Effect of Octacalcium Phosphate on Osteogenic Differentiation of Induced Pluripotent Stem Cells in a 3D Hybrid Spheroid Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Oxy Chip and Non-Oxy Chip for 3D Cell Culture
2.2. iPS Cell Culture
2.3. Three-Dimensional Culture of iPSC on Oxy Chip and Non-Oxy Chip
2.4. Culture of iPSCs in the Presence of CaP on Oxy Chip
2.4.1. Preparation of CaP Granules
2.4.2. Culture of iPSC Spheroids with CaP Granules with Different Diameters
2.5. Morphological Observation of iPSC Spheroids in 3D Culture
2.6. Measurements of DNA Concentrations and Alkaline Phosphatase (ALP) Activities of Spheroids
2.7. Measurement of Ion Concentrations in Osteogenic Medium
2.8. Calculation of Degree of Supersaturation (DS) with Respect to CaP in Osteogenic Medium
2.9. Structural Analysis of CaP Incubated in the Spheroids
2.10. Statistical Analysis
3. Results
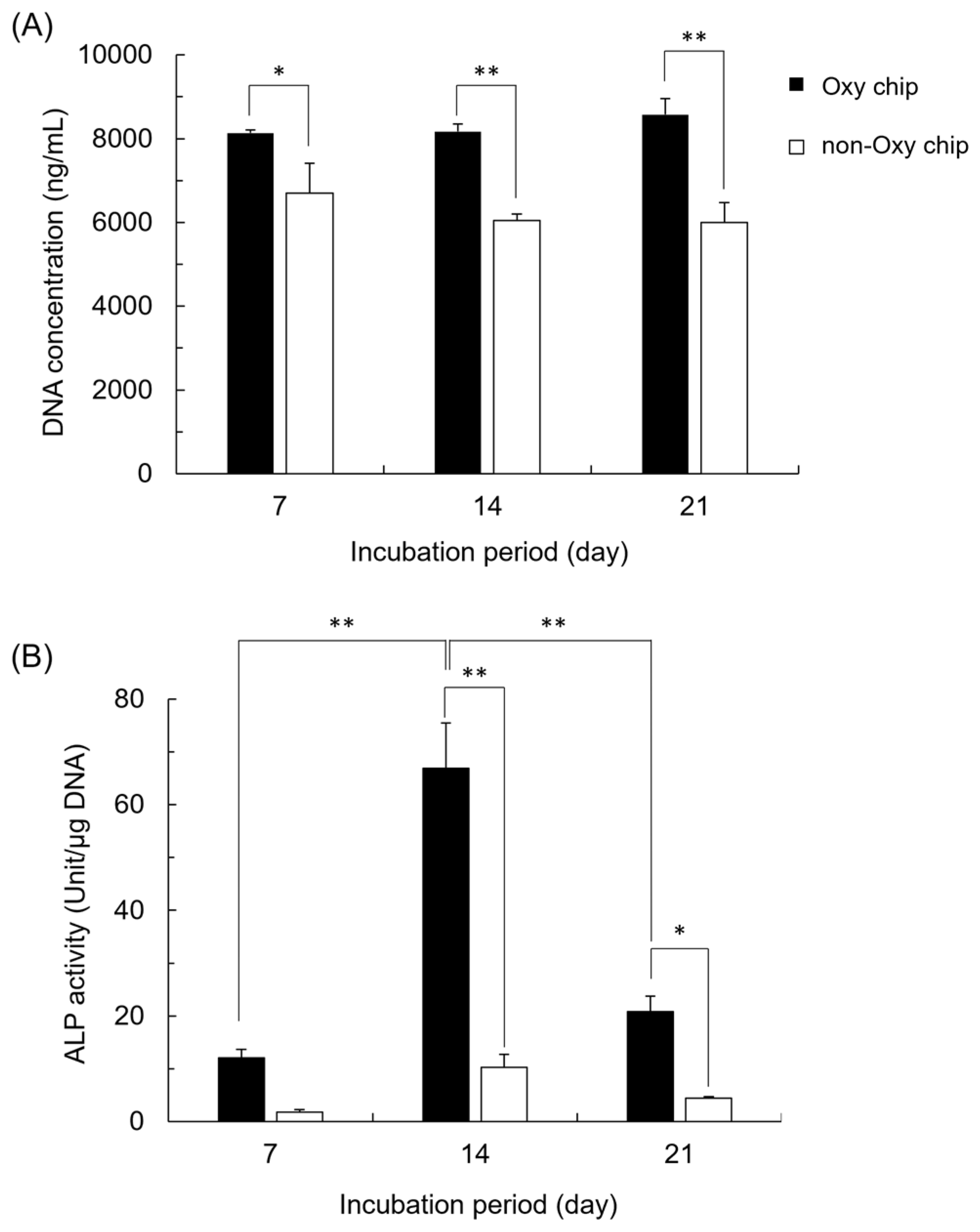
3.1. Morphological Observations of iPSCs on Oxy Chip and Non-Oxy Chip
3.2. DNA Concentration and ALP Activity of iPSCs Cultured on Oxy Chip and Non-Oxy Chip
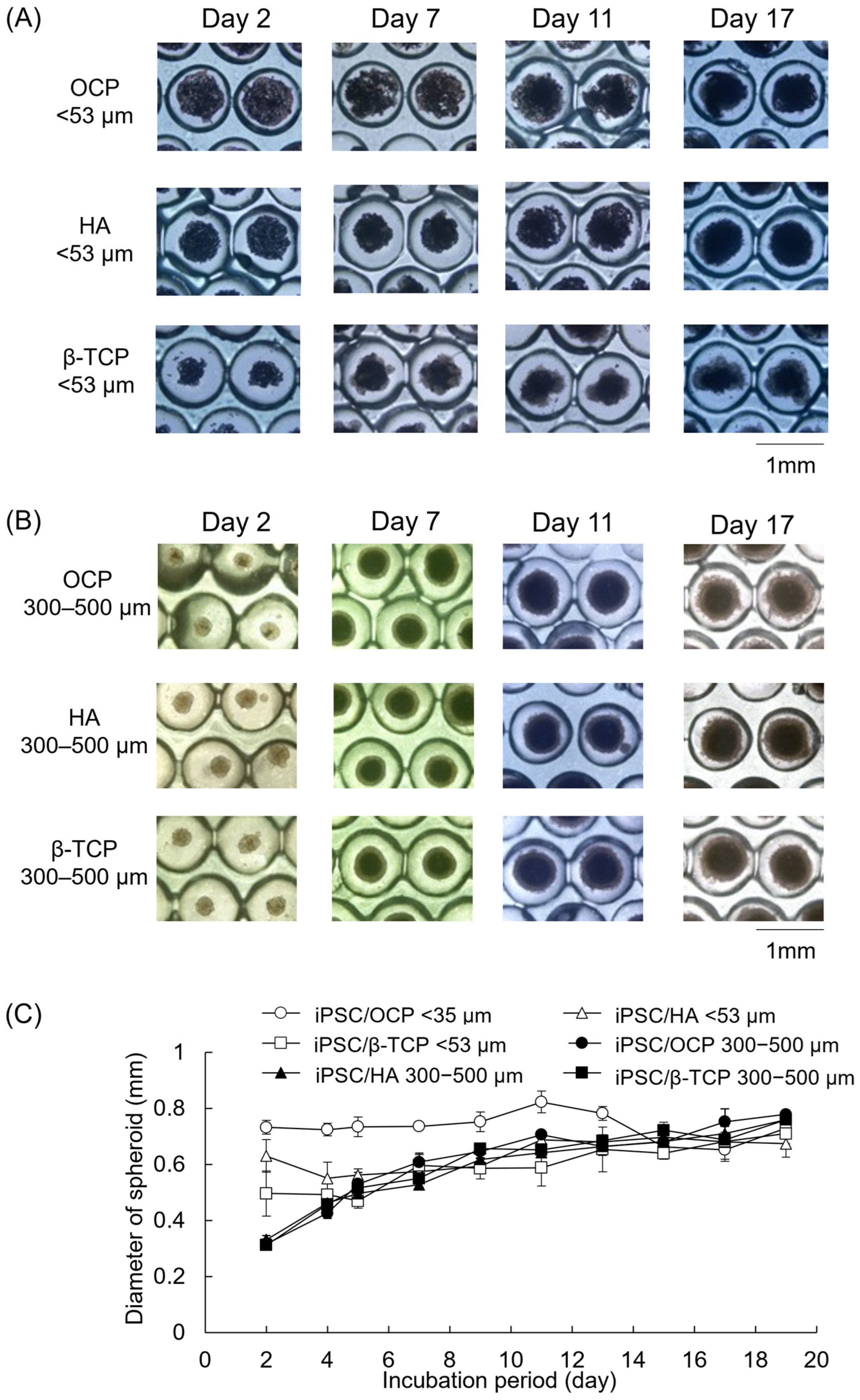
3.3. Morphological Observations of iPSCs Cultured with Various CaP Granules on Oxy Chip
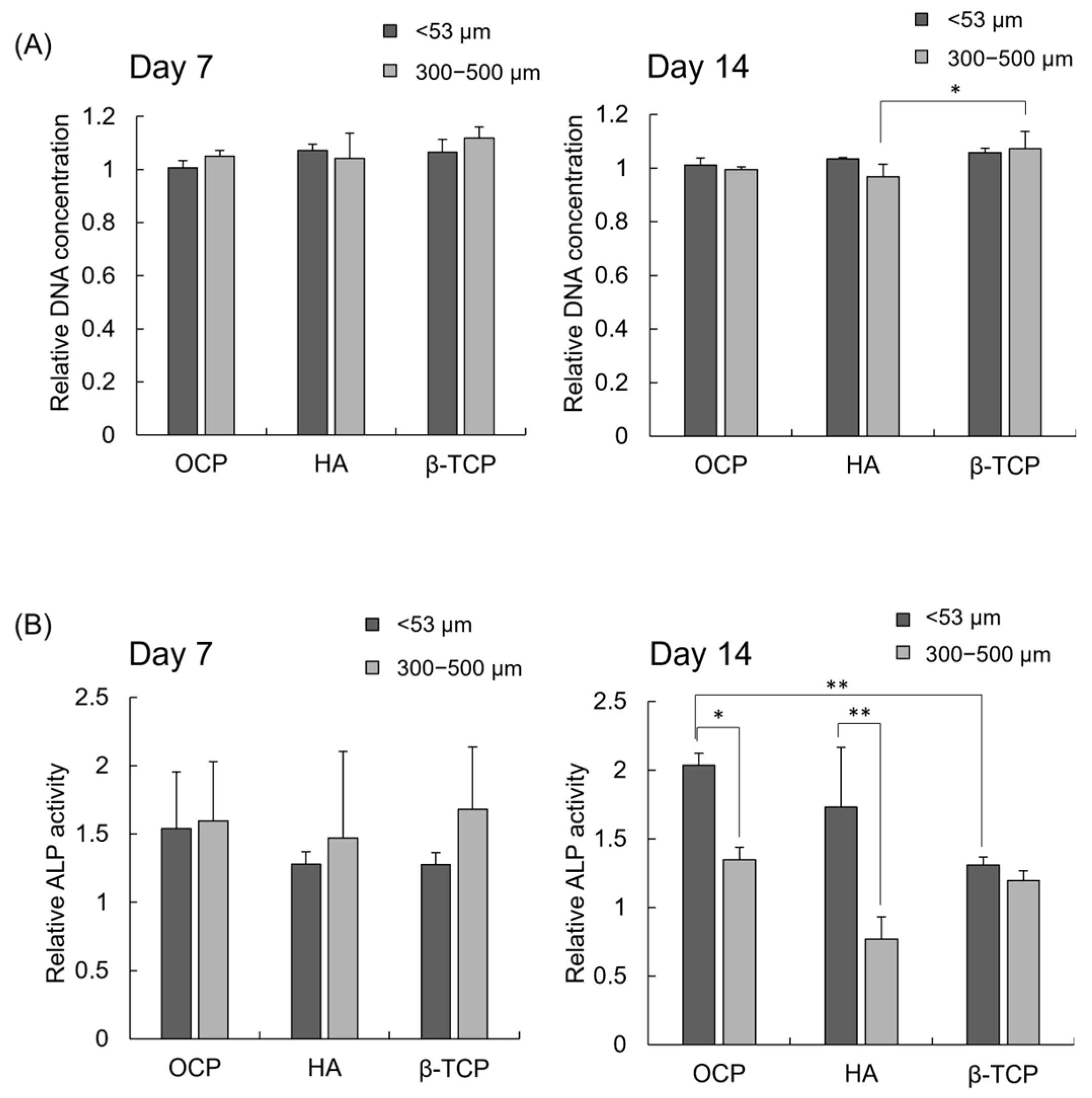
3.4. DNA Concentration and ALP Activity of iPSCs Cultured with Various CaP Granules
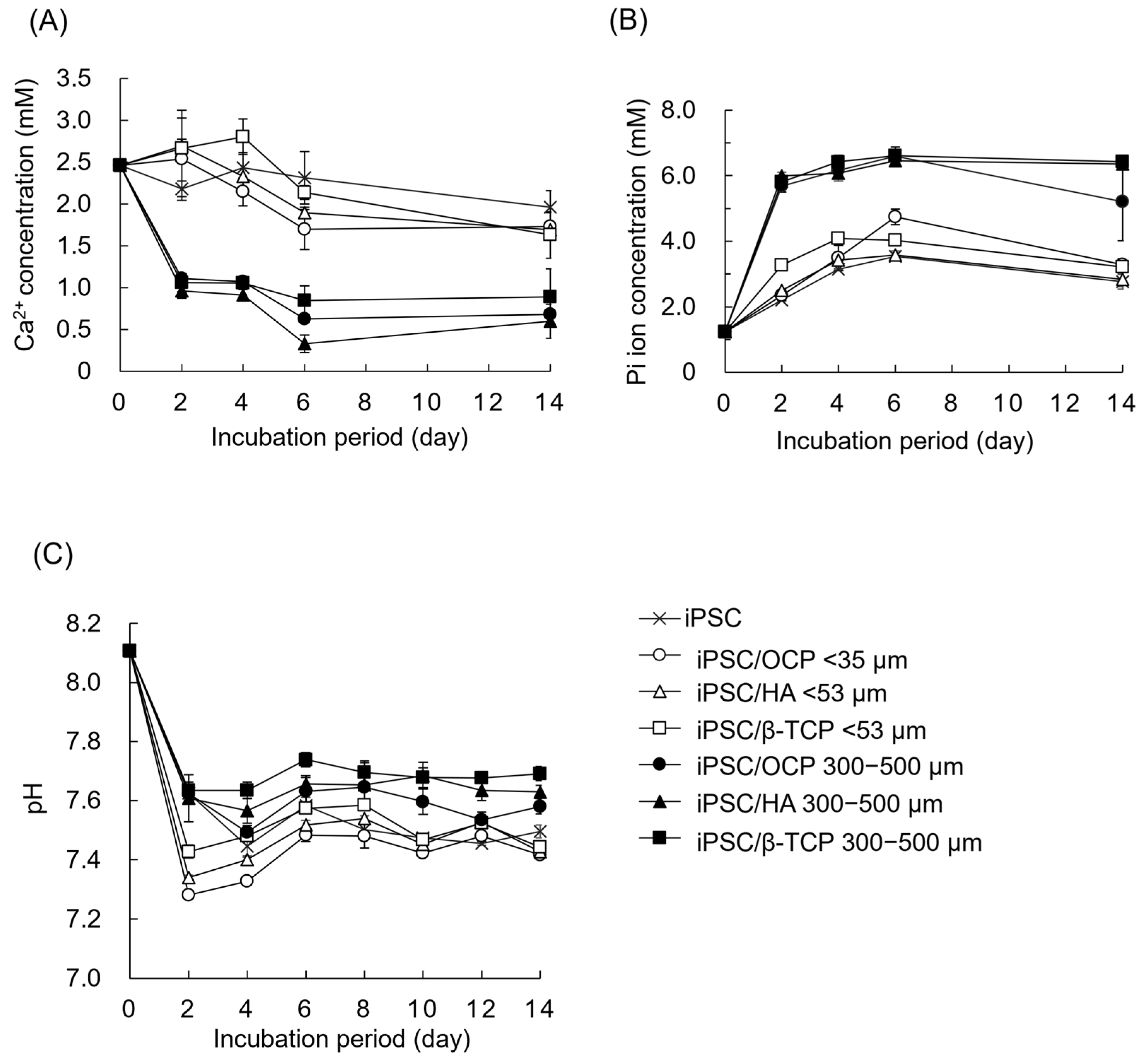
3.5. Changes in Ion Concentrations and DS with Respect to Various CaP Materials in Culture Media
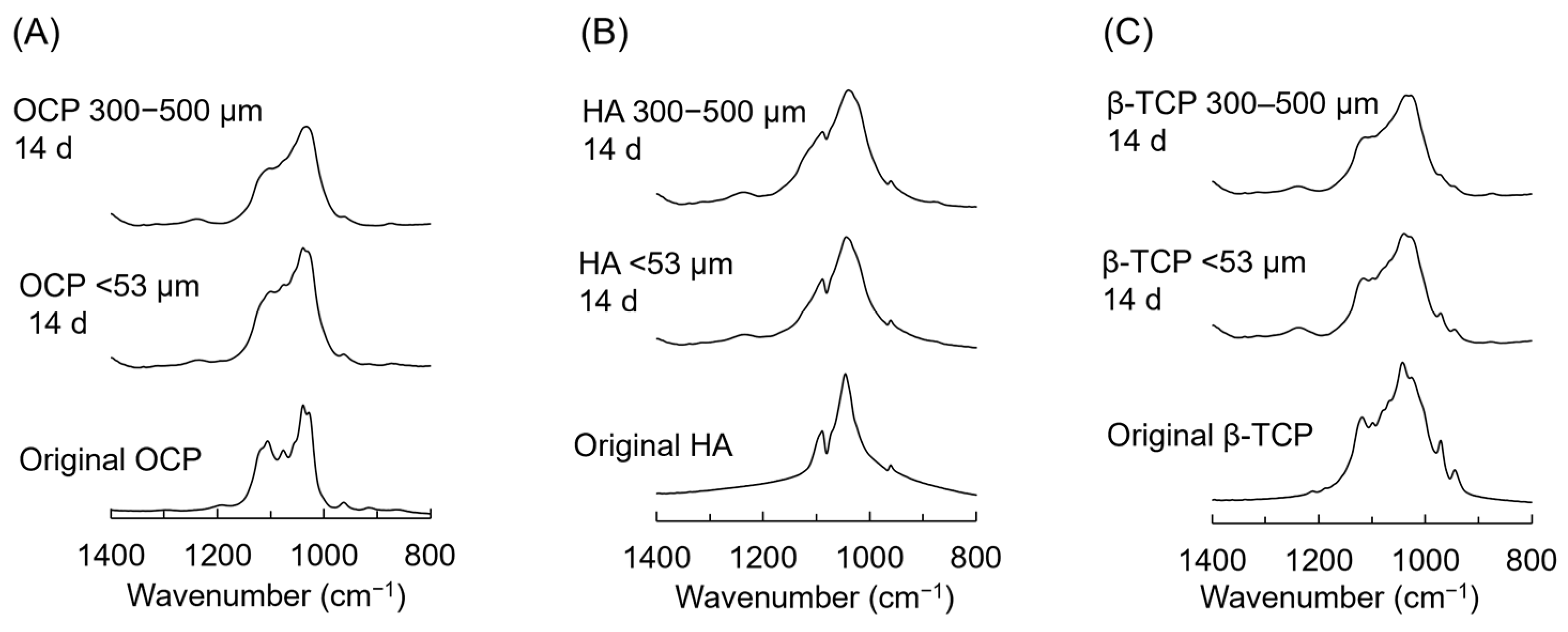
3.6. FTIR Analysis of CaP Granules Incubated with iPSC Spheroids
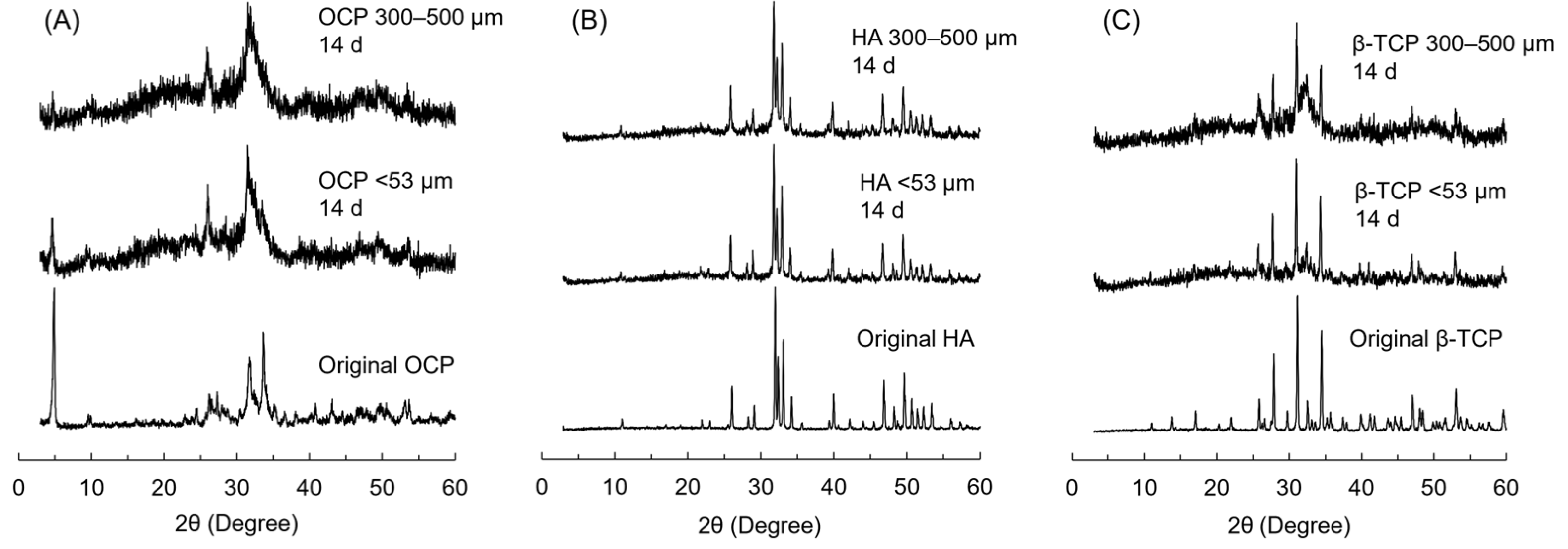
3.7. XRD Patterns of CaP Granules Incubated with iPSC Spheroids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kawai, T.; Kamakura, S.; Matsui, K.; Fukuda, M.; Takano, H.; Iino, M.; Ishikawa, S.; Kawana, H.; Soma, T.; Imamura, E.; et al. Clinical study of octacalcium phosphate and collagen composite in oral and maxillofacial surgery. J. Tissue Eng. 2020, 11, 2041731419896449. [Google Scholar] [CrossRef] [PubMed]
- Komlev, V.S.; Barinov, S.M.; Bozo, I.I.; Deev, R.V.; Eremin, I.I.; Fedotov, A.Y.; Gurin, A.N.; Khromova, N.V.; Kopnin, P.B.; Kuvshinova, E.A.; et al. Bioceramics composed of octacalcium phosphate demonstrate enhanced biological behavior. ACS Appl. Mater. Interfaces 2014, 6, 16610–16620. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, O.; Nakamura, M.; Miyasaka, Y.; Kagayama, M.; Sakurai, M. Bone formation on synthetic precursors of hydroxyapatite. Tohoku J. Exp. Med. 1991, 164, 37–50. [Google Scholar] [CrossRef]
- Suzuki, O.; Kamakura, S.; Katagiri, T.; Nakamura, M.; Zhao, B.H.; Honda, Y.; Kamijo, R. Bone formation enhanced by implanted octacalcium phosphate involving conversion into Ca-deficient hydroxyapatite. Biomaterials 2006, 27, 2671–2681. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, N.; Kishimoto, K.N.; Anada, T.; Imaizumi, H.; Itoi, E.; Suzuki, O. Effect of partial hydrolysis of octacalcium phosphate on its osteoconductive characteristics. Biomaterials 2009, 30, 1005–1014. [Google Scholar] [CrossRef]
- Sato, T.; Anada, T.; Hamai, R.; Shiwaku, Y.; Tsuchiya, K.; Sakai, S.; Baba, K.; Sasaki, K.; Suzuki, O. Culture of hybrid spheroids composed of calcium phosphate materials and mesenchymal stem cells on an oxygen-permeable culture device to predict in vivo bone forming capability. Acta Biomater. 2019, 88, 477–490. [Google Scholar] [CrossRef]
- Suzuki, O.; Hamai, R.; Sakai, S. The material design of octacalcium phosphate bone substitute: Increased dissolution and osteogenecity. Acta Biomater. 2023, 158, 1–11. [Google Scholar] [CrossRef]
- Brown, W.E.; Lehr, J.R.; Smith, J.P.; Frazier, A.W. Crystallography of octacalcium phosphate. J. Am. Chem. Soc. 1957, 79, 5318–5319. [Google Scholar] [CrossRef]
- Brown, W.E.; Smith, J.P.; Frazier, A.W.; Lehr, J.R. Crystallographic and chemical relations between octacalcium phosphate and hydroxyapatite. Nature 1962, 196, 1050–1055. [Google Scholar] [CrossRef]
- Brown, W.E. Crystal growth of bone mineral. Clin. Orthop. Relat. Res. 1966, 44, 205–220. [Google Scholar]
- Lotsari, A.; Rajasekharan, A.K.; Halvarsson, M.; Andersson, M. Transformation of amorphous calcium phosphate to bone-like apatite. Nat. Commun. 2018, 9, 4170. [Google Scholar] [CrossRef] [PubMed]
- Onuma, K.; Ito, A. Cluster growth model for hydroxyapatite. Chem. Mater. 1998, 10, 3346–3351. [Google Scholar] [CrossRef]
- Posner, A.S.; Betts, F. Synthetic amorphous calcium-phosphate and its relation to bone-mineral structure. Acc. Chem. Res. 1975, 8, 273–281. [Google Scholar] [CrossRef]
- Treboux, G.; Layrolle, P.; Kanzaki, N.; Onuma, K.; Ito, A. Symmetry of posner’s cluster. J. Am. Chem. Soc. 2000, 122, 8323–8324. [Google Scholar] [CrossRef]
- Meyer, J.L.; Eanes, E.D. Thermodynamic analysis of secondary transition in spontaneous precipitation of calcium-phosphate. Calcif. Tissue Res. 1978, 25, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.; Tao, J.H.; Brylka, L.J.; Friedrich, H.; Bertinetti, L.; Schenk, A.S.; Verch, A.; Dmitrovic, V.; Bomans, P.H.H.; Frederik, P.M.; et al. Ion-association complexes unite classical and non-classical theories for the biomimetic nucleation of calcium phosphate. Nat. Commun. 2013, 4, 1507. [Google Scholar] [CrossRef]
- Simon, P.; Grüner, D.; Worch, H.; Pompe, W.; Lichte, H.; Khassawna, T.; Heiss, C.; Wenisch, S.; Kniep, R. First evidence of octacalcium phosphate @ osteocalcin nanocomplex as skeletal bone component directing collagen triple-helix nanofibril mineralization. Sci. Rep. 2018, 8, 13696. [Google Scholar] [CrossRef]
- Suzuki, O.; Shiwaku, Y.; Hamai, R. Octacalcium phosphate bone substitute materials: Comparison between properties of biomaterials and other calcium phosphate materials. Dent. Mater. J. 2020, 39, 187–199. [Google Scholar] [CrossRef]
- Liu, X.C.; Shiwaku, Y.; Hamai, R.; Tsuchiya, K.; Takahashi, T.; Suzuki, O. Effect of octacalcium phosphate crystals on the osteogenic differentiation of tendon stem/progenitor cells in vitro. Int. J. Mol. Sci. 2023, 24, 1235. [Google Scholar] [CrossRef]
- Brown, W.E.; Mathew, M.; Tung, M.S. Crystal chemistry of octacalcium phosphate. Prog. Cryst. Growth Charact. 1981, 4, 59–87. [Google Scholar] [CrossRef]
- Mathew, M.; Brown, W.E.; Schroeder, L.W.; Dickens, B. Crystal structure of octacalcium bis(hydrogenphosphate) tetrakis(phosphate)pentahydrate, Ca8(HPO4)2(PO4)4⸱5H2O. J. Crystallogr. Spectroscop Res. 1988, 18, 235–250. [Google Scholar] [CrossRef]
- Sakai, S.; Anada, T.; Tsuchiya, K.; Yamazaki, H.; Margolis, H.C.; Suzuki, O. Comparative study on the resorbability and dissolution behavior of octacalcium phosphate, β-tricalcium phosphate, and hydroxyapatite under physiological conditions. Dent. Mater. J. 2016, 35, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Legeros, R.Z.; Daculsi, G.; Orly, I.; Abergas, T.; Torres, W. Solution mediated transformation of octacalcium phosphate (OCP) to apatite. Scanning Microsc. 1989, 3, 129–138. [Google Scholar]
- Tomazic, B.B.; Tung, M.S.; Gregory, T.M.; Brown, W.E. Mechanism of hydrolysis of octacalcium phosphate. Scanning Microsc. 1989, 3, 119–127. [Google Scholar]
- Suzuki, O.; Kamakura, S.; Katagiri, T. Surface chemistry and biological responses to synthetic octacalcium phosphate. J. Biomed. Mater. Res. Part B 2006, 77B, 201–212. [Google Scholar] [CrossRef]
- Bellows, C.G.; Heersche, J.N.M.; Aubin, J.E. Inorganic phosphate added exogenously or released from β-glycerophosphate initiates mineralization of osteoid nodules invitro. Bone Miner. 1992, 17, 15–29. [Google Scholar] [CrossRef]
- Meleti, Z.; Shapiro, I.M.; Adams, C.S. Inorganic phosphate induces apoptosis of osteoblast-like cells in culture. Bone 2000, 27, 359–366. [Google Scholar] [CrossRef]
- Dvorak, M.M.; Siddiqua, A.; Ward, D.T.; Carter, D.H.; Dallas, S.L.; Nemeth, E.F.; Riccardi, D. Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc. Natl. Acad. Sci. USA 2004, 101, 5140–5145. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Pierschbacher, M.D. New perspectives in cell adhesion: RGD and integrins. Science 1987, 238, 491–497. [Google Scholar] [CrossRef]
- Takeichi, M. Functional correlation between cell adhesive properties and some cell-surface proteins. J. Cell Biol. 1977, 75, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induced pluripotent stem cells in medicine and biology. Development 2013, 140, 2457–2461. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.Z.; Kim, T.H.; Kim, J.H.; Won, J.E.; Yoo, S.Y.; Choi, S.J.; Hyun, J.K.; Kim, H.W. Bone tissue engineering of induced pluripotent stem cells cultured with macrochanneled polymer scaffold. J. Biomed. Mater. Res. Part A 2013, 101, 1283–1291. [Google Scholar] [CrossRef]
- Holzwarth, J.M.; Ma, P.X. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 2011, 32, 9622–9629. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, W.C.; Zhao, Z.H.; Xu, H.H.K. Reprogramming of mesenchymal stem cells derived from iPSCs seeded on biofunctionalized calcium phosphate scaffold for bone engineering. Biomaterisals 2013, 34, 7862–7872. [Google Scholar] [CrossRef]
- TheinHan, W.W.; Liu, J.; Tang, M.H.; Chen, W.C.; Cheng, L.Z.; Xu, H.H.K. Induced pluripotent stem cell-derived mesenchymal stem cell seeding on biofunctionalized calcium phosphate cements. Bone Res. 2013, 1, 371–384. [Google Scholar] [CrossRef]
- Ozasa, R.; Matsugaki, A.; Matsuzaka, T.; Ishimoto, T.; Yun, H.S.; Nakano, T. Superior alignment of human iPSC-osteoblasts associated with focal adhesion formation stimulated by oriented collagen scaffold. Int. J. Mol. Sci. 2021, 22, 6232. [Google Scholar] [CrossRef]
- Hua, Z.Y.; Li, S.; Liu, Q.Z.; Yu, M.X.; Liao, M.L.; Zhang, H.M.; Xiang, X.R.; Wu, Q.Q. Low-intensity pulsed ultrasound promotes osteogenic potential of ipsc-derived mscs but fails to simplify the iPSC-EB-MSC differentiation process. Front. Bioeng. Biotechnol. 2022, 10, 841778. [Google Scholar] [CrossRef]
- Manokawinchoke, J.; Osathanon, T.; Egusa, H.; Pavasant, P. Hypoxia enhances osteogenic differentiation in retinoic acid-treated murine-induced pluripotent stem cells. Tissue Eng. Regen. Med. 2016, 13, 547–553. [Google Scholar] [CrossRef]
- Sugimoto, K.; Yoshizawa, Y.; Yamada, S.; Igawa, K.; Hayashi, Y.; Ishizaki, H. Effects of hypoxia on pluripotency in murine iPS cells. Microsc. Res. Tech. 2013, 76, 1084–1092. [Google Scholar] [CrossRef]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2017, 16, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Okano, H.; Morimoto, S.; Kato, C.; Nakahara, J.; Takahashi, S. Induced pluripotent stem cells-based disease modeling, drug screening, clinical trials, and reverse translational research for amyotrophic lateral sclerosis. J. Neurochem. 2023, 167, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Groebe, K.; MuellerKlieser, W. On the relation between size of necrosis and diameter of tumor spheroids. Int. J. Radiat. Oncol. Biol. Phys. 1996, 34, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef]
- Anada, T.; Fukuda, J.; Sai, Y.; Suzuki, O. An oxygen-permeable spheroid culture system for the prevention of central hypoxia and necrosis of spheroids. Biomaterials 2012, 33, 8430–8441. [Google Scholar] [CrossRef]
- Egusa, H.; Okita, K.; Kayashima, H.; Yu, G.; Fukuyasu, S.; Saeki, M.; Matsumoto, T.; Yamanaka, S.; Yatani, H. Gingival fibroblasts as a promising source of induced pluripotent stem cells. PLoS ONE 2010, 5, e12743. [Google Scholar] [CrossRef]
- Hamai, R.; Tsuchiya, K.; Suzuki, O. Enhancement of the cytokine adsorption capacity of octacalcium phosphate by interaction-controlled glycosaminoglycan modification to promote osteoblastic differentiation. Langmuir 2024, 40, 27253–27269. [Google Scholar] [CrossRef]
- Moreno, E.C.; Kresak, M.; Zahradni, R.T. Fluoridated hydroxyapatite solubility and caries formation. Nature 1974, 247, 64–65. [Google Scholar] [CrossRef]
- Tung, M.S.; Eidelman, N.; Sieck, B.; Brown, W.E. Octacalcium phosphate solubility product from 4 to 37 °C. J. Res. Nat. Bur. Stand. 1988, 93, 613–624. [Google Scholar] [CrossRef]
- Moreno, E.C.; Brown, W.E.; Osborn, G. Solubility of dicalcium phosphate dihydrate in aqueous systems. Soil. Sci. Soc. Am. J. 1960, 24, 94–98. [Google Scholar]
- Moreno, E.C.; Margolis, H.C. Composition of human plaque fluid. J. Dent. Res. 1988, 67, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Aoba, T.; Fukae, M.; Tanabe, T.; Shimizu, M.; Moreno, E.C. Selective adsorption of porcine-amelogenins onto hydroxyapatite and their inhibitory activity on hydroxyapatite growth in supersaturated solutions. Calcif. Tissue Int. 1987, 41, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Fowler, B.O.; Markovic, M.; Brown, W.E. Octacalcium phosphate 3. infrared and raman vibrational-spectra. Chem. Mater. 1993, 5, 1417–1423. [Google Scholar] [CrossRef]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef]
- Sinusaite, L.; Popov, A.; Antuzevics, A.; Mazeika, K.; Baltrunas, D.; Yang, J.C.; Horng, J.L.; Shi, S.; Sekino, T.; Ishikawa, K.; et al. Fe and Zn co-substituted beta-tricalcium phosphate (β-TCP): Synthesis, structural, magnetic, mechanical and biological properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110918. [Google Scholar] [CrossRef]
- Lennon, D.P.; Edmison, J.M.; Caplan, A.I. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: Effects on in vitro and in vivo osteochondrogenesis. J. Cell Physiol. 2001, 187, 345–355. [Google Scholar] [CrossRef]
- Grayson, W.L.; Zhao, F.; Izadpanah, R.; Bunnell, B.; Ma, T. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J. Cell Physiol. 2006, 207, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Pattappa, G.; Heywood, H.K.; De Bruijn, J.D.; Lee, D.A. The Metabolism of human mesenchymal stem cells during proliferation and differentiation. J. Cell Physiol. 2011, 226, 2562–2570. [Google Scholar] [CrossRef]
- Anada, T.; Sato, T.; Kamoya, T.; Shiwaku, Y.; Tsuchiya, K.; Takano-Yamamoto, T.; Sasaki, K.; Suzuki, O. Evaluation of bioactivity of octacalcium phosphate using osteoblastic cell aggregates on a spheroid culture device. Regen. Ther. 2016, 3, 58–62. [Google Scholar] [CrossRef]
- Yang, S.C.; Liu, J.J.; Wang, C.K.; Lin, Y.T.; Tsai, S.Y.; Chen, W.J.; Huang, W.K.; Tu, P.W.A.; Lin, Y.C.; Chang, C.F.; et al. Down-regulation of ATF1 leads to early neuroectoderm differentiation of human embryonic stem cells by increasing the expression level of SOX2. FASEB J. 2019, 33, 10577–10592. [Google Scholar] [CrossRef]
- Okamura, K.; Inagaki, Y.; Matsui, T.K.; Matsubayashi, M.; Komeda, T.; Ogawa, M.; Mori, E.; Tanaka, Y. RT-qPCR analyses on the osteogenic differentiation from human iPS cells: An investigation of reference genes. Sci. Rep. 2020, 10, 11748. [Google Scholar] [CrossRef]
- Jong, J.; Yoo, J.E.; Lee, J.A.; Lee, D.R.; Kim, J.Y.; Huh, Y.J.; Kim, D.S.; Park, C.Y.; Hwang, D.Y.; Kim, H.S.; et al. Disease-specific induced pluripotent stem cells: A platform for human disease modeling and drug discovery. Exp. Mol. Med. 2012, 44, 202–213. [Google Scholar] [CrossRef]
- Nieden, N.I.; Kempka, G.; Ahr, H.J. In vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation 2003, 71, 18–27. [Google Scholar] [CrossRef]
- Kawaguchi, J.; Mee, P.J.; Smith, A.G. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone 2005, 36, 758–769. [Google Scholar] [CrossRef]
- Duplomb, L.; Dagouassat, M.; Jourdon, P.; Heymann, D. Concise review: Embryonic stem cells: A new tool to study osteoblast and osteoclast differentiation. Stem Cells 2007, 25, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ma, T.; Guo, D.; Hu, K.; Shu, Y.; Xu, H.H.K.; Schneider, A. Metformin induces osteoblastic differentiation of human induced pluripotent stem cell-derived mesenchymal stem cells. J. Tissue Eng. Regener Med. 2018, 12, 437–446. [Google Scholar]
- Chen, X.R.; Hansen, D.K.; Merry, G.; DeJarnette, C.; Nolen, G.; Sloper, D.; Fisher, J.E.; Harrouk, W.; Tassinari, M.S.; Inselman, A.L. Developing osteoblasts as an endpoint for the mouse embryonic stem cell test. Reprod. Toxicol. 2015, 53, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.J.; Yang, Y.; Zhang, B.; Bai, X.F.; Fei, Q.; Zhang, L. Rapid human-derived iPSC osteogenesis combined with three-dimensionally printed Ti6Al4V scaffolds for the repair of bone defects. J. Cell Physiol. 2020, 235, 9763–9772. [Google Scholar] [CrossRef]
- Jeon, O.H.; Panicker, L.M.; Lu, Q.Z.; Chae, J.J.; Feldman, R.A.; Elisseeff, J.H. Human iPSC-derived osteoblasts and osteoclasts together promote bone regeneration in 3D biomaterials. Sci. Rep. 2016, 6, 26761. [Google Scholar] [CrossRef]
- Sato, M.; Aoki, H.; Nakamura, T.; Onodera, S.; Yamaguchi, A.; Saito, A.; Azuma, T. Effects of intermittent treatment with parathyroid hormone (PTH) on osteoblastic differentiation and mineralization of mouse induced pluripotent stem cells in a 3D culture model. J. Peri Res. 2020, 55, 734–743. [Google Scholar] [CrossRef]
- Sasaki, J.I.; Matsumoto, T.; Egusa, H.; Matsusaki, M.; Nishiguchi, A.; Nakano, T.; Akashi, M.; Imazato, S.; Yatani, H. In vitro reproduction of endochondral ossification using a 3D mesenchymal stem cell construct. Integrative Biology 2012, 4, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, X.H.; Baker, C.; Murphy, W.L.; McDevitt, T.C. Mineral particles modulate osteo-chondrogenic differentiation of embryonic stem cell aggregates. Acta Biomater. 2016, 29, 42–51. [Google Scholar] [CrossRef]
- Dang, P.N.; Dwivedi, N.; Phillips, L.M.; Yu, X.H.; Herberg, S.; Bowerman, C.; Solorio, L.D.; Murphy, W.L.; Alsberg, E. Controlled dual growth factor delivery from microparticles incorporated within human bone marrow-derived mesenchymal stem cell aggregates for enhanced bone tissue engineering via endochondral ossification. Stem Cells Transl. Med. 2016, 5, 206–217. [Google Scholar] [CrossRef]
- Wang, L.J.; Nancollas, G.H. Calcium orthophosphates: Crystallization and dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.C. Next generation calcium phosphate-based biomaterials. Dent. Mater. J. 2009, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.R.V.; Hwang, Y.; Phadke, A.; Kang, H.; Hwang, N.S.; Caro, E.J.; Nguyen, S.; Siu, M.; Theodorakis, E.A.; Gianneschi, N.C.; et al. Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 990–995. [Google Scholar] [CrossRef]
- Kim, H.M.; Himeno, T.; Kokubo, T.; Nakamura, T. Process and kinetics of bonelike apatite formation on sintered hydroxyapatite in a simulated body fluid. Biomaterials 2005, 26, 4366–4373. [Google Scholar] [CrossRef]
- Obata, A.; Fujimoto, T.; Kasuga, T. Enhancement of bone-like apatite forming abilities of calcium phosphate ceramics in SBF by autoclaving. J. Ceram. Soc. Jpn. 2006, 114, 63–66. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef]
- Jung, G.Y.; Park, Y.J.; Han, J.S. Effects of HA released calcium ion on osteoblast differentiation. J. Mater. Sci. Mater. Med. 2010, 21, 1649–1654. [Google Scholar] [CrossRef]
- Ma, S.; Yang, Y.; Carnes, D.L.; Kim, K.; Park, S.; Oh, S.H.; Ong, J.L. Effects of dissolved calcium and phosphorous on osteoblast responses. J. Oral. Implantol. 2005, 31, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Germaini, M.M.; Detsch, R.; Grünewald, A.; Magnaudeix, A.; Lalloue, F.; Boccaccini, A.R.; Champion, E. Osteoblast and osteoclast responses to A/B type carbonate-substituted hydroxyapatite ceramics for bone regeneration. Biomed. Mater. 2017, 12, 035008. [Google Scholar] [CrossRef]
- Suzuki, K.; Tamazawa, M.; Onuma, E.; Honda, M.; Aizawa, M. Preferred orientation of hydroxyapatite ceramics along the c-axis promotes osteoblast differentiation. Int. J. Mol. Sci. 2024, 25, 12926. [Google Scholar] [CrossRef]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef]
- Li, N.; Dai, X.F.; Yang, F.; Sun, Y.; Wu, X.W.; Zhou, Q.R.; Chen, K.; Sun, J.; Bi, W.; Shi, L.; et al. Spontaneous spheroids from alveolar bone-derived mesenchymal stromal cells maintain pluripotency of stem cells by regulating hypoxia-inducible factors. Biol. Res. 2023, 56, 17. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.B.; Yu, S.J.; Kim, J.; Ko, U.H.; Park, E.Y.; Choung, J.S.; Choi, G.; Kim, D.; Lee, E.; Im, S.G.; et al. Remodeling of adhesion network within cancer spheroids via cell-polymer interaction. ACS Biomater. Sci. Eng. 2020, 6, 5632–5644. [Google Scholar] [CrossRef]
- Adenis, L.; Gontran, E.; Deroulers, C.; Grammaticos, B.; Juchaux, M.; Seksek, O.; Badoual, M. Experimental and modeling study of the formation of cell aggregates with differential substrate adhesion. PLoS ONE 2020, 15, e0222371. [Google Scholar] [CrossRef]
- Lin, R.-Z.; Chang, H.Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [PubMed]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2001, 22, 87–96. [Google Scholar] [CrossRef]
- Hong, M.H.; Kim, S.M.; Han, M.H.; Kim, Y.H.; Lee, Y.K.; Oh, D.S. Evaluation of microstructure effect of the porous spherical β-tricalcium phosphate granules on cellular responses. Ceram. Int. 2014, 40, 6095–6102. [Google Scholar] [CrossRef]
- Beck, G.R. Inorganic phosphate as a signaling molecule in osteoblast differentiation. J. Cell Biochem. 2003, 90, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Yoshiko, Y.; Candeliere, G.A.; Maeda, N.; Aubin, J.E. Osteoblast autonomous Pi regulation via Pit1 plays a role in bone mineralization. Mol. Cellular Biol. 2007, 27, 4465–4474. [Google Scholar] [CrossRef]
- Anisimov, R.A.; Gorin, D.A.; Abalymov, A.A. 3D cell spheroids as a tool for evaluating the effectiveness of carbon nanotubes as a drug delivery and photothermal therapy agents. C 2022, 8, 56. [Google Scholar] [CrossRef]
- Yun, C.; Kim, S.H.; Kim, K.M.; Yang, M.H.; Byun, M.R.; Kim, J.H.; Kwon, D.; Pham, H.T.M.; Kim, H.S.; Kim, J.H.; et al. Advantages of using 3D spheroid culture systems in toxicological and pharmacological assessment for osteogenesis research. Int. J. Mol. Sci. 2024, 25, 2512. [Google Scholar] [CrossRef]
- Fang, Y.; Eglen, R.M. Three-dimensional cell cultures in drug discovery and development. SLAS Discov. 2017, 22, 456–472. [Google Scholar] [CrossRef] [PubMed]
- Boscaro, D.; Sikorski, P. Spheroids as a 3D in vitro model to study bone and bone mineralization. Biomater. Adv. 2024, 157, 213727. [Google Scholar] [CrossRef] [PubMed]
- Nashimoto, Y.; Konno, A.; Imaizumi, T.; Nishikawa, K.; Ino, K.; Hori, T.; Kaji, H.; Shintaku, H.; Goto, M.; Shiku, H. Microfluidic vascular formation model for assessing angiogenic capacities of single islets. Biotechnol. Bioeng. 2024, 121, 1050–1059. [Google Scholar] [CrossRef]
- Yao, Q.G.; Cheng, S.; Pan, Q.L.; Yu, J.; Cao, G.Q.; Li, L.J.; Cao, H.C. Organoids: Development and applications in disease models, drug discovery, precision medicine, and regenerative medicine. MedComm 2024, 5, e735. [Google Scholar] [CrossRef]
- Hiramoto, K.; Konno, A.; Nashimoto, Y.; Hirano-Iwata, A.; Ino, K.; Shiku, H. Osteogenic differentiation of mesenchymal stem cell spheroids: A microfluidic device and electrochemiluminescence imaging study. Electrochim. Acta 2024, 491, 144291. [Google Scholar] [CrossRef]
- Maffioletti, S.; Sarcar, S.; Henderson, A.; Mannhardt, I.; Pinton, L.; Moyle, L.; Steele-Stallard, H.; Cappellari, O.; Wells, K.; Ferrari, G.; et al. Three-dimensional human ipsc-derived artificial skeletal muscles model muscular dystrophies and enable multilineage tissue engineering. Cell Rep. 2018, 23, 899–908. [Google Scholar] [CrossRef]
- Smith, A.; Macadangdang, J.; Leung, W.; Laflamme, M.; Kim, D. Human iPSC-derived cardiomyocytes and tissue engineering strategies for disease modeling and drug screening. Biotechnol. Adv. 2017, 35, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Eyckmans, J.; Chen, C. 3D culture models of tissues under tension. J. Cell Sci. 2017, 130, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Csobonyeiova, M.; Polak, S.; Zamborsky, R.; Danisovic, L. iPS cell technologies and their prospect for bone regeneration and disease modeling: A mini review. J. Adv. Res. 2017, 8, 321–327. [Google Scholar] [CrossRef]
- Bordoni, M.; Rey, F.; Fantini, V.; Pansarasa, O.; Di Giulio, A.; Carelli, S.; Cereda, C. From neuronal differentiation of ipscs to 3D neuro-organoids: Modelling and therapy of neurodegenerative diseases. Int. J. Mol. Sci. 2018, 19, 3972. [Google Scholar] [CrossRef] [PubMed]
- Di Buduo, C.; Laurent, P.; Zaninetti, C.; Lordier, L.; Soprano, P.; Ntai, A.; Barozzi, S.; La Spada, A.; Biunno, I.; Raslova, H.; et al. Miniaturized 3D bone marrow tissue model to assess response to Thrombopoietin-receptor agonists in patients. eLife 2021, 10, e58775. [Google Scholar] [CrossRef]
- Lamandé, S.L.; Ng, E.S.; Cameron, T.L.; Kung, L.H.W.; Sampurno, L.; Rowley, L.; Lilianty, J.; Patria, Y.N.; Stenta, T.; Hanssen, E.; et al. Modeling human skeletal development using human pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2023, 120, e2211510120. [Google Scholar] [CrossRef]
- Singh, U.; Quintanilla, R.H.; Grecian, S.; Gee, K.R.; Rao, M.S.; Lakshmipathy, U. Novel live alkaline phosphatase substrate for identification of pluripotent stem cells. Stem Cell Rev. Rep. 2012, 8, 1021–1029. [Google Scholar] [CrossRef]
- Hamada, S.; Mori, Y.; Shiwaku, Y.; Hamai, R.; Tsuchiya, K.; Baba, K.; Oizumi, I.; Kanabuchi, R.; Miyatake, N.; Aizawa, T.; et al. Octacalcium phosphate/gelatin composite (OCP/Gel) enhances bone repair in a critical-sized transcortical femoral defect rat model. Clin. Orthop. Relat. Res. 2022, 480, 2043–2055. [Google Scholar] [CrossRef]


| Supernatants | Periods (Days) | DS at 37 °C | ||
|---|---|---|---|---|
| HA | OCP | DCPD | ||
| Osteogenic medium | 0 | 5.30 × 1014 | 1.11 × 105 | 1.04 × 100 |
| iPSCs only (Oxy chip) | 2 | 6.97 × 1012 | 2.02 × 104 | 1.41 × 100 |
| OCP < 53 μm | 2 | 5.97 × 1012 | 2.36 × 104 | 1.65 × 100 |
| OCP 300–500 μm | 2 | 3.31 × 1013 | 6.66 × 104 | 1.86 × 100 |
| HA < 53 μm | 2 | 1.74 × 1013 | 4.94 × 104 | 1.89 × 100 |
| HA 300–500 μm | 2 | 1.50 × 1013 | 3.85 × 104 | 1.69 × 100 |
| β-TCP < 53 μm | 2 | 8.37 × 1013 | 1.63 × 105 | 2.48 × 100 |
| β-TCP300–500 μm | 2 | 3.05 × 1013 | 6.18 × 104 | 1.82 × 100 |
| iPSC (Oxy chip) | 4 | 1.80 × 1013 | 6.02 × 104 | 2.13 × 100 |
| OCP < 53 μm | 4 | 1.27 × 1013 | 4.88 × 104 | 2.08 × 100 |
| OCP 300–500 μm | 4 | 8.22 × 1012 | 3.30 × 104 | 1.85 × 100 |
| HA < 53 μm | 4 | 3.70 × 1013 | 9.48 × 105 | 2.27 × 100 |
| HA 300–500 μm | 4 | 7.95 × 1012 | 2.60 × 104 | 1.60 × 100 |
| β-TCP < 53 μm | 4 | 3.09 × 1014 | 4.66 × 105 | 3.24 × 100 |
| β-TCP 300–500 μm | 4 | 3.76 × 1013 | 7.75 × 104 | 1.97 × 100 |
| iPSCs only (Oxy chip) | 6 | 7.78 × 1013 | 1.47 × 105 | 2.37 × 100 |
| OCP < 53 μm | 6 | 3.88 × 1013 | 9.88 × 104 | 2.30 × 100 |
| OCP 300–500 μm | 6 | 2.56 × 1012 | 9.48 × 103 | 1.19 × 100 |
| HA < 53 μm | 6 | 5.09 × 1013 | 9.33 × 104 | 2.02 × 100 |
| HA 300–500 μm | 6 | 1.43 × 1011 | 8.69 × 102 | 6.33 × 10−1 |
| β-TCP < 53 μm | 6 | 2.03 × 1014 | 2.66 × 105 | 2.56 × 100 |
| β-TCP 300–500 μm | 6 | 3.55 × 1013 | 5.82 × 104 | 1.66 × 100 |
| iPSC only (Oxy chip) | 14 | 9.25 × 1012 | 2.77 × 104 | 1.59 × 100 |
| OCP < 53 μm | 14 | 9.68 × 1012 | 3.04 × 104 | 1.66 × 100 |
| OCP 300–500 μm | 14 | 1.50 × 1012 | 6.11 × 103 | 1.06 × 100 |
| HA < 53 μm | 14 | 6.58 × 1012 | 1.99 × 104 | 1.43 × 100 |
| HA 300–500 μm | 14 | 2.02 × 1012 | 7.67 × 103 | 1.12 × 100 |
| β-TCP < 53 μm | 14 | 8.44 × 1012 | 2.56 × 104 | 1.55 × 100 |
| β-TCP 300–500 μm | 14 | 2.58 × 1013 | 5.04 × 104 | 1.68 × 100 |
| Supernatants | Periods (Days) | Ca (mM) | Pi (mM) | pH | DS at 37 °C | ||
|---|---|---|---|---|---|---|---|
| HA | OCP | DCPD | |||||
| Original medium | 0 | 2.46 | 1.23 | 7.92 | 5.30 × 1014 | 1.11 × 105 | 1.04 × 100 |
| OCP < 53 μm | 2 | 1.56 | 0.81 | 7.74 | 3.24 × 1012 | 2.40 × 103 | 4.41 × 10−1 |
| 14 | 1.61 | 0.95 | 7.36 | 1.27 × 1011 | 5.24 × 102 | 4.71 × 10−1 | |
| OCP 300–500 μm | 2 | 1.40 | 0.77 | 7.83 | 4.02 × 1012 | 2.19 × 103 | 3.86 × 10−1 |
| 14 | 1.33 | 0.93 | 7.37 | 5.26 × 1010 | 2.51 × 102 | 3.87 × 10−1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugai, Y.; Hamai, R.; Shiwaku, Y.; Anada, T.; Tsuchiya, K.; Kimura, T.; Tadano, M.; Yamauchi, K.; Takahashi, T.; Egusa, H.; et al. Effect of Octacalcium Phosphate on Osteogenic Differentiation of Induced Pluripotent Stem Cells in a 3D Hybrid Spheroid Culture. Biomimetics 2025, 10, 205. https://doi.org/10.3390/biomimetics10040205
Sugai Y, Hamai R, Shiwaku Y, Anada T, Tsuchiya K, Kimura T, Tadano M, Yamauchi K, Takahashi T, Egusa H, et al. Effect of Octacalcium Phosphate on Osteogenic Differentiation of Induced Pluripotent Stem Cells in a 3D Hybrid Spheroid Culture. Biomimetics. 2025; 10(4):205. https://doi.org/10.3390/biomimetics10040205
Chicago/Turabian StyleSugai, Yuki, Ryo Hamai, Yukari Shiwaku, Takahisa Anada, Kaori Tsuchiya, Tai Kimura, Manami Tadano, Kensuke Yamauchi, Tetsu Takahashi, Hiroshi Egusa, and et al. 2025. "Effect of Octacalcium Phosphate on Osteogenic Differentiation of Induced Pluripotent Stem Cells in a 3D Hybrid Spheroid Culture" Biomimetics 10, no. 4: 205. https://doi.org/10.3390/biomimetics10040205
APA StyleSugai, Y., Hamai, R., Shiwaku, Y., Anada, T., Tsuchiya, K., Kimura, T., Tadano, M., Yamauchi, K., Takahashi, T., Egusa, H., & Suzuki, O. (2025). Effect of Octacalcium Phosphate on Osteogenic Differentiation of Induced Pluripotent Stem Cells in a 3D Hybrid Spheroid Culture. Biomimetics, 10(4), 205. https://doi.org/10.3390/biomimetics10040205







