dual-film containing E. coli reporter cells shows faster increase in the presence of the signaling molecule AI-2 compared with its absence. Reproduced with permission from [54], published by John Wiley and Sons, 2017.
Figure 9a also shows that a dual-film system is used to interface these E. coli reporter cells adjacent to an electrode. Specifically, these cells are entrapped within a Ca2+-alginate bio-hydrogel film that is electroaddressed on top of the catechol–chitosan redox-capacitor film [100,101,102]. The redox-inactive PAPG substrate is purposefully added to the system, and it can diffuse into the dual-film system. When this dual film system is exposed to AI-2, the reporter cells express the β-gal enzyme that converts PAPG into the redox-active PAP product that can diffuse into the redox-capacitor where it can undergo oxidative redox-cycling reactions. Figure 9b shows experimental results demonstrating that the oxidative charge transfer is considerably larger when the dual-film was exposed to AI-2 (compared to the control which the dual-film was not exposed to AI-2).
In summary, the dual-film system serves to process the chemical information of AI-2 into an electrical signal by first using a synbio construct to transduce the AI-2 chemical input into a redox intermediate (PAP), and then using the catechol–chitosan redox-capacitor to convert this redox intermediate into an amplified electrical output. There are two broad features of this work. First, the work demonstrates the coupling of synthetic biology, thin film technology, and electrochemistry to convert chemical information into electrical signals. Second, this coupling is enabled by the use of redox as an intermediate modality that bridges the chemical modality of biology and the electrical modality of devices. Redox can bridge these modalities because it shares features of both the molecular and electrical modalities.
6. Conclusions and Future Perspectives
Over the past half-century, microelectronics and information technology transformed the way we process information, but these advances have had a relatively small impact on accessing and understanding the nature of chemical information. A key limitation to interfacing advanced electronic technology with biology is to identify a suitable means to span the chemical modalities of biology and the electrical modality of modern devices. We suggest redox provides a means to span these modalities and propose a new paradigm of interactively probing a local environment for redox-based chemical information in a manner that is analogous to sonar. In this approach, we impose electrical inputs and purposely add redox mediators that can diffuse into the environment and transduce electrical inputs into redox signals (e.g., redox transmission). These mediators probe for redox information in the environment and this information is converted at the electrode into electrical signals that can be “decoded” by signal processing strategies to extract the chemical information. In this approach, thin hydrogel films coated onto an electrode can perform important information processing operations.
We summarize several examples to show that a catechol-based redox-capacitor film can serve to process the redox information generated from various biological systems. We envision the catechol–based redox-capacitor could be useful for processing redox-based chemical information because: (1) it facilitates electron exchange with a broad range of oxidants and reductants, and thus may provide a means to globally sample redox information; (2) it is easily assembled on the electrode by chitosan electrodeposition and electrochemical grafting of catechols; and (3) it possesses unique molecular electronic properties (amplification, partial rectification, gating, switching, and steady response) that serve to process electrochemical information.
We anticipate that this new paradigm for accessing chemical information using a signal processing approach could provide insights on: (1) the oxidative/reductive stresses being exerted on a biological system (e.g., due to inflammation or tumor therapy); (2) the oxidative/reductive actions being taken by a biological system (e.g., oxidative burst or redox signaling); (3) the redox-mediated or regulation process (e.g., for disulfide bond formation/cleavage); and (4) the redox interactions that occur among various chemical components (e.g., between redox-cycling drugs and antioxidants in our diet). Obviously, more tests are needed to validate this proposed signal processing approach and also to understand the utility of the information gained from this interactive redox-probing.
Acknowledgments
This work has been supported by National Science Foundation (NSF) (DMREF-1435957) and Defense Threat Reduction Agency (DTRA) (HDTRA1-13-1- 0037).
Author Contributions
This review describes results from a multi-investigator collaboration. E.K. and G.F.P. were responsible for the redox-capacitor studies; Z.L. was responsible for the interactive redox-probing of biothiols; Y.L. was responsible for coupling redox-probing with synthetic biology; W.E.B. and G.F.P. were responsible for the conceptualization of many of the advances described in this paper; and G.F.P. and E.K. wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Hong, S.; Lee, H. Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomater. 2015, 27, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 2007, 448, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Wilker, J.J. The iron-fortified adhesive system of marine mussels. Angew. Chem. Int. Ed. 2010, 49, 8076–8078. [Google Scholar] [CrossRef] [PubMed]
- Klupfel, L.; Piepenbrock, A.; Kappler, A.; Sander, M. Humic substances as fully regenerable electron acceptors in recurrently anoxic environments. Nat. Geosci. 2014, 7, 195–200. [Google Scholar] [CrossRef]
- Scott, D.T.; McKnight, D.M.; Blunt-Harris, E.L.; Kolesar, S.E.; Lovley, D.R. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ. Sci. Technol. 1998, 32, 2984–2989. [Google Scholar] [CrossRef]
- Lovley, D.R.; Coates, J.D.; Blunt-Harris, E.L.; Phillips, E.J.P.; Woodward, J.C. Humic substances as electron acceptors for microbial respiration. Nature 1996, 382, 445–448. [Google Scholar] [CrossRef]
- Jacobson, E.S.; Hong, J.D. Redox buffering by melanin and Fe(II) in Cryptococcus neoformans. J. Bacteriol. 1997, 179, 5340–5346. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G. Metabolic signalling in defence and stress: The central roles of soluble redox couples. Plant Cell Environ. 2006, 29, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Sarna, T.; Plonka, P.M. Biophysical studies of melanin. In Biomedical EPR, Part A: Free Radicals, Metals, Medicine, and Physiology; Eaton, S.R., Eaton, G.R., Berliner, L.J., Eds.; Springer: Boston, MA, USA, 2005; pp. 125–146. [Google Scholar]
- Schweigert, N.; Zehnder, A.J.B.; Eggen, R.I.L. Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environ. Microbiol. 2001, 3, 81–91. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox signaling in plants. Antioxid Redox Signal. 2013, 18, 2087–2090. [Google Scholar] [CrossRef]
- Sarewicz, M.; Osyczka, A. Electronic connection between the quinone and cytochrome c redox pools and its role in regulation of mitochondrial electron transport and redox signaling. Physiol. Rev. 2014, 95, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Ernster, L.; Dallner, G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys. Acta 1995, 1271, 195–204. [Google Scholar] [CrossRef]
- Maurer, F.; Christl, I.; Hoffmann, M.; Kretzschmar, R. Reduction and reoxidation of humic acid: Influence on speciation of cadmium and silver. Environ. Sci. Technol. 2012, 46, 8808–8816. [Google Scholar] [CrossRef] [PubMed]
- Aeschbacher, M.; Graf, C.; Schwarzenbach, R.P.; Sander, M. Antioxidant properties of humic substances. Environ. Sci. Technol. 2012, 46, 4916–4925. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, M.; Park, C.B. Polydopamine as a biomimetic electron gate for artificial photosynthesis. Angew. Chem. Int. Ed. 2014, 53, 6364–6368. [Google Scholar] [CrossRef]
- Steinfeld, J.I.; Wormhoudt, J. Explosives detection: A challenge for physical chemistry. Annu. Rev. Phys. Chem. 1998, 49, 203–232. [Google Scholar] [CrossRef] [PubMed]
- Madsen, R.; Lundstedt, T.; Trygg, J. Chemometrics in metabolomics—A review in human disease diagnosis. Anal. Chim. Acta 2010, 659, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Bosque-Sendra, J.M.; Cuadros-Rodríguez, L.; Ruiz-Samblás, C.; de la Mata, A.P. Combining chromatography and chemometrics for the characterization and authentication of fats and oils from triacylglycerol compositional data—A review. Anal. Chim. Acta 2012, 724, 1–11. [Google Scholar] [CrossRef]
- Karoui, R.; Downey, G.; Blecker, C. Mid-infrared spectroscopy coupled with chemometrics: A tool for the analysis of intact food systems and the exploration of their molecular structure−quality relationships—A review. Chem. Rev. 2010, 110, 6144–6168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kim, E.; Li, J.; Kang, M.; Bentley, W.E.; Payne, G.F. Electrochemistry for bio-device molecular communication: The potential to characterize, analyze and actuate biological systems. Nano Commun. Netw. 2017, 11, 76–89. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Kim, E.; March, J.C.; Bentley, W.E.; Payne, G.F. Electrochemical reverse engineering: A systems-level tool to probe the redox-based molecular communication of biology. Free Radic. Biol. Med. 2017, 105, 110–131. [Google Scholar] [CrossRef] [PubMed]
- Röck, F.; Barsan, N.; Weimar, U. Electronic nose: Current status and future trends. Chem. Rev. 2008, 108, 705–725. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Suslick, K.S. Portable optoelectronic nose for monitoring meat freshness. ACS Sens. 2016, 1, 1330–1335. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Kim, E.; Bentley, W.E.; Payne, G.F. Electrochemical probing through a redox capacitor to acquire chemical information on biothiols. Anal. Chem. 2016, 88, 7213–7221. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Liu, Y.; Ben-Yoav, H.; Winkler, T.E.; Yan, K.; Shi, X.; Shen, J.; Kelly, D.L.; Ghodssi, R.; Bentley, W.E.; et al. Fusing sensor paradigms to acquire chemical information: An integrative role for smart biopolymeric hydrogels. Adv. Healthc. Mater. 2016, 5, 2595–2616. [Google Scholar] [CrossRef] [PubMed]
- Giner-Sanz, J.J.; Ortega, E.M.; Pérez-Herranz, V. Total harmonic distortion based method for linearity assessment in electrochemical systems in the context of EIS. Electrochim. Acta 2015, 186, 598–612. [Google Scholar] [CrossRef]
- Lisdat, F.; Schäfer, D. The use of electrochemical impedance spectroscopy for biosensing. Anal. Bioanal. Chem. 2008, 391, 1555. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Leverage, W.T.; Liu, Y.; White, I.M.; Bentley, W.E.; Payne, G.F. Redox-capacitor to connect electrochemistry to redox-biology. Analyst 2014, 139, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.M.; Kim, E.; Wu, L.-Q.; Liu, Y.; Bentley, W.E.; Payne, G.F. Biomimetic fabrication of information-rich phenolic-chitosan films. Soft Matter 2011, 7, 9601–9615. [Google Scholar] [CrossRef]
- Wu, L.Q.; McDermott, M.K.; Zhu, C.; Ghodssi, R.; Payne, G.F. Mimicking biological phenol reaction cascades to confer mechanical function. Adv. Funct. Mater. 2006, 16, 1967–1974. [Google Scholar] [CrossRef]
- Wu, L.Q.; Ghodssi, R.; Elabd, Y.A.; Payne, G.F. Biomimetic pattern transfer. Adv. Funct. Mater. 2005, 15, 189–195. [Google Scholar] [CrossRef]
- Morrow, B.H.; Payne, G.F.; Shen, J. pH-Responsive self-assembly of polysaccharide through a rugged energy landscape. J. Am. Chem. Soc. 2015, 137, 13024–13030. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-Q.; Gadre, A.P.; Yi, H.; Kastantin, M.J.; Rubloff, G.W.; Bentley, W.E.; Payne, G.F.; Ghodssi, R. Voltage-dependent assembly of the polysaccharide chitosan onto an electrode surface. Langmuir 2002, 18, 8620–8625. [Google Scholar] [CrossRef]
- Fernandes, R.; Wu, L.-Q.; Chen, T.; Yi, H.; Rubloff, G.W.; Ghodssi, R.; Bentley, W.E.; Payne, G.F. Electrochemically induced deposition of a polysaccharide hydrogel onto a patterned surface. Langmuir 2003, 19, 4058–4062. [Google Scholar] [CrossRef]
- Sugumaran, M.; Hennigan, B.; Obrien, J. Tyrosinase catalyzed protein polymerization as an in vitro model for quinone tanning of insect cuticle. Arch. Insect Biochem. Physiol. 1987, 6, 9–25. [Google Scholar] [CrossRef]
- Kramer, K.J.; Kanost, M.R.; Hopkins, T.L.; Jiang, H.; Zhu, Y.C.; Xu, R.; Kerwin, J.L.; Turecek, F. Oxidative conjugation of catechols with proteins in insect skeletal systems. Tetrahedron 2001, 57, 385–392. [Google Scholar] [CrossRef]
- Aberg, C.M.; Chen, T.; Olumide, A.; Raghavan, S.R.; Payne, G.F. Enzymatic grafting of peptides from casein hydrolysate to chitosan. Potential for value-added byproducts from food-processing wastes. J. Agric. Food Chem. 2004, 52, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, J.L.; Whitney, D.L.; Sheikh, A. Mass spectrometric profiling of glucosamine, glucosamine polymers and their catecholamine adducts: Model reactions and cuticular hydrolysates of Toxorhynchites amboinensis (Culicidae) pupae. Insect Biochem. Mol. Biol. 1999, 29, 599–607. [Google Scholar] [CrossRef]
- McGinness, J.; Corry, P.; Proctor, P. Amorphous semiconductor switching in melanins. Science 1974, 183, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Meredith, P.; Sarna, T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006, 19, 572–594. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.K.; Kolter, R. A role for excreted quinones in extracellular electron transfer. Nature 2000, 405, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.E.; Newman, D.K. Extracellular electron transfer. Cell. Mol. Life Sci. 2001, 58, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Liu, Y.; Bentley, W.E.; Payne, G.F. Redox capacitor to establish bio-device redox-connectivity. Adv. Funct. Mater. 2012, 22, 1409–1416. [Google Scholar] [CrossRef]
- Kim, E.; Liu, Y.; Shi, X.-W.; Yang, X.; Bentley, W.E.; Payne, G.F. Biomimetic approach to confer redox activity to thin chitosan films. Adv. Funct. Mater. 2010, 20, 2683–2694. [Google Scholar] [CrossRef]
- Kim, E.; Xiong, Y.; Cheng, Y.; Wu, H.-C.; Liu, Y.; Morrow, H.B.; Ben-Yoav, H.; Ghodssi, R.; Rubloff, W.G.; Shen, J.; et al. Chitosan to connect biology to electronics: Fabricating the bio-device interface and communicating across this interface. Polymers 2015, 7, 1–46. [Google Scholar] [CrossRef]
- Kim, E.; Liu, Y.; Baker, C.J.; Owens, R.; Xiao, S.; Bentley, W.E.; Payne, G.F. Redox-cycling and H2O2 generation by fabricated catecholic films in the absence of enzymes. Biomacromolecules 2011, 12, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Gordonov, T.; Bentley, W.E.; Payne, G.F. Amplified and in situ detection of redox-active metabolite using a biobased redox capacitor. Anal. Chem. 2013, 85, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Leverage, W.T.; Liu, Y.; Panzella, L.; Alfieri, M.L.; Napolitano, A.; Bentley, W.E.; Payne, G.F. Paraquat–melanin redox-cycling: Evidence from electrochemical reverse engineering. ACS Chem. Neurosci. 2016, 7, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yoav, H.; Winkler, T.E.; Kim, E.; Chocron, S.E.; Kelly, D.L.; Payne, G.F.; Ghodssi, R. Redox cycling-based amplifying electrochemical sensor for in situ clozapine antipsychotic treatment monitoring. Electrochim. Acta 2014, 130, 497–503. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, E.; White, I.M.; Bentley, W.E.; Payne, G.F. Information processing through a bio-based redox capacitor: Signatures for redox-cycling. Bioelectrochemistry 2014, 98, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tsao, C.-Y.; Kim, E.; Tschirhart, T.; Terrell, J.L.; Bentley, W.E.; Payne, G.F. Using a redox modality to connect synthetic biology to electronics: Hydrogel-based chemo-electro signal transduction for molecular communication. Adv. Healthc. Mater. 2017, 6, 1600908. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Winkler, T.E.; Kitchen, C.; Kang, M.; Banis, G.; Bentley, W.E.; Kelly, D.L.; Ghodssi, R.; Payne, G.F. Redox probing for chemical information of oxidative stress. Anal. Chem. 2017, 89, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microb. 2009, 7, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Scherer, S. Do photosynthetic and respiratory electron transport chains share redox proteins? Trends Biochem. Sci. 1990, 15, 458–462. [Google Scholar] [CrossRef]
- Deplancke, B.; Gaskins, H.R. Redox control of the transsulfuration and glutathione biosynthesis pathways. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Tenhaken, R.; Dixon, R.; Lamb, C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 1994, 79, 583–593. [Google Scholar] [CrossRef]
- Nürnberger, T.; Scheel, D. Signal transmission in the plant immune response. Trends Plant Sci. 2001, 6, 372–379. [Google Scholar] [CrossRef]
- Nathan, C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006, 6, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Boudsocq, M.; Willmann, M.R.; McCormack, M.; Lee, H.; Shan, L.; He, P.; Bush, J.; Cheng, S.-H.; Sheen, J. Differential innate immune signalling via Ca2+ sensor protein kinases. Nature 2010, 464, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Klomsiri, C.; Karplus, P.A.; Poole, L.B. Cysteine-based redox switches in enzymes. Antioxid. Redox Signal. 2010, 14, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Brandes, N.; Schmitt, S.; Jakob, U. Thiol-based redox switches in eukaryotic proteins. Antioxid. Redox Signal. 2008, 11, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Groitl, B.; Jakob, U. Thiol-based redox switches. Biochim. Biophys. Acta 2014, 1844, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Levonen, A.-L.; Hill, B.G.; Kansanen, E.; Zhang, J.; Darley-Usmar, V.M. Redox regulation of antioxidants, autophagy, and the response to stress: Implications for electrophile therapeutics. Free Radic. Biol. Med. 2014, 71, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Keaney, J.F. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000, 62, 649–671. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, H.; Lu, C.; Zu, Y. Oxidation of l-cysteine at a fluorosurfactant-modified gold electrode: Lower overpotential and higher selectivity. Langmuir 2007, 23, 10816–10822. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Chen, J.; Nie, L.; Yao, S.; Kuang, Y. Electrochemical oxidation of glutathione at well-aligned carbon nanotube array electrode. Electrochim. Acta 2006, 51, 3046–3051. [Google Scholar] [CrossRef]
- Leopold, M.C.; Bowden, E.F. Influence of gold substrate topography on the voltammetry of cytochrome c adsorbed on carboxylic acid terminated self-assembled monolayers. Langmuir 2002, 18, 2239–2245. [Google Scholar] [CrossRef]
- Boubour, E.; Lennox, R.B. Insulating properties of self-assembled monolayers monitored by impedance spectroscopy. Langmuir 2000, 16, 4222–4228. [Google Scholar] [CrossRef]
- Janek, R.P.; Fawcett, W.R.; Ulman, A. Impedance spectroscopy of self-assembled monolayers on Au(111): Sodium ferrocyanide charge transfer at modified electrodes. Langmuir 1998, 14, 3011–3018. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.N.; St. John, A.L. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 2010, 10, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Faoro, F. Review of innate and specific immunity in plants and animals. Mycopathologia 2007, 164, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Price-Whelan, A.; Dietrich, L.E.P.; Newman, D.K. Rethinking ‘secondary’ metabolism: Physiological roles for phenazine antibiotics. Nat. Chem. Biol. 2006, 2, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Okegbe, C.; Sakhtah, H.; Sekedat, M.D.; Price-Whelan, A.; Dietrich, L.E.P. Redox eustress: Roles for redox-active metabolites in bacterial signaling and behavior. Antioxid. Redox Signal. 2011, 16, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.; Jamier, V.; Ba, L.A. Redox active secondary metabolites. Curr. Opin. Chem. Biol. 2011, 15, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, L.E.P.; Price-Whelan, A.; Petersen, A.; Whiteley, M.; Newman, D.K. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol. Microbiol. 2006, 61, 1308–1321. [Google Scholar] [CrossRef] [PubMed]
- Price-Whelan, A.; Dietrich, L.E.P.; Newman, D.K. Pyocyanin alters redox homeostasis and carbon flux through central metabolic pathways in Pseudomonas aeruginosa PA14. J. Bacteriol. 2007, 189, 6372–6381. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, L.E.P.; Okegbe, C.; Price-Whelan, A.; Sakhtah, H.; Hunter, R.C.; Newman, D.K. Bacterial community morphogenesis is intimately linked to the intracellular redox state. J. Bacteriol. 2013, 195, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, L.E.P.; Teal, T.K.; Price-Whelan, A.; Newman, D.K. Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science 2008, 321, 1203. [Google Scholar] [CrossRef] [PubMed]
- Branski, L.K.; Al-Mousawi, A.; Rivero, H.; Jeschke, M.G.; Sanford, A.P.; Herndon, D.N. Emerging infections in burns. Surg. Infect. 2009, 10, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, J.B.A.; McManus, A.T.; Kim, S.H.; Goodwin, C.W. Burn wound infections: Current status. World J. Surg. 1998, 22, 135–145. [Google Scholar] [PubMed]
- Tredget, E.E.; Shankowsky, H.A.; Rennie, R.; Burrell, R.E.; Logsetty, S. Pseudomonas infections in the thermally injured patient. Burns 2004, 30, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Gordonov, T.; Liu, Y.; Bentley, W.E.; Payne, G.F. Reverse engineering to suggest biologically relevant redox activities of phenolic materials. ACS Chem. Biol. 2013, 8, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.J.; Mock, N.M.; Smith, J.M.; Aver’yanov, A.A. The dynamics of apoplast phenolics in tobacco leaves following inoculation with bacteria. Front. Plant Sci. 2015, 6, 649. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.J.; Whitaker, B.D.; Roberts, D.P.; Mock, N.M.; Rice, C.P.; Deahl, K.L.; Aver’yanov, A.A. Induction of redox sensitive extracellular phenolics during plant–bacterial interactions. Physiol. Mol. Plant Pathol. 2005, 66, 90–98. [Google Scholar] [CrossRef]
- Jacyn Baker, C.; Roberts, D.P.; Mock, N.M.; Whitaker, B.D.; Deahl, K.L.; Aver’yanov, A.A. Apoplastic redox metabolism: Synergistic phenolic oxidation and a novel oxidative burst. Physiol. Mol. Plant Pathol. 2005, 67, 296–303. [Google Scholar] [CrossRef]
- Espey, M.G. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic. Biol. Med. 2013, 55, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-W.; Tsao, C.-Y.; Yang, X.; Liu, Y.; Dykstra, P.; Rubloff, G.W.; Ghodssi, R.; Bentley, W.E.; Payne, G.F. Electroaddressing of cell populations by co-deposition with calcium alginate hydrogels. Adv. Funct. Mater. 2009, 19, 2074–2080. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, X.; Tsao, C.-Y.; Wu, H.-C.; Betz, J.; Payne, G.F.; Bentley, W.E.; Rubloff, G.W. Biocompatible multi-address 3D cell assembly in microfluidic devices using spatially programmable gel formation. Lab On A Chip 2011, 11, 2316–2318. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kim, E.; Ghodssi, R.; Rubloff, G.W.; Culver, J.N.; Bentley, W.E.; Payne, G.F. Biofabrication to build the biology–device interface. Biofabrication 2010, 2, 022002. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).