Collagen Formulation in Xenogeneic Bone Substitutes Influences Cellular Responses in Periodontal Regeneration: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Isolation and Culture
2.2. Characterization of hDPSCs
2.3. Preparation of Bone Substitutes
2.4. Evaluation of hDPSC Viability/Proliferation-MTT Assay
2.5. Evaluation of hDPSCs Viability-Cell Staining Assay
2.6. Evaluation of In Vitro Cell Adhesion and Morphology by Scanning Electron Microscopy (SEM)
2.7. Evaluation of In Vitro Cell Migration-Wound Heal Assay
2.8. Quantitative Real-Time Reverse-Transcriptase Polymerase Chain Reaction (qRT-PCR)
2.9. Evaluation of In Vitro Biomineralization-Alizarin Red S Staining Assay
2.10. Statistical Analysis
3. Results
3.1. Effects on hDPSC Viability/Proliferation-MTT Assay
3.2. Effects on hDPSC Viability-Cell Staining Assay
3.3. Effects on hDPSCs Cell Adhesion and Morphology by Scanning Electron Microscopy (SEM)
3.4. Effects on Cell Migration-Wound Heal Assay
3.5. Real-Time RT-PCR Assays-Adhesion, Migration, Proliferation, and Angiogenic Gene Expression
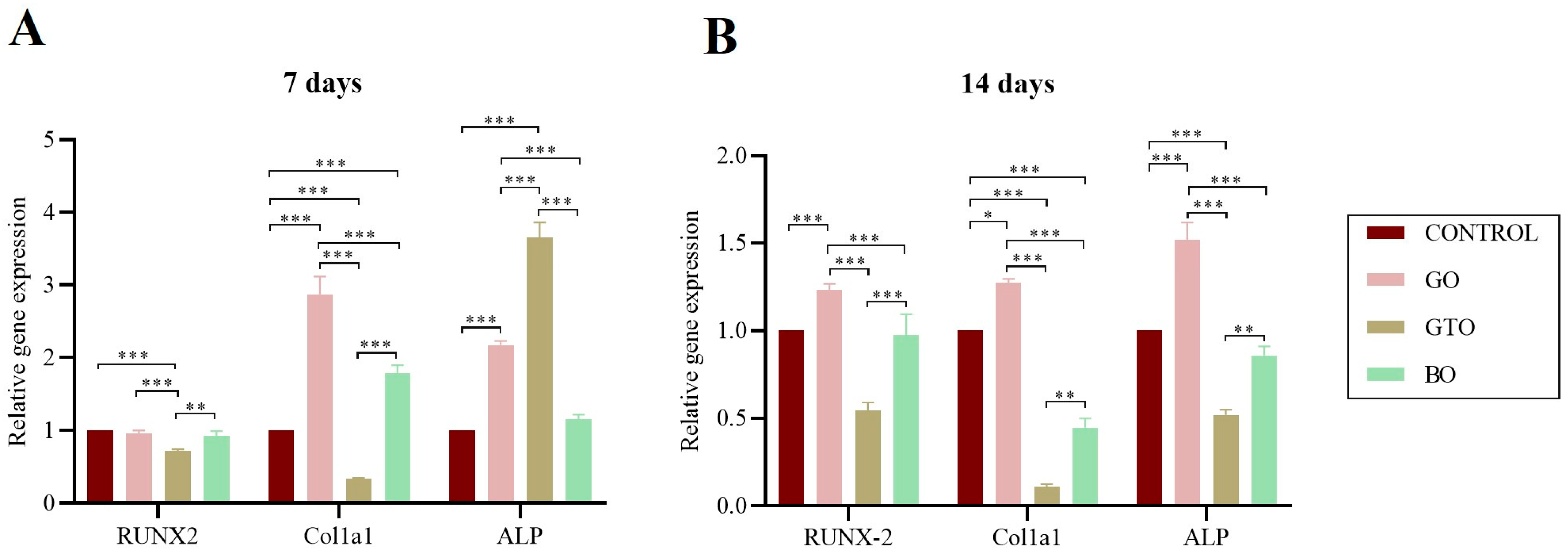
3.6. Real-Time RT-PCR Assays-Osteogenic Differentiation Gene Expression
3.7. Effects on In Vitro Biomineralization-Alizarin Red S Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solakoglu, Ö.; Heydecke, G.; Amiri, N.; Anitua, E. The use of plasma rich in growth factors (PRGF) in guided tissue regeneration and guided bone regeneration. A review of histological, immunohistochemical, histomorphometrical, radiological and clinical results in humans. Ann. Anat. 2020, 231, 151528. [Google Scholar] [CrossRef]
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9. [Google Scholar] [CrossRef]
- Dos Santos, R.L.; Ahmed, A.; Hunn, B.E.; Addison, A.E.; Marques, D.W.; Bruce, K.A.; Martin, J.R. Oxidation-responsive, settable bone substitute composites for regenerating critically-sized bone defects. Biomater. Sci. 2025, 13, 1975–1992. [Google Scholar] [CrossRef]
- Brunelli, G.; Sollazzo, V.; Carinci, F.; Palmieri, A.; Girardi, A.; Monguzzi, R. Osteobiol® Influences Osteogenic Differentiation of Adipose Derived Stem Cells. Eur. J. Inflamm. 2011, 9, 103–107. [Google Scholar]
- Lee, S.S.; Du, X.; Kim, I.; Ferguson, S.J. Scaffolds for bone-tissue engineering. Matter 2022, 5, 2722–2759. [Google Scholar] [CrossRef]
- Jeanneau, C.; Le Fournis, C.; About, I. Xenogeneic bone filling materials modulate mesenchymal stem cell recruitment: Role of the Complement C5a. Clin. Oral Investig. 2020, 24, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Jeanneau, C.; Catherine, J.-H.; Giraud, T.; Lan, R.; About, I. The Added Value of a Collagenated Thermosensitive Bone Substitute as a Scaffold for Bone Regeneration. Materials 2024, 17, 625. [Google Scholar] [CrossRef]
- Mizuno, M.; Fujisawa, R.; Kuboki, Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-?2?1 integrin interaction. J. Cell. Physiol. 2000, 184, 207–213. [Google Scholar] [CrossRef]
- Ortiz-Arrabal, O.; Rodriguez, M.A.; Chato-Astrain, J.; Martín-Piedra, M.Á.; Garzón, I.; Carriel, V.; Fernández-Valadés, R.; España-López, A.; Alaminos, M.; Rodriguez, I.A.; et al. A comprehensive analysis of two types of xenogeneic bone particles for use in maxillofacial bone regeneration therapies. PLoS ONE 2025, 20, e0323754. [Google Scholar] [CrossRef]
- Rombouts, C.; Jeanneau, C.; Camilleri, J.; Laurent, P.; About, I. Characterization and angiogenic potential of xenogeneic bone grafting materials: Role of periodontal ligament cells. Dent. Mater. J. 2016, 35, 900–907. [Google Scholar] [CrossRef][Green Version]
- Cheng, Y.; Ramos, D.; Lee, P.; Liang, D.; Yu, X.; Kumbar, S.G. Collagen Functionalized Bioactive Nanofiber Matrices for Osteogenic Differentiation of Mesenchymal Stem Cells: Bone Tissue Engineering. J. Biomed. Nanotechnol. 2014, 10, 287–298. [Google Scholar] [CrossRef]
- da Silva, H.F.; Goulart, D.R.; Sverzut, A.T.; Olate, S.; de Moraes, M. Comparison of two anorganic bovine bone in maxillary sinus lift: A split-mouth study with clinical, radiographical, and histomorphometrical analysis. Int. J. Implant. Dent. 2020, 6, 1–11. [Google Scholar] [CrossRef]
- Romasco, T.; Tumedei, M.; Inchingolo, F.; Pignatelli, P.; Montesani, L.; Iezzi, G.; Petrini, M.; Piattelli, A.; Di Pietro, N. A Narrative Review on the Effectiveness of Bone Regeneration Procedures with OsteoBiol® Collagenated Porcine Grafts: The Translational Research Experience over 20 Years. J. Funct. Biomater. 2022, 13, 12–14. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Horii, A.; Wang, X.; Gelain, F.; Zhang, S.; Isalan, M. Biological Designer Self-Assembling Peptide Nanofiber Scaffolds Significantly Enhance Osteoblast Proliferation, Differentiation and 3-D Migration. PLoS ONE 2007, 2, e190. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, H.-J.; Kim, M.-K.; Kim, H.J.; Kim, Y.-I.; Bae, S.-K.; Bae, M.-K. Effects of Hispidulin on the Osteo/Odontogenic and Endothelial Differentiation of Dental Pulp Stem Cells. Pharmaceuticals 2024, 17, 1740. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, G.; Codispoti, B.; Marrelli, M.; Rengo, C.; Rengo, S.; Tatullo, M. Commitment of Oral-Derived Stem Cells in Dental and Maxillofacial Applications. Dent. J. 2018, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.-T.; Lee, W.-F.; Chen, S.-M.; Hao, L.-T.; Hung, Y.-T.; Lai, P.-C.; Feng, S.-W. Effect of Different Bone Grafting Materials and Mesenchymal Stem Cells on Bone Regeneration: A Micro-Computed Tomography and Histomorphometric Study in a Rabbit Calvarial Defect Model. Int. J. Mol. Sci. 2021, 22, 8101. [Google Scholar] [CrossRef]
- Bai, X.; Cao, R.; Wu, D.; Zhang, H.; Yang, F.; Wang, L.; Chimenti, I. Dental Pulp Stem Cells for Bone Tissue Engineering: A Literature Review. Stem Cells Int. 2023, 2023, 1–15. [Google Scholar] [CrossRef]
- Hossein-Khannazer, N.; Hashemi, S.M.; Namaki, S.; Ghanbarian, H.; Sattari, M.; Khojasteh, A. Study of the immunomodulatory effects of osteogenic differentiated human dental pulp stem cells. Life Sci. 2019, 216, 111–118. [Google Scholar] [CrossRef]
- Sweeney, S.M.; DiLullo, G.; Slater, S.J.; Martinez, J.; Iozzo, R.V.; Lauer-Fields, J.L.; Fields, G.B.; Antonio, J.D.S. Angiogenesis in Collagen I Requires α2β1 Ligation of a GFP*GER Sequence and Possibly p38 MAPK Activation and Focal Adhesion Disassembly. J. Biol. Chem. 2003, 278, 30516–30524. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.-G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Schmidt, S.; Friedl, P. Interstitial cell migration: Integrin-dependent and alternative adhesion mechanisms. Cell Tissue Res. 2010, 339, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef] [PubMed]
- Sapudom, J.; Rubner, S.; Martin, S.; Kurth, T.; Riedel, S.; Mierke, C.T.; Pompe, T. The phenotype of cancer cell invasion controlled by fibril diameter and pore size of 3D collagen networks. Biomaterials 2015, 52, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Li, R.; Bai, H.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Liu, H.; Wang, J.; et al. Collagen-based biomaterials for bone tissue engineering. Mater. Des. 2021, 210, 110049. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Hannink, G.; Arts, J.J.C. Bioresorbability, porosity and mechanical strength of bone substitutes: What is optimal for bone regeneration? Injury 2011, 42, S22–S25. [Google Scholar] [CrossRef]
- Hu, P.; Lun, D.; Wang, P.; Tu, Z. Effect of Particle Size Ratios on the Physical and Chemical Properties of Surgical-Grade Calcium Sulfate Hemihydrate. Orthop. Surg. 2020, 12, 295–303. [Google Scholar] [CrossRef]
- Galliera, E.; Massaccesi, L.; Banfi, G.; De Vecchi, E.; Ragone, V.; Romanelli, M.M.C. Effect of Oxidative Stress on Bone Remodeling in Periprosthetic Osteolysis. Clin. Rev. Bone Miner. Metab. 2021, 19, 14–23. [Google Scholar] [CrossRef]
- Milkovic, L.; Gasparovic, A.C.; Cindric, M.; Mouthuy, P. Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef]
- Mukasheva, F.; Adilova, L.; Dyussenbinov, A.; Yernaimanova, B.; Abilev, M.; Akilbekova, D. Optimizing scaffold pore size for tissue engineering: Insights across various tissue types. Front. Bioeng. Biotechnol. 2024, 12, 1444986. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Du, C.; Liu, S.; Liu, J.; Yang, Y.; Dong, L.; Zhao, W.; Huang, W.; Lei, Y. Progress in biomaterials inspired by the extracellular matrix. Giant 2024, 19, 100323. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Orlando, F.; Ottobelli, M.; Fiori, D.; Garagiola, U. A comparison between anorganic bone and collagen-preserving bone xenografts for alveolar ridge preservation: Systematic review and future perspectives. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 24. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.B.; Stein, G.S. Concepts of osteoblast growth and differentiation: Basis for modulation of bone cell development and tissue formation. Crit. Rev. Oral Biol. Med. 1992, 3, 269–305. [Google Scholar] [CrossRef]
- Cheng, Z.; Teo, G.; Krueger, S.; Rock, T.M.; Koh, H.W.; Choi, H.; Vogel, C. Differential dynamics of the mammalian mRNA and protein expression response to misfolding stress. Mol. Syst. Biol. 2016, 12, 855. [Google Scholar] [CrossRef]
- Hoffman-Kim, D.; Mitchel, J.A.; Bellamkonda, R.V. Topography, cell response, and nerve regeneration. Annu. Rev. Biomed. Eng. 2010, 12, 203–231. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.W.; Oreffo, R.O.C. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef]
- Olausson, M.; Esfahani, N.; Östlin, J.; Hägglin, C. Native-born versus foreign-born patients’ perception of communication and care in Swedish dental service. Swed. Dent. J. 2016, 40, 91–100. [Google Scholar]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Di Tinco, R.; Consolo, U.; Pisciotta, A.; Orlandi, G.; Bertani, G.; Nasi, M.; Bertacchini, J.; Carnevale, G. Characterization of Dental Pulp Stem Cells Response to Bone Substitutes Biomaterials in Dentistry. Polymers 2022, 14, 2223. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, W.; Li, P.; Luo, Q.; Li, A.; Zhang, X. Comparison of the osteogenic effectiveness of an autogenous demineralised dentin matrix and Bio-Oss® in bone augmentation: A systematic review and meta-analysis. Br. J. Oral Maxillofac. Surg. 2022, 60, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Łobacz, M.; Wieczorek, K.; Mertowska, P.; Mertowski, S.; Kos, M.; Grywalska, E.; Hajduk, G.; Rahnama-Hezavah, M. Evaluation of Peri-Implantitis Bone Defect Healing: Comparing the Efficacy of Small-Particle Dentin and Bio-Oss in Bone Density Attenuation. J. Clin. Med. 2024, 13, 4638. [Google Scholar] [CrossRef]
- Mladenović, Ž.; Sahlin-Platt, A.; Andersson, B.; Johansson, A.; Björn, E.; Ransjö, M. In vitro study of the biological interface of Bio-Oss: Implications of the experimental setup. Clin. Oral Implant. Res. 2013, 24, 329–335. [Google Scholar] [CrossRef]
- Peláez-Cruz, P.; López Jornet, P.; Tatullo, M.; Pons-Fuster López, E. Epigallocatechin-3-gallate improves the biocompatibility of bone substitutes in dental pulp stem cells. Ann. Anat. 2023, 246, 152045. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.A.N.; Zheng, Z.; Li, Z. Bio-Oss ® modified by calcitonin gene-related peptide promotes osteogenesis in vitro. Exp. Ther. Med. 2017, 14, 4001–4008. [Google Scholar] [CrossRef]
- Rosa, V.; Cavalcanti, B.N.; Nör, J.E.; Tezvergil-Mutluay, A.; Silikas, N.; Bottino, M.C.; Kishen, A.; Soares, D.G.; Franca, C.M.; Cooper, P.R.; et al. Guidance for evaluating biomaterials’ properties and biological potential for dental pulp tissue engineering and regeneration research. Dent. Mater. 2024, 41, 248–264. [Google Scholar] [CrossRef]

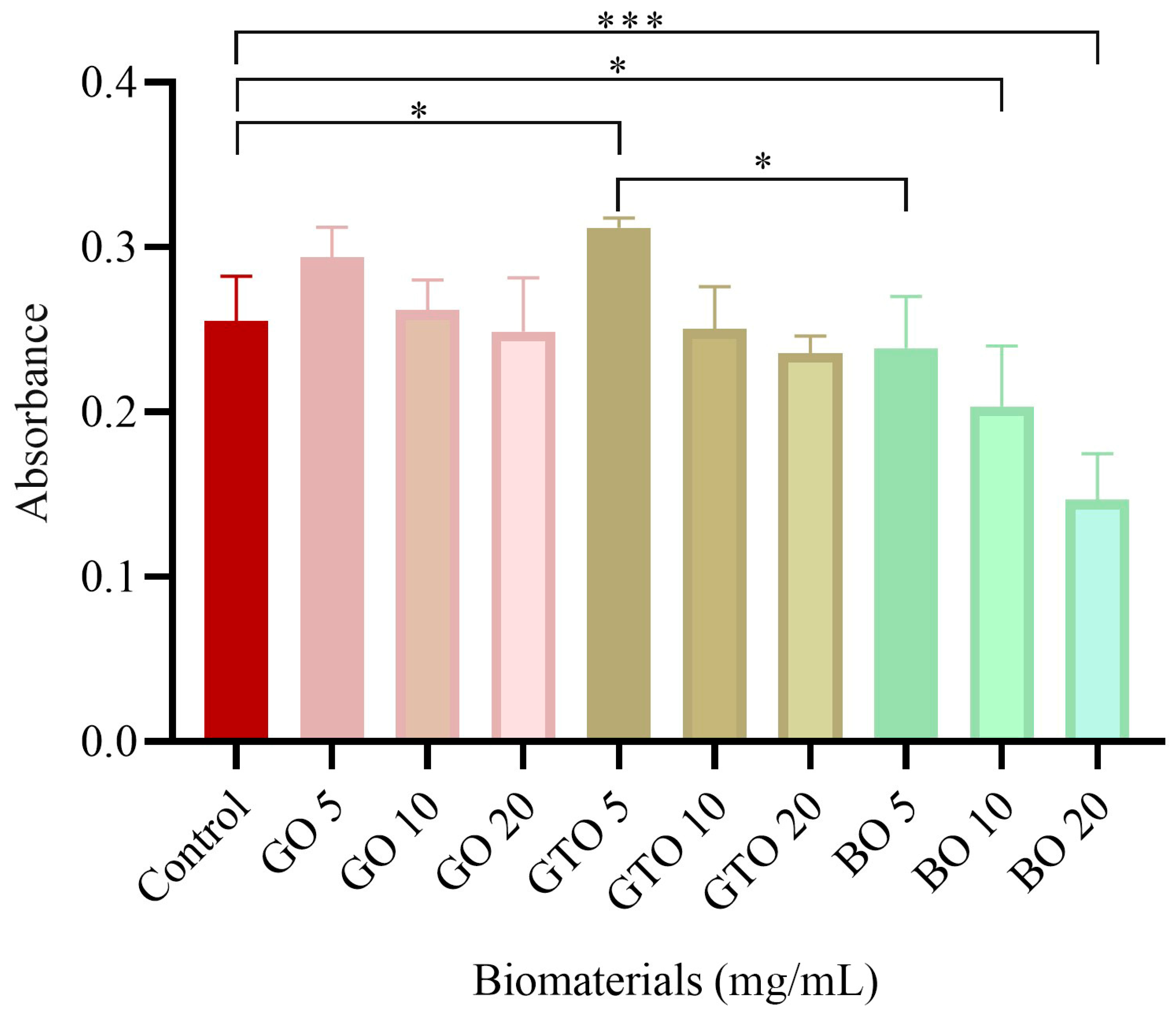

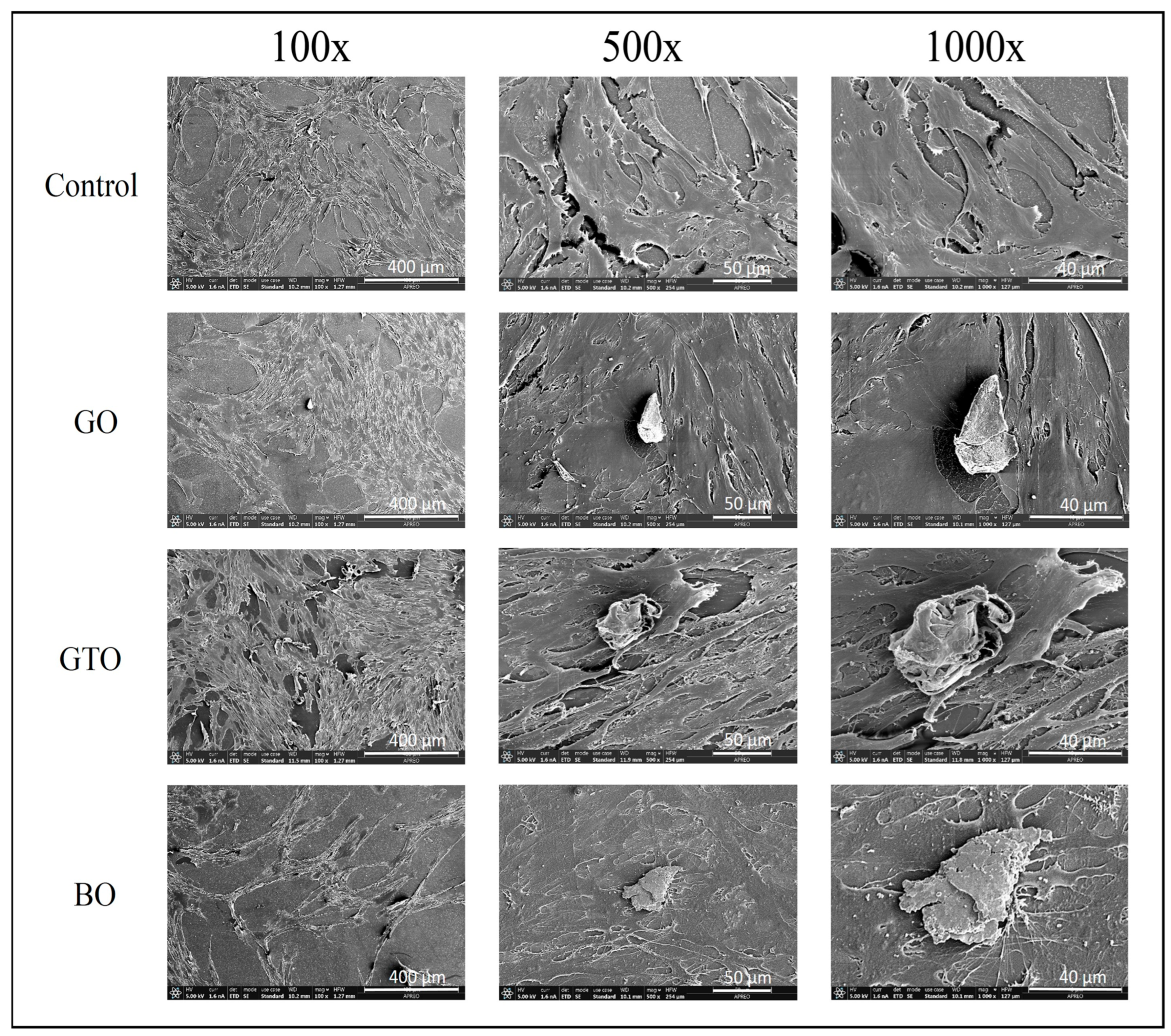
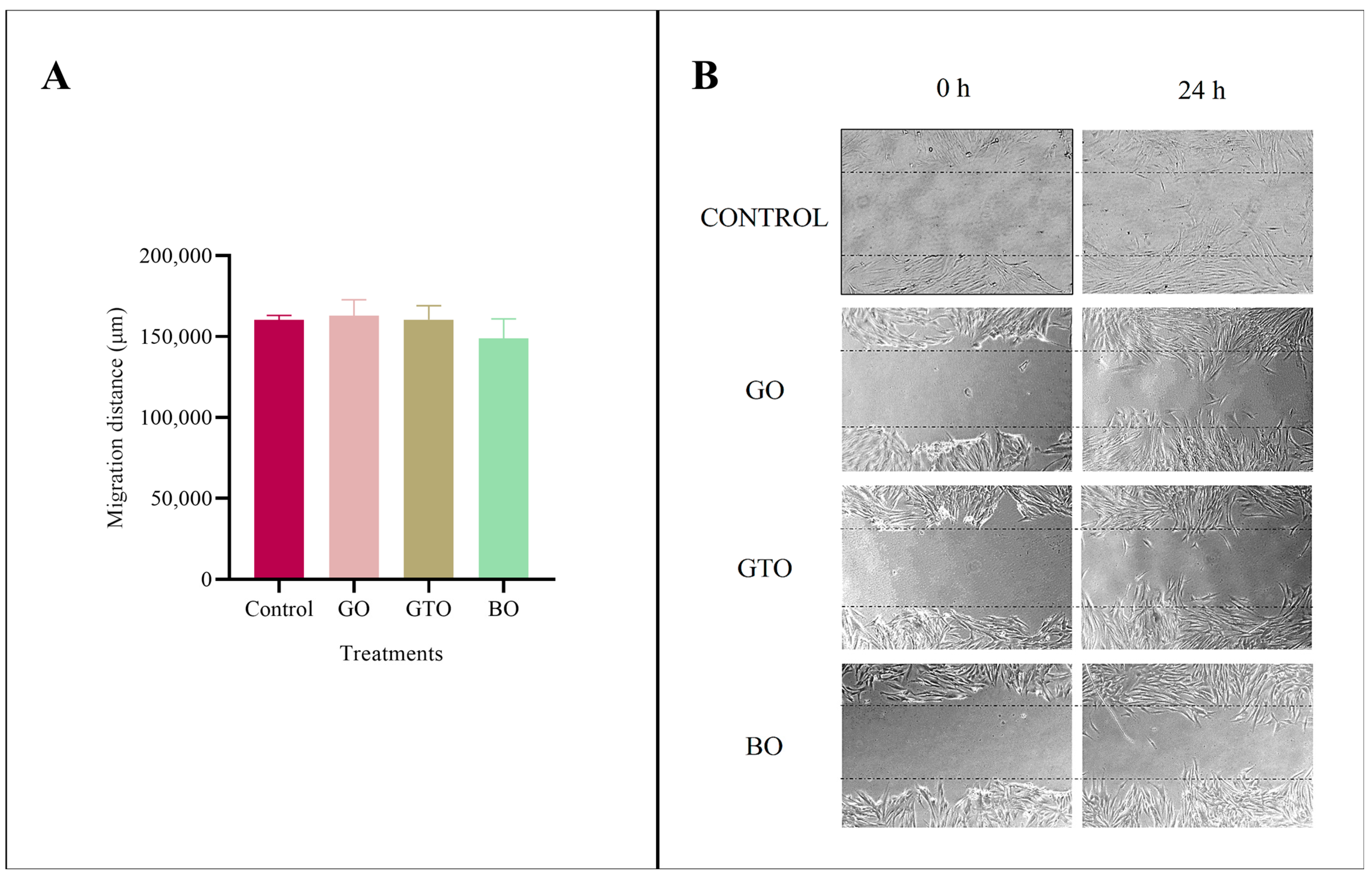
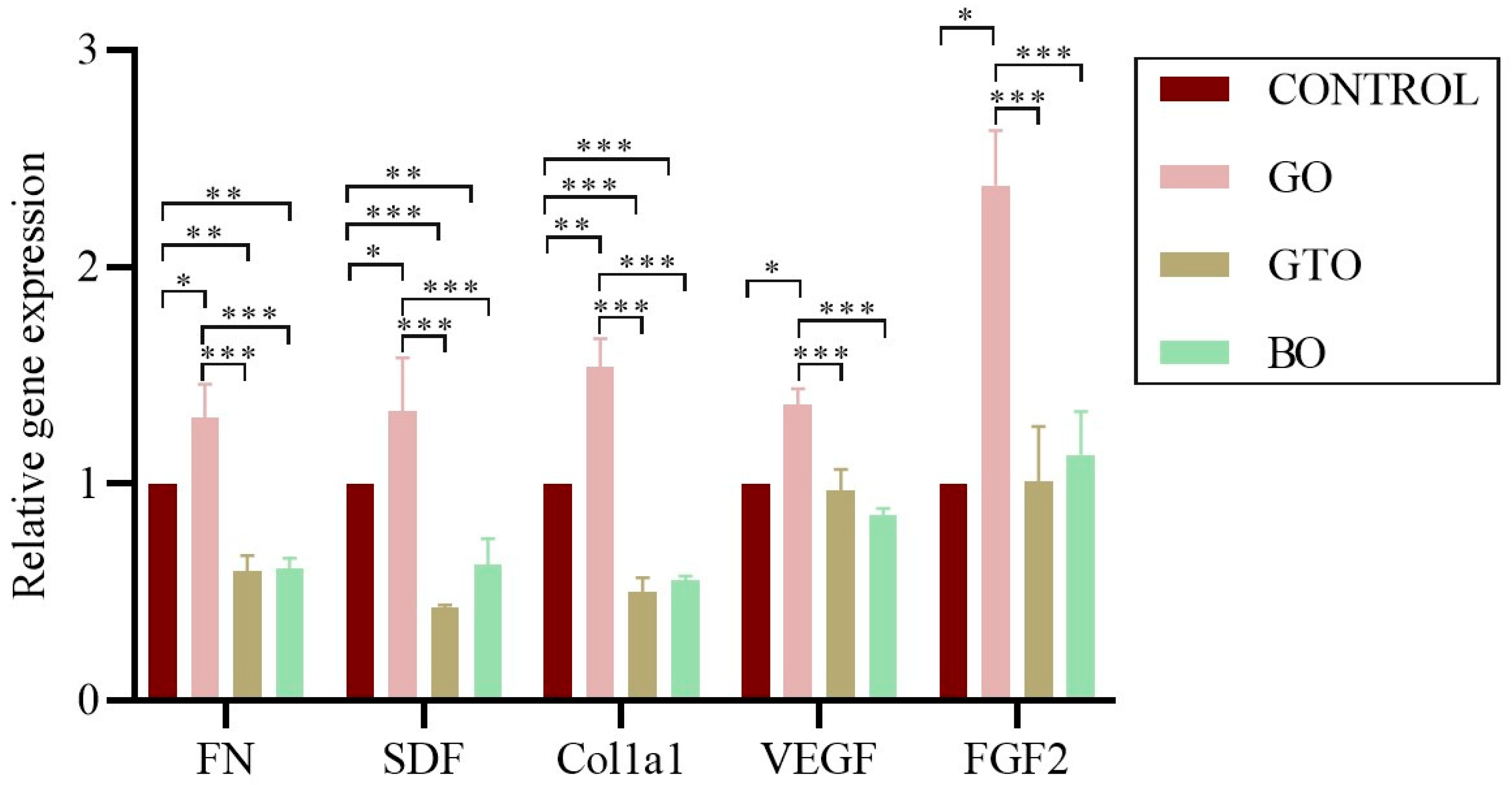

| Biomaterial/Batch | Manufacturer | Type/ Mechanism of Bone Formation | Origin/ Composition | Clinical Use |
|---|---|---|---|---|
| OsteoBiol® GTO® (GTO) 600–1000 µm/240129 | Tecnoss®, Giaveno, Italy | Xenograft/ Osteoconductive | Porcine/80% Cortico-cancellous bone granules combined with 20% OsteoBiol® TSV gel | Adaptable to any bone defect, horizontal augmentation procedures, and socket preservation |
| OsteoBiol® Gen-Os® (GO) 250–1000 μm/240146 | Tecnoss®, Giaveno, Italy | Xenograft/ Osteoconductive | Collagenated cortico-cancellous bone mix with tissue collagen preserved | Alveolar ridge preservation, lateral access maxillary sinus lift, dehiscence regeneration, periodontal regeneration. |
| Geistlich Bio-Oss® (BO) 0.25 mm–1 mm/82400382 | Geistlich Pharma AG, Wolhusen, Switzerland | Xenograft/ Osteoconductive | Bovine/Deproteinized cancellous bone | Sinus lifts, periodontal defects, and long-term preservation of graft volume. |
| Assays | Gene | Primer Sequences (Forward/Reverse) |
|---|---|---|
| Migration and adhesion | Human Fibronectin (FN) | 5′-TCCTTGCTGGTATCATGGCAG-3′/5′-AGACCCAGGCTTCTCATACTTGA-3′ |
| Collagen type 1 (COL1A1) | 5′-CTACCTCCACCATGCCAAGT-3′/5′-GCAGTAGCTGCGCTGATAGA-3′ | |
| Human Stromal cell-derived factor-1 (SDF-1) | 5′-CGTGCTATGAAGGAAGATGGA-3′/5′-TGCCCAGTTCGTTTCAGT-3′ | |
| Angiogenesis and proliferation | Human Vascular endothelial growth factor (VEGF) | 5′-GTCAGCCTGAGCTACAGATGC-3′/5′-CACTTTAGCTTCGGGTCAATG-3′ |
| Human Fibroblast growth factor 2 (FGF2) | 5′-CGATGGATTCCAGTTCGAGTATG-3′/5′-TGTTCTTGCAGTGGTAGGTGATG-3′ | |
| Osteogenic differentiation | Alkaline phosphatase (ALP) | 5′-TCAGAAGCTCAACACCAACG-3′/5′-TTGTACGTCTTGGAGAGGGC-3′ |
| Collagen type 1 (COL1A1) | 5′-CGATGGATTCCAGTTCGAGTATG-3′/5′-TGTTCTTGCAGTGGTAGGTGATG-3′ | |
| Runt-related transcription factor 2 (RUNX2) | 5′-TCCACACCATTAGGGACCATC-3′/5′-TGCTAATGCTTCGTGTTTCCA-3′ | |
| Endogenous Gene | Human GAPDH (GAPDH) | 5′-CCTGCACCACCAACTGCTTA-3′/5′-GGCCATCCACAGTCTTCTGAG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelaez-Cruz, P.; López Jornet, P.; Pons-Fuster, E. Collagen Formulation in Xenogeneic Bone Substitutes Influences Cellular Responses in Periodontal Regeneration: An In Vitro Study. Biomimetics 2025, 10, 608. https://doi.org/10.3390/biomimetics10090608
Pelaez-Cruz P, López Jornet P, Pons-Fuster E. Collagen Formulation in Xenogeneic Bone Substitutes Influences Cellular Responses in Periodontal Regeneration: An In Vitro Study. Biomimetics. 2025; 10(9):608. https://doi.org/10.3390/biomimetics10090608
Chicago/Turabian StylePelaez-Cruz, Priscilla, Pia López Jornet, and Eduardo Pons-Fuster. 2025. "Collagen Formulation in Xenogeneic Bone Substitutes Influences Cellular Responses in Periodontal Regeneration: An In Vitro Study" Biomimetics 10, no. 9: 608. https://doi.org/10.3390/biomimetics10090608
APA StylePelaez-Cruz, P., López Jornet, P., & Pons-Fuster, E. (2025). Collagen Formulation in Xenogeneic Bone Substitutes Influences Cellular Responses in Periodontal Regeneration: An In Vitro Study. Biomimetics, 10(9), 608. https://doi.org/10.3390/biomimetics10090608







