Geomimetics and Extreme Biomimetics Inspired by Hydrothermal Systems—What Can We Learn from Nature for Materials Synthesis?
Abstract
:1. Introduction
2. Discussion
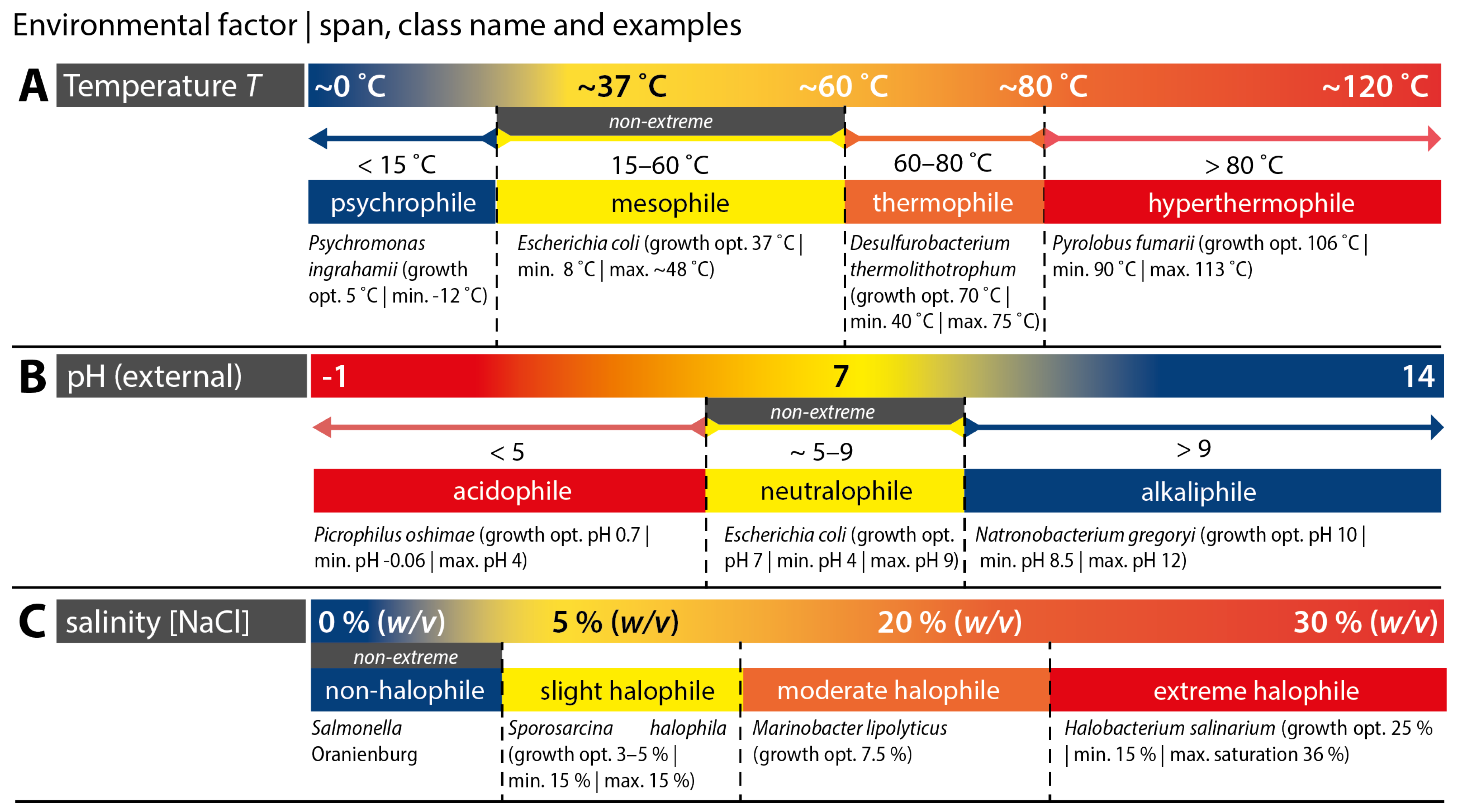
2.1. ‘Extreme’ Biological Conditions
2.1.1. Extremophile Microorganisms
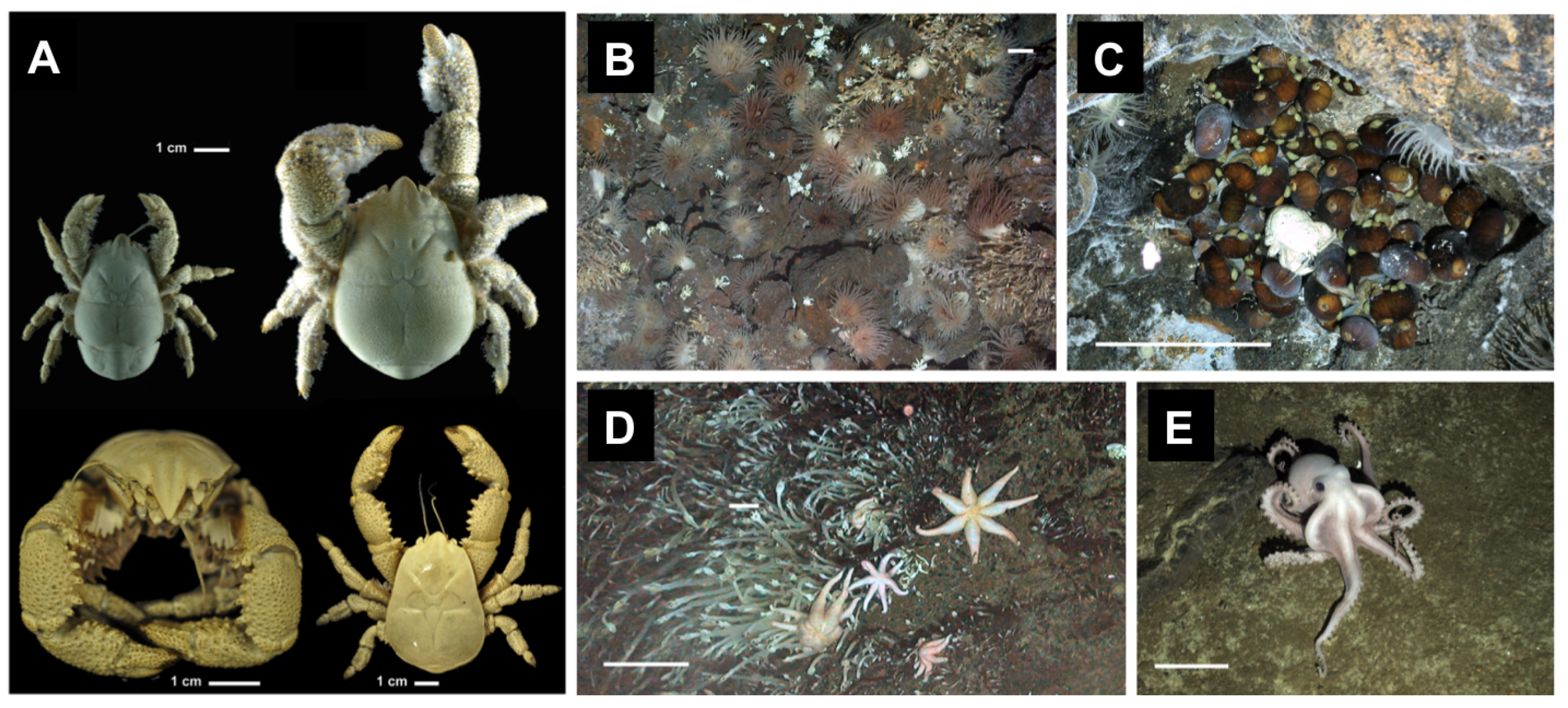
2.1.2. Hydrothermal Vent Fauna
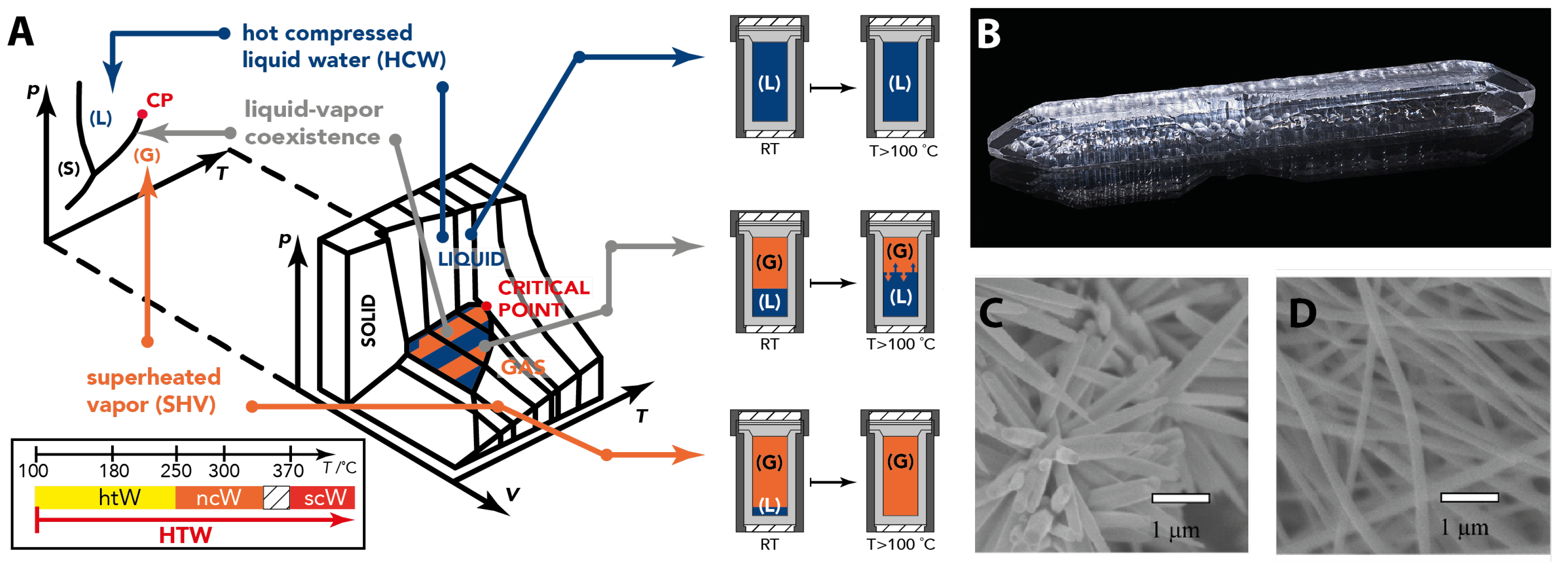
2.2. Geological Materials Formation Processes
2.3. Geomimetics for Materials Synthesis
2.3.1. Geomimetic Hydrothermal Synthesis of Inorganic Materials
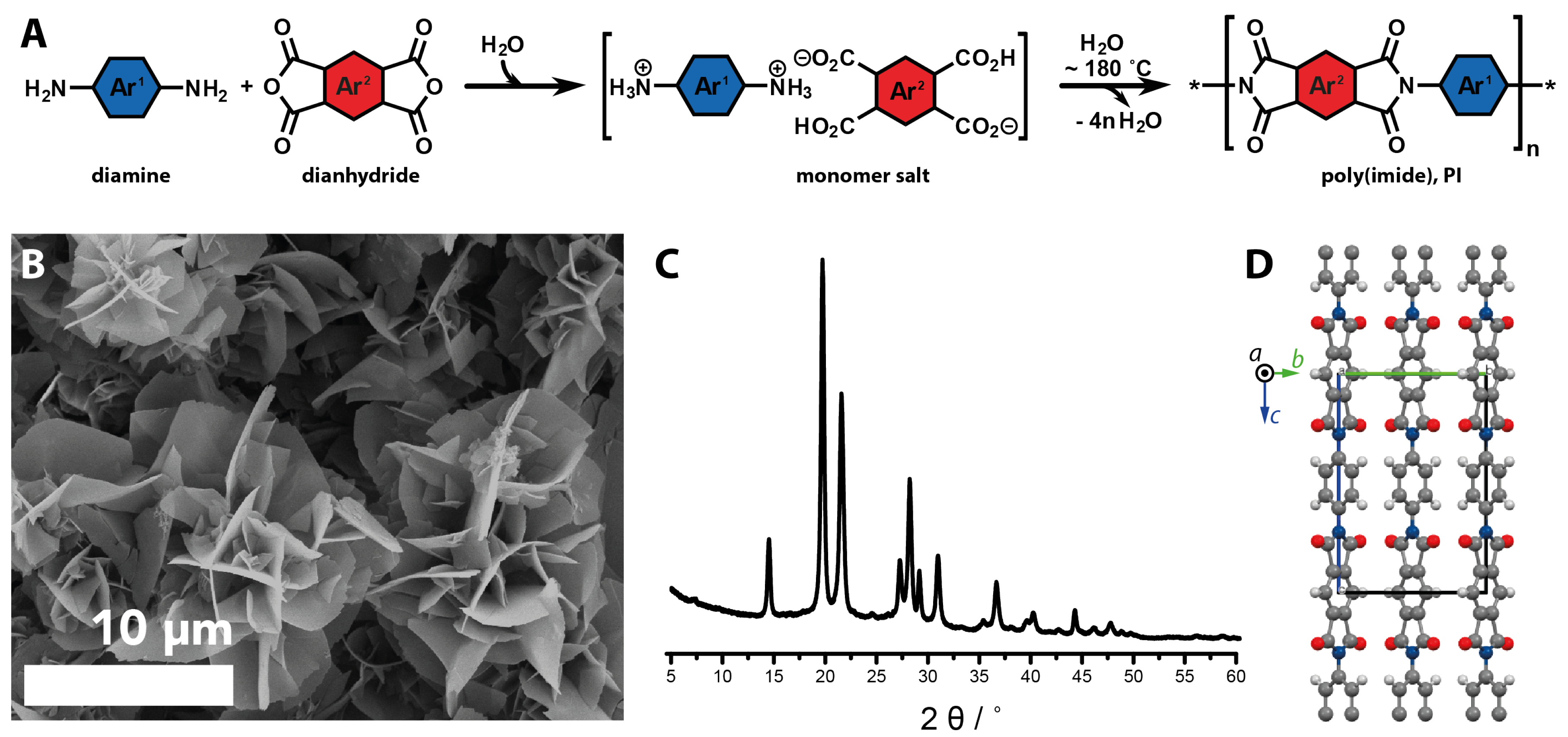
2.3.2. Geomimetic Hydrothermal Synthesis of Organic Materials
2.4. Synthesis of Hybrid Materials by Extreme Biomimetics
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Tang, S.L.; Smith, R.L.; Poliakoff, M. Principles of green chemistry: PRODUCTIVELY. Green Chem. 2005, 7, 761–762. [Google Scholar] [CrossRef]
- Merkel, C.; Deuschle, J.; Griesshaber, E.; Enders, S.; Steinhauser, E.; Hochleitner, R.; Brand, U.; Schmahl, W.W. Mechanical properties of modern calcite-(Mergerlia truncata) and phosphate-shelled brachiopods (Discradisca stella and Lingula anatina) determined by nanoindentation. J. Struct. Biol. 2009, 168, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Schubert, U.; Hüsing, N. Synthesis of Inorganic Materials; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Bhushan, B. Biomimetics: Bioinspired Hierarchical-Structured Surfaces for Green Science and Technology; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Byrappa, K.; Yoshimura, M. Handbook of Hydrothermal Technology; William Andrew: Norwich, NY, USA, 2012. [Google Scholar]
- Titirici, M.M.; Antonietti, M. Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem. Soc. Rev. 2010, 39, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Unterlass, M.M. Creating geomimetic polymers. Mater. Today 2015, 5, 242–243. [Google Scholar] [CrossRef]
- Lalena, J.N.; Cleary, D.A. Principles of Inorganic Materials Design; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Livage, J. Chimie douce: from shake-and-bake processing to wet chemistry. New J. Chem. 2001, 25. [Google Scholar] [CrossRef]
- Rainey, F.A.; Oren, A. 1 Extremophile microorganisms and the methods to handle them. Methods Microbiol. 2006, 35, 1–25. [Google Scholar]
- Rothschild, L.J.; Mancinelli, R.L. Life in extreme environments. Nature 2001, 409, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Rampelotto, P.H. Resistance of microorganisms to extreme environmental conditions and its contribution to astrobiology. Sustainability 2010, 2, 1602–1623. [Google Scholar] [CrossRef]
- Zeikus, J.G.; Vieille, C.; Savchenko, A. Thermozymes: Biotechnology and structure–function relationships. Extremophiles 1998, 2, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Russell, N.J.; Cowan, D.A. Handling of psychrophilic microorganisms. Extremophiles 2006, 35, 371–393. [Google Scholar]
- Shaw, M.K.; Marr, A.G.; Ingraham, J.L. Determination of the minimal temperature for growth of Escherichia coli. J. Bacteriol. 1971, 105, 683–684. [Google Scholar] [PubMed]
- Rudolph, B.; Gebendorfer, K.M.; Buchner, J.; Winter, J. Evolution of Escherichia coli for growth at high temperatures. J. Biol. Chem. 2010, 285, 19029–19034. [Google Scholar] [CrossRef] [PubMed]
- L’haridon, S.; Cilia, V.; Messner, P.; Raguenes, G.; Gambacorta, A.; Sleytr, U.; Prieur, D.; Jeanthon, C. Desulfurobacterium thermolithotrophum gen. nov., sp. nov., a novel autotrophic, sulphur-reducing bacterium isolated from a deep-sea hydrothermal vent. Int. J. Syst. Evol. Microbiol. 1998, 48, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Krulwich, T.A.; Sachs, G.; Padan, E. Molecular aspects of bacterial pH sensing and homeostasis. Nat. Rev. Microbiol. 2011, 9, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Presser, K.; Ratkowsky, D.; Ross, T. Modelling the growth rate of Escherichia coli as a function of pH and lactic acid concentration. Appl. Environ. Microbiol. 1997, 63, 2355–2360. [Google Scholar] [PubMed]
- Madigan, M.T.; Orent, A. Thermophilic and halophilic extremophiles. Curr. Opin. Microbiol. 1999, 2, 265–269. [Google Scholar] [CrossRef]
- Larsen, H. Halophilic and halotolerant microorganisms—An overview and historical perspective. FEMS Microbiol. Lett. 1986, 39, 3–7. [Google Scholar] [CrossRef]
- Claus, D.; Fahmy, F.; Rolf, H.; Tosunoglu, N. Sporosarcina halophila sp. nov., an obligate, slightly halophilic bacterium from salt marsh soils. Syst. Appl. Microbiol. 1983, 4, 496–506. [Google Scholar] [CrossRef]
- Martin, S.; Márquez, M.; Sánchez-Porro, C.; Mellado, E.; Arahal, D.; Ventosa, A. Marinobacter lipolyticus sp. nov., a novel moderate halophile with lipolytic activity. Int. J. Syst. Evol. Microbiol. 2003, 53, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Gerday, C.; Glansdorff, N. Physiology and Biochemistry of Extremophiles; ASM Press: Washington, DC, USA, 2007. [Google Scholar]
- Beeby, M.; D O’Connor, B.; Ryttersgaard, C.; Boutz, D.R.; Perry, L.J.; Yeates, T.O. The genomics of disulfide bonding and protein stabilization in thermophiles. PLoS Biol. 2005, 3, e309. [Google Scholar] [CrossRef] [PubMed]
- Corliss, J.B.; Dymond, J.; Gordon, L.I.; Edmond, J.M. on the Galapagos Rift. Science 1979, 203, 16. [Google Scholar]
- Rogers, A.D.; Tyler, P.A.; Connelly, D.P.; Copley, J.T.; James, R.; Larter, R.D.; Linse, K.; Mills, R.A.; Garabato, A.N.; Pancost, R.D.; et al. The discovery of new deep-sea hydrothermal vent communities in the Southern Ocean and implications for biogeography. PLoS Biol. 2012, 10, e1001234. [Google Scholar] [CrossRef] [PubMed]
- Tunnicliffe, V.; Fowler, C.M.R. Influence of sea-floor spreading on the global hydrothermal vent fauna. Nature 1996, 379, 531. [Google Scholar] [CrossRef]
- López-García, P.; Gaill, F.; Moreira, D. Wide bacterial diversity associated with tubes of the vent worm Riftia pachyptila. Environ. Microbiol. 2002, 4, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, E.; Jones, W.; Segonzac, M. A new squat lobster family of Galatheoidea (Crustacea, Decapoda, Anomura) from the hydrothermal vents of the Pacific-Antarctic Ridge. Zoosystema 2005, 27, 709–723. [Google Scholar]
- Thatje, S.; Marsh, L.; Roterman, C.N.; Mavrogordato, M.N.; Linse, K. Adaptations to hydrothermal vent life in Kiwa tyleri, a new species of yeti crab from the East Scotia Ridge, Antarctica. PLoS ONE 2015, 10, e0127621. [Google Scholar] [CrossRef] [PubMed]
- Desbruyères, D.; Segonzac, M. Handbook of Deep-Sea Hydrothermal Vent Fauna; Editions Quae: Versailles, France, 1997. [Google Scholar]
- Lunina, A.A.; Vereshchaka, A.L. Distribution of hydrothermal alvinocaridid shrimps: Effect of geomorphology and specialization to extreme biotopes. PLoS ONE 2014, 9, e92802. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.; Schiaparelli, S.; Ereskovsky, A.; Schupp, P.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Biol. Macromol. 2010, 47, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Plath, M.; Tobler, M.; Riesch, R. Extremophile Fishes: An Introduction. In Extremophile Fishes; Springer: Cham, Switzerland, 2015; pp. 1–7. [Google Scholar]
- Wysokowski, M.; Kaiser, S.; Jesionowski, T. Hydrothermal Synthesis of Advanced Chitin-Based Materials. In Extreme Biomimetics; Springer: Basel, Switzerland, 2017; pp. 223–249. [Google Scholar]
- Stawski, D.; Rabiej, S.; Herczyńska, L.; Draczyński, Z. Thermogravimetric analysis of chitins of different origin. J. Therm. Anal. Calorim. 2008, 93, 489–494. [Google Scholar] [CrossRef]
- Aida, T.M.; Oshima, K.; Abe, C.; Maruta, R.; Iguchi, M.; Watanabe, M.; Smith, R.L. Dissolution of mechanically milled chitin in high temperature water. Carbohydr. Polym. 2014, 106, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry; Oxford University Press on Demand: Oxford, UK, 2001; Volume 5. [Google Scholar]
- Cöelfen, H.; Antonietti, M. Mesocrystals and Nonclassical Crystallization; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Lowenstam, H. Lepidocrocite, an apatite mineral, and magnetite in teeth of chitons (Polyplacophora). Science 1967, 156, 1373–1375. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Krautter, M.; Hanke, T.; Simon, P.; Knieb, C.; Heinemann, S.; Worch, H. First evidence of the presence of chitin in skeletons of marine sponges. Part II. Glass sponges (Hexactinellida: Porifera). J. Exp. Zool. Part B Mol. Dev. Evol. 2007, 308, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Brunner, E.; Richthammer, P.; Ehrlich, H.; Paasch, S.; Simon, P.; Ueberlein, S.; van Pée, K.H. Chitin-based organic networks: an integral part of cell wall biosilica in the diatom Thalassiosira pseudonana. Angew. Chem. Int. Ed. 2009, 48, 9724–9727. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; Lineweaver, C. Pressure-temperature phase diagram of the Earth. arXiv, 2010; arXiv:1005.2440. [Google Scholar]
- Lutgens, F.K.; Tarbuck, E.J.; Tasa, D. Essentials of Geology; Pearson New International Edition: Lodon, UK, 2014. [Google Scholar]
- Mizan, T.I.; Savage, P.E.; Ziff, R.M. Temperature dependence of hydrogen bonding in supercritical water. J. Phys. Chem. 1996, 100, 403–408. [Google Scholar] [CrossRef]
- Cengel, Y.A.; Boles, M.A. Thermodynamics: An engineering approach. Sea 2002, 1000, 8862. [Google Scholar]
- Sengers, J.; Watson, J.T.R. Improved international formulations for the viscosity and thermal conductivity of water substance. J. Phys. Chem. Ref. Data 1986, 15, 1291–1314. [Google Scholar] [CrossRef]
- Uematsu, M.; Frank, E. Static dielectric constant of water and steam. J. Phys. Chem. Ref. Data 1980, 9, 1291–1306. [Google Scholar] [CrossRef]
- Marshall, W.L.; Franck, E. Ion product of water substance, 0–1000 C, 1–10,000 bars new international formulation and its background. J. Phys. Chem. Ref. Data 1981, 10, 295–304. [Google Scholar] [CrossRef]
- Savage, P.E.; Rebacz, N.A. Water Under Extreme Conditions for Green Chemistry. Handb. Green Chem. 2009, 11, 1948–1954. [Google Scholar]
- Akiya, N.; Savage, P.E. Roles of water for chemical reactions in high-temperature water. Chem. Rev. 2002, 102, 2725–2750. [Google Scholar] [CrossRef] [PubMed]
- Savage, P.E. Organic chemical reactions in supercritical water. Chem. Rev. 1999, 99, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Quarch, K.; Kind, M. Inorganic precipitated silica gel. Part 1: Gelation kinetics and gel properties. Chem. Eng. Technol. 2010, 33, 1034–1039. [Google Scholar] [CrossRef]
- Siskin, M.; Katritzky, A.R. Reactivity of organic compounds in superheated water: General background. Chem. Rev. 2001, 101, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Kind, M. Influence of pH, temperature and sample size on natural and enforced syneresis of precipitated silica. Polymers 2015, 7, 2504–2521. [Google Scholar] [CrossRef]
- Scheel, H.J. Historical aspects of crystal growth technology. J. Cryst. Growth 2000, 211, 1–12. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2011, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Rabenau, A. The role of hydrothermal synthesis in preparative chemistry. Angew. Chem. Int. Ed. Engl. 1985, 24, 1026–1040. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, W.; Xu, L.; Peng, S. Hydrothermal synthesis of various hierarchical ZnO nanostructures and their methane sensing properties. Sensors 2013, 13, 6171–6182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Chen, X.; Wan, J.; Qian, Y. A simple hydrothermal route to large-scale synthesis of uniform silver nanowires. Chem. Eur. J. 2005, 11, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Wu, H.L.; Kuo, C.H.; Huang, M.H. Hydrothermal synthesis of monodispersed octahedral gold nanocrystals with five different size ranges and their self-assembled structures. Chem. Mater. 2008, 20, 7570–7574. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Liang, J.; Hu, Z.; Li, S.; Peng, S.; Qian, Y. Synthesis of copper nanowires via a complex-surfactant-assisted hydrothermal reduction process. J. Phys. Chem. B 2003, 107, 12658–12661. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: history and development from the earliest days to the present time. Chem. Rev. 2003, 103, 663–702. [Google Scholar] [CrossRef] [PubMed]
- Laudise, R.; Ballman, A. Hydrothermal Synthesis of Sapphire1. J. Am. Chem. Soc. 1958, 80, 2655–2657. [Google Scholar] [CrossRef]
- Walker, A. Hydrothermal synthesis of quartz crystals. J. Am. Ceram. Soc. 1953, 36, 250–256. [Google Scholar] [CrossRef]
- Chiefari, J.; Dao, B.; Groth, A.M.; Hodgkin, J.H. Water as solvent in polyimide synthesis: Thermoset and thermoplastic examples. High Perform. Polym. 2003, 15, 269–279. [Google Scholar] [CrossRef]
- Chiefari, J.; Dao, B.; Groth, A.M.; Hodgkin, J.H. Water as solvent in polyimide synthesis II: Processable aromatic polyimides. High Perform. Polym. 2006, 18, 31–44. [Google Scholar] [CrossRef]
- Chiefari, J.; Dao, B.; Groth, A.M.; Hodgkin, J.H. Water as solvent in polyimide synthesis III: Towards the synthesis of polyamideimides. High Perform. Polym. 2006, 18, 437–451. [Google Scholar] [CrossRef]
- Dao, B.N.; Groth, A.M.; Hodgkin, J.H. Microwave-assisted aqueous polyimidization using high-throughput techniques. Macromol. Rapid Commun. 2007, 28, 604–607. [Google Scholar] [CrossRef]
- Brunel, R.; Marestin, C.; Martin, V.; Mercier, R. Water-borne polyimides via microwave-assisted polymerization. High Perform. Polym. 2009, 22, 82–94. [Google Scholar] [CrossRef]
- Unterlass, M.M.; Kopetzki, D.; Antonietti, M.; Weber, J. Mechanistic study of hydrothermal synthesis of aromatic polyimides. Polym. Chem. 2011, 2, 1744–1753. [Google Scholar] [CrossRef]
- Kriechbaum, K.; Cerron-Infantes, D.A.; Støger, B.; Unterlass, M.M. Shape-anisotropic polyimide particles by solid-state polycondensation of monomer salt single crystals. Macromolecules 2015, 48, 8773–8780. [Google Scholar] [CrossRef]
- Baumgartner, B.; Bojdys, M.J.; Unterlass, M.M. Geomimetics for green polymer synthesis: Highly ordered polyimides via hydrothermal techniques. Polym. Chem. 2014, 5, 3771–3776. [Google Scholar] [CrossRef]
- Baumgartner, B.; Puchberger, M.; Unterlass, M.M. Towards a general understanding of hydrothermal polymerization of polyimides. Polym. Chem. 2015, 6, 5773–5781. [Google Scholar] [CrossRef]
- Baumgartner, B.; Bojdys, M.J.; Skrinjar, P.; Unterlass, M.M. Design strategies in hydrothermal polymerization of polyimides. Macromol. Chem. Phys. 2016, 217, 485–500. [Google Scholar] [CrossRef]
- Holleman, A.F.; Wiberg, E. Lehrbuch der Anorganischen Chemie, 91–100 ed.; Walter de Gruyter: Berlin, Germany, 1985. [Google Scholar]
- Tashiro, K. Molecular theory of mechanical properties of crystalline polymers. Prog. Polym. Sci. 1993, 18, 377–435. [Google Scholar] [CrossRef]
- Carraher, C.E.; Swift, G. Functional Condensation Polymers; Springer: New York, NY, USA, 2002. [Google Scholar]
- Leimhofer, L.; Baumgartner, B.; Puchberger, M.; Prochaska, T.; Konegger, T.; Unterlass, M.M. Green one-pot synthesis and processing of polyimide–silica hybrid materials. J. Mater. Chem. A 2017. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Nichols, D.A.; Siskin, M.; Murugan, R.; Balasubramanian, M. Reactions in high-temperature aqueous media. Chem. Rev. 2001, 101, 837–892. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, B.; Arnett, E.M.; Siskin, M. Classical organic reactions in pure superheated water. J. Org. Chem. 1994, 59, 3098–3101. [Google Scholar] [CrossRef]
- Dudd, L.M.; Venardou, E.; Garcia-Verdugo, E.; Licence, P.; Blake, A.J.; Wilson, C.; Poliakoff, M. Synthesis of benzimidazoles in high-temperature water. Green Chem. 2003, 5, 187–192. [Google Scholar] [CrossRef]
- Baumgartner, B.; Svirkova, A.; Bintinger, J.; Hametner, C.; Marchetti-Deschmann, M.; Unterlass, M.M. Green and highly efficient synthesis of perylene and naphthalene bisimides in nothing but water. Chem. Commun. 2017, 53, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Extreme Biomimetics; Springer: Basel, Switzerland, 2017. [Google Scholar]
- Wysokowski, M.; Petrenko, I.; Stelling, A.L.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan chitin as a versatile template for extreme biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef]
- Wysokowski, M.; Piasecki, A.; Bazhenov, V.V.; Paukszta, D.; Born, R.; Schupp, P.; Petrenko, I.; Jesionowski, T. Poriferan chitin as the scaffold for nanosilica deposition under hydrothermal synthesis conditions. J. Chitin Chitosan Sci. 2013, 1, 26–33. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Stöcker, H.; Bazhenov, V.V.; Langer, E.; Dobrowolska, A.; Czaczyk, K.; Galli, R.; Stelling, A.L.; Behm, T.; et al. An extreme biomimetic approach: Hydrothermal synthesis of β-chitin/ZnO nanostructured composites. J. Mater. Chem. B 2013, 1, 6469–6476. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Bazhenov, V.V.; Stawski, D.; Petrenko, I.; Ehrlich, A.; Behm, T.; Kljajic, Z.; Stelling, A.L.; Jesionowski, T.; et al. Poriferan chitin as a template for hydrothermal zirconia deposition. Front. Mater. Sci. 2013, 7, 248–260. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Beyer, J.; Makarova, A.; Stöcker, H.; Walter, J.; Galli, R.; Kaiser, S.; Vyalikh, D.; Bazhenov, V.V.; et al. Extreme biomimetic approach for developing novel chitin-GeO2. Nano Res. 2015, 8, 2288–2301. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Walter, J.; Lota, G.; Wojciechowski, J.; Stöcker, H.; Galli, R.; Stelling, A.L.; Himcinschi, C.; Niederschlag, E.; et al. Synthesis of nanostructured chitin–hematite composites under extreme biomimetic conditions. RSC Adv. 2014, 4, 61743–61752. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Rafaja, D.; Koltsov, I.; Stöcker, H.; Szalaty, T.J.; Bazhenov, V.V.; Stelling, A.L.; Beyer, J.; Heitmann, J.; et al. Extreme biomimetic approach for synthesis of nanocrystalline chitin-(Ti, Zr)O2 multiphase composites. Mater. Chem. Phys. 2017, 188, 115–124. [Google Scholar] [CrossRef]
- Fei, X.; Shao, Z.; Chen, X. Hematite nanostructures synthesized by a silk fibroin-assisted hydrothermal method. J. Mater. Chem. B 2013, 1, 213–220. [Google Scholar] [CrossRef]
- Fei, X.; Li, W.; Shao, Z.; Seeger, S.; Zhao, D.; Chen, X. Protein biomineralized nanoporous inorganic mesocrystals with tunable hierarchical nanostructures. J. Am. Chem. Soc. 2014, 136, 15781–15786. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Li, S.M.; Ma, M.G.; Sun, R.C.; Zhu, J.F. Hydrothermal fabrication, characterization, and biological activity of cellulose/CaCO3 bionanocomposites. Carbohydr. Polym. 2012, 88, 179–184. [Google Scholar] [CrossRef]
- Ma, M.G.; Zhu, J.F.; Li, S.M.; Jia, N.; Sun, R.C. Nanocomposites of cellulose/iron oxide: Influence of synthesis conditions on their morphological behavior and thermal stability. Mater. Sci. Eng. C 2012, 32, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.H.; Zhao, C.; Liu, X. Paper-based piezoelectric touch pads with hydrothermally grown zinc oxide nanowires. ACS Appl. Mater. Interfaces 2014, 6, 22004–22012. [Google Scholar] [CrossRef] [PubMed]
- Szatkowski, T.; Wysokowski, M.; Lota, G.; Pęziak, D.; Bazhenov, V.V.; Nowaczyk, G.; Walter, J.; Molodtsov, S.L.; Stöcker, H.; Himcinschi, C.; et al. Novel nanostructured hematite–spongin composite developed using an extreme biomimetic approach. RSC Adv. 2015, 5, 79031–79040. [Google Scholar] [CrossRef]
- Szatkowski, T.; Siwińska-Stefańska, K.; Wysokowski, M.; Stelling, A.L.; Joseph, Y.; Ehrlich, H.; Jesionowski, T. Immobilization of titanium (IV) oxide onto 3D spongin scaffolds of marine sponge origin according to extreme biomimetics principles for removal of CI Basic Blue 9. Biomimetics 2017, 2, 4. [Google Scholar] [CrossRef]


© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unterlass, M.M. Geomimetics and Extreme Biomimetics Inspired by Hydrothermal Systems—What Can We Learn from Nature for Materials Synthesis? Biomimetics 2017, 2, 8. https://doi.org/10.3390/biomimetics2020008
Unterlass MM. Geomimetics and Extreme Biomimetics Inspired by Hydrothermal Systems—What Can We Learn from Nature for Materials Synthesis? Biomimetics. 2017; 2(2):8. https://doi.org/10.3390/biomimetics2020008
Chicago/Turabian StyleUnterlass, Miriam M. 2017. "Geomimetics and Extreme Biomimetics Inspired by Hydrothermal Systems—What Can We Learn from Nature for Materials Synthesis?" Biomimetics 2, no. 2: 8. https://doi.org/10.3390/biomimetics2020008
APA StyleUnterlass, M. M. (2017). Geomimetics and Extreme Biomimetics Inspired by Hydrothermal Systems—What Can We Learn from Nature for Materials Synthesis? Biomimetics, 2(2), 8. https://doi.org/10.3390/biomimetics2020008





