Structure Design and Performance Study of Bionic Electronic Nasal Cavity
Abstract
1. Introduction
2. Materials and Methods
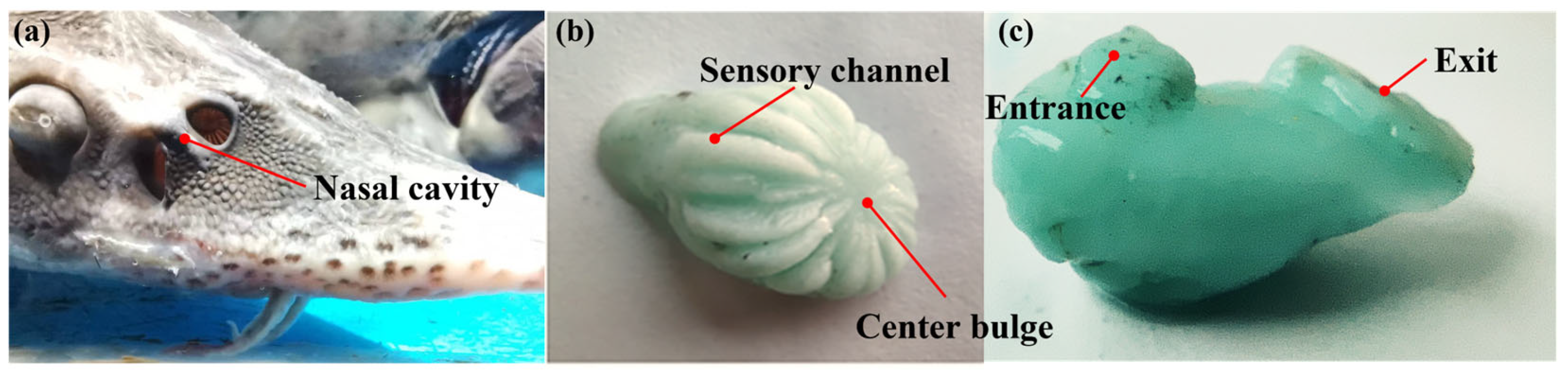
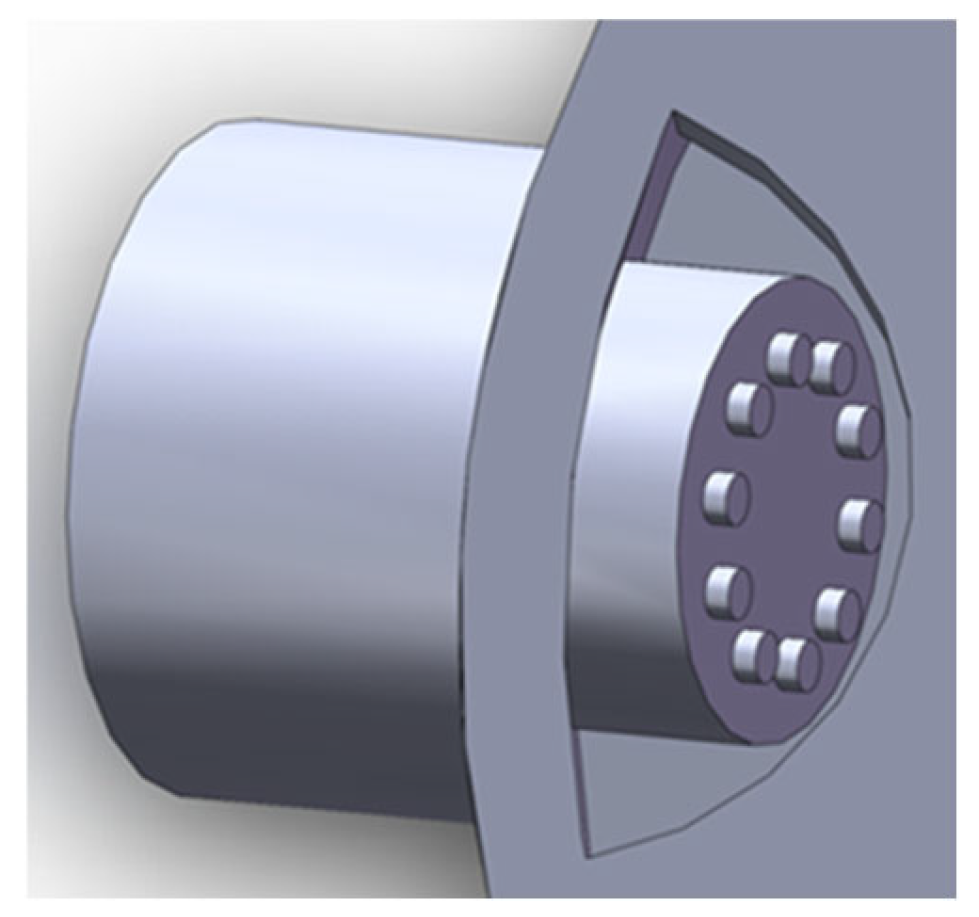
2.1. Bionic Nasal Cavity Design
2.1.1. Bionic Nasal Cavity External Structure Design
2.1.2. Bionic Nasal Cavity Internal Structure Design
2.2. Aerodynamic Simulation of Nasal Structure
2.3. Bionic Electronic Nose Performance Test
3. Results and Discussion
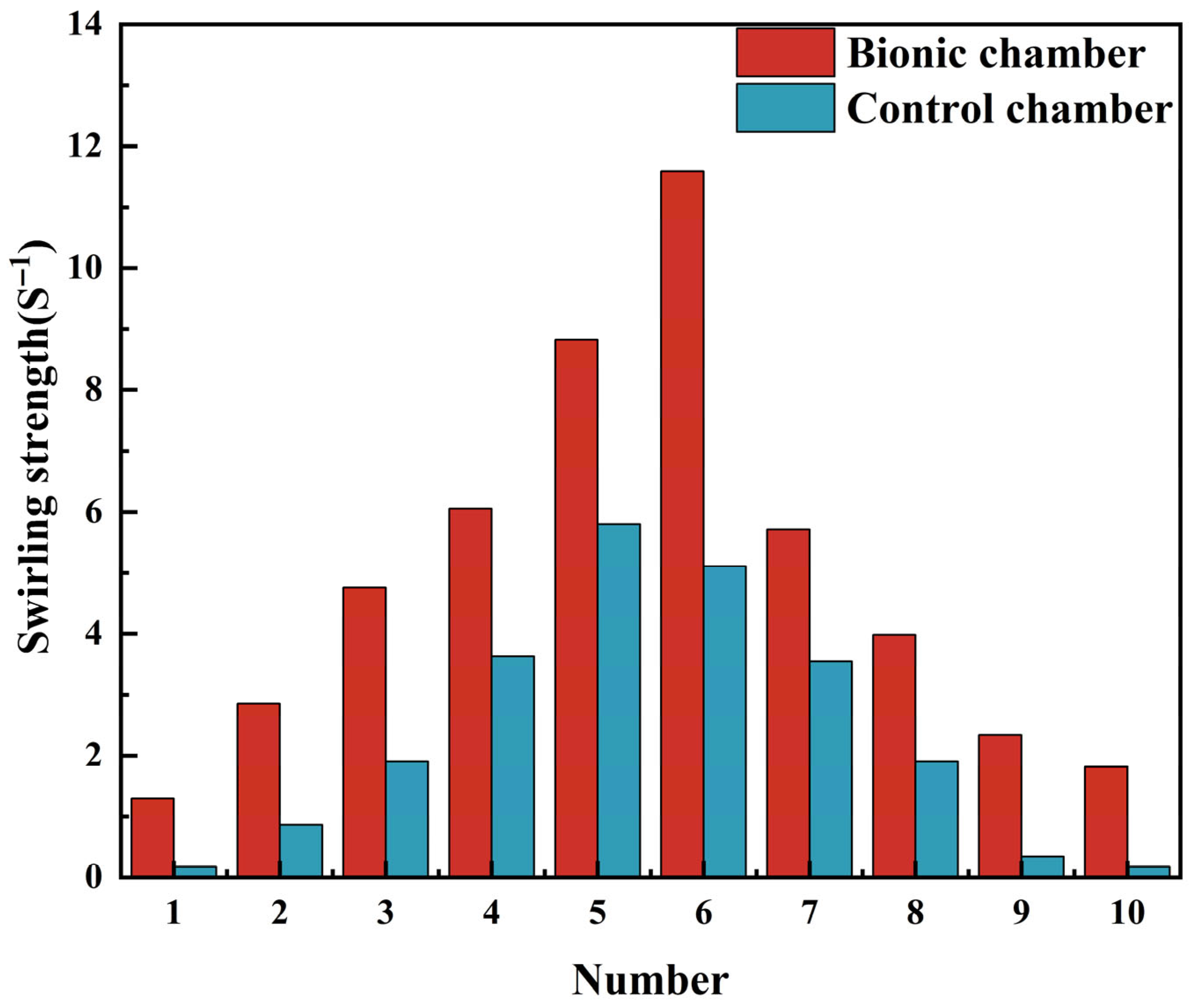
3.1. CFD Analysis Results
3.2. Performance Test Result
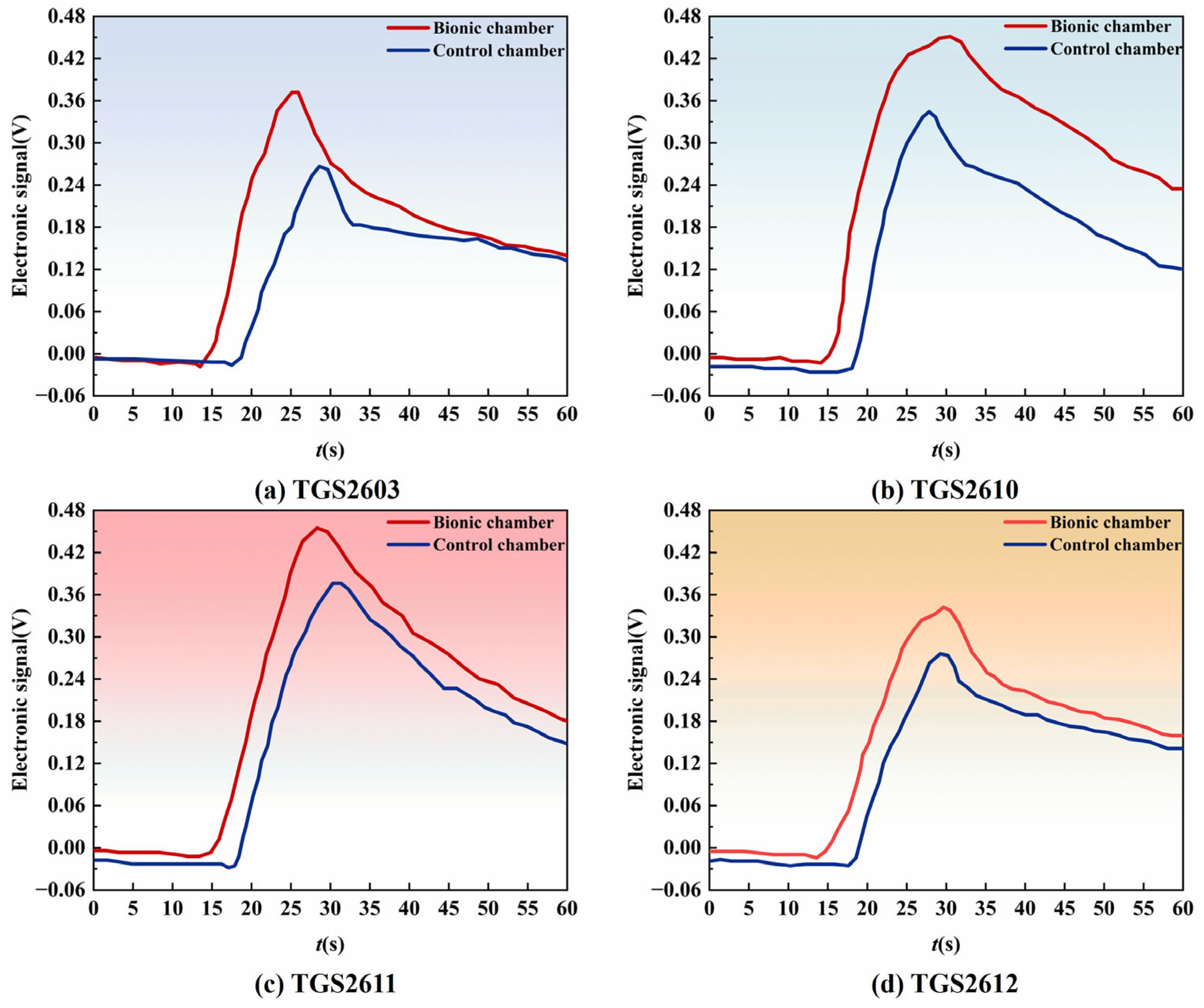
3.2.1. Sensor Response Comparison
3.2.2. Algorithm Recognition Rate Comparison
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, H.; Kumar, R.; Sankhla, M.S. Residues of pesticides and heavy metals in crops resulting in toxic effects on living organism. J. Seybold Rep. 2020, 15, 1527–1541. [Google Scholar]
- Rachna; Singh, M.P.; Goswami, S.; Singh, U.K. Pesticide pollution: Toxicity, sources and advanced remediation approaches. Environ. Sci. Pollut. Res. 2024, 31, 64385–64418. [Google Scholar] [CrossRef]
- Masía, A.; Blasco, C.; Picó, Y. Last trends in pesticide residue determination by liquid chromatography–mass spectrometry. Trends Environ. Anal. Chem. 2014, 2, 11–24. [Google Scholar] [CrossRef]
- Kim, Y.K.; Baek, E.J.; Na, T.W.; Sim, K.S.; Kim, H.Y.; Kim, H.J. LC–MS/MS and GC–MS/MS Cross-Checking Analysis Method for 426 Pesticide Residues in Agricultural Products: A Method Validation and Measurement of Uncertainty. Agric. Food Chem. 2024, 72, 22814–22821. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, G.; Atreya, K.; Scheepers, P.T.J.; Geissen, V. Concentration and Distribution of Pesticide Residues in Soil: Non-Dietary Human Health Risk Assessment. Chemosphere 2020, 253, 126594. [Google Scholar] [CrossRef]
- Li, Z.J.; Fantke, P. Toward harmonizing global pesticide regulations for Surface freshwaters in support of protecting human health. J. Environ. Manag. 2022, 301, 113909. [Google Scholar] [CrossRef]
- Kumar, A.; Castro, M.; Feller, J.-F. Review on sensor array-based analytical technologies for quality control of food and beverages. Sensors 2023, 23, 4017. [Google Scholar] [CrossRef]
- Viccione, G.; Zarra, T.; Giuliani, S.; Naddeo, V.; Belgiorno, V. Performance study of e-nose measurement chamber for environmental odour monitoring. Chem. Eng. Trans. 2012, 30, 483–488. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, Y.-B.; Meng, Q.-H. A novel e-nose chamber design for vocs detection in automobiles. In Proceedings of the 39th Chinese Control Conference, Shenyang, China, 27–29 July 2020. [Google Scholar]
- Jaeschke, C.; Glöckler, J.; El Azzi, O.; Gonzalez, O.; Padilla, M.; Mitrovics, J.; Mizaikoff, B. An innovative modular enose system based on a unique combination of analog and digital metal oxide sensors. ACS Sens. 2019, 4, 2277–2281. [Google Scholar] [CrossRef]
- Wesoły, M.; Przewodowski, W.; Ciosek-Skibińska, P. Electronic noses and electronic tongues for agricultural purposes. TRAC-Trends Anal. Chem. 2023, 164, 117082. [Google Scholar] [CrossRef]
- Falcitella, M.; Benassai, A.; Di Francesco, F.; Domenici, C.; Marano, L.; Pioggia, G. Fluid dynamic simulation of a measurement chamber for electronic noses. Sens. Actuators B Chem. 2002, 85, 166–174. [Google Scholar] [CrossRef]
- Villarreal, B.L.; Gordillo, J.L. Bioinspired smell sensor: Nostril model and design. IEEE/ASME Trans. Mechatron. 2016, 21, 912–921. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Meng, Q.-H.; Jin, X.-W.; Sun, Z.-H. Design of handheld electronic nose bionic chambers for chinese liquors recognition. Measurement 2021, 172, 108856. [Google Scholar] [CrossRef]
- Chang, Z.; Sun, Y.; Zhang, Y.; Gao, Y.; Weng, X.; Chen, D.; David, L.; Xie, J. Erratum to: Bionic optimization design of electronic nose chamber for oil and gas detection. J. Bionic Eng. 2018, 15, 939. [Google Scholar] [CrossRef]
- Weng, X.-H.; Sun, Y.-H.; Zhang, S.-J.; Xie, J.; Chang, Z.-Y. Oil and gas detection method and experimental new technology based on bionic nasal chamber optimization. J. Jilin Univ. (Eng. Technol. Ed.) 2020, 50, 382–388. [Google Scholar]
- Shooshtari, M.; Salehi, A. An electronic nose based on carbon nanotube-titanium dioxide hybrid nanostructures for detection and discrimination of volatile organic compounds. Sens. Actuators B Chem. 2022, 357, 131418. [Google Scholar] [CrossRef]
- Abel, R.L.; Maclaine, J.S.; Cotton, R.; Bui Xuan, V.; Nickels, T.B.; Clark, T.H.; Wang, Z.; Cox, J.P.L. Functional morphology of the nasal region of a hammerhead shark. Comp. Biochem. Phys. D Part A Mol. Integr. Physiol. 2010, 155, 464–475. [Google Scholar] [CrossRef]
- Jin, H.Y.; Chang, X.Z.; Luan, X.Y.; Chen, Z.H.; Zhang, T.; Dang, J.M.; Weng, X.H.; Chang, Z.Y. A bio-inspired gas sensing sampling chamber inspired by the sensitive olfaction of sturgeons: Achieving low-power inspection and high-sensitivity sensing. Sens. Actuators B Chem. 2025, 433, 137536. [Google Scholar] [CrossRef]
- Ni, W.; Wang, T.; Wu, Y.; Chen, L.; Zeng, M.; Yang, J.; Hu, N.; Zhang, B.; Xuan, F.; Yang, Z. Robust Odor Detection in Electronic Nose Using Transfer-Learning Powered Scentformer Model. ACS Sens. 2025, 10, 3704–3712. [Google Scholar] [CrossRef]
- Mohammed, A.; Abdullah, A. Scanning electron microscopy (sem): A review. In Proceedings of the 2018 International Conference on Hydraulics and Pneumatics—HERVEX, Băile Govora, Romania, 7–9 November 2019. [Google Scholar]
- Kong, C.; Ren, L.; Zhang, T.; Sun, Y.; Chang, Z. Rapid identification of pesticides in soil by bionic sniffing sensing system with unknown category detection function. Comput. Electron. Agric. 2024, 217, 108667. [Google Scholar] [CrossRef]
- Qian, J.; Tian, F.; Zhang, S.; Liu, R. A novel conformal design for multi-sensor system synthesis. IEEE Trans. Circuits Syst. II Express Br. 2021, 68, 1532–1536. [Google Scholar] [CrossRef]
- Zeng, Y.; Fang, J. Numerical simulation and experimental study on gas mixing in a gas chamber for sensor evaluation. Meas. Sens. 2021, 18, 100338. [Google Scholar] [CrossRef]
- Roy, R.; Miller, J. Miniaturization of image sensors: The role of innovations in complementary technologies in overcoming technological trade-offs associated with product innovation. J. Eng. Technol. Manag. 2017, 44, 58–69. [Google Scholar] [CrossRef]
- Garwood, R.J.; Behnsen, J.; Haysom, H.K.; Hunt, J.N.; Dalby, L.J.; Quilter, S.K.; Maclaine, J.S.; Cox, J.P.L. Olfactory flow in the sturgeon is externally driven. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 235, 211–225. [Google Scholar] [CrossRef]
- Liu, Y.; Johnson, M.R.; Matida, E.A.; Kherani, S.; Marsan, J. Creation of a standardized geometry of the human nasal cavity. J. Appl. Physiol. 2009, 106, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; He, X.; Yang, Q.; Gu, Q.; Zhang, X.; Sui, X.; Zhou, R.; Feng, W. Numerical simulation of the influence of nasal cycle on nasal airflow. Sci. Rep. 2024, 14, 12161. [Google Scholar] [CrossRef] [PubMed]
- Lawson, M.J.; Li, Y.; Sale, D.C. Development and verification of a computational fluid dynamics model of a horizontal-axis tidal current turbine. In Proceedings of the ASME 2011 30th International Conference on Ocean, Offshore and Arctic Engineering (OMAE2011), Rotterdam, The Netherlands, 19–24 June 2011. Paper No.: OMAE2011-49863. [Google Scholar] [CrossRef]
- Craven, B.A.; Paterson, E.G.; Settles, G.S.; Lawson, M.J. Development and verification of a high-fidelity computational fluid dynamics model of canine nasal airflow. J. Biomech. Eng. 2009, 131, 091002. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Dalton, P.; Yang, G.C.; Scherer, P.W. Numerical modeling of turbulent and laminar airflow and odorant transport during sniffing in the human and rat nose. Chem. Senses 2006, 31, 107–118. [Google Scholar] [CrossRef]
- Bleckmann, H. The lateral line system of fish. In Fish Physiology; Academic Press: San Diego, CA, USA, 2006; Volume 25, pp. 411–453. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, Y.; Yuan, X.; Ma, S.; Dong, S. Modular assembly of tensegrity structures with diverse mesh division forms. Eng. Struct. 2024, 315, 118491. [Google Scholar] [CrossRef]
- Luo, S.; Liu, Y.; Du, M.; Gao, S.; Wang, P.; Liu, X. The influence of spatial grid division on the layout analysis of urban functional areas. ISPRS Int. J. Geo-Inf. 2021, 10, 189. [Google Scholar] [CrossRef]
- Garmella, S.V.M.; Kalyani Radha, K. Modelling and cfd simulation of turbocharger’s centrifugal compressor for a marine diesel engine. Int. J. Emerg. Technol. Innov. Res. 2019, 6, 213–219. [Google Scholar]
- Yoon, S.H.; Tak, N.-i.; Lim, H.S. Validation of a system code (gamma+) using standard k-ε model for multi-dimensional turbulent flows in various geometries. Nucl. Eng. Des. 2025, 439, 114079. [Google Scholar] [CrossRef]
- Waldrop, L.D.; Hann, M.; Henry, A.K.; Kim, A.; Punjabi, A.; Koehl, M.A. Ontogenetic changes in the olfactory antennules of the shore crab, hemigrapsus oregonensis, maintain sniffing function during growth. J. R. Soc. Interface 2015, 12, 20141077. [Google Scholar] [CrossRef] [PubMed]
- Konda, L.N.; Pásztor, Z. Environmental distribution of acetochlor, atrazine, chlorpyrifos, and propisochlor under field conditions. J. Agric. Food Chem. 2001, 49, 3859–3863. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. The history and current status of glyphosate. Pest Manag. Sci. 2018, 74, 1027–1034. [Google Scholar] [CrossRef]
- Solomon, K.R.; Williams, W.M.; Mackay, D.; Purdy, J.; Giddings, J.M.; Giesy, J.P. Properties and uses of chlorpyrifos in the united states. Rev. Environ. Contam. Toxicol. 2014, 231, 13–34. [Google Scholar] [CrossRef]
- Babić, K.; Tomašić, V.; Gilja, V.; Le Cunff, J.; Gomzi, V.; Pintar, A.; Žerjav, G.; Kurajica, S.; Duplančić, M.; Zelić, I.E.; et al. Photocatalytic degradation of imidacloprid in the flat-plate photoreactor under uva and simulated solar irradiance conditions—The influence of operating conditions, kinetics and degradation pathway. J. Environ. Chem. Eng. 2021, 9, 105611. [Google Scholar] [CrossRef]
- Agbesi, M.P.K.; Borsuk, H.S.; Hunt, J.N.; Maclaine, J.S.; Abel, R.L.; Sykes, D.; Ramsey, A.T.; Wang, Z.; Cox, J.P.L. Motion-driven flow in an unusual piscine nasal region. Zoology 2016, 119, 500–510. [Google Scholar] [CrossRef]
- Shao, C.; Zhong, G.; Zhou, J. Study on gas–liquid two-phase flow in the suction chamber of a centrifugal pump and its dimensionless characteristics. Nucl. Eng. Des. 2021, 380, 111298. [Google Scholar] [CrossRef]
- Muck, K.C.; Hoffmann, P.H.; Bradshaw, P. The effect of convex surface curvature on turbulent boundary layers. J. Fluid Mech. 1985, 161, 347–369. [Google Scholar] [CrossRef]
- Anandhi, G.; Iyapparaja, M. Systematic approaches to machine learning models for predicting pesticide toxicity. Heliyon 2024, 10, e28752. [Google Scholar] [CrossRef]








| Level | Factors | |||
|---|---|---|---|---|
| A/mm | B/mm | C/(m∙s−1) | D/s | |
| 1 | 3.6 | 80 | 0.5 | 60 |
| 2 | 4.0 | 90 | 0.8 | 90 |
| 3 | 4.4 | 100 | 1.2 | 120 |
| Sum of deviation square | 0.189 | 0.059 | 0.111 | 0.002 |
| Mean square | 0.119 | 0.056 | 0.079 | 0.024 |
| F test value | 5.133 | 1.195 | 3.171 | 0.024 |
| Level | Figures | |||
|---|---|---|---|---|
| E/mm | F/mm | G/mm | H | |
| 1 | 10 | 20 | 10 | 8 |
| 2 | 15 | 22 | 15 | 12 |
| 3 | 20 | 24 | 20 | 16 |
| Sum of deviation square | 0.193 | 0.057 | 0.132 | 0.001 |
| Mean square | 0.124 | 0.063 | 0.086 | 0.021 |
| F test value | 5.253 | 1.251 | 2.986 | 0.021 |
| Reagent Name | Recommended Usage | Proportioning Concentration | Concentration Value (mg/L) | |
|---|---|---|---|---|
| 29% acetochlor 26% atrazine | Dilute 100–150 times | Low | Dilute 100 times | 2900 (acetochlor) + 2600 (atrazine) |
| Medium | Dilute 40 times | 7250 (acetochlor) + 6500 (atrazine) | ||
| High | Dilute 20 times | 14,500 (acetochlor) + 13,000 (atrazine) | ||
| 30%glyphosate | Dilute 95–285 times | Low | Dilute 100 times | 3000 |
| Medium | Dilute 40 times | 7500 | ||
| High | Dilute 20 times | 15,000 | ||
| 45% chlorpyrifos | Dilute 283–353 times | Low | Dilute 300 times | 1500 |
| Medium | Dilute 120 times | 3750 | ||
| High | Dilute 60 times | 7500 | ||
| 5% imidacloprid | Dilute 375–500 times | Low | Dilute 350 times | 143 |
| Medium | Dilute 140 times | 357 | ||
| High | Dilute 70 times | 714 | ||
| Sensor Serial Number | Sensor Type | Target Gas | Detection Range (ppm) |
|---|---|---|---|
| 1 | TGS2603 | Organic solvents, organic gases (methane, methyl mercaptan, etc.) | 1–30 |
| 2 | TGS2610 | Ethanol, Hydrogen, Methane, Isobutane, Propane | 500–10,000 |
| 3 | TGS2611 | Ethanol, Hydrogen, Isobutane, Methane, Natural Gas | 500–10,000 |
| 4 | TGS2612 | Methane, Propane, Isobutane | 500–10,000 |
| Classification Method | Explain |
|---|---|
| Divided into 4 kinds | Divided into four pesticides (acetochlor-atrazine, glyphosate, imidacloprid, chlorpyrifos) |
| Divided into 13 kinds | Divided into 13 samples (pesticide type + concentration), such as [acetochlor-atrazine − low concentration], [imidacloprid − medium concentration] |
| Classifier | Feature Extraction Method | Training Set Recognition Rate | Test Set Recognition Rate | ||
|---|---|---|---|---|---|
| Bionic Chamber | Contrast Chamber | Bionic Chamber | Contrast Chamber | ||
| KNN | IV | 99.7% | 100% | 99.2% | 98.2% |
| MAX | 100% | 100% | 99.2% | 99.2% | |
| Mean | 100% | 100% | 99.4% | 97.9% | |
| WT | 98.2% | 89.4% | 97.9% | 87.6% | |
| RF | IV | 100% | 100% | 98.9% | 97.6% |
| MAX | 100% | 100% | 99.2% | 96.4% | |
| Mean | 100% | 100% | 99.4% | 98.2% | |
| WT | 100% | 93.0% | 97.4% | 83.0% | |
| SVM | IV | 100% | 100% | 100% | 99.4% |
| MAX | 99.7% | 99.6% | 99.7% | 98.7% | |
| Mean | 100% | 100% | 100% | 99.7% | |
| WT | 99.7% | 92.5% | 98.9% | 88.4% | |
| Classifier | Feature Extraction Method | Training Set Recognition Rate | Test Set Recognition Rate | ||
|---|---|---|---|---|---|
| Bionic Chamber | Contrast Chamber | Bionic Chamber | Contrast Chamber | ||
| KNN | IV | 100% | 99.8% | 97.9% | 94.8% |
| MAX | 100% | 99.7% | 93.8% | 94.6% | |
| Mean | 100% | 99.5% | 97.6% | 94.6% | |
| WT | 100% | 86.9% | 93.0% | 78.9% | |
| RF | IV | 100% | 99.7% | 95.6% | 95.1% |
| MAX | 100% | 99.7% | 95.3% | 95.1% | |
| Mean | 100% | 99.7% | 95.3% | 94.6% | |
| WT | 100% | 92.8% | 92.5% | 77.9% | |
| SVM | IV | 99.7% | 98.8% | 98.2% | 96.6% |
| MAX | 97.2% | 99.3% | 96.6% | 93.8% | |
| Mean | 99.7% | 98.8% | 98.2% | 97.1% | |
| WT | 97.7% | 90.2% | 95.1% | 81.2% | |
| Classifier | KNN | RF | SVM | |||
|---|---|---|---|---|---|---|
| Chamber Type | Bionic Chamber | Contrast Chamber | Bionic Chamber | Contrast Chamber | Bionic Chamber | Contrast Chamber |
| Recognition rate | 99.2% | 96.5% | 99.4% | 96.0% | 99.75% | 97.3% |
| classifier | KNN | RF | SVM | |||
|---|---|---|---|---|---|---|
| Chamber Type | Bionic Chamber | Contrast Chamber | Bionic Chamber | Contrast Chamber | Bionic Chamber | Contrast Chamber |
| Recognition rate | 97.8% | 93.6% | 97.3% | 94.3% | 98.0% | 94.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Yin, Z.; Xu, S.; Wang, P.; Yang, L.; Lv, Y. Structure Design and Performance Study of Bionic Electronic Nasal Cavity. Biomimetics 2025, 10, 555. https://doi.org/10.3390/biomimetics10080555
Chen P, Yin Z, Xu S, Wang P, Yang L, Lv Y. Structure Design and Performance Study of Bionic Electronic Nasal Cavity. Biomimetics. 2025; 10(8):555. https://doi.org/10.3390/biomimetics10080555
Chicago/Turabian StyleChen, Pu, Zhipeng Yin, Shun Xu, Pengyu Wang, Lianjun Yang, and You Lv. 2025. "Structure Design and Performance Study of Bionic Electronic Nasal Cavity" Biomimetics 10, no. 8: 555. https://doi.org/10.3390/biomimetics10080555
APA StyleChen, P., Yin, Z., Xu, S., Wang, P., Yang, L., & Lv, Y. (2025). Structure Design and Performance Study of Bionic Electronic Nasal Cavity. Biomimetics, 10(8), 555. https://doi.org/10.3390/biomimetics10080555





