Silanised Fluoride Hydrotalcites as Functional and Multicomponent Fillers for Dental Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of HTlc in Nitrate Form (HTlc-NO3)
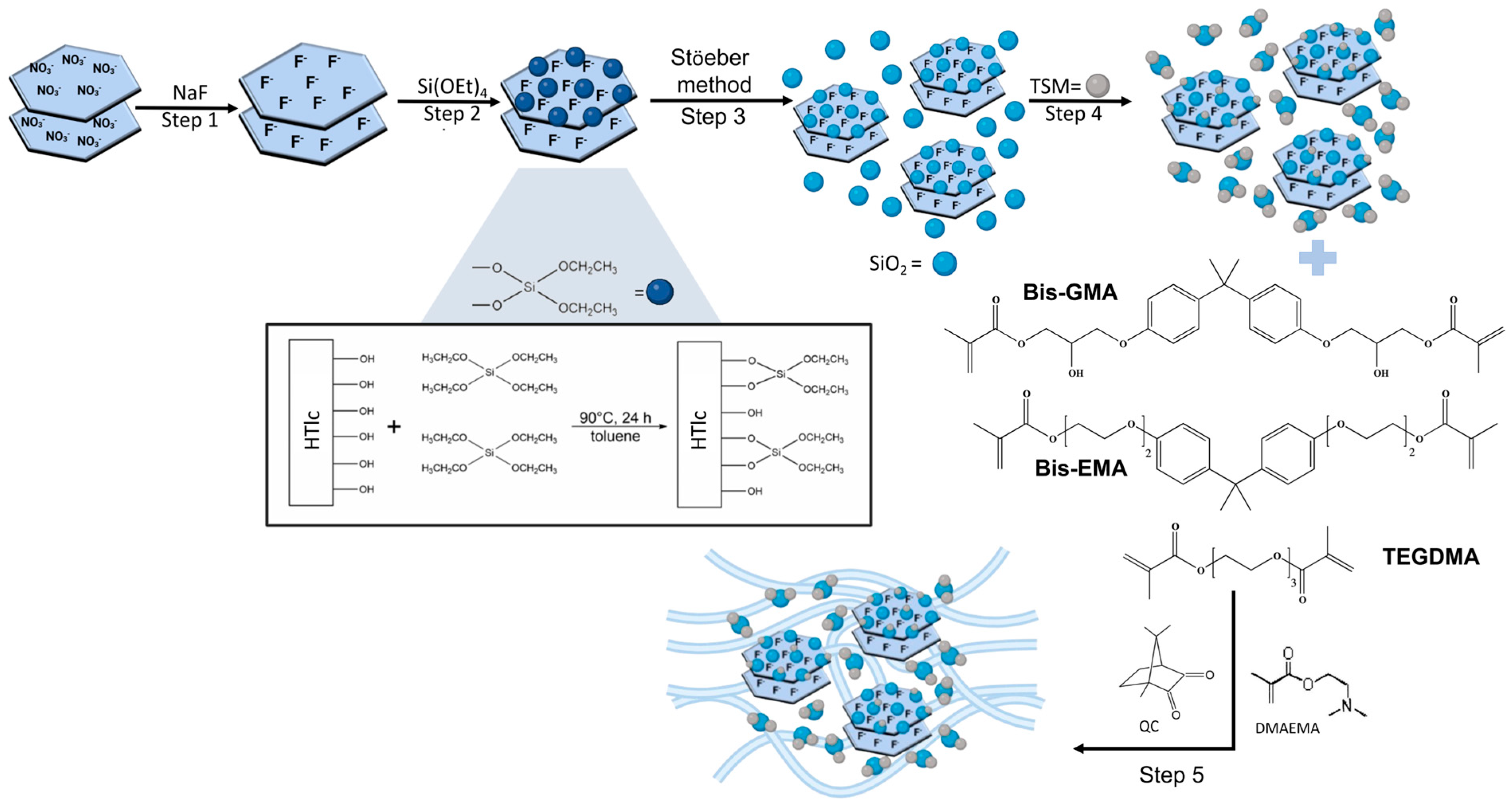
2.3. Preparation of HTlc/F
2.4. Silanisation of the HTlc/F Surface
2.5. Precipitation of Silica Nanoparticles on Silanisated HTlc/F by Stöeber Method
2.6. Surface Functionalisation of SiO2@HTlc/F with TSM
2.7. Synthesis of Acrylic Resin and Composite Resins
2.8. In Vitro Release Studies
2.9. Degree of Conversion (DC)
2.10. Instrumentation
3. Results and Discussion
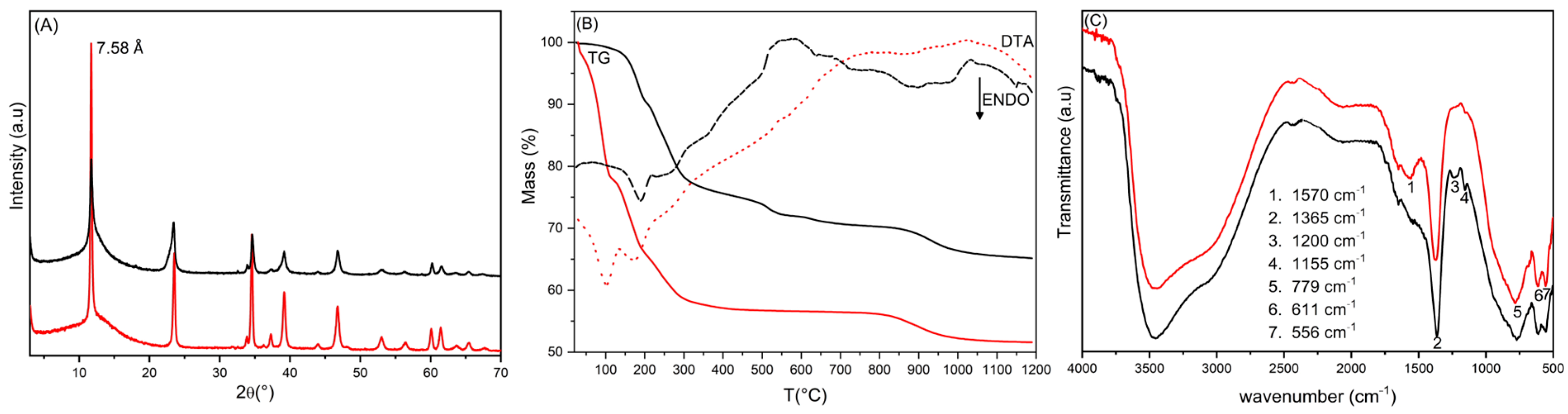
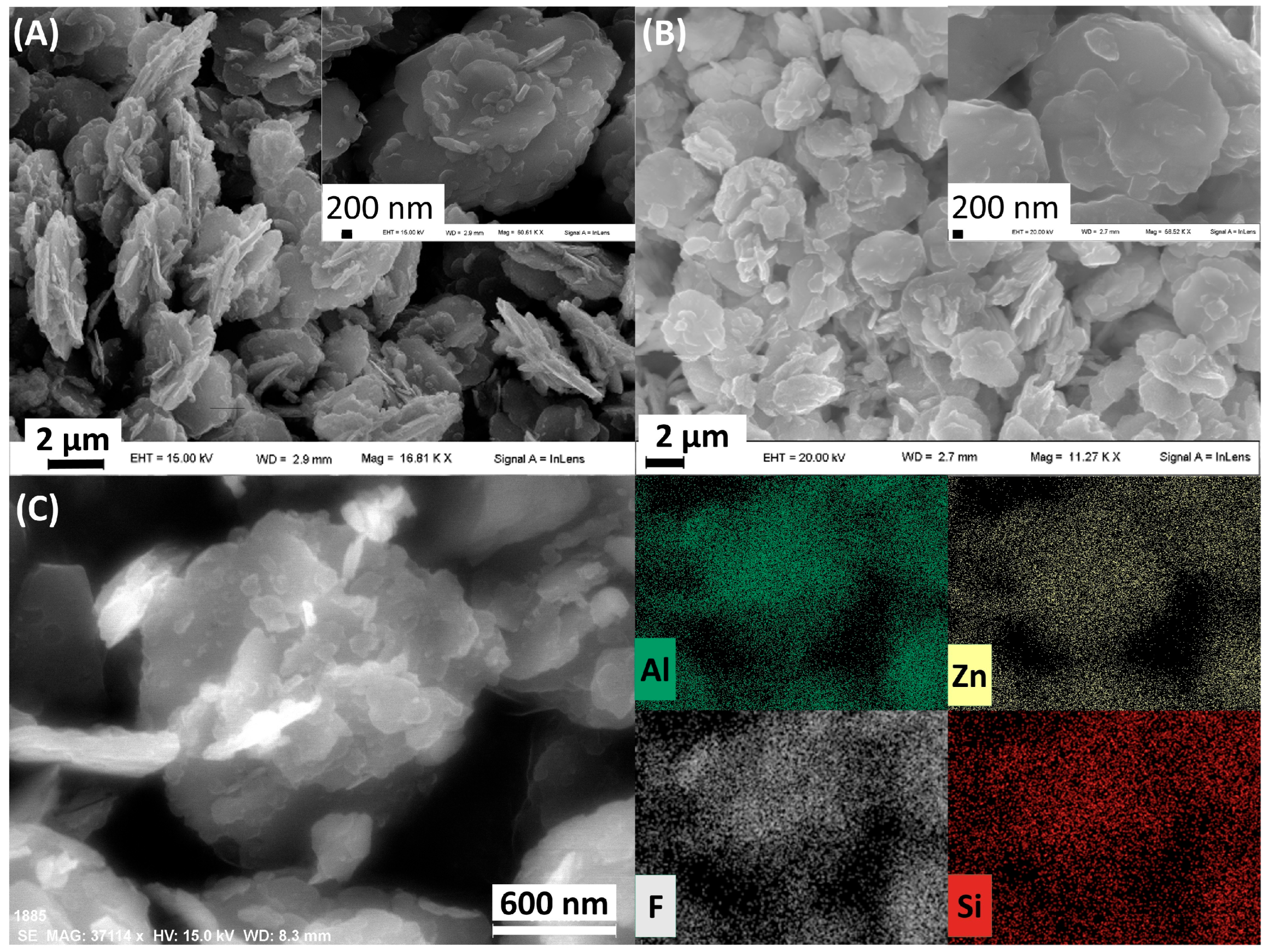
3.1. Silanisated HTlc/F (Steps 1 and 2)
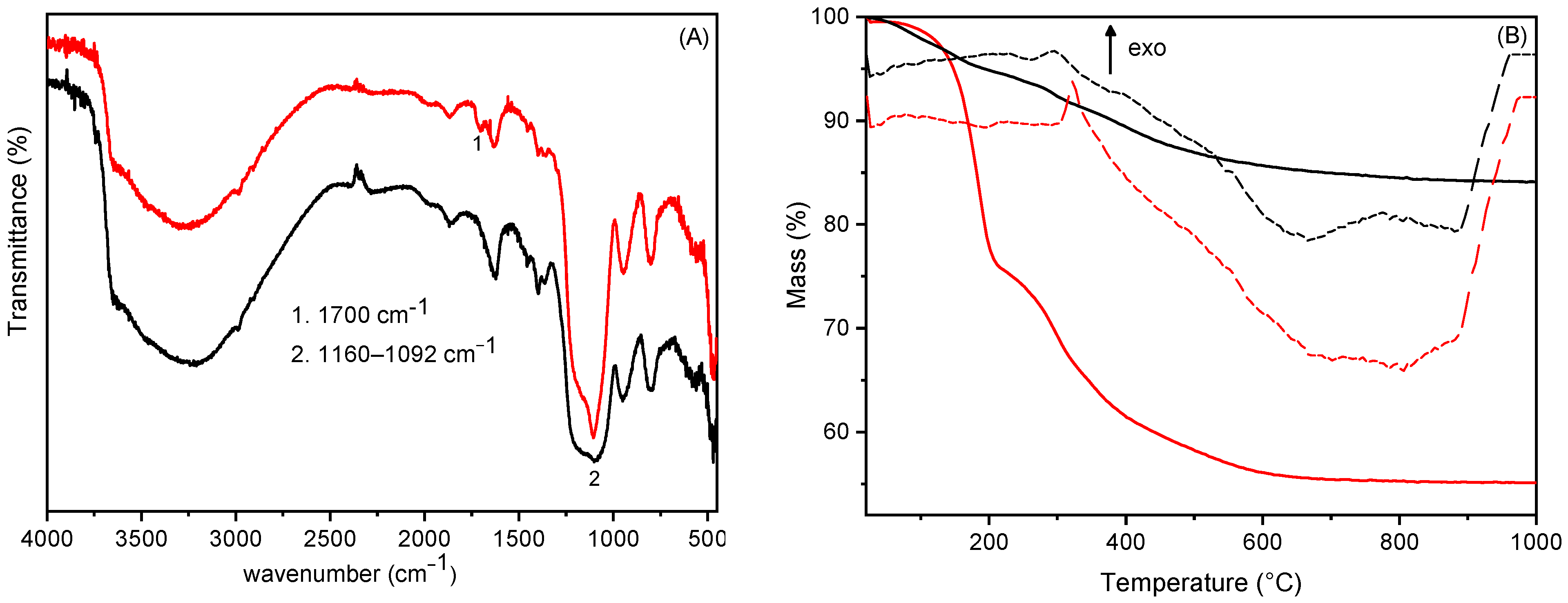
3.2. Silica Growth on Silanisated HTlc/F and Functionalisation with TSM (Steps 3 and 4)
3.3. Preparation of Acrylic Resin Composites Containing TSM/SiO2@HTlc/F as a Filler (Step 5)
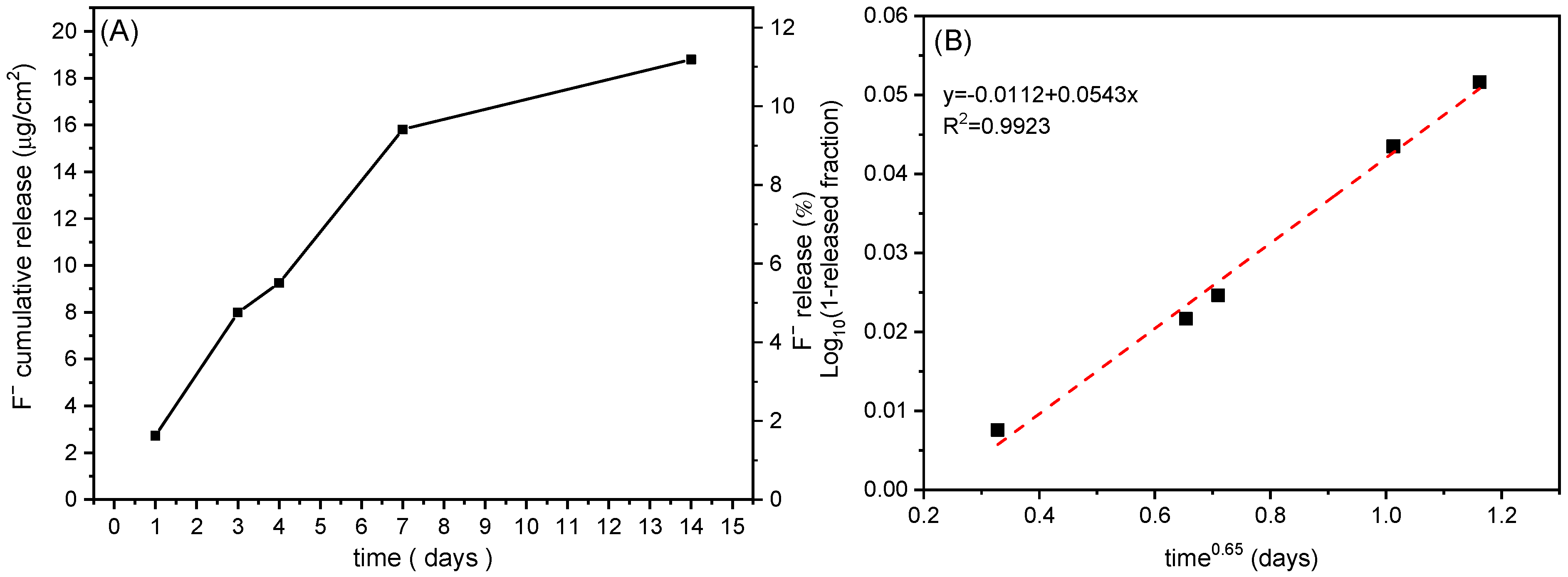
3.4. Release Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerdolle, D.A.; Mortier, E.; Droz, D. Microleakage and polymerization shrinkage of various polymer restorative materials. J. Dent. Child. 2008, 75, 125–133. [Google Scholar]
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Habib, E.; Wang, R.; Wang, Y.; Zhu, M.; Zhu, X.X. Inorganic fillers for dental resin composites: Present and future. ACS Biomater. Sci. Eng. 2016, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nourian, A.; Rostamizadeh, K.; Haghi, F.; Aghajanzadeh, M. Fabrication of acrylic nanocomposites containing different percentages of TiO2 nanoparticles and their antimicrobial activity compared to other mineral nanoparticles. Int. J. Mol. Clin. Microbiol. 2024, 14, 1981–1990. [Google Scholar]
- Kazemi, M.; Navarchian, A.H.; Ahangaran, F. Effects of silica surface modification with silane and poly (ethylene glycol) on flexural strength, protein-repellent, and antibacterial properties of acrylic dental nanocomposites. Dent. Mater. 2023, 39, 863–871. [Google Scholar] [CrossRef]
- Karkanis, S.; Nikolaidis, A.K.; Koulaouzidou, E.A.; Achilias, D.S. Effect of silica nanoparticles silanized by functional/functional or functional/non-functional silanes on the physicochemical and mechanical properties of dental nanocomposite resins. Appl. Sci. 2021, 12, 159. [Google Scholar] [CrossRef]
- Nikolaidis, A.K.; Koulaouzidou, E.A.; Gogos, C.; Achilias, D.S. Synthesis of novel dental nanocomposite resins by incorporating polymerizable, quaternary ammonium silane-modified silica nanoparticles. Polymers 2021, 13, 1682. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, M.; Zhu, X.X. Functional fillers for dental resin composites. Acta Biomater. 2021, 122, 50–65. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Bahador, A. Effects of incorporation of nanoparticles into dental acrylic resins on antimicrobial and physico-mechanical properties: A meta-analysis of in vitro studies. J. Oral Biol. Craniofacial Res. 2022, 12, 557–568. [Google Scholar] [CrossRef]
- Mankar, N.; Kumbhare, S.; Nikhade, P.; Mahapatra, J.; Agrawal, P. Role of fluoride in dentistry: A narrative review. Cureus 2023, 15, e50884. [Google Scholar] [CrossRef]
- Piszko, P.J.; Piszko, A.; Kiryk, J.; Lubojański, A.; Dobrzyński, W.; Wiglusz, R.J.; Matys, J.; Dobrzyński, M. The influence of fluoride gels on the physicochemical properties of tooth tissues and dental materials—A systematic review. Gels 2024, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Nocchetti, M.; Costantino, U. Progress in Layered Double Hydroxides from Synthesis to New Applications. In Series on Chemistry, Energy and the Environment; Kadish, K.M., Guilard, R., Eds.; World Scientific Publishing: Singapore, 2022; Volume 8. [Google Scholar]
- Costantino, U.; Vivani, R.; Bastianini, M.; Costantino, F.; Nocchetti, M. Ion exchange and intercalation properties of layered double hydroxides towards halide anions. Dalton Trans. 2014, 43, 11587–11596. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, A.; Karpukhina, N.; Gillam, D.G.; Bushby, A.J.; Patel, M.P. Fluoride rechargeable layered double hydroxide powders for dental applications. Appl. Clay Sci. 2021, 200, 105863. [Google Scholar] [CrossRef]
- Hoxha, A.; Gillam, D.G.; Agha, A.; Karpukhina, N.; Bushby, A.J.; Patel, M.P. Novel fluoride rechargeable dental composites containing MgAl and CaAl layered double hydroxide (LDH). Dent. Mater. 2020, 36, 973–986. [Google Scholar] [CrossRef]
- Hoxha, A.; Gillam, D.G.; Bushby, A.J.; Agha, A.; Patel, M.P. Layered double hydroxide fluoride release in dental applications: A systematic review. Dent. J. 2019, 7, 87. [Google Scholar] [CrossRef]
- Tammaro, L.; Vittoria, V.; Calarco, A.; Petillo, O.; Riccitiello, F.; Peluso, G. Effect of layered double hydroxide intercalated with fluoride ions on the physical, biological and release properties of a dental composite resin. J. Dent. 2014, 42, 60–67. [Google Scholar] [CrossRef]
- Calarco, A.; Di Salle, A.; Tammaro, L.; De Luca, I.; Mucerino, S.; Petillo, O.; Riccitiello, F.; Vittoria, V.; Peluso, G. Long-term fluoride release from dental resins affects STRO-1+ cell behavior. J. Dent. Res. 2015, 94, 1099–1105. [Google Scholar] [CrossRef]
- Su, L.W.; Lin, D.J.; Uan, J.Y. Novel dental resin composites containing LiAl-F layered double hydroxide (LDH) filler: Fluoride release/recharge, mechanical properties, color change, and cytotoxicity. Dent. Mater. 2019, 35, 663–672. [Google Scholar] [CrossRef]
- Bini, M.; Ambrogi, V.; Donnadio, A.; Di Michele, A.; Ricci, P.; Nocchetti, M. Layered double hydroxides intercalated with fluoride and methacrylate anions as multifunctional filler of acrylic resins for dental composites. App. Clay Sci. 2020, 197, 105796. [Google Scholar] [CrossRef]
- Tammaro, L.; Di Salle, A.; Calarco, A.; De Luca, I.; Riccitiello, F.; Peluso, G.; Vittoria, V.; Sorrentino, A. Multifunctional Bioactive Resin for Dental Restorative Materials. Polymers 2020, 12, 332. [Google Scholar] [CrossRef]
- Di Michele, A.; Boccalon, E.; Costantino, F.; Bastianini, M.; Vivani, R.; Nocchetti, M. Insight into the synthesis of LDH using the urea method: Morphology and intercalated anion control. Dalton Trans. 2024, 53, 12543–12553. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.M.; Choi, S.J.; Lee, G.E.; Han, S.H.; Choy, J.H. Inorganic drug-delivery nanovehicle conjugated with cancer-cell-specific ligand. Adv. Funct. Mater. 2009, 19, 1617–1624. [Google Scholar] [CrossRef]
- Stöeber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Bogush, G.H.; Tracy, M.A.; Zukoski IV, C.F. Preparation of monodisperse silica particles: Control of size and mass fraction. J. Non Cryst. Solids 1988, 104, 95–106. [Google Scholar] [CrossRef]
- Ambrogi, V.; Pietrella, D.; Marmottini, F.; Riva, F.; Tiralti, M.C.; Ricci, M. Chlorhexidine-loaded functionalized mesoporous MCM-41 poly (methylmethacrylate) based composites with Candida antibiofilm activity. RSC Adv. 2015, 5, 84827–84835. [Google Scholar] [CrossRef]
- Pagano, C.; Perioli, L.; Marmottini, F.; Ramella, D.; Tiralti, M.C.; Ricci, M. Dentifrice Based on Fluoride–Hydrotalcite Compounds: Characterization and Release Capacity Evaluation by Novel In Vitro Methods. AAPS PharmSciTech 2019, 20, 248. [Google Scholar] [CrossRef]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials 2002, 23, 1819–1829. [Google Scholar] [CrossRef]
- Chen, C.; Buffet, J.C.; O’Hare, D. Surface modification of aqueous miscible organic layered double hydroxides (AMO-LDHs). Dalton Trans. 2020, 49, 8498–8503. [Google Scholar] [CrossRef]
- Samuel, S.P.; Li, S.; Mukherjee, I.; Guo, Y.; Patel, A.C.; Baran, G.; Wei, Y. Mechanical properties of experimental dental composites containing a combination of mesoporous and nonporous spherical silica as fillers. Dent. Mater. 2009, 25, 296–301. [Google Scholar] [CrossRef]
- Roman, A.; Páll, E.; Moldovan, M.; Rusu, D.; Şoriţău, O.; Feştilă, D.; Lupşe, M. Cytotoxicity of Experimental Resin Composites on Mesenchymal Stem Cells Isolated from Two Oral Sources. Microsc. Microanal. 2016, 22, 1018–1033. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M. Structure–property relationships in dimethacrylate networks based on Bis-GMA, UDMA and TEGDMA. Dent. Mater. 2009, 25, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Burgess, J.O. Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials 2003, 24, 2451–2461. [Google Scholar] [CrossRef] [PubMed]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S., Jr. The exchange adsorption of ions from aqueous solutions by organic zeolites, II: Kinetics. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, R.; Murthy, S.R.S.; Miglani, B.D.; Viswanathan, K. Novel method to evaluate diffusion controlled release of drug from resinate. Int. J. Pharm. 1986, 28, 59–66. [Google Scholar] [CrossRef]
- Ambrogi, V.; Fardella, G.; Grandolini, G.; Perioli, L.; Tiralti, M.C. Intercalation compounds of hydrotalcite-like anionic clays with anti-inflammatory agents, II: Uptake of diclofenac for a controlled release formulation. AAPS Pharmscitech 2002, 3, 77–82. [Google Scholar] [CrossRef]
- Guida, A.; Hill, R.G.; Towler, M.R.; Eramo, S. Fluoride release from model glass ionomer cements. J. Mater. Sci. Mater. Med. 2002, 13, 645–649. [Google Scholar] [CrossRef]
- Furtos, G.; Cosma, V.; Prejmerean, C.; Moldovan, M.; Brie, M.; Colceriu, A.; Vezsenyi, l.; Silaghi-dumitrescu, l.; Sirbu, C. Fluoride release from dental resin composite. Mater. Sci. Eng. C 2005, 25, 231–236. [Google Scholar] [CrossRef]
- NaKabo, S.; Torii, Y.; Itota, T.; Ishikawa, K.; Suzuki, K. Regulation of NaF release from bis-GMA/TEGDMA resin using g-methacryloxypropyltrimethoxysilane. Dent. Mater. 2002, 18, 81–87. [Google Scholar] [CrossRef]
- Imazato, S. Antibacterial properties of resin composites and dentin bonding systems. Dent. Mater. 2003, 19, 449–457. [Google Scholar] [CrossRef]
- Ebi, N.; Imazato, S.; Noiri, Y.; Ebisu, S. Inhibitory effects of resin composite containing bactericide-immobilized filler on plaque accumulation. Dent. Mater. 2001, 17, 485–491. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nocchetti, M.; Piccinini, M.; Scafuri, A.; Di Michele, A.; Ambrogi, V. Silanised Fluoride Hydrotalcites as Functional and Multicomponent Fillers for Dental Composites. Biomimetics 2025, 10, 398. https://doi.org/10.3390/biomimetics10060398
Nocchetti M, Piccinini M, Scafuri A, Di Michele A, Ambrogi V. Silanised Fluoride Hydrotalcites as Functional and Multicomponent Fillers for Dental Composites. Biomimetics. 2025; 10(6):398. https://doi.org/10.3390/biomimetics10060398
Chicago/Turabian StyleNocchetti, Morena, Michela Piccinini, Antonio Scafuri, Alessandro Di Michele, and Valeria Ambrogi. 2025. "Silanised Fluoride Hydrotalcites as Functional and Multicomponent Fillers for Dental Composites" Biomimetics 10, no. 6: 398. https://doi.org/10.3390/biomimetics10060398
APA StyleNocchetti, M., Piccinini, M., Scafuri, A., Di Michele, A., & Ambrogi, V. (2025). Silanised Fluoride Hydrotalcites as Functional and Multicomponent Fillers for Dental Composites. Biomimetics, 10(6), 398. https://doi.org/10.3390/biomimetics10060398








