State-of-the-Art Organ-on-Chip Models and Designs for Medical Applications: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Inclusion Criteria
2.3. Exclusion Criteria
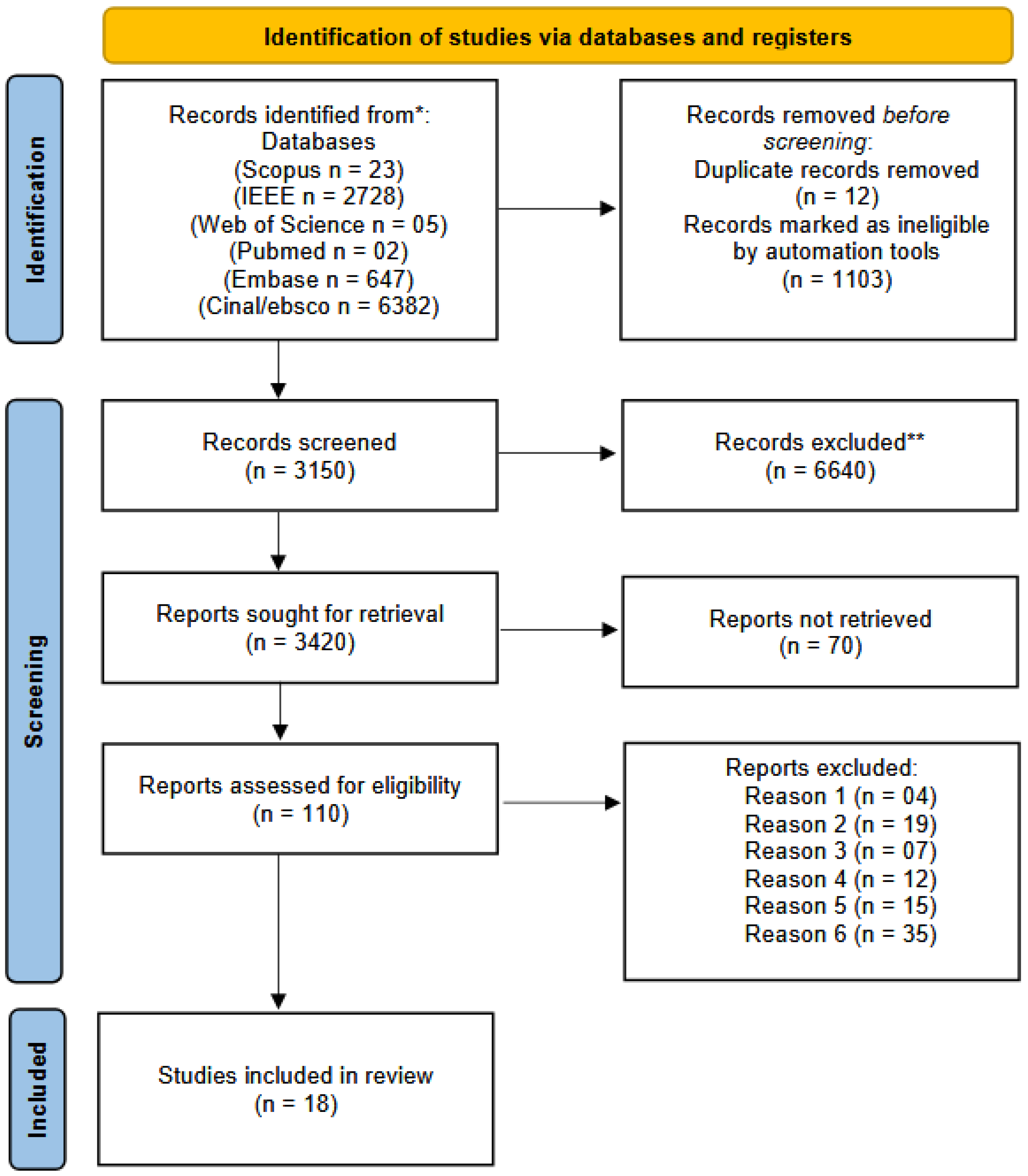
2.4. Search Strategy and Information Sources
2.5. Bias Risks and Quality in Individual Studies
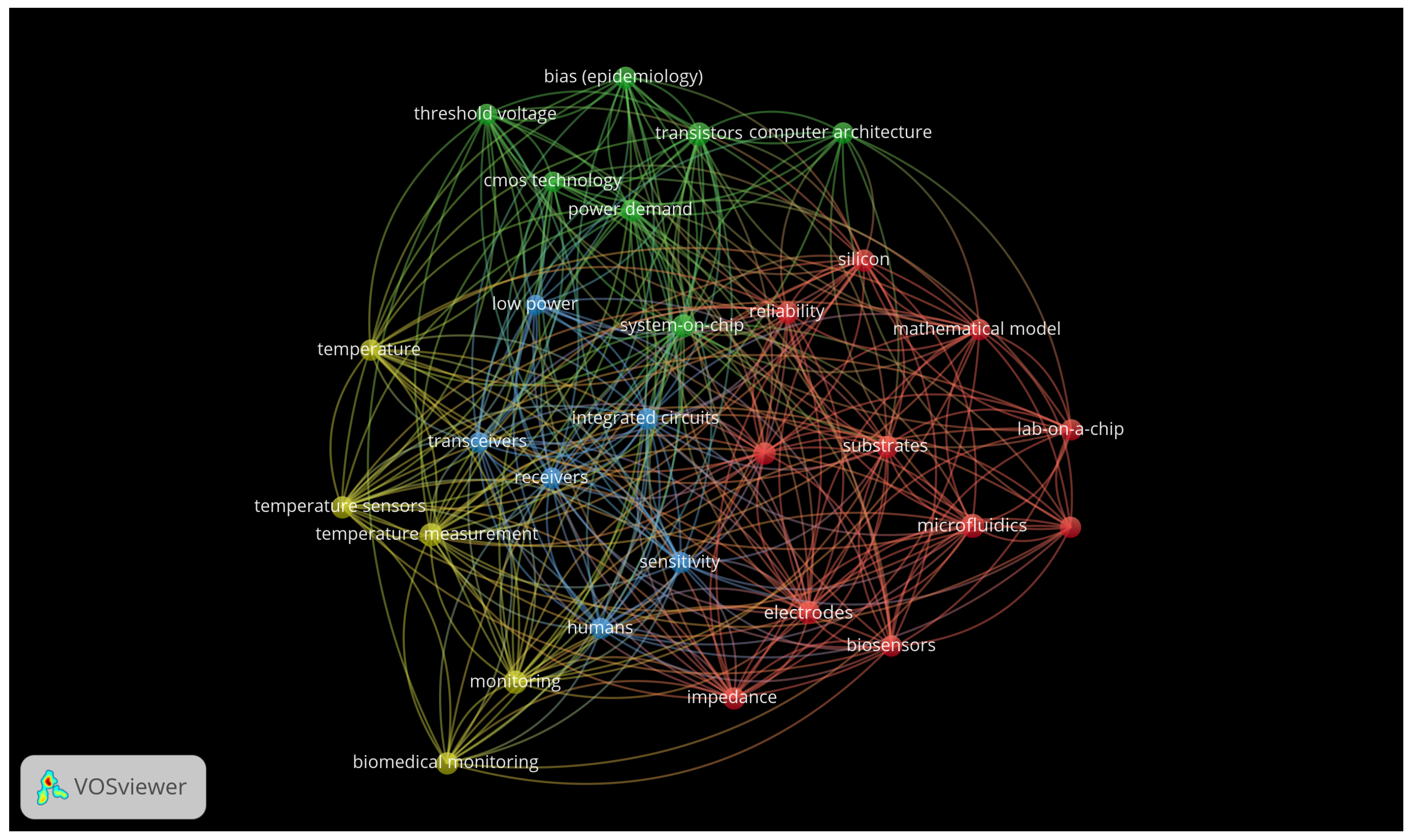
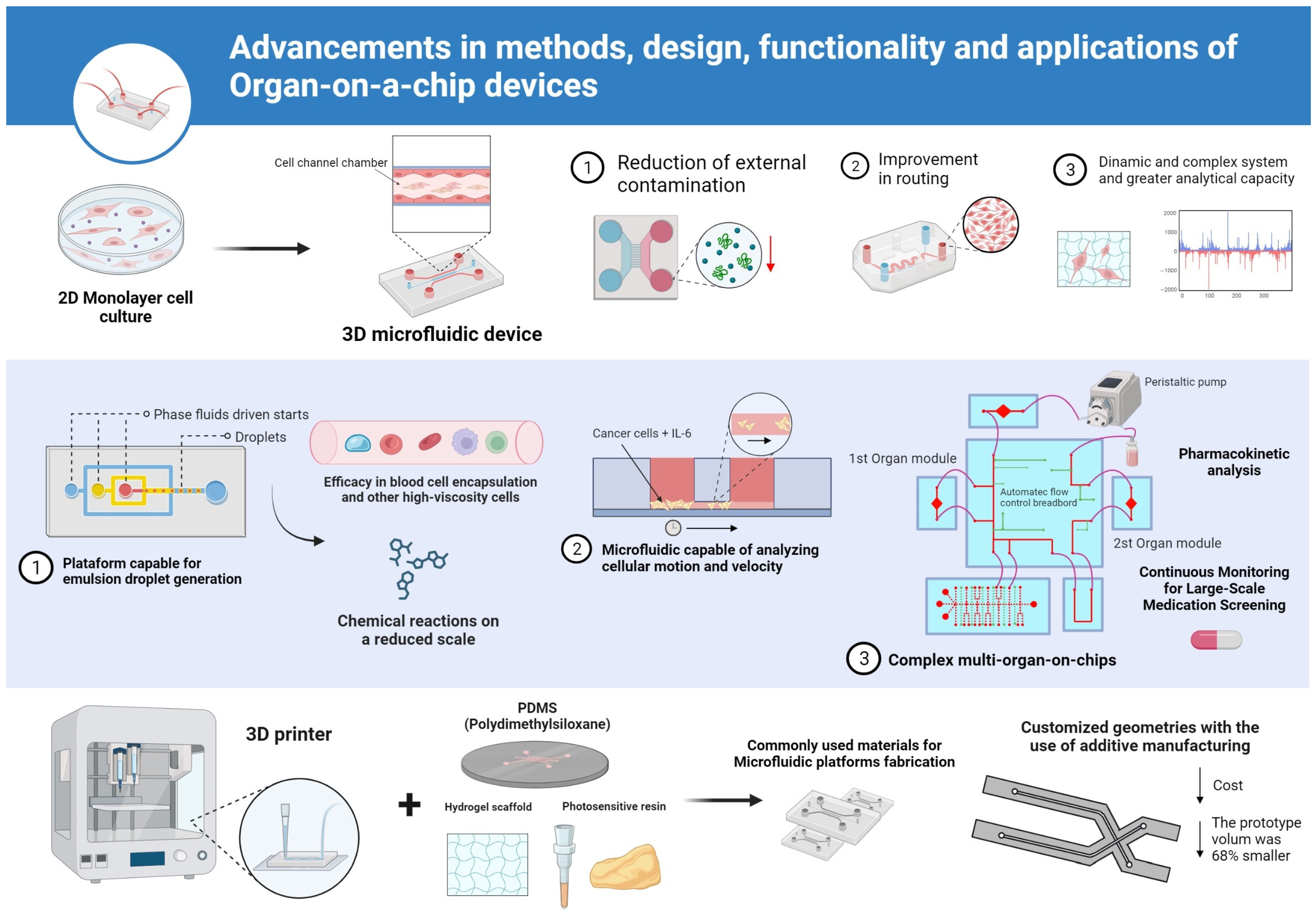
3. Results
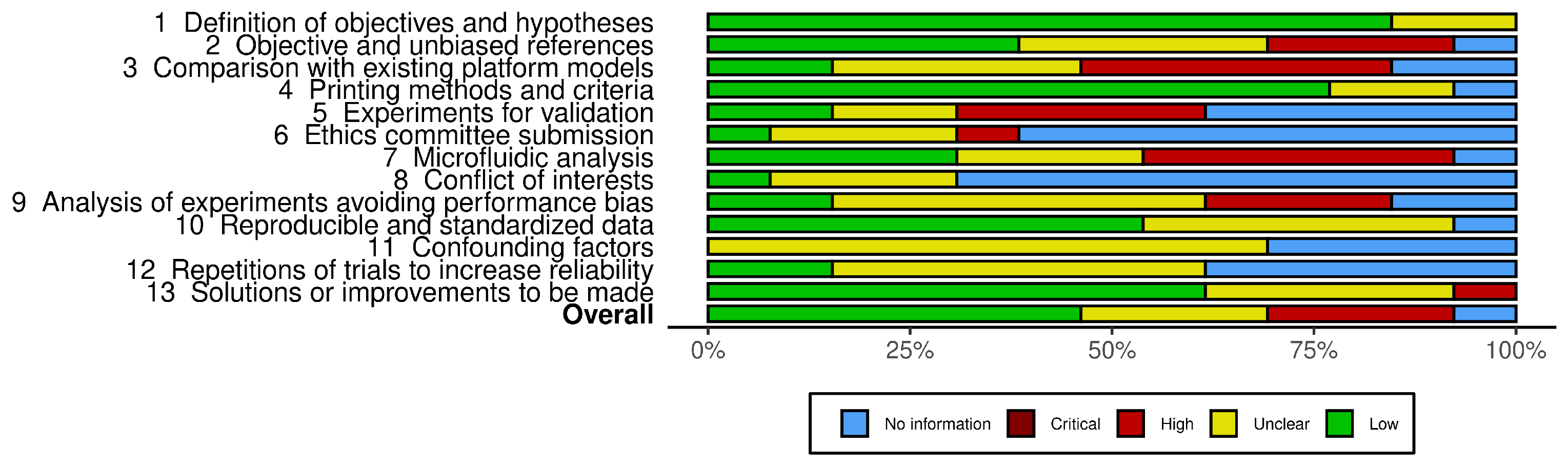
Analysis of Methodological Quality in Articles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balijepalli, A.; Sivaramakrishan, V. Organs-on-chips: Research and commercial perspectives. Drug Discov. Today 2017, 22, 397–403. [Google Scholar] [CrossRef]
- Chliara, M.A.; Elezoglou, S.; Zergioti, I. Bioprinting on Organ-on-Chip: Development and Applications. Biosensors 2022, 12, 1135. [Google Scholar] [CrossRef] [PubMed]
- Jodat, Y.A.; Kang, M.G.; Kiaee, K.; Kim, G.J.; Martinez, A.F.; Rosenkranz, A.; Bae, H.; Shin, S.R. Human-derived organ-on-a-chip for personalized drug development. Curr. Pharm. Des. 2018, 24, 5471–5486. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.; Gonçalves, I.; Lage, T.; Rodrigues, R.O.; Minas, G.; Teixeira, S.F.; Moita, A.S.; Hori, T.; Kaji, H.; Lima, R.A. 3D printing techniques and their applications to organ-on-a-chip platforms: A systematic review. Sensors 2021, 21, 3304. [Google Scholar] [CrossRef] [PubMed]
- Funamoto, K.; Zervantonakis, I.K.; Liu, Y.; Ochs, C.J.; Kim, C.; Kamm, R.D. A novel microfluidic platform for high-resolution imaging of a three-dimensional cell culture under a controlled hypoxic environment. Lab Chip 2012, 12, 4855–4863. [Google Scholar] [CrossRef] [PubMed]
- Fort, D.G.; Herr, T.M.; Shaw, P.L.; Gutzman, K.E.; Starren, J.B. Mapping the evolving definitions of translational research. J. Clin. Transl. Sci. 2017, 1, 60–66. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Sousa, P.C.; Gaspar, J.; Bañobre-López, M.; Lima, R.; Minas, G. Organ-on-a-chip: A preclinical microfluidic platform for the progress of nanomedicine. Small 2020, 16, 2003517. [Google Scholar] [CrossRef]
- Wang, K.; Man, K.; Liu, J.; Liu, Y.; Chen, Q.; Zhou, Y.; Yang, Y. Microphysiological systems: Design, fabrication, and applications. ACS Biomater. Sci. Eng. 2020, 6, 3231–3257. [Google Scholar] [CrossRef]
- Cecen, B.; Bal-Ozturk, A.; Yasayan, G.; Alarcin, E.; Kocak, P.; Tutar, R.; Kozaci, L.D.; Shin, S.R.; Miri, A.K. Selection of natural biomaterials for micro-tissue and organ-on-chip models. J. Biomed. Mater. Res. Part A 2022, 110, 1147–1165. [Google Scholar] [CrossRef]
- Wu, H.; Shi, S.; Liu, Y.; Zhang, Q.; Lam, R.H.; Lim, C.T.; Hu, J. Recent progress of organ-on-a-chip towards cardiovascular diseases: Advanced design, fabrication, and applications. Biofabrication 2023, 15, 042001. [Google Scholar] [CrossRef]
- Virumbrales-Muñoz, M.; Ayuso, J.M. From microfluidics to microphysiological systems: Past, present, and future. Organs-on-a-Chip 2022, 4, 100015. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Saeb, M.R.; Stadler, F.J.; Yazdi, M.K.; Nezhad, M.N.; Mohebbi, S.; Seidi, F.; Ganjali, M.R.; Mozafari, M. Human Organs-on-Chips: A Review of the State-of-the-Art, Current Prospects, and Future Challenges. Adv. Biol. 2022, 6, 2000526. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef]
- Busek, M.; Aizenshtadt, A.; Koch, T.; Frank, A.; Delon, L.; Martinez, M.A.; Golovin, A.; Dumas, C.; Stokowiec, J.; Gruenzner, S.; et al. Pump-less, recirculating organ-on-a-chip (rOoC) platform. Lab Chip 2023, 23, 591–608. [Google Scholar] [CrossRef]
- National Institute for Health Research. PROSPERO: International Prospective Register of Systematic Reviews. Registration Number: CRD42022352569. 2022. Available online: https://www.crd.york.ac.uk/PROSPERO/view/CRD42022352569 (accessed on 23 June 2025).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Santos, C.M.d.C.; Pimenta, C.A.d.M.; Nobre, M.R.C. A estratégia PICO para a construção da pergunta de pesquisa e busca de evidências. Rev. Lat.-Am. Enferm. 2007, 15, 508–511. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Hong, C.C. A disposable emulsion droplet generation lab chips driven by vacuum module for manipulation of blood cells. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milano, Italy, 25–29 August 2015; pp. 8010–8013. [Google Scholar]
- Yeh, C.C.; Goh, A.; Lei, K.F. Development of a Microfluidic Chip for 3D Cancer Cell Migration Assay. In Proceedings of the 2019 IEEE 13th International Conference on Nano/Molecular Medicine & Engineering (NANOMED), Gwangju, Republic of Korea, 21–24 November 2019; pp. 167–170. [Google Scholar]
- Ko, P.L.; Lee, T.A.; Hsu, H.H.; Liao, W.H.; Tung, Y.C. Development of in Vitro Microfluidic Circulatory System. In Proceedings of the 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS), Vancouver, BC, Canada, 18–22 January 2020; pp. 1028–1031. [Google Scholar]
- Ma, H.L.; Urbaczek, A.C.; Zeferino Ribeiro de Souza, F.; Augusto Gomes Garrido Carneiro Leão, P.; Rodrigues Perussi, J.; Carrilho, E. Rapid fabrication of microfluidic devices for biological mimicking: A survey of materials and biocompatibility. Micromachines 2021, 12, 346. [Google Scholar] [CrossRef]
- Roy, P.; Howladar, P.; Dastidar, R.; Rahaman, H.; Dasgupta, P. 3D integration in biochips: New proposed architectures for 3D applications in ATDA based digital microfluidic biochips. In Proceedings of the 2015 10th International Conference on Design & Technology of Integrated Systems in Nanoscale Era (DTIS), Napoli, Italy, 21–23 April 2015; pp. 1–6. [Google Scholar]
- Kato, Y.; Hirai, Y.; Kamei, K.; Tsuchiya, T.; Tabata, O. Microfluidic device to interconnect multiple organs via fluidic circulation: Towards body-on-a-chip. In Proceedings of the 2015 Transducers-2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, AK, USA, 21–25 June 2015; pp. 1549–1552. [Google Scholar]
- Ono, T.; Fujii, T.; Sakai, Y.; Kimura, H. Development of organs-on-a-chip with metabolism model. In Proceedings of the 2016 International Symposium on Micro-NanoMechatronics and Human Science (MHS), Nagoya, Japan, 28–30 November 2016; pp. 1–3. [Google Scholar]
- Hipolito, T.M.; Rodrigues, L.R.; da Silva, G.C.; del Conte, E.G. Additive manufacturing of microfluidic devices. IEEE Lat. Am. Trans. 2016, 14, 4652–4656. [Google Scholar] [CrossRef]
- Poorreza, E.; Vafaie, R.H.; Tayyebi, A.; Ghavifekr, H.B. A miniaturized electrokinetic bioparticle microseparator based on travelling wave dielectrophoresis for lab on a chip applications. In Proceedings of the 2013 First RSI/ISM International Conference on Robotics and Mechatronics (ICRoM), Tehran, Iran, 13–15 February 2013; pp. 540–545. [Google Scholar]
- Kyung, R.; Zhao, A. Biomechanics of Bio-Fluid in the Microfluidic Channels Using Computer Simulations. In Proceedings of the 2020 IEEE International IOT, Electronics and Mechatronics Conference (IEMTRONICS), Vancouver, BC, Canada, 9–12 September 2020; pp. 1–4. [Google Scholar]
- Clement, E.; Knowlton, S.; Mandelkern, T.; Tasoglu, S. Engineering a microfluidic organ model using 3-dimensional micropatterned cellular constructs. In Proceedings of the 2015 41st Annual Northeast Biomedical Engineering Conference (NEBEC), Troy, NY, USA, 17–19 April 2015; pp. 1–2. [Google Scholar]
- Zhang, Y.S. Modular multi-organ-on-chips platform with physicochemical sensor integration. In Proceedings of the 2017 IEEE 60th International Midwest Symposium on Circuits and Systems (MWSCAS), Boston, MA, USA, 6–9 August 2017; pp. 80–83. [Google Scholar]
- Gaio, N.; Waafi, A.; Vlaming, M.; Boschman, E.; Dijkstra, P.; Nacken, P.; Braam, S.; Boucsein, C.; Sarro, P.M.; Dekker, R. A multiwell plate Organ-on-Chip (OOC) device for in-vitro cell culture stimulation and monitoring. In Proceedings of the 2018 IEEE Micro Electro Mechanical Systems (MEMS), Belfast, UK, 21–25 January 2018; pp. 314–317. [Google Scholar]
- Bischel, L.L.; Young, E.W.; Mader, B.R.; Beebe, D.J. Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials 2013, 34, 1471–1477. [Google Scholar] [CrossRef]
- Mastrangeli, M.; van den Eijnden-van Raaij, J. Organs-on-chip: The way forward. Stem Cell Rep. 2021, 16, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; Oelgeschlaeger, M.; Burgdorf, T.; van Meer, P.; Theunissen, P.; Kienhuis, A.S.; Piersma, A.H.; Vandebriel, R.J. Applicability of organ-on-chip systems in toxicology and pharmacology. Crit. Rev. Toxicol. 2021, 51, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Park, S.E.; Huh, D.D. Organ-on-a-chip technology for nanoparticle research. Nano Converg. 2021, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Dornhof, J.; Kieninger, J.; Muralidharan, H.; Maurer, J.; Urban, G.A.; Weltin, A. Microfluidic organ-on-chip system for multi-analyte monitoring of metabolites in 3D cell cultures. Lab Chip 2022, 22, 225–239. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Aleman, J.; Shin, S.R.; Kilic, T.; Kim, D.; Mousavi Shaegh, S.A.; Massa, S.; Riahi, R.; Chae, S.; Hu, N.; et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, E2293–E2302. [Google Scholar] [CrossRef]
- Kilic, T.; Navaee, F.; Stradolini, F.; Renaud, P.; Carrara, S. Organs-on-chip monitoring: Sensors and other strategies. Microphysiol. Syst. 2018, 2, 1–32. [Google Scholar] [CrossRef]
- Emmerich, M.; Ebner, P.; Wille, R. Design Automation for Organs-on-Chip. In Proceedings of the 2024 Design, Automation & Test in Europe Conference & Exhibition (DATE), Valencia, Spain, 25–27 March 2024; pp. 1–6. [Google Scholar]
- Emmerich, M.; Ebner, P.; Wille, R. Automated Design for Multi-Organ-on-Chip Geometries. IEEE Trans. Comput.-Aided Des. Integr. Circuits Syst. 2024, 44, 2287–2299. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, C.; Wang, X. Innovative Stamp-Structured Organ-on-a-Chip Platform for Vascularized Tumor and Colon Models. In Proceedings of the 2025 IEEE 38th International Conference on Micro Electro Mechanical Systems (MEMS), Kaohsiung, Taiwan, 19–23 January 2025; pp. 531–534. [Google Scholar]
- Xu, X.; Chen, C.Y.; Wen, Z.; Young, O.M.; Felix, B.M.; Bandyopadhyay, B.C.; Bentley, W.E.; Sochol, R.D. 3D-Microprinted PDMS-Based Microfluidic Vessels for Organ-on-a-Chip Applications. In Proceedings of the 2024 IEEE 37th International Conference on Micro Electro Mechanical Systems (MEMS), Austin, TX, USA, 21–25 January 2024; pp. 221–224. [Google Scholar]
- Brusina, K.E.; Nikiforov, A.I.; Fomina, E.A.; Testov, D.O.; Gareev, K.G.; Sitkov, N.O. Assessing the Propagation of Magnetic Nanoparticles in a Microfluidic Channel and their Behavior at the Suspension-Hydrogel Interface for On-Chip Modeling of Organs and Tissues. In Proceedings of the 2024 Conference of Young Researchers in Electrical and Electronic Engineering (ElCon), St. Petersburg, Russia, 29–31 January 2024; pp. 937–941. [Google Scholar]
| Study | Device Type | Droplet Diameter (µm) | Flow Rate (µL/s) | Cell Type | Shear Stress (dyn/cm2) | Culture Duration (h) |
|---|---|---|---|---|---|---|
| Lee & Hong, 2015 [19] | Droplet generator chip | 200–240 | ≈0.0375 | Blood cells | Not reported | Not reported |
| Yeh et al., 2019 [20] | 3D migration chip | Not reported | Not reported | HeLa-GFP | Variable (IL-6-induced) | Not reported |
| Ko et al., 2020 [21] | Blood–air barrier chip | Not reported | Simulated (pulsatile) | A549 + HUVEC | Pulsatile flow | 24 |
| Ma et al., 2021 [22] | Shear stress microchip | Not reported | Controlled | Endothelial | Present | 24 |
| Roy et al., 2015 [23] | 3D microfluidic biochip | Not reported | Not reported | Not specified | Not reported | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, G.A.M.d.A.; da Silva, A.K.A.; Faria, R.M.; Santos, K.S.; Aguiar, A.d.C.; Barreto Mota da Costa, L.; Luz, G.V.d.S.; Carneiro, M.L.B.; Rosa, M.F.F.; Joanitti, G.A.; et al. State-of-the-Art Organ-on-Chip Models and Designs for Medical Applications: A Systematic Review. Biomimetics 2025, 10, 524. https://doi.org/10.3390/biomimetics10080524
Nunes GAMdA, da Silva AKA, Faria RM, Santos KS, Aguiar AdC, Barreto Mota da Costa L, Luz GVdS, Carneiro MLB, Rosa MFF, Joanitti GA, et al. State-of-the-Art Organ-on-Chip Models and Designs for Medical Applications: A Systematic Review. Biomimetics. 2025; 10(8):524. https://doi.org/10.3390/biomimetics10080524
Chicago/Turabian StyleNunes, Gustavo Adolfo Marcelino de Almeida, Ana Karoline Almeida da Silva, Rafael Mendes Faria, Klériston Silva Santos, Arthur da Costa Aguiar, Lindemberg Barreto Mota da Costa, Glécia Virgolino da Silva Luz, Marcella Lemos Brettas Carneiro, Mário Fabrício Fleury Rosa, Graziella Anselmo Joanitti, and et al. 2025. "State-of-the-Art Organ-on-Chip Models and Designs for Medical Applications: A Systematic Review" Biomimetics 10, no. 8: 524. https://doi.org/10.3390/biomimetics10080524
APA StyleNunes, G. A. M. d. A., da Silva, A. K. A., Faria, R. M., Santos, K. S., Aguiar, A. d. C., Barreto Mota da Costa, L., Luz, G. V. d. S., Carneiro, M. L. B., Rosa, M. F. F., Joanitti, G. A., Ibiapina, K. M., Gomes, A. K. G. d. B., da Rocha, A. F., & Fleury Rosa, S. d. S. R. (2025). State-of-the-Art Organ-on-Chip Models and Designs for Medical Applications: A Systematic Review. Biomimetics, 10(8), 524. https://doi.org/10.3390/biomimetics10080524







