A Coral- and Goose Down-Inspired Coating with Integrated Anti-Scaling and Heat Retention for Energy Conservation
Abstract
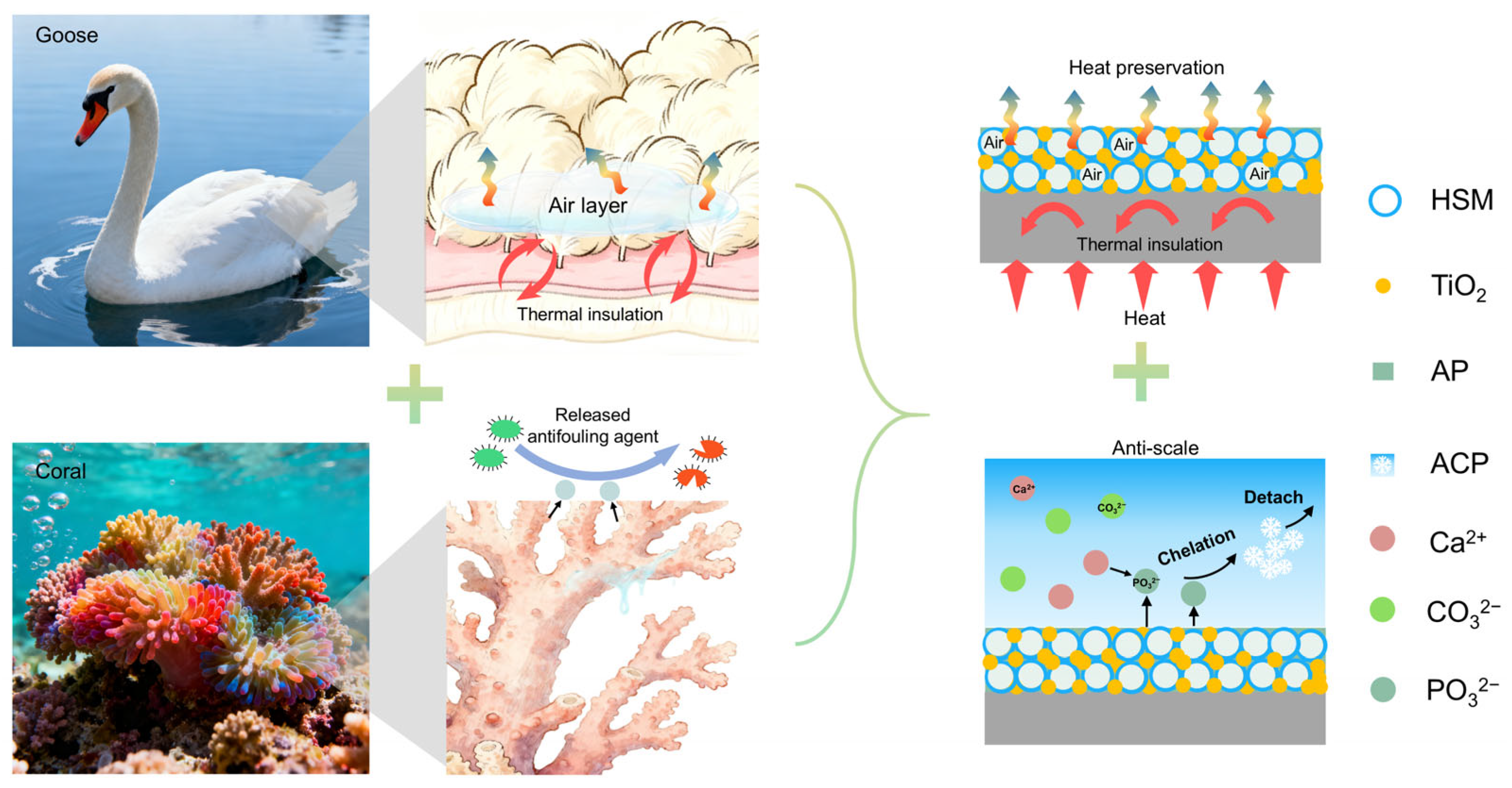
1. Introduction
2. Materials and Methods
2.1. Fabrication of BHSM
2.2. Experiment for Scale Deposition
2.3. Characterization
3. Results and Discussion
3.1. Preparation and Characterization of BHSM Coating
3.2. Anti-Scale Mechanism and Performance of BHSM
3.3. Thermal Insulation of BHSM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BHSM | Bioinspired hollow silica microsphere-based |
| HSM | Hollow silica microsphere |
| AP | Aluminum phosphate |
| TiO2 | Titanium dioxide |
| ACP | Amorphous calcium phosphate |
| SS 304 | Stainless steel 304 |
References
- AlJanabi, A.; Malayeri, M.R. Innovative non-metal heat transfer surfaces to mitigate crystallization fouling. Int. J. Therm. Sci. 2019, 138, 384–392. [Google Scholar] [CrossRef]
- Olajire, A.A. A review of oilfield scale management technology for oil and gas production. J. Pet. Sci. Eng. 2015, 135, 723–737. [Google Scholar] [CrossRef]
- Jitka, M.; Simon, A.P. Calcium carbonate scale formation and control. Rev. Environ. Sci. Bio. 2004, 3, 159–169. [Google Scholar]
- Chaussemier, M.; Pourmohtasham, E.; Gelus, D.; Pécoul, N.; Perrot, H.; Lédion, J.; Cheap-Charpentier, H.; Horner, O. State of art of natural inhibitors of calcium carbonate scaling. A review article. Desalination 2015, 356, 47–55. [Google Scholar] [CrossRef]
- Liang, B.; Sun, Y.; Bai, Z.; Li, H.; Shi, Y.; Lin, D.; Pei, L.; Zhu, Y.; Wang, H. A novel UV-curable amphiphobic coating with dynamic air-layer copolymer brush offering stable dual-functional anti-scaling and anti-corrosion properties. Prog. Org. Coat. 2024, 194, 108607. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, H.; Zhu, M.; Wang, H.; Li, Z. Dynamic and active antiscaling via scale inhibitor pre-stored superhydrophobic coating. Chem. Eng. J. 2021, 403, 126467. [Google Scholar] [CrossRef]
- Sosa, M.D.; Levy, I.K.; Butt, H.-J.; Kappl, M. Nanofilament-coated membranes with enhanced scaling and biofouling resistance for membrane distillation. ACS Appl. Mater. Interfaces 2025, 17, 24588–24600. [Google Scholar] [CrossRef]
- Zuo, K.; Zhang, X.; Huang, X.; Oliveira, E.F.; Guo, H.; Zhai, T.; Wang, W.; Alvarez, P.J.J.; Elimelech, M.; Ajayan, P.M.; et al. Ultrahigh resistance of hexagonal boron nitride to mineral scale formation. Nat. Commun. 2022, 13, 4523. [Google Scholar] [CrossRef]
- Jia, Y.; Guan, K.; Zhang, P.; Shen, Q.; Li, Z.; Istirokhatun, T.; Matsuyama, H. Asymmetric superwetting Janus structure for fouling- and scaling-resistant membrane distillation. J. Membr. Sci. 2022, 657, 120697. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W.; Ma, S.; Liu, H.; Wang, X.; Zhao, X.; Yu, B.; Cai, M.; Zhou, F. Modulus adaptive lubricating prototype inspired by instant muscle hardening mechanism of catfish skin. Nat. Commun. 2022, 13, 377. [Google Scholar] [CrossRef]
- Yao, X.; Lin, W.; Wang, M.; Wang, S. Nature-inspired high temperature scale-resistant slippery lubricant-induced porous surfaces (HTS-SLIPS). Small 2022, 18, 2203615. [Google Scholar] [CrossRef]
- Masoudi, A.; Irajizad, P.; Farokhnia, N.; Kashyap, V.; Ghasemi, H. Antiscaling Magnetic Slippery Surfaces. ACS Appl. Mater. Interfaces 2017, 9, 21025–21033. [Google Scholar] [CrossRef] [PubMed]
- Subramanyam, S.B.; Azimi, G.; Varanasi, K.K. Designing lubricant-impregnated textured surfaces to resist scale formation. Adv. Mater. Interfaces 2014, 1, 1300068. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Wang, Z.; Pan, S.; Yi, B.; Ai, L.; Gao, J.; Mugele, F.; Yao, X. Wetting ridge assisted programmed magnetic actuation of droplets on ferrofluid-infused surface. Nat. Commun. 2021, 12, 7136. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, X.; Chen, L.; Liu, S.; Xu, X.; Zhao, S.; Huang, S.; Tian, X. Dynamic poly(dimethylsiloxane) brush coating shows even better antiscaling capability than the low-surface-energy fluorocarbon counterpart. Environ. Sci. Technol. 2021, 55, 8839–8847. [Google Scholar] [CrossRef]
- Saul, A.; Barker, R.; Baraka-Lokmane, S.; Le Beulze, A.; Charpentier, T.; Tangparitkul, S.; Ordonez-Varela, J.-R.; Taleb, W.; Neville, A. Corrosion derived lubricant infused surfaces on X65 carbon steel for improved inorganic scaling performance. J. Adhes. Sci. Technol. 2021, 36, 632–653. [Google Scholar] [CrossRef]
- Li, C.; Wang, F.; Wang, Y.; Guo, M.; Li, J.; Deng, C.; Song, F.; Yang, W.; Wang, Y. Deformation resistant monolithic hierarchical textures inducing stretchable superamphiphobicity with environmental adaptability and flame retardancy. Nat. Commun. 2025, 16, 2729. [Google Scholar] [CrossRef]
- Wei, J.; Mao, M.; Li, B.; Zhang, J. Roles of adhesives in forming mechanically robust superhydrophobic coatings. Chem. Sci. 2025, 16, 19048–19071. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, H.; Liu, Y.; Zhong, Y.; Chen, H. Engineered ceramic composite coatings via multiscale binder synergy: Achieving dynamic icephobicity and mechanical robustness. Prog. Org. Coat. 2025, 209, 109565. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, S.; Li, Y.; Li, Q.; Wu, F. Metallurgical refractory lining-guided inorganic binder for stable lithium storage in silicon microparticle anodes. Energy Environ. Sci. 2026. [Google Scholar] [CrossRef]
- Liu, M.; Li, J.; Hou, Y.; Guo, Z. Inorganic adhesives for robust superwetting surfaces. ACS Nano 2017, 11, 1113–1119. [Google Scholar] [CrossRef]
- Liu, W.; Cui, M.; Shen, Y.; Mu, P.; Yang, Y.; Li, J. Efficient separation of crude oil-in-water emulsion based on a robust underwater superoleophobic titanium dioxide-coated mesh. New J. Chem. 2020, 44, 2705–2713. [Google Scholar] [CrossRef]
- Wang, M.; Liu, J.; Du, H.; Hou, F.; Guo, A.; Zhao, Y.; Zhang, J. A new practical inorganic phosphate adhesive applied under both air and argon atmosphere. J. Alloys Compd. 2014, 617, 219–221. [Google Scholar] [CrossRef]
- Rulliere, C.; Perenes, L.; Senocq, D.; Dodi, A.; Marchesseau, S. Heat treatment effect on polyphosphate chain length in aqueous and calcium solutions. Food Chem. 2012, 134, 712–716. [Google Scholar] [CrossRef]
- Guo, C.; Wu, K.; Chen, Y.; Chen, K.; Liu, R.; Luo, J. Efficient fabrication of hollow microspheres by photopolymerization and their application in thermal insulation coatings. Ind. Eng. Chem. Res. 2025, 64, 7723–7731. [Google Scholar] [CrossRef]
- Jia, Z.; Lyu, H.; Bao, Y.; Qi, X.; Guo, S. Incorporation of hollow TiO2 microspheres in cement-based waterborne coatings for synergistic enhancement of thermal barrier and infrared reflective properties. Constr. Build. Mater. 2024, 439, 137330. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Z.; Jiao, R.; Chen, Y.; Cao, X.; Sun, H.; Li, J.; Li, A. Preparation of DOPO-KH550 modified hollow glass microspheres/PVA composite aerogel for thermal insulation and flame retardancy. J. Colloid Interface Sci. 2024, 654, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Huang, C.; Wu, Y.; Wang, J.; Situ, Y.; Huang, H. Silica/Cesium tungsten bronze composite nanospheres with synergistically enhanced thermal insulation properties for transparent coatings. ACS Appl. Nano Mater. 2023, 6, 18934–18944. [Google Scholar] [CrossRef]
- Jin, H.; Tian, L.; Bing, W.; Zhao, J.; Ren, L. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Meng, T.; Zhu, L.; Stone, D.; Armstrong, J.N.; Ren, S. Bioinspired dry-steam superinsulation straw foam. Small 2025, 21, 2503511. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Jing, J.; Zhu, L.; Ning, H.; Liu, H.; Li, M.; Jin, H.; Wang, H.; Wang, H.; et al. Combination of chemical anchoring and microcapsules for durable slippery coating with anti-corrosion/scaling property. Prog. Org. Coat. 2025, 200, 108992. [Google Scholar] [CrossRef]
- Bartlett, M.D.; Case, S.W.; Kinloch, A.J.; Dillard, D.A. Peel tests for quantifying adhesion and toughness: A review. Prog. Mater. Sci. 2023, 137, 101086. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Y.; Ding, X.; Li, W.; Zhao, Y.; Han, S.; Liu, M.; Wang, S. Photothermal and electrothermal superhydrophobic anti-icing/de-icing coating based on straw-derived sorbitol. Chem. Eng. J. 2025, 521, 166517. [Google Scholar] [CrossRef]
- Kumar, D.; Alam, M.; Zou, P.X.W.; Sanjayan, J.G.; Memon, R.A. Comparative analysis of building insulation material properties and performance. Renew. Sustain. Energy Rev. 2020, 131, 110038. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Zhao, R.; Shang, Z.; Deng, X.; Lan, J.; Meng, J. A Coral- and Goose Down-Inspired Coating with Integrated Anti-Scaling and Heat Retention for Energy Conservation. Biomimetics 2026, 11, 22. https://doi.org/10.3390/biomimetics11010022
Zhao R, Shang Z, Deng X, Lan J, Meng J. A Coral- and Goose Down-Inspired Coating with Integrated Anti-Scaling and Heat Retention for Energy Conservation. Biomimetics. 2026; 11(1):22. https://doi.org/10.3390/biomimetics11010022
Chicago/Turabian StyleZhao, Ran, Zhihao Shang, Xiaosong Deng, Jinze Lan, and Jingxin Meng. 2026. "A Coral- and Goose Down-Inspired Coating with Integrated Anti-Scaling and Heat Retention for Energy Conservation" Biomimetics 11, no. 1: 22. https://doi.org/10.3390/biomimetics11010022
APA StyleZhao, R., Shang, Z., Deng, X., Lan, J., & Meng, J. (2026). A Coral- and Goose Down-Inspired Coating with Integrated Anti-Scaling and Heat Retention for Energy Conservation. Biomimetics, 11(1), 22. https://doi.org/10.3390/biomimetics11010022






