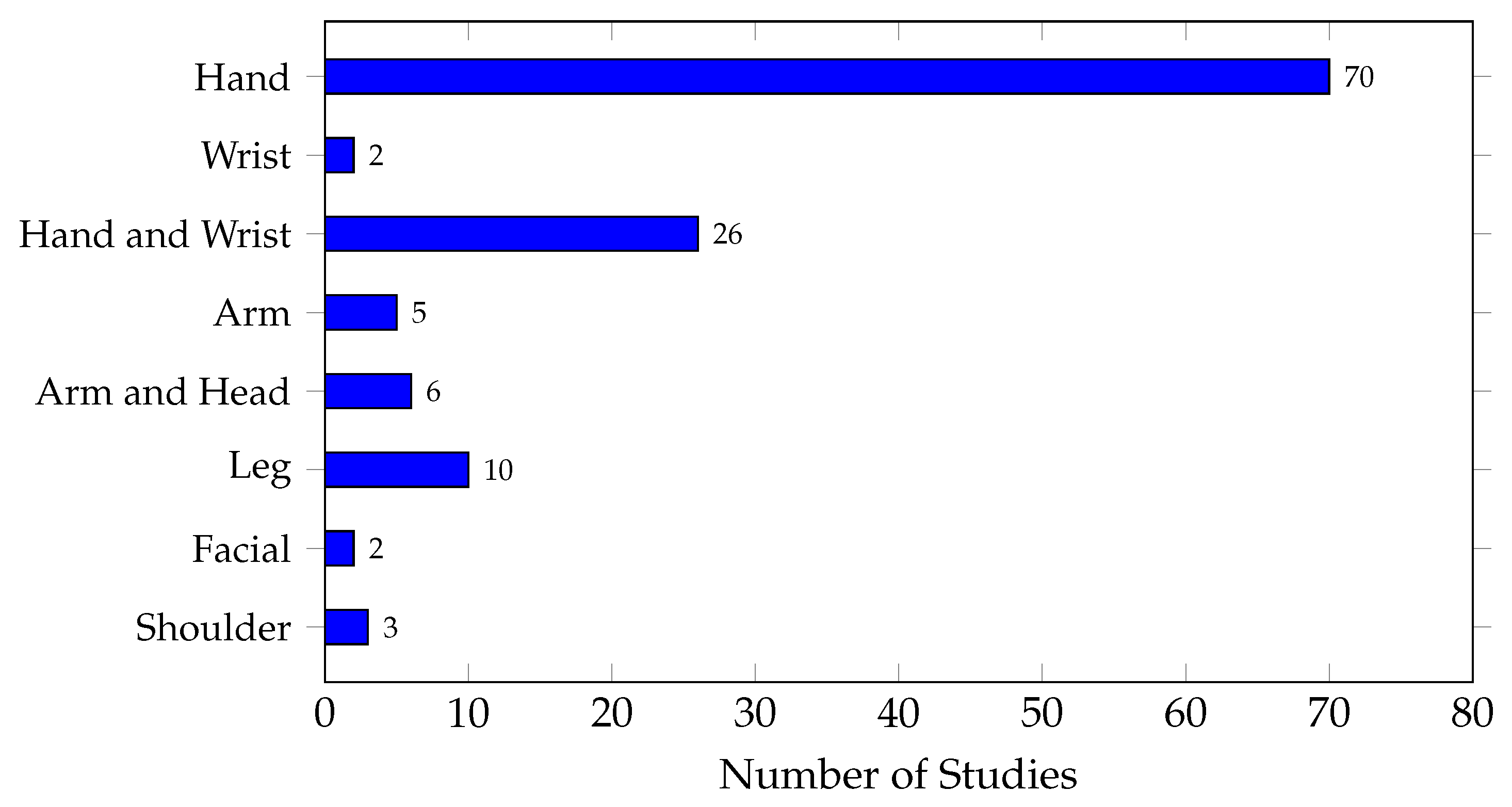1. Introduction
Recently, multiple studies on the use of bioelectric signals from the human body have aimed to develop new technologies for their analysis and interpretation and thus achieve the development or optimization of processes or models [
1]. One of the most important types of biosignals is electromyography (EMG) signals, which have been extensively studied as they provide data for analyzing muscle activity. An EMG signal is a biopotential representing the electrical currents generated during muscle contraction and relaxation. However, since these signals originate from muscle activity controlled by the nervous system, they are very complex in their raw representation [
2].
EMG signals are a fundamental tool in the development of biomimetic technologies, whose purpose is to replicate the natural behavior of human body movements [
3]. In this context, an EMG gesture is defined as a sequence of voluntary muscle activations resulting in distinguishable movements captured in EMG signals [
4]. The analysis of these signals enables the interpretation of different gestures, which is crucial for applications such as intelligent prosthetics, exoskeletons, and control systems based on human–machine interfaces.
Due to their complexity, classifying EMG signals is crucial to correctly interpreting the information and obtaining useful data. To achieve this, certain features representing the signal need to be extracted, and artificial intelligence algorithms are usually used for classification. Among the most prevalent techniques are supervised learning algorithms such as support vector machine (SVM) and k-nearest neighbor (KNN) algorithms. Other techniques that have recently gained popularity are neural networks, such as multilayer perceptron networks (MLPs), which are among the most common. Convolutional neural networks (CNNs) are frequently used, as shown in [
5]. Recently, classifiers with recurrent neural networks (RNNs) have been used, as the authors of [
6] show. These and other artificial intelligence methods aim to achieve adequate performance in motion detection. However, achieving such performance comes at a high computational cost due to the model’s training process and hyperparameters. These external parameters define important aspects such as the model’s architecture and learning rate [
2]. Therefore, implementing these methods in responsive applications, such as real-time systems, often requires powerful and costly computing equipment. In addition, these systems are difficult to transport due to their size and power requirements [
1,
7].
This situation raises a challenge when integrating EMG signal classification into wearable systems. In this context, accurate and efficient classification of EMG signals on embedded devices has gained significant importance, especially for real-time and field applications such as smart prostheses, rehabilitation devices, and gesture-based control systems. Thus, for extracting features and classifying these signals, it is fundamental to find technologies that allow an efficient implementation in terms of time and energy consumption. Some devices and processors have already been used for the above applications. For example, microcontrollers are used for feature extraction and AI model inference for classification in [
8,
9]. In [
10], digital signal processors (DSPs) or field-programmable gate arrays (FPGAs) are used. In [
11], feature extraction and classification are performed on System-on-Chip (SoC) devices such as Raspberry Pi
®, which the UK-based Raspberry Pi Foundation manufacture, or Jetson
® GPUs, which is manufactured by NVIDIA, an American company based in Santa Clara, California, USA. Recently, neuromorphic systems have been used for these applications, as shown in [
12].
This work aims to review studies on implementing embedded devices for processing and classifying EMG signals and the techniques used. It analyzes the processing times and energy consumption reported in these studies. The focus is on a detailed analysis of device implementations such as microcontrollers, DSPs, FPGAs, SoCs, and neuromorphic chips. In terms of processing, the study is primarily concerned with extracting features representing the EMG signal and shows which features are most commonly implemented and on which devices. The precision metrics obtained in the studies are presented in terms of classification, and the preferred classification models for each type of embedded architecture are analyzed. This article provides an overview of embedded technologies used to analyze EMG signals for motion discrimination, with potential applications in portable devices that can be integrated into daily life.
The most important contributions of the work are as follows:
Novel approach: few studies take this analytical perspective and emphasize using embedded systems to implement artificial intelligence algorithms in classifying EMG signals.
State-of-the-art vision: This work provides a comprehensive overview of the state of the art in embedding EMG classification models for potential use in portable applications, such as smart prostheses. It serves as a reference for selecting the appropriate device for future studies.
Identification of challenges and opportunities: The study identifies the challenges associated with current technologies and areas of opportunity for research, such as improving portability and reducing costs.
This paper is structured as follows:
Section 2 contains the methodology for article selection.
Section 3 gives an overview of the features used to identify gestures with different embedded devices.
Section 4 gives an overview of works in which embedded devices were used for classification and presents the results obtained and the study’s implications.
Section 5 contains a discussion and comparison of the use of different devices. Finally, the study’s conclusions are presented in
Section 6.
2. Methodology
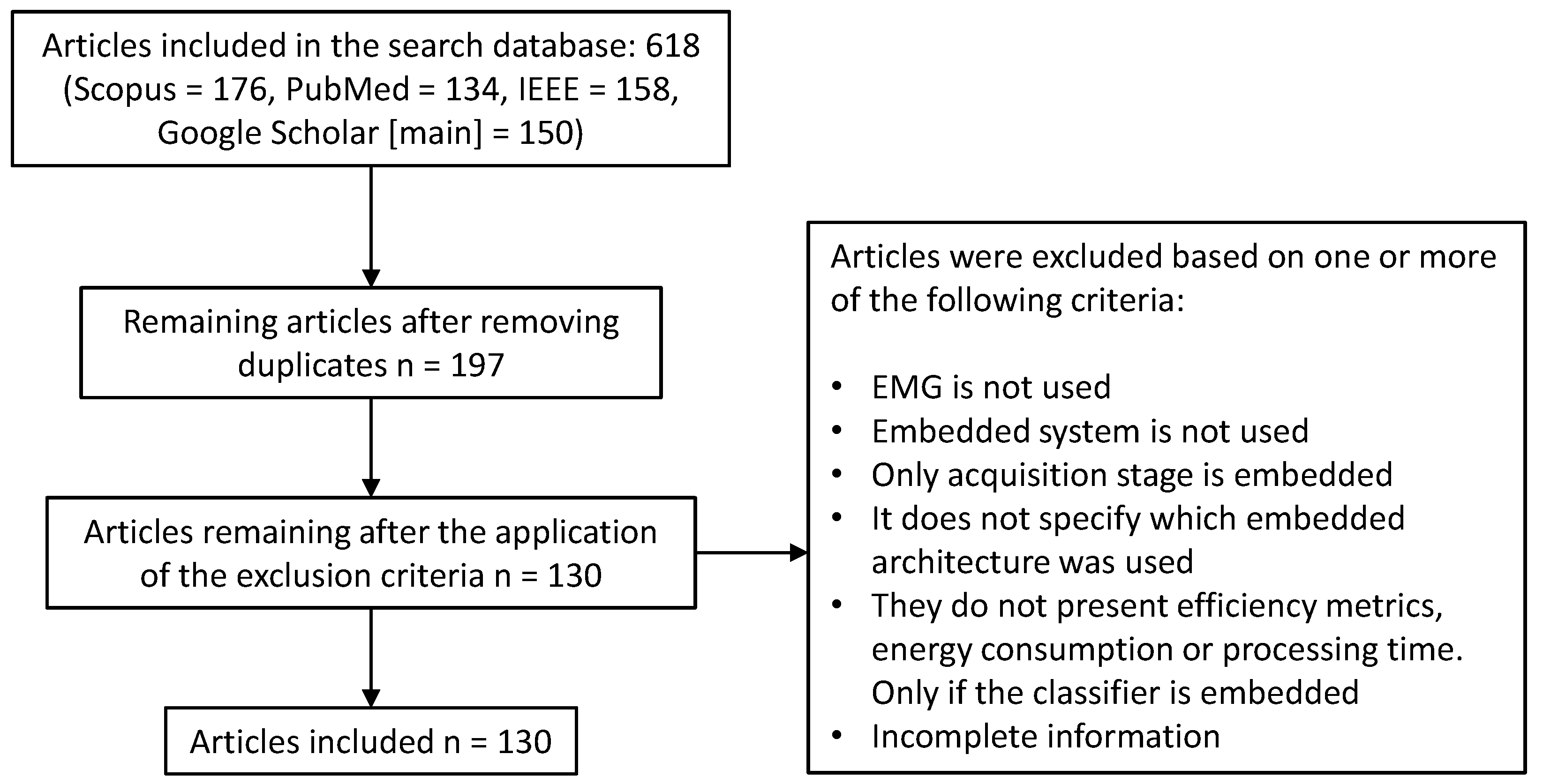
This section describes the methodology for article selection and exclusion. To conduct this review, the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were used [
13]. An extensive search for indexed articles was conducted in scientific databases such as Scopus, IEEE Xplore, PubMed, and Google Scholar. Publications from 2010 to 2025 were considered, with some exceptions for particularly relevant studies.
Keywords such as “EMG signals”, “embedded systems”, “classification”, “neuromorphic systems”, “FPGA”, “SoC”, “GPU”, “Raspberry”, “DSP”, “microcontroller”, and “artificial intelligence”, as well as combinations of these, were used. Initially, the articles were screened by reviewing their titles and abstracts to determine their inclusion. Duplicate articles were discarded. Subsequently, the full texts were reviewed to assess the relevance of the content and finalize the selection. The following criteria were applied for article selection:
Feature extraction of EMG signals on embedded devices.
Classification of EMG signals on embedded devices.
Works that did not describe the architecture used in detail were excluded.
Works in which the embedded device was only used to capture the EMG signal were excluded.
The results were recorded in tables summarizing the key features of each study. A total of 130 articles were analyzed in this work. The article selection process is illustrated in
Figure 1.
3. EMG Signals
EMG signals provide information about muscle strength, movement, and fatigue. This muscle activity is recorded using electrodes that are either invasive (e.g., with needles inserted into the muscle) or non-invasive (on the skin) [
7,
14]. The EMG signal represents the action potentials of the muscle fibers [
7].
4. EMG Signal Classification in Embedded Devices
The classification of EMG signals is an important task in biomedical engineering. Artificial intelligence algorithms are often used for this purpose, as they have proven effective in identifying and distinguishing patterns in this type of signal.
Table 3 lists the acronyms for the classification algorithms used in the studies examined.
A high level of computation is usually required to implement these algorithms, which is why computing systems that consume much power and, in some cases, cooling systems are used to maintain their operation, as is the case with servers and high-performance workstations [
7,
21].
The need for these large and energy-intensive systems is a limitation for applying these solutions in everyday life. This applies to real-time and field applications like intelligent prosthetics or gesture-controlled systems. For this reason, the trend is towards more efficient and compact alternatives, such as embedded devices and specialized hardware platforms [
17]. A good practice in embedded systems, which has yielded positive results in processing times and classification accuracy, is to perform only model inference [
63]. Training, on the other hand, is usually the most computationally intensive phase.
4.1. Inference of Algorithms
Algorithm inference on embedded devices, also known as edge inference or edge computing, refers to the process of running pre-trained artificial intelligence models on portable devices such as microcontrollers, FPGAs, SoCs, and other embedded devices [
64,
65]. Using inference eliminates the reliance on remote servers or the cloud, which was previously necessary for systems with higher processing capacity and larger size to perform operations [
66]. This means that the logic and arithmetic of the algorithms are executed directly on the device into which the data are input.
Several platforms stand out in terms of model inference for the classification of EMG signals.
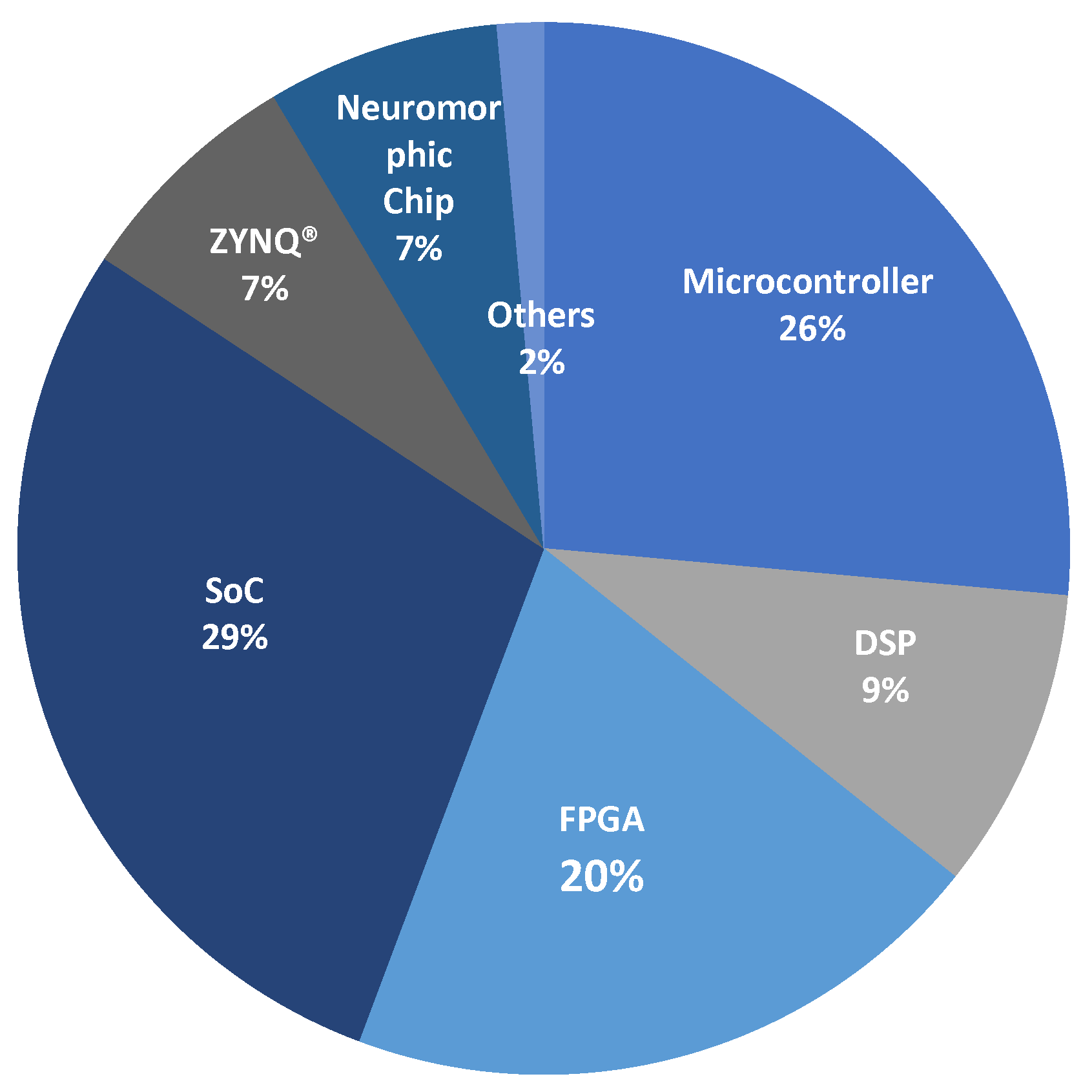
Figure 3 shows the percentage of use of these platforms based on the literature reviewed. SoCs lead with 29% of implementations due to their ease of executing artificial intelligence algorithms, thanks to programming capabilities in languages like Python. Microcontrollers, with 26% of implementations, follow them as they are the oldest technology on the market. In contrast, neuromorphic chips and ZYNQ devices were the architectures with the fewest model inference studies, at only 7%. The other platforms have similar percentages. In practice, there is no significant difference in preference for a particular technology, and all aim to enable portable real-time systems.
The following subsections report on the reviewed works that use different architectures for interpreting and discriminating EMG signals in embedded systems.
4.2. Microcontroller
Microcontrollers are an option for classifying EMG signals due to their low cost and good energy efficiency [
9]. Although these devices have limited memory and processing power compared to an FPGA or DSP, they can still run classification algorithms and process signals using optimization techniques and less computationally intensive algorithms. In [
9,
11,
19], LDA is used to classify signals as a lightweight algorithm compared to other techniques.
Table 4 shows some relevant studies that use microcontrollers to process EMG signals and give the accuracy percentage in motion prediction.
The methods used for signal classification via microcontrollers highlight the use of MLP, SVM, and LDA algorithms. These algorithms are less demanding in processing power, making them an excellent choice for using microcontrollers.
Microcontrollers are a suitable option for energy consumption when low power consumption is required. In [
9], for example, an energy consumption of 29.7 mW was measured during the execution of an SVM classifier. In terms of processing time, microcontrollers also show reasonable performance for applications that do not require decision speeds below 100 ms.
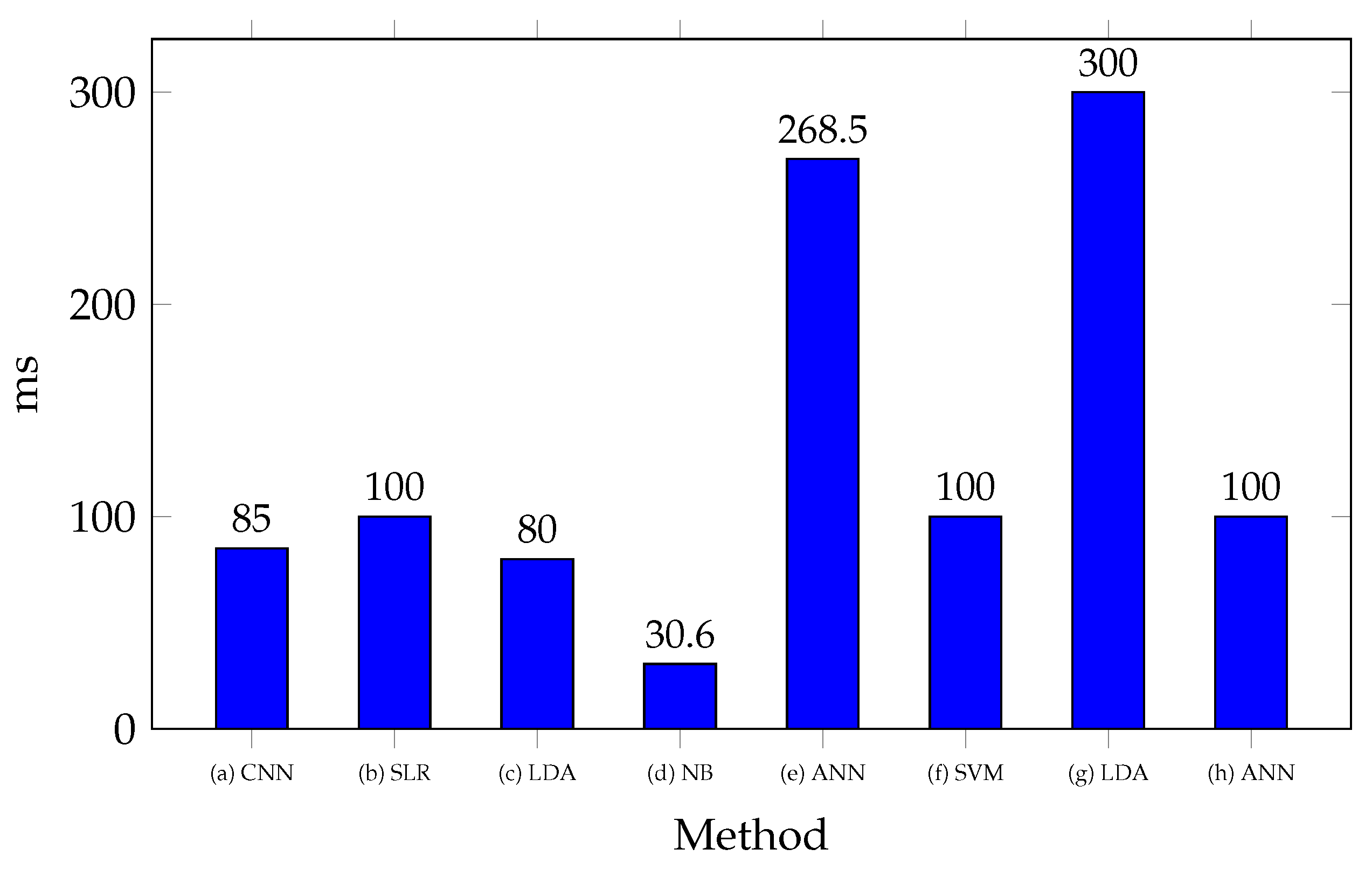
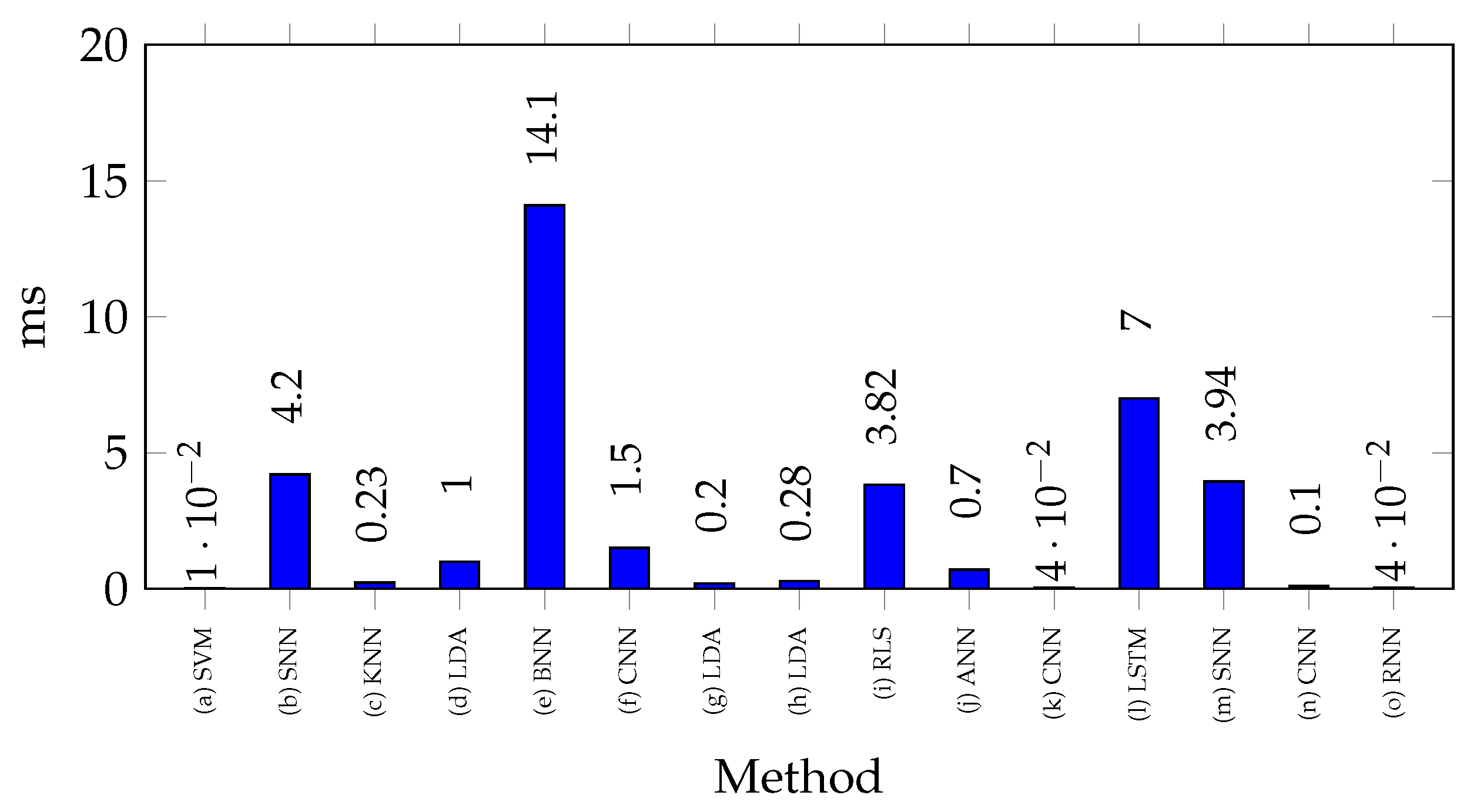
Figure 4 shows the processing times reported for classifying a gesture with microcontrollers. Variations are highlighted based on algorithm complexity. In [
42], the shortest processing time is reported using the NB method, with a time of 30.6 ms, making it a strong candidate for applications requiring high processing speed. Meanwhile, in [
74], 80 ms is reported using LDA, in [
70], 85 ms with CNN, in [
78], 100 ms with ANN, and in [
77], 100 ms with SVM. These represent moderate processing times due to the use of more robust algorithms. In contrast, in [
47], ANN was used with a processing time of 268.5 ms, while in [
55], LDA was used with a processing time of 300 ms. These require significantly higher processing times, suggesting that more complex models may suffer from increased latency in real-time applications on microcontrollers. It is worth noting that in [
55,
74], the LDA method was employed, but with a significant difference in processing times. This can be primarily attributed to the fact that in [
55], a higher number of channels were processed. Additionally, differences in implementation techniques or microcontroller architecture variations may have influenced the results. Similarly, in [
47,
78], ANN models were implemented, showing a considerable difference in processing times, mainly due to the specific neural network architecture used in each study.
These results indicate that lightweight models are preferable for microcontroller implementation, while complex models may require more powerful hardware to minimize latency.
4.3. Digital Signal Processor
DSPs are designed to process real-time signals and perform filtering, analysis, and transformation operations [
33]. They are more efficient than general-purpose processors, although they are more expensive. DSPs are a choice for processing EMG signals due to their precision and speed. In addition, they are reconfigurable devices, so their performance can be optimized according to the specific requirements of each application.
Table 5 shows relevant studies that use DSPs to classify EMG signals.
Regarding power consumption, [
31] reported a power consumption of 40.3 mW for extracting four features in the time domain and 26.6 mW for performing classification with LDA. The same study documented that the device required 75 ms to complete the task from feature extraction to classification of a signal pattern. In contrast, in [
33], it was reported that the device took between 200 and 300 ms to complete the task under the same conditions with eight movements and six sensors for detection, also using LDA for classification.
4.4. Field Programmable Gate Array
FPGAs are reconfigurable devices that enable the parallel implementation of algorithms as they are programmed at the hardware or gate level. This feature is advantageous for applications that require high processing speed [
84]. These devices are suitable for classifying EMG signals due to their ability to process large amounts of real-time data and flexibility in implementing different classification algorithms. Due to their parallel programming structure, FPGAs are well suited for implementing neural networks, which are also based on a parallel architecture [
85].
Table 6 shows some studies using FPGAs to classify EMG signals.
In [
86], an FPGA was used to extract muscle synergies, which were then used in motion classification, achieving high precision in motion discrimination and low execution time. On the other hand, in [
20], an SVM algorithm was derived on a Kintex 7
® FPGA for the classification of EMG signal, which showed significant improvements in execution time compared to the software-implemented model.
In the reported studies, the processing time for classification using FPGAs stands out as a design specifically tailored to the task of significantly optimizing the latency in model execution.
Figure 5 shows the processing times recorded by the authors when performing inference on FPGAs. The figure indicates that most studies report processing times of less than 1 ms, which underlines the efficiency of FPGAs for the inference of classification models in real-time. Higher processing times for some classifiers may indicate higher computational complexity or a less optimized hardware design [
26]. The highest reported processing time is found in [
87], a time of 14.1 ms due to the model used, as it uses a binarized neural network approach. This approach is new and has the potential for further optimization. Other studies, such as [
1,
88], reported processing times of around 4 ms using spiking neural network (SNN) models. Since these models belong to the neural network category, they require longer processing times compared to other classifiers, such as the research of [
86], which reported 0.01 ms using SVM, or [
10], which recorded 0.23 ms with KNN.
Overall, using FPGAs for optimized EMG classification leveraging parallelism enables significantly faster processing times than architectures like microcontrollers or SoCs, making them well suited for real-time applications.
Regarding the energy efficiency of FPGAs, power consumption data usually refer to FPGA development boards and not to the chip alone. For example, in [
86], a power consumption of 3.1 W was reported for running a classifier based on non-negative matrix factorization on a Pynq-Z1 board. Another reported power consumption was 3.8 W when running an SVM classifier on a Zynq-7000 board, as shown in [
18].
4.5. System on a Chip
SoCs integrate multiple system components into a single chip, including CPU, GPU, memory, and communication interfaces [
106]. Platforms such as the Raspberry Pi
® and Jetson Nano
® are widely used in signal processing because they can run complex algorithms in real-time with moderate power consumption. For example, the Raspberry Pi 3
® requires 4 W of power to operate, and a Jetson Nano
® was reported in [
107] to consume 3.005 W when running a CNN model. These characteristics make SoCs suitable for classifying EMG signals, as they enable the efficient implementation of powerful machine learning algorithms, such as CNN, CRNN, and RVFLN. All this is achieved in compact and portable environments.
Table 7 shows several studies using SoCs to classify EMG signals.
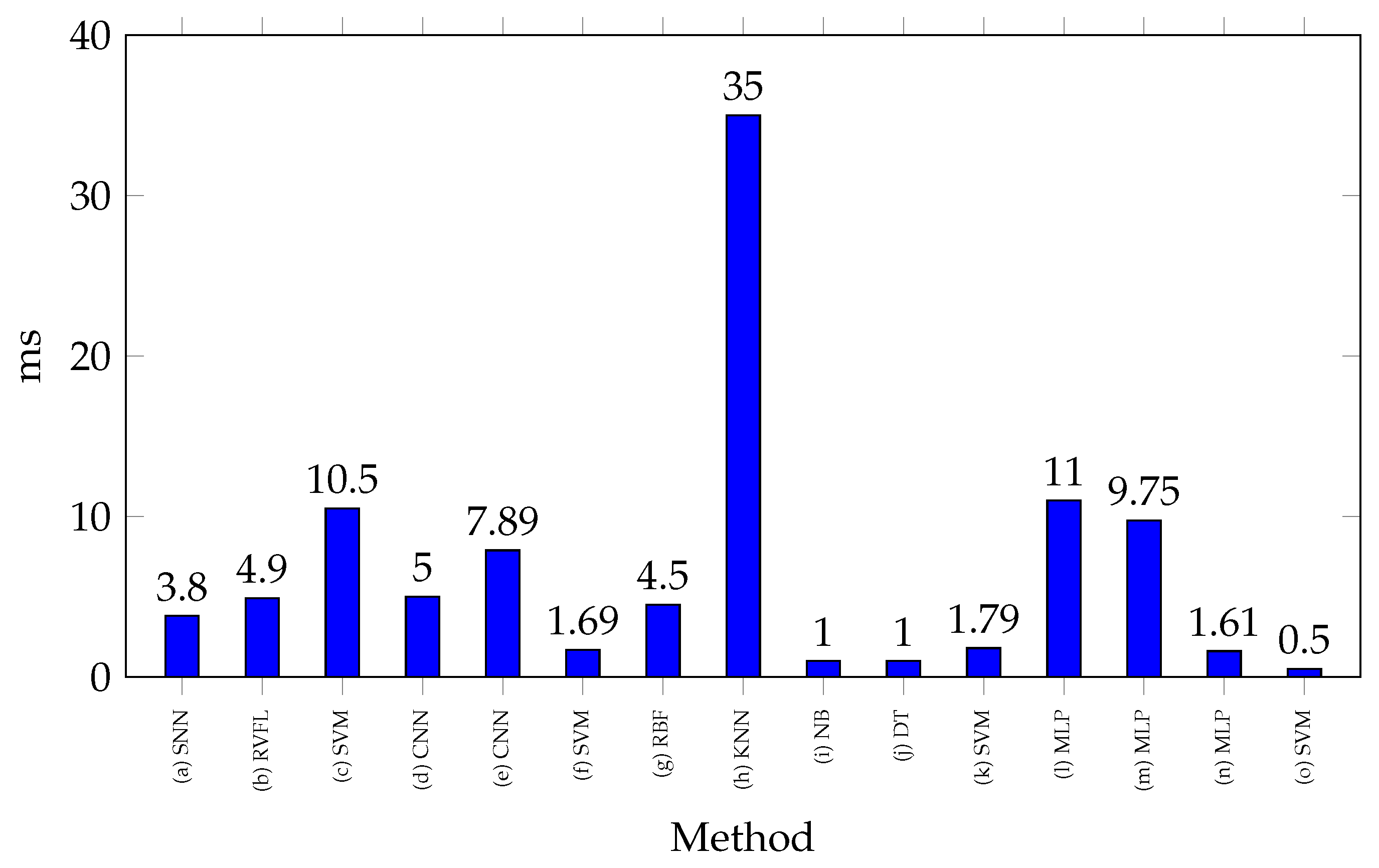
Figure 6 shows the processing times reported for classifying a gesture with SoCs. Most of the processing times recorded in these studies do not show significant differences, as they are around 5 ms, highlighting the use of complex classification methods, such as neural networks. For example, in [
111], a processing time of 7.89 ms is reported using CNN, while in [
37], 11 ms is recorded when using an MLP. Significant variations in processing times can also be observed even when using the same method. In [
46,
113], MLP-based classification reports processing times of 9.75 ms and 1.61 ms, respectively. This discrepancy is mainly attributed to the model’s architecture, as in neural networks, factors such as the number of neurons, activation functions, input size, and other parameters influence processing performance. In contrast, the study of [
32] stands out in the Figure for reporting the highest processing time, with 35 ms. This is due to its use of a sliding window classification process, executing 10 predictions at 3.5 ms each.
4.6. Neuromorphic System
Neuromorphic chips are devices whose architecture is designed to replicate the functioning of the human brain [
1,
130]. They enable the efficient implementation of algorithms for neural networks, as they process information similarly to the biological nervous system [
12]. These chips, such as ODIN
® and Loihi
®, are designed to process large amounts of data in parallel, making them ideal for applications that require high-performance signal processing, such as the classification of EMG signals when multiple sensors acquire the signals [
131]. These devices are well suited for artificial intelligence tasks as they can process large amounts of data in real-time while having low energy consumption. In [
132], it was reported that the DYNAP-SE
® chip requires only 0.05 W to classify three movements with an SNN model.
Table 8 presents several studies using neuromorphic chips to classify EMG signals.
Figure 7 shows some of the recorded results; the results of these studies demonstrate the fast response of these architectures, even when using more complex models and larger datasets in [
1]—using Loihi
®, a response time of just 5.89 ms was achieved with 96% accuracy in motion prediction. A similar time was reported in [
136], where the same neuromorphic device was used, recording 5.7 ms. In [
1], using ODIN
®, and in [
132], using DYNAP-SE
®, the classification time was approximately 25 ms, which remains remarkably fast considering the low energy consumption. In [
137], a processing time of 50 ms was reported; however, it was not included in the graph since this time includes classification signal acquisition and processing stages.
In general, neuromorphic devices provide a suitable processing time for real-time applications. However, architectures such as that in [
88] using FPGAs and in [
1] using SoCs have shown even lower processing times when using the SNN method, which is popular in neuromorphic chips. Additionally, these devices stand out for their extremely low energy consumption compared to edge computing devices.
5. Which Processor to Use in EMG Signal Classification?
The choice of the appropriate device for classifying EMG signals does not directly affect the classification accuracy. Instead, it influences the efficiency and complexity of the model during implementation. Robust or complex classification models require computing systems that meet the processing requirements for real-time applications. This relationship results in two approaches: Complex classification algorithms require robust hardware devices. At the same time, optimized embedded systems enable the execution of algorithms tailored to their resources in portable or everyday-use environments. The challenge for the user is, therefore, to find a balance between the complexity of the model, which affects classification accuracy, and its ability to be embedded in devices that meet the application’s requirements.
The embedded architectures analyzed in this study offer specific advantages and limitations. These depend on processing speed, energy consumption, and device costs.
Table 9 summarizes each architecture’s main advantages and limitations.
It is important to note that the performance in terms of classification accuracy shows minimal differences when the same algorithm is implemented on different devices. These minor variations are usually due to implementation-specific factors, such as hardware configuration, optimization techniques, or data processing. Therefore, architectural limitations have little to no impact on classification accuracy. With this in mind, the choice of processor should focus on application-specific requirements such as the processing time required to make a decision, energy consumption, and portability rather than focusing solely on higher classification accuracy.
Table 10 presents relevant research involving different devices and their characteristics.
Edge inference is becoming increasingly popular for portable applications, emphasizing the importance of embedded devices for real-time EMG signal classification and similar applications. SoCs are characterized by their ability to execute complex artificial intelligence algorithms efficiently. Meanwhile, neuromorphic chips represent an emerging option with promising latency and energy efficiency characteristics. Ultimately, the choice of device depends on how the needs and specific application requirements are assessed. These include the required classification speed, the number of sensors used for signal acquisition, the number of movements to be identified, the robustness of the model used, and the budget available for the study. In addition, it is important to consider the level of programming or design knowledge required for the device when selecting the most suitable solution. For a project that requires a robust algorithm and a limited budget, for example, a Raspberry Pi® could be the right choice. For applications that require multiple tasks at the same time, such as using multiple sensors and high processing speed, an FPGA would be a better choice.
Although the choice of device varies by application, the following guidelines can be considered based on the reviewed research:
For low-cost and low-power applications: microcontrollers or DSPs.
For high-speed and parallel processing: FPGAs.
For flexibility and simple programming with complex models: SoCs.
For applications with extremely low power consumption, high energy efficiency, and parallel processing: neuromorphic chips.
Evolution of Devices Used in EMG Analysis and Types of Gestures
The trend in the use of embedded devices for EMG classification over the years provides a global overview of the amount of literature available on this topic. This allows users to determine whether the solution they are looking for is already mature and to take advantage of previous research.
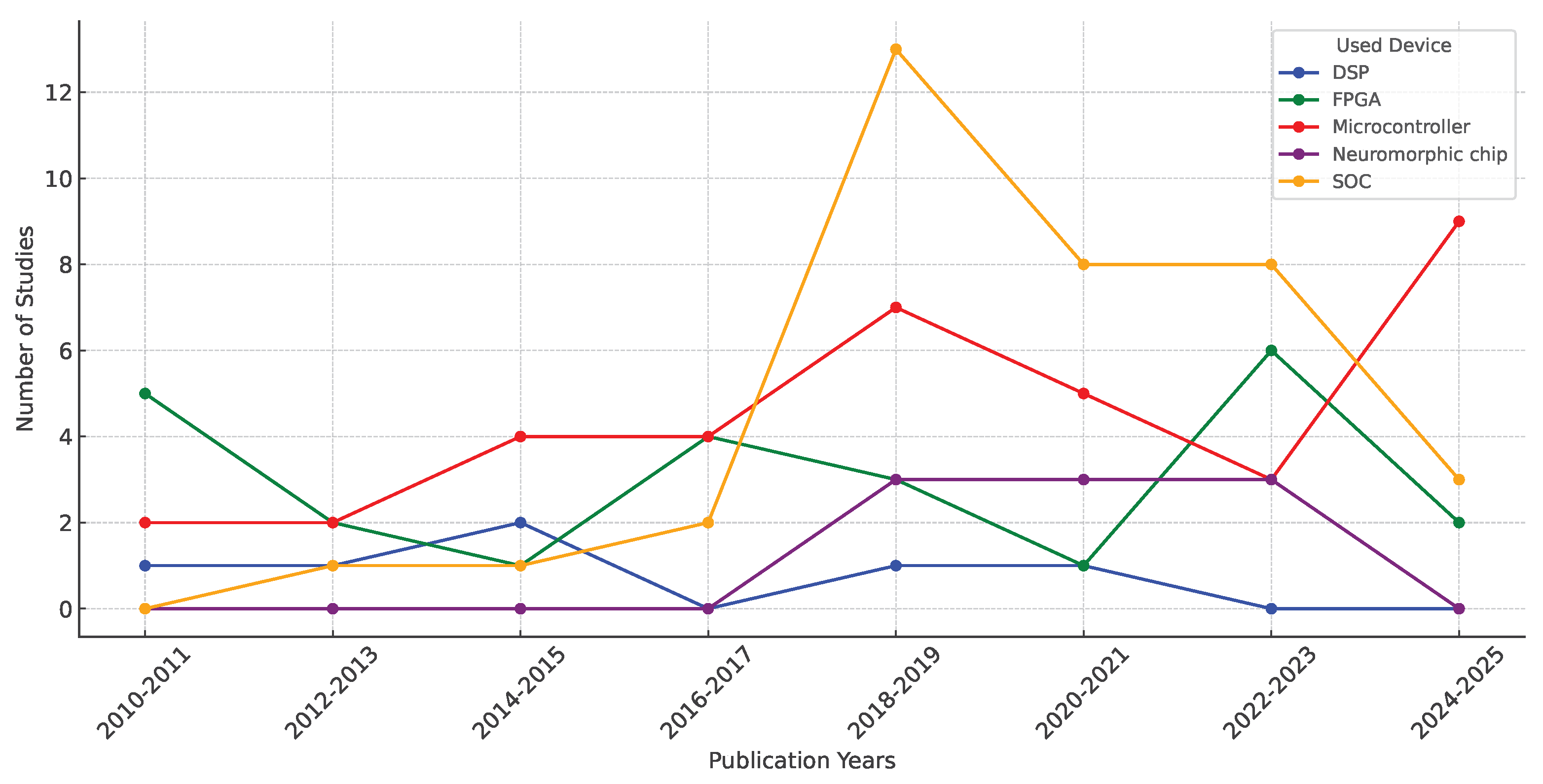
Figure 8 shows a line chart illustrating the evolution of the use of embedded devices.
Figure 8 provides an overview of the use of embedded devices in EMG analysis over the years. A continuous use of microcontrollers can be observed, with a significant increase in recent years, indicating a growing demand for portable technologies. This suggests that microcontrollers are the most widely used technology due to their extensive documentation and lower cost compared to other technologies. In contrast, DSPs have only been used sporadically, and there have been times when their use has not been reported, suggesting that they are one of the least widely used technologies for this type of application. This is mainly due to the integration of DSP functions into other architectures, such as microcontrollers and FPGAs, which incorporate DSP modules into their internal structure. On the other hand, a gradual increase in FPGA usage can be observed, especially in recent years, indicating an increasing demand for parallel processing and optimization of energy consumption in embedded applications. SoCs have grown significantly since 2018 and have become the most popular technology this year. This suggests a shift in preference towards highly integrated devices where programming complex algorithms for wearable applications is more accessible, highlighting the increasing use of artificial intelligence algorithms in EMG signal processing. Meanwhile, neuromorphic chips have seen a limited number of trials, particularly between 2018 and 2023, indicating ongoing development compared to other technologies. However, their application in EMG may expand as neuromorphic architectures continue to improve.
Another important aspect to highlight is the type of gestures or movements analyzed in the reviewed studies.
Figure 9 shows the distribution of EMG gesture types analyzed in embedded implementations. It can be observed that most studies focused their analysis on hand movements, with a total of 70 studies, followed by combined hand and wrist gestures with 26 studies. This suggests a clear trend in the analysis of upper limb gestures. This result is justified as the hand is the most important body part used in human–machine interfaces and in the control of prostheses and rehabilitation applications. In contrast, leg movements (10 studies), shoulder movements (3 studies), arm movements with the elbow (5 studies), and facial gestures (2 studies) were studied significantly less.
Since embedded devices rely on their processing capacity to implement complex classification procedures, the complexity of the recorded signal source can influence the classification process. Therefore, it is important to consider the type of movements analyzed when selecting a device to ensure efficient and accurate classification. Regarding comparability, gestures from different body parts, such as hand, arm, and leg, can use similar signal processing, feature extraction techniques, and classification algorithms. However, their biomechanical properties and noise distribution must be accounted for [
139]. Hand and arm signals are primarily associated with fine motor tasks that require precision. EMG signals from the legs may have a higher noise level as they are influenced by external factors such as ground contact. In addition, these signals originate from a larger group of muscles than the arm, resulting in more significant variability. On the other hand, facial gestures tend to be less comparable as they originate from smaller muscles with lower-amplitude activations [
139,
140].
6. Conclusions
This article gives an overview of the implementation of EMG signal classifiers in different embedded systems. It shows that each architecture has advantages and disadvantages regarding accuracy, processing time, power consumption, and cost. Microcontrollers and DSPs are suitable for low-cost and low-power applications, while FPGAs and SoCs are ideal for tasks requiring high speed and reconfiguration flexibility. In addition, FPGAs offer parallel processing, which can be helpful for a more significant number of sensors. On the other hand, neuromorphic chips provide a promising solution for applications that require energy efficiency and real-time processing. However, as these are new technologies, the steep learning curve and the price are high. The choice of the appropriate device depends on the specific requirements of the application and the limitations of the intended use. The following recommendations can serve as a guide for developers of embedded systems working with EMG-based applications:
Use microcontrollers or DSPs for portable and low-cost projects.
Use FPGAs for high-speed applications with multiple inputs.
Use SoCs for simple implementation of complex algorithms.
Use neuromorphic chips for environments with extreme power constraints.
As a recommendation, it is suggested to avoid the use of certain architectures based on their main limitations, as outlined below:
The computational capacity of microcontrollers is limited compared to the other analyzed devices.
The multitasking capability of DSPs is restricted, and as algorithm complexity increases, energy consumption also rises.
FPGAs are complex to program without a high-level compiler, and their internal resources are constrained based on cost.
SoCs have the highest energy consumption compared to the other analyzed devices.
The market availability of neuromorphic systems is limited, and specialized programming skills are required.
Finally, this work emphasizes the importance of understanding the necessary balance between device resources and model complexity when selecting an embedded architecture for EMG signal classification. The analysis provides a valuable reference for identifying the most suitable device for a given application, such as smart prostheses, portable medical devices, or wearable gesture control systems.
This review addresses current and emerging techniques as well as the challenges related to portability, resource optimization, and cost reduction. It contributes to the development of real-time and energy-efficient solutions in fields such as biomedical engineering and provides a guide for future research and innovation in this area.
Author Contributions
Conceptualization, J.F.C.-L.; data curation, J.F.C.-L. and M.A.; formal analysis, J.F.C.-L., M.A. and D.C.T.-P.; investigation, J.F.C.-L., M.A., J.R.-R. and D.C.T.-P.; methodology, J.F.C.-L., M.A., J.R.-R. and D.C.T.-P.; project administration, M.A. and J.R.-R.; resources, J.R.-R. and I.M.-S.; supervision, J.F.C.-L. and M.A.; validation J.F.C.-L. and M.A.; visualization, M.A. and J.R.-R.; writing—original draft, J.F.C.-L., M.A., J.R.-R. and D.C.T.-P.; writing—review and editing, J.F.C.-L., M.A., J.R.-R., D.C.T.-P. and I.M.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Consejo Nacional de Humanidades, Ciencia y Tecnología (CONAHCYT), and Universidad Autónoma de Querétaro (UAQ) for the resources provided.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A. Device Manufacturers
This appendix shows the manufacturers, cities and countries of the devices named in this work, which are listed in
Table A1.
Table A1.
Device manufacturers mentioned in the article.
Table A1.
Device manufacturers mentioned in the article.
| Device | Manufacturer | City | Country |
|---|
| Arduino Nano 33 BLE Sense® | Arduino® | Ivrea | Italy |
| Arduino Nano® | Arduino® | Ivrea | Italy |
| Arduino UNO® | Arduino® | Ivrea | Italy |
| ATSAML21E18B® Microcontroller | Microchip® | Chandler | USA |
| DSP dsPIC33FJ25® | Microchip® | Chandler | USA |
| DSP TMS320VC5509A® | Texas Instruments® | Dallas | USA |
| FPGA Cyclone V® | Intel® (Altera®) | San José | USA |
| FPGA Kintex 7® | Xilinx® | San José | USA |
| FPGA MAX 10® | Intel® | Santa Clara | USA |
| FPGA Stratix III® | Intel® (Altera®) | San José | USA |
| Freescale MPC5566® | Freescale® Semiconductor | Austin | USA |
| Intel Atom Z530® | Intel® | Santa Clara | USA |
| Intel Loihi® | Intel® | Santa Clara | USA |
| Jetson Nano® | NVIDIA® | Santa Clara | USA |
| Jetson TX2® | NVIDIA® | Santa Clara | USA |
| Pynq-Z1® | Xilinx® | San José | USA |
| Raspberry Pi 3® | Raspberry Pi Ltd® | Cambridge | United Kingdom |
| Raspberry Pi 3B+® | Raspberry Pi Ltd® | Cambridge | United Kingdom |
| SoC PULP® | ETH Zurich® | Zürich | Switzerland |
| Spartan-3® FPGA | Xilinx® | San José | USA |
| STM32F429® Microcontroller | STMicroelectronics® | Geneva | Switzerland |
| TMS320F28335® DSP | Texas Instruments® | Dallas | USA |
| Zynq-7000® FPGA | Xilinx® | San José | USA |
References
- Azghadi, M.R.; Lammie, C.; Eshraghian, J.K.; Payvand, M.; Donati, E.; Linares-Barranco, B.; Indiveri, G. Hardware implementation of deep network accelerators towards healthcare and biomedical applications. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 1138–1159. [Google Scholar] [CrossRef] [PubMed]
- Aviles, M.; Rodríguez-Reséndiz, J.; Ibrahimi, D. Optimizing EMG classification through metaheuristic algorithms. Technologies 2023, 11, 87. [Google Scholar] [CrossRef]
- Zheng, M.; Crouch, M.S.; Eggleston, M.S. Surface electromyography as a natural human–machine interface: A review. IEEE Sens. J. 2022, 22, 9198–9214. [Google Scholar] [CrossRef]
- Han, M.; Zandigohar, M.; Günay, S.Y.; Schirner, G.; Erdoğmuş, D. Inference of Upcoming Human Grasp Using EMG During Reach-to-Grasp Movement. Front. Neurosci. 2022, 16, 849991. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.; Boukadoum, M.; Campeau-Lecours, A.; Gosselin, B. Intuitive real-time control strategy for high-density myoelectric hand prosthesis using deep and transfer learning. Sci. Rep. 2021, 11, 11275. [Google Scholar] [CrossRef]
- Aviles, M.; Alvarez-Alvarado, J.M.; Robles-Ocampo, J.B.; Sevilla-Camacho, P.Y.; Rodríguez-Reséndiz, J. Optimizing RNNs for EMG Signal Classification: A Novel Strategy Using Grey Wolf Optimization. Bioengineering 2024, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Reaz, M.B.I.; Hussain, M.S.; Mohd-Yasin, F. Techniques of EMG signal analysis: Detection, processing, classification and applications. Biol. Proced. Online 2006, 8, 11–35. [Google Scholar] [CrossRef]
- Mastinu, E.; Ahlberg, J.; Lendaro, E.; Hermansson, L.; Håkansson, B.; Ortiz-Catalan, M. An alternative myoelectric pattern recognition approach for the control of hand prostheses: A case study of use in daily life by a dysmelia subject. IEEE J. Transl. Eng. Health Med. 2018, 6, 2600112. [Google Scholar] [CrossRef]
- Benatti, S.; Casamassima, F.; Milosevic, B.; Farella, E.; Schönle, P.; Fateh, S.; Burger, T.; Huang, Q.; Benini, L. A versatile embedded platform for EMG acquisition and gesture recognition. IEEE Trans. Biomed. Circuits Syst. 2015, 9, 620–630. [Google Scholar] [CrossRef]
- Kerdjidj, O.; Amara, K.; Harizi, F.; Boumridja, H. Implementing hand gesture recognition using EMG on the Zynq circuit. IEEE Sens. J. 2023, 23, 10054–10061. [Google Scholar] [CrossRef]
- Bonilla, D.; Bravo, M.; Bonilla, S.P.; Iragorri, A.M.; Mendez, D.; Mondragon, I.F.; Alvarado-Rojas, C.; Colorado, J.D. Progressive Rehabilitation Based on EMG Gesture Classification and an MPC-Driven Exoskeleton. Bioengineering 2023, 10, 770. [Google Scholar] [CrossRef] [PubMed]
- Bezugam, S.S.; Shaban, A.; Suri, M. Low power neuromorphic emg gesture classification. arXiv 2022, arXiv:2206.02061. [Google Scholar]
- Grueso, S.; Viejo-Sobera, R. Machine learning methods for predicting progression from mild cognitive impairment to Alzheimer’s disease dementia: A systematic review. Alzheimer’s Res. Ther. 2021, 13, 162. [Google Scholar] [CrossRef]
- Toledo-Pérez, D.C.; Rodríguez-Reséndiz, J.; Gómez-Loenzo, R.A.; Jauregui-Correa, J. Support vector machine-based EMG signal classification techniques: A review. Appl. Sci. 2019, 9, 4402. [Google Scholar] [CrossRef]
- Mereu, F.; Morosato, F.; Cordella, F.; Zollo, L.; Gruppioni, E. Exploring the EMG transient: The muscular activation sequences used as novel time-domain features for hand gestures classification. Front. Neurorobot. 2023, 17, 1264802. [Google Scholar] [CrossRef] [PubMed]
- Sanipatín-Díaz, P.A.; Rosero-Montalvo, P.D.; Hernandez, W. Portable Facial Expression System Based on EMG Sensors and Machine Learning Models. Sensors 2024, 24, 3350. [Google Scholar] [CrossRef]
- Raurale, S.; McAllister, J.; del Rincon, J.M. EMG wrist-hand motion recognition system for real-time embedded platform. In Proceedings of the ICASSP 2019—2019 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Brighton, UK, 12–17 May 2019; IEEE: New York, NY, USA, 2019; pp. 1523–1527. [Google Scholar]
- Boschmann, A.; Thombansen, G.; Witschen, L.; Wiens, A.; Platzner, M. A Zynq-based dynamically reconfigurable high density myoelectric prosthesis controller. In Proceedings of the Design, Automation & Test in Europe Conference & Exhibition (DATE), Lausanne, Switzerland, 27–31 March 2017; IEEE: New York, NY, USA, 2017; pp. 1002–1007. [Google Scholar]
- Acevedo, C.M.D.; Duarte, J.E.J. Development of an embedded system for classification of EMG signals. In Proceedings of the 2014 III International Congress of Engineering Mechatronics and Automation (CIIMA), Cartagena, Colombia, 22–24 October 2014; IEEE: New York, NY, USA, 2014; pp. 1–5. [Google Scholar]
- Majolo, M.; Balbinot, A. Proposal of a hardware SVM implementation for fast semg classification. In Proceedings of the XXVI Brazilian Congress on Biomedical Engineering: CBEB 2018, Armação de Buzios, RJ, Brazil, 21–25 October 2018; Springer: Berlin/Heidelberg, Germany, 2019; Volume 2, pp. 381–386. [Google Scholar]
- Choi, H.S. Electromyogram (EMG) signal classification based on light-weight neural network with FPGAs for wearable application. Electronics 2023, 12, 1398. [Google Scholar] [CrossRef]
- Cedeño, C.; Cordova-Garcia, J.; Asanza, V.; Ponguillo, R.; Muñoz, L. K-NN-based emg recognition for gestures communication with limited hardware resources. In Proceedings of the 2019 IEEE SmartWorld, Ubiquitous Intelligence & Computing, Advanced & Trusted Computing, Scalable Computing & Communications, Cloud & Big Data Computing, Internet of People and Smart City Innovation (SmartWorld/SCALCOM/UIC/ATC/CBDCom/IOP/SCI), Leicester, UK, 19–23 August 2019; IEEE: New York, NY, USA, 2019; pp. 812–817. [Google Scholar]
- Javaid, H.A.; Tiwana, M.I.; Alsanad, A.; Iqbal, J.; Riaz, M.T.; Ahmad, S.; Almisned, F.A. Classification of hand movements using MYO armband on an embedded platform. Electronics 2021, 10, 1322. [Google Scholar] [CrossRef]
- Manalo, K.D.; Linsangan, N.B.; Torres, J.L. Classification of myoelectric signals using multilayer perceptron neural network with back propagation algorithm in a wireless surface myoelectric prosthesis. Int. J. Inf. Educ. Technol. 2016, 6, 686. [Google Scholar] [CrossRef]
- Guzmán-Quezada, E.; Mancilla-Jiménez, C.; Rosas-Agraz, F.; Romo-Vázquez, R.; Vélez-Pérez, H. Embedded Machine Learning System for Muscle Patterns Detection in a Patient with Shoulder Disarticulation. Sensors 2024, 24, 3264. [Google Scholar] [CrossRef]
- Boschmann, A.; Agne, A.; Thombansen, G.; Witschen, L.; Kraus, F.; Platzner, M. Zynq-based acceleration of robust high density myoelectric signal processing. J. Parallel Distrib. Comput. 2019, 123, 77–89. [Google Scholar] [CrossRef]
- Suppiah, R.; Noori, K.; Abidi, K.; Sharma, A. Real-time edge computing design for physiological signal analysis and classification. Biomed. Phys. Eng. Express 2024, 10, 045034. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ke, A.; Ma, X.; He, J. SoC-based architecture for robotic prosthetics control using surface electromyography. In Proceedings of the 2016 8th International Conference on Intelligent Human-Machine Systems and Cybernetics (IHMSC), Hangzhou, China, 27–28 August 2016; IEEE: New York, NY, USA, 2016; Volume 1, pp. 134–137. [Google Scholar]
- Cene, V.H.; Balbinot, A. Enhancing the classification of hand movements through sEMG signal and non-iterative methods. Health Technol. 2019, 9, 561–577. [Google Scholar] [CrossRef]
- Garouche, M.; Thamsuwan, O. Development of a low-cost portable EMG for measuring the muscular activity of workers in the field. Sensors 2023, 23, 7873. [Google Scholar] [CrossRef] [PubMed]
- Pancholi, S.; Joshi, A.M. Electromyography-based hand gesture recognition system for upper limb amputees. IEEE Sens. Lett. 2019, 3, 5500304. [Google Scholar] [CrossRef]
- Bisi, S.; De Luca, L.; Shrestha, B.; Yang, Z.; Gandhi, V. Development of an EMG-controlled mobile robot. Robotics 2018, 7, 36. [Google Scholar] [CrossRef]
- Pancholi, S.; Joshi, A.M. Advanced energy kernel-based feature extraction scheme for improved EMG-PR-based prosthesis control against force variation. IEEE Trans. Cybern. 2020, 52, 3819–3828. [Google Scholar] [CrossRef]
- Roland, T. Motion artifact suppression for insulated EMG to control myoelectric prostheses. Sensors 2020, 20, 1031. [Google Scholar] [CrossRef]
- Choi, H.S. Siamese Neural Network for User Authentication in Field-Programmable Gate Arrays (FPGAs) for Wearable Applications. Electronics 2023, 12, 4030. [Google Scholar] [CrossRef]
- Triwiyanto, T.; Caesarendra, W.; Purnomo, M.H.; Sułowicz, M.; Wisana, I.D.G.H.; Titisari, D.; Lamidi, L.; Rismayani, R. Embedded machine learning using a multi-thread algorithm on a Raspberry Pi platform to improve prosthetic hand performance. Micromachines 2022, 13, 191. [Google Scholar] [CrossRef]
- de Souza, J.O.d.O.; Bloedow, M.D.; Rubo, F.C.; de Figueiredo, R.M.; Pessin, G.; Rigo, S.J. Investigation of different approaches to real-time control of prosthetic hands with electromyography signals. IEEE Sens. J. 2021, 21, 20674–20684. [Google Scholar] [CrossRef]
- Geethanjali, P.; Ray, K. A low-cost real-time research platform for EMG pattern recognition-based prosthetic hand. IEEE/ASME Trans. Mechatron. 2014, 20, 1948–1955. [Google Scholar] [CrossRef]
- Triwiyanto, T.; Pawana, I.P.A.; Irianto, B.G.; Indrato, T.B.; Wisana, I.D.G.H. Embedded system for upper-limb exoskeleton based on electromyography control. TELKOMNIKA (Telecommun. Comput. Electron. Control.) 2019, 17, 2992–3002. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, H.; Yang, Q. A special purpose embedded system for neural machine interface for artificial legs. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; IEEE: New York, NY, USA, 2011; pp. 5207–5210. [Google Scholar]
- Zhang, X.; Huang, H.; Yang, Q. Design and implementation of a special purpose embedded system for neural machine interface. In Proceedings of the 2010 IEEE International Conference on Computer Design, Amsterdam, The Netherlands, 3–6 October 2010; IEEE: New York, NY, USA, 2010; pp. 166–172. [Google Scholar]
- Mantilla-Brito, J.; Pozo-Espín, D.; Solórzano, S.; Morales, L. Embedded system for hand gesture recognition using EMG signals: Effect of size in the analysis windows. In Proceedings of the International Conference on Computer Science, Electronics and Industrial Engineering (CSEI), Ambato, Ecuador, 28–31 October 2019; Springer: Cham, Switzerland, 2019; pp. 214–225. [Google Scholar]
- Ayaz, M.; Ayub, M.W.; Qureshi, I.A. Arduino based fatigue level measurement in muscular activity using RMS technique. In Proceedings of the 2020 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania, 29–30 October 2020; IEEE: New York, NY, USA, 2020; pp. 1–4. [Google Scholar]
- Zhao, L.; Yuan, P.; Xiao, L.; Meng, Q. Application of digital signal processor in EMG-based human machine interface. In Proceedings of the 29th Chinese Control Conference, Beijing, China, 29–31 July 2010; IEEE: New York, NY, USA, 2010; pp. 2788–2791. [Google Scholar]
- Triwiyanto, T.; Luthfiyah, S.; Caesarendra, W.; Ahmed, A.A. Implementation of Supervised Machine Learning on Embedded Raspberry Pi System to Recognize Hand Motion as Preliminary Study for Smart Prosthetic Hand. Indones. J. Electr. Eng. Inform. (IJEEI) 2023, 11, 685–699. [Google Scholar] [CrossRef]
- Raurale, S.A.; McAllister, J.; del Rincon, J.M. Real-time embedded EMG signal analysis for wrist-hand pose identification. IEEE Trans. Signal Process. 2020, 68, 2713–2723. [Google Scholar] [CrossRef]
- Mongardi, A.; Ros, P.M.; Rossi, F.; Roch, M.R.; Martina, M.; Demarchi, D. A low-power embedded system for real-time sEMG based event-driven gesture recognition. In Proceedings of the 2019 26th IEEE International Conference on Electronics, Circuits and Systems (ICECS), Genoa, Italy, 27–29 November 2019; IEEE: New York, NY, USA, 2019; pp. 65–68. [Google Scholar]
- Cene, V.H.; Dos Santos, R.R.; Balbinot, A. Using antonyan vardan transform and extreme learning machines for accurate sEMG signal classification. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; IEEE: New York, NY, USA, 2018; pp. 5224–5227. [Google Scholar]
- Díaz, S.A.A.; Sanchez, A.D.; Gaspariano, L.A.S.; Uribe, A.Z.E. 4 channel signal based FPGA architecture for myolectric features extraction by HOS. In Proceedings of the 2010 2nd Circuits and Systems for Medical and Environmental Applications Workshop (CASME), Merida, Mexico, 13–15 December 2010; IEEE: New York, NY, USA, 2010; pp. 1–4. [Google Scholar]
- Mahaphonchaikul, K.; Sueaseenak, D.; Pintavirooj, C.; Sangworasil, M.; Tungjitkusolmun, S. EMG signal feature extraction based on wavelet transform. In Proceedings of the ECTI-CON2010: The 2010 ECTI International Confernce on Electrical Engineering/Electronics, Computer, Telecommunications and Information Technology, Chiang Mai, Thailand, 19–21 May 2010; IEEE: New York, NY, USA, 2010; pp. 327–331. [Google Scholar]
- Chanwimalueang, T.; Sueaseenak, D.; Laoopugsin, N.; Pintavirooj, C. Robotic arm controller using muscular contraction classification based on independent component analysis. In Proceedings of the 2008 5th International Conference on Electrical Engineering/Electronics, Computer, Telecommunications and Information Technology, Krabi, Thailand, 14–17 May 2008; IEEE: New York, NY, USA, 2008; Volume 2, pp. 641–644. [Google Scholar]
- Güler, N.F.; Hardalaç, F. Design of microcontroller-based EMG and the analysis of EMG signals. J. Med. Syst. 2002, 26, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.; Yang, Q.; Huang, H.; Zhang, F.; Zhang, X. Design and implementation of a low power mobile CPU based embedded system for artificial leg control. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; IEEE: New York, NY, USA, 2013; pp. 5769–5772. [Google Scholar]
- Cerina, L.; Santambrogio, M.D. Reconfigurable embedded systems applications for versatile biomedical measurements. In Proceedings of the Design, Automation & Test in Europe Conference & Exhibition (DATE), Lausanne, Switzerland, 27–31 March 2017; IEEE: New York, NY, USA, 2017; pp. 1420–1425. [Google Scholar]
- Lin, A.; Zhang, X.; Huang, H.; Yang, Q. Design and implementation of an embedded system for neural-controlled artificial legs. In Proceedings of the 2010 IEEE Workshop on Health Care Management (WHCM), Venice, Italy, 18–20 February 2010; IEEE: New York, NY, USA, 2010; pp. 1–6. [Google Scholar]
- Morón, J.; DiProva, T.; Cochrane, J.R.; Ahn, I.S.; Lu, Y. EMG-based hand gesture control system for robotics. In Proceedings of the 2018 IEEE 61st International Midwest Symposium on Circuits and Systems (MWSCAS), Windsor, ON, Canada, 5–8 August 2018; IEEE: New York, NY, USA, 2018; pp. 664–667. [Google Scholar]
- Praveen, L.; Nagananda, S.; Shankapal, P. Design and development of real time bionic hand control using emg signal. In Proceedings of the 2018 IEEE International Conference on Electronics, Computing and Communication Technologies (CONECCT), Bangalore, India, 16–17 March 2018; IEEE: New York, NY, USA, 2018; pp. 1–4. [Google Scholar]
- Liu, Y.H.; Huang, H.P.; Weng, C.H. Recognition of electromyographic signals using cascaded kernel learning machine. IEEE/ASME Trans. Mechatron. 2007, 12, 253–264. [Google Scholar] [CrossRef]
- Chang, G.C.; Kang, W.J.; Luh, J.J.; Cheng, C.K.; Lai, J.S.; Chen, J.J.J.; Kuo, T.S. Real-time implementation of electromyogram pattern recognition as a control command of man-machine interface. Med. Eng. Phys. 1996, 18, 529–537. [Google Scholar] [CrossRef]
- Huang, H.P.; Liu, Y.H.; Wong, C.S. Automatic EMG feature evaluation for controlling a prosthetic hand using supervised feature mining method: An intelligent approach. In Proceedings of the 2003 IEEE International Conference on Robotics and Automation (Cat. No. 03CH37422), Taiperi, Taiwan, 14–19 September 2003; IEEE: New York, NY, USA, 2003; Volume 1, pp. 220–225. [Google Scholar]
- Huang, H.P.; Chiang, C.Y. DSP-based controller for a multi-degree prosthetic hand. In Proceedings of the 2000 ICRA. Millennium Conference. IEEE International Conference on Robotics and Automation. Symposia Proceedings (Cat. No. 00CH37065), San Francisco, CA, USA, 24–28 April 2000; IEEE: New York, NY, USA, 2000; Volume 2, pp. 1378–1383. [Google Scholar]
- Alkim, E.; Kiliç, E. Chip design for intelligent data classification algorithms and implementation on an fpga: A case study to classify emg signals. In Proceedings of the 2011 IEEE 19th Signal Processing and Communications Applications Conference (SIU), Antalya, Turkey, 20–22 April 2011; IEEE: New York, NY, USA, 2011; pp. 307–310. [Google Scholar]
- Marco, V.S.; Taylor, B.; Wang, Z.; Elkhatib, Y. Optimizing deep learning inference on embedded systems through adaptive model selection. ACM Trans. Embed. Comput. Syst. (TECS) 2020, 19, 1–28. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, J.; Wang, Y.; Liu, Y.; Liu, Q.; Luo, J.; Wang, C.; Ren, F.; Huang, L. A review of algorithm & hardware design for AI-based biomedical applications. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 145–163. [Google Scholar]
- Li, E.; Zeng, L.; Zhou, Z.; Chen, X. Edge AI: On-demand accelerating deep neural network inference via edge computing. IEEE Trans. Wirel. Commun. 2019, 19, 447–457. [Google Scholar] [CrossRef]
- Xu, X.; Ding, Y.; Hu, S.X.; Niemier, M.; Cong, J.; Hu, Y.; Shi, Y. Scaling for edge inference of deep neural networks. Nat. Electron. 2018, 1, 216–222. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, H.; Yang, Q. Real-time implementation of a self-recovery EMG pattern recognition interface for artificial arms. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; IEEE: New York, NY, USA, 2013; pp. 5926–5929. [Google Scholar]
- Xu, K.; Guo, W.; Hua, L.; Sheng, X.; Zhu, X. A prosthetic arm based on EMG pattern recognition. In Proceedings of the 2016 IEEE International Conference on Robotics and Biomimetics (ROBIO), Qingdao, China, 3–7 December 2016; IEEE: New York, NY, USA, 2016; pp. 1179–1184. [Google Scholar]
- Lu, C.; Xu, X.; Liu, Y.; Li, D.; Wang, Y.; Xian, W.; Chen, C.; Wei, B.; Tian, J. An Embedded Electromyogram Signal Acquisition Device. Sensors 2024, 24, 4106. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Zhang, J.; Shen, H.; Wang, Y. Arm movements recognition by implementing CNN on microcontrollers. In Proceedings of the 2021 9th International Conference on Control, Mechatronics and Automation (ICCMA), Belval, Luxembourg, 11–14 November 2021; IEEE: New York, NY, USA, 2021; pp. 171–176. [Google Scholar]
- Dadi, P.S.; Rajasekar, B.; Surendran, R.; Rahasekar, B.; Surendran, R. Exoskeleton Pysiotherapy and Assistive Robotic Arm. In Proceedings of the 2024 2nd International Conference on Sustainable Computing and Smart Systems (ICSCSS), Coimbatore, India, 10–12 July 2024; IEEE: New York, NY, USA, 2024; pp. 138–141. [Google Scholar]
- Bezrukov, V.A.; Vakhitov, R.R.; Chuykin, S.A.; Anuchin, P.Y.; Kruglov, A.V.; Siziakova, A.Y. Evaluating the accuracy of knn classifier for gesture detection based on forearm emg signal. In Proceedings of the 2024 6th International Youth Conference on Radio Electronics, Electrical and Power Engineering (REEPE), Moscow, Russian, 29 February–2 March 2024; IEEE: New York, NY, USA, 2024; pp. 1–5. [Google Scholar]
- Malesevic, N.; Björkman, A.; Andersson, G.S.; Cipriani, C.; Antfolk, C. Evaluation of simple algorithms for proportional control of prosthetic hands using intramuscular electromyography. Sensors 2022, 22, 5054. [Google Scholar] [CrossRef]
- Huang, H.; Sun, Y.; Yang, Q.; Zhang, F.; Zhang, X.; Liu, Y.; Ren, J.; Sierra, F. Integrating neuromuscular and cyber systems for neural control of artificial legs. In Proceedings of the 1st ACM/IEEE International Conference on Cyber-Physical Systems, Stockholm, Sweden, 13–15 April 2010; pp. 129–138. [Google Scholar]
- Calderon, C.; Jaramillo, L.; Zuñiga, J.; Hernandez, W.; Rivas-Echeverría, F. A Neural Network embedded system for real-time identification of EMG signals. In Proceedings of the 2018 IEEE International Conference on Automation/XXIII Congress of the Chilean Association of Automatic Control (ICA-ACCA), Concepcion, Chile, 17–19 October 2018; IEEE: New York, NY, USA, 2018; pp. 1–7. [Google Scholar]
- Benatti, S.; Milosevic, B.; Casamassima, F.; Schönle, P.; Bunjaku, P.; Fateh, S.; Huang, Q.; Benini, L. EMG-based hand gesture recognition with flexible analog front end. In Proceedings of the 2014 IEEE Biomedical Circuits and Systems Conference (BioCAS) Proceedings, Lausanne, Switzerland, 22–24 October 2014; IEEE: New York, NY, USA, 2014; pp. 57–60. [Google Scholar]
- Yang, D.; Chhatre, N.; Campi, F.; Menon, C. Feasibility of Support Vector Machine gesture classification on a wearable embedded device. In Proceedings of the 2016 IEEE Canadian Conference on Electrical and Computer Engineering (CCECE), Vancouver, BC, Canada, 15–18 May 2016; IEEE: New York, NY, USA, 2016; pp. 1–4. [Google Scholar]
- Rafiq, A.; Talukder, Z.; Hamim, A.; Firdous, J.; Ahmed, K.I.U. sEMG Signal-Based Prosthetic Hand Control Using Embedded Machine Learning with Deep Neural Network. In Proceedings of the 2024 6th International Conference on Electrical Engineering and Information & Communication Technology (ICEEICT), Dhaka, Bangladesh, 2–4 May 2024; IEEE: New York, NY, USA, 2024; pp. 382–386. [Google Scholar]
- Zanghieri, M.; Benatti, S.; Burrello, A.; Kartsch, V.; Conti, F.; Benini, L. Robust real-time embedded EMG recognition framework using temporal convolutional networks on a multicore IoT processor. IEEE Trans. Biomed. Circuits Syst. 2019, 14, 244–256. [Google Scholar] [CrossRef]
- Cisnal, A.; Pérez-Turiel, J.; Fraile, J.C.; Sierra, D.; de la Fuente, E. Robhand: A hand exoskeleton with real-time emg-driven embedded control. quantifying hand gesture recognition delays for bilateral rehabilitation. IEEE Access 2021, 9, 137809–137823. [Google Scholar] [CrossRef]
- Bitar, F.; Madi, N.; Ramly, E.; Saghir, M.; Karameh, F. A portable MIDI controller using EMG-based individual finger motion classification. In Proceedings of the 2007 IEEE Biomedical Circuits and Systems Conference, Montreal, QC, Canada, 27–30 November 2007; IEEE: New York, NY, USA, 2007; pp. 138–141. [Google Scholar]
- Tenore, F.; Armiger, R.S.; Vogelstein, R.J.; Wenstrand, D.S.; Harshbarger, S.D.; Englehart, K. An embedded controller for a 7-degree of freedom prosthetic arm. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; IEEE: New York, NY, USA, 2008; pp. 185–188. [Google Scholar]
- Salem, F.H.; Mohamed, K.S.; Mohamed, S.B.; El Gehani, A.A. The Development of Body-Powered Prosthetic Hand Controlled by EMG Signals Using DSP Processor with Virtual Prosthesis Implementation. Conf. Pap. Sci. 2013, 2013, 598945. [Google Scholar] [CrossRef]
- Ahsan, M.; Ibrahimy, M.; Khalifa, O.; Ullah, M. VHDL modeling of EMG signal classification using artificial neural network. J. Appl. Sci. 2012, 12, 244–253. [Google Scholar] [CrossRef][Green Version]
- Liu, W.; Guo, Q.; Chen, S.; Chang, S.; Wang, H.; He, J.; Huang, Q. A fully-mapped and energy-efficient FPGA accelerator for dual-function AI-based analysis of ECG. Front. Physiol. 2023, 14, 1079503. [Google Scholar] [CrossRef]
- Franco, G.; Cancian, P.; Cerina, L.; Besana, E.; Beretta, N.; Santambrogio, M.D. FPGA-based muscle synergy extraction for surface EMG gesture classification. In Proceedings of the 2017 IEEE Biomedical Circuits and Systems Conference (BioCAS), Turin, Italy, 19–21 October 2017; IEEE: New York, NY, USA, 2017; pp. 1–4. [Google Scholar]
- Kang, S.; Kim, H.; Park, C.; Sim, Y.; Lee, S.; Jung, Y. sEMG-based hand gesture recognition using binarized neural network. Sensors 2023, 23, 1436. [Google Scholar] [CrossRef]
- Scrugli, M.A.; Leone, G.; Busia, P.; Raffo, L.; Meloni, P. Real-Time sEMG Processing with Spiking Neural Networks on a Low-Power 5K-LUT FPGA. IEEE Trans. Biomed. Circuits Syst. 2024, 19, 68–81. [Google Scholar] [CrossRef]
- Wöhrle, H.; Tabie, M.; Kim, S.K.; Kirchner, F.; Kirchner, E.A. A hybrid FPGA-based system for EEG-and EMG-based online movement prediction. Sensors 2017, 17, 1552. [Google Scholar] [CrossRef]
- Reyes, D.; López, M.; Duarte, J.; Loaiza, H. Implementación en FPGA de un clasificador de movimientos de la mano usando señales EMG. Redes Ing. 2015, 6, 85–94. [Google Scholar] [CrossRef]
- Proaño-Guevara, D.; Blanco Valencia, X.P.; Rosero-Montalvo, P.D.; Peluffo-Ordóñez, D.H. Electromiographic Signal Processing Using Embedded Artificial Intelligence: An Adaptive Filtering Approach. Int. J. Interact. Multimed. Artif. Intell. 2022, 7, 40–50. [Google Scholar] [CrossRef]
- Góra, J.; Szecówka, P.M.; Wolczowski, A.R. Control of dexterous hand-algorithm implementation issues. In Proceedings of the 2009 9th International Conference on Information Technology and Applications in Biomedicine, Larnaka, Cyprus, 4–7 November 2009; IEEE: New York, NY, USA, 2009; pp. 1–4. [Google Scholar]
- Góra, J.; Szecówka, P.M.; Wołczowski, A.R. Fft based emg signals analysis on fpgas for dexterous hand prosthesis control. In Man-Machine Interactions; Springer: Berlin/Heidelberg, Germany, 2009; pp. 655–662. [Google Scholar]
- Szecówka, P.M.; Spyra, A.; Pçdzińska-Rzany, J.; Wołczowski, A.R. Artificial hand control by myoelectric signals—Reduced DFT approach. In Proceedings of the 2010 15th International Conference on Methods and Models in Automation and Robotics, Miedzyzdroje, Poland, 23–26 August 2010; IEEE: New York, NY, USA, 2010; pp. 170–173. [Google Scholar]
- Glette, K.; Gruber, T.; Kaufmann, P.; Torresen, J.; Sick, B.; Platzner, M. Comparing evolvable hardware to conventional classifiers for electromyographic prosthetic hand control. In Proceedings of the 2008 NASA/ESA Conference on Adaptive Hardware and Systems, Noordwijk, The Netherlands, 22–25 June 2008; IEEE: New York, NY, USA, 2008; pp. 32–39. [Google Scholar]
- De Venuto, D.; Mezzina, G. Neuromuscular disorders assessment by FPGA-based SVM classification of synchronized EEG/EMG. In Applications in Electronics Pervading Industry, Environment and Society: APPLEPIES 2018; Springer: Cham, Switzerland, 2019; pp. 37–44. [Google Scholar]
- Annese, V.F.; De Venuto, D. FPGA based architecture for fall-risk assessment during gait monitoring by synchronous EEG/EMG. In Proceedings of the 2015 6th International Workshop on Advances in Sensors and Interfaces (IWASI), Gallipoli, Italy, 18–19 June 2015; IEEE: New York, NY, USA, 2015; pp. 116–121. [Google Scholar]
- Guo, Y.; Gou, G.; Yao, P.; Gao, F.; Ma, T.; Sun, J.; Han, M.; Cheng, J.; Liu, C.; Zhao, M.; et al. FPGA-based Lightweight QDS-CNN System for sEMG Gesture and Force Level Recognition. IEEE Trans. Biomed. Circuits Syst. 2024, 1–14. [Google Scholar] [CrossRef]
- Choi, H.S. Drowsy driving detection using neural network with backpropagation algorithm implemented by FPGA. Concurr. Comput. Pract. Exp. 2020, 32, e5471. [Google Scholar] [CrossRef]
- Shima, K.; Tsuji, T. FPGA implementation of a probabilistic neural network using delta-sigma modulation for pattern discrimination of EMG signals. In Proceedings of the 2007 IEEE/ICME International Conference on Complex Medical Engineering, Beijing, China, 23–27 May 2007; IEEE: New York, NY, USA, 2007; pp. 402–407. [Google Scholar]
- Choi, H.S. Simple Siamese Model with Long Short-Term Memory for User Authentication with Field-Programmable Gate Arrays. Electronics 2024, 13, 2584. [Google Scholar] [CrossRef]
- De Venuto, D.; Mezzina, G. Multisensing System for Parkinson’s Disease Stage Assessment Based on FPGA-Embedded Serial SVM Classifier. IEEE Des. Test 2019, 38, 44–51. [Google Scholar] [CrossRef]
- Glette, K.; Kaufmann, P.; Assad, C.; Wolf, M.T. Investigating evolvable hardware classification for the biosleeve electromyographic interface. In Proceedings of the 2013 IEEE International Conference on Evolvable Systems (ICES), Singapore, 16–19 April 2013; IEEE: New York, NY, USA, 2013; pp. 73–80. [Google Scholar]
- Amelin, D.; Potapov, I.; Audí, J.C.; Kogut, A.; Rupp, R.; Ruff, R.; Hoffmann, K.P. Real-time classification of hand movements as a basis for intuitive control of grasp neuroprostheses. Curr. Dir. Biomed. Eng. 2020, 6, 20202011. [Google Scholar] [CrossRef]
- Ogbodo, M.I.; Dang, K.N.; Abdallah, A.B. Study of a multi-modal neurorobotic prosthetic arm control system based on recurrent spiking neural network. SHS Web Conf. 2022, 139, 03019. [Google Scholar] [CrossRef]
- Triwiyanto, T.; Caesarendra, W.; Abdullayev, V.; Ahmed, A.A.; Herianto, H. Single Lead EMG signal to Control an Upper Limb Exoskeleton Using Embedded Machine Learning on Raspberry Pi. J. Robot. Control. (JRC) 2023, 4, 35–45. [Google Scholar] [CrossRef]
- Tam, S.; Boukadoum, M.; Campeau-Lecours, A.; Gosselin, B. A fully embedded adaptive real-time hand gesture classifier leveraging HD-sEMG and deep learning. IEEE Trans. Biomed. Circuits Syst. 2019, 14, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Attenberger, A.; Buchenrieder, K. MATLAB/simulink-supported EMG classification on the raspberry Pi. In Proceedings of the Computer Aided Systems Theory–EUROCAST 2015: 15th International Conference, Las Palmas de Gran Canaria, Spain, 8–13 February 2015; Revised Selected Papers 15. Springer: Cham, Switzerland, 2015; pp. 449–456. [Google Scholar]
- Savithri, C.; Priya, E.; Sudharsanan, J. Classification of semg signal-based arm action using convolutional neural network. In Signal and Image Processing Techniques for the Development of Intelligent Healthcare Systems; Springer: Singapore, 2021; pp. 241–259. [Google Scholar]
- Kim, E.; Shin, J.; Kwon, Y.; Park, B. EMG-based dynamic hand gesture recognition using edge AI for human–robot interaction. Electronics 2023, 12, 1541. [Google Scholar] [CrossRef]
- Hartwell, A.; Kadirkamanathan, V.; Anderson, S. Compact Deep Neural Networks for Computationally Efficient Gesture Classification From Electromyography Signals. arXiv 2018, arXiv:1806.08641. [Google Scholar]
- Jafarzadeh, M.; Hussey, D.C.; Tadesse, Y. Deep learning approach to control of prosthetic hands with electromyography signals. In Proceedings of the 2019 IEEE International Symposium on Measurement and Control in Robotics (ISMCR), Houston, TX, USA, 19–21 September 2019; IEEE: New York, NY, USA, 2019; pp. A1–A4. [Google Scholar]
- Raurale, S.A.; McAllister, J.; Del Rincón, J.M. EMG biometric systems based on different wrist-hand movements. IEEE Access 2021, 9, 12256–12266. [Google Scholar] [CrossRef]
- Benatti, S.; Rovere, G.; Bösser, J.; Montagna, F.; Farella, E.; Glaser, H.; Schönle, P.; Burger, T.; Fateh, S.; Huang, Q.; et al. A sub-10mW real-time implementation for EMG hand gesture recognition based on a multi-core biomedical SoC. In Proceedings of the 2017 7th IEEE International Workshop on Advances in Sensors and Interfaces (IWASI), Vieste, Italy, 15–16 June 2017; IEEE: New York, NY, USA, 2017; pp. 139–144. [Google Scholar]
- Espinoza, D.L.; Velasco, L.E.S. Comparison of emg signal classification algorithms for the control of an upper limb prosthesis prototype. In Proceedings of the 2020 17th International Conference on Electrical Engineering, Computing Science and Automatic Control (CCE), Mexico City, Mexico, 11–13 November 2020; IEEE: New York, NY, USA, 2020; pp. 1–4. [Google Scholar]
- Cárdenas-Valdez, J.R.; Valdez-Luis, D.; de Jesús García-Ortega, M.; Corral-Domínguez, A.H.; Galaviz-Aguilar, J.A. SVM Classifier and evaluation of muscle power of EMG signals and Python implementation. In Proceedings of the 2020 International Conference on Mechatronics, Electronics and Automotive Engineering (ICMEAE), Cuernavaca, Mexico, 16–21 November 2020; IEEE: New York, NY, USA, 2020; pp. 20–22. [Google Scholar]
- Falih, A.D.I.; Dharma, W.A.; Sumpeno, S. Classification of EMG signals from forearm muscles as automatic control using Naive Bayes. In Proceedings of the 2017 International Seminar on Intelligent Technology and Its Applications (ISITIA), Surabaya, Indonesia, 28–29 August 2017; IEEE: New York, NY, USA, 2017; pp. 346–351. [Google Scholar]
- Akmal, M.; Qureshi, M.F.; Amin, F.; Rehman, M.Z.U.; Niazi, I.K. SVM-based real-time classification of prosthetic fingers using myo armband-acquired electromyography data. In Proceedings of the 2021 IEEE 21st International Conference on Bioinformatics and Bioengineering (BIBE), Kragujevac, Serbia, 25–27 October 2021; IEEE: New York, NY, USA, 2021; pp. 1–5. [Google Scholar]
- Kumar, B.; Paul, Y.; Jaswal, R.A. Development of emg controlled electric wheelchair using svm and knn classifier for sci patients. In Proceedings of the Advanced Informatics for Computing Research: Third International Conference, ICAICR 2019, Shimla, India, 15–16 June 2019; Revised Selected Papers, Part II 3. Springer: Singapore, 2019; pp. 75–83. [Google Scholar]
- de Freitas, R.C.; Alves, R.; da Silva Filho, A.G.; de Souza, R.E.; Bezerra, B.L.; dos Santos, W.P. Electromyography-controlled car: A proof of concept based on surface electromyography, Extreme Learning Machines and low-cost open hardware. Comput. Electr. Eng. 2019, 73, 167–179. [Google Scholar] [CrossRef]
- Babu, R.D.; Adithya, S.S.; Dhanalakshmi, M. Design and development of an EMG controlled transfemoral prosthesis. Meas. Sensors 2024, 36, 101399. [Google Scholar] [CrossRef]
- Ferdiansyah, F.A.; Prajitno, P.; Wijaya, S.K. EEG-EMG based bio-robotics elbow orthotics control. J. Phys. Conf. Ser. 2020, 1528, 012033. [Google Scholar] [CrossRef]
- Laganà, F.; Pratticò, D.; Angiulli, G.; Oliva, G.; Pullano, S.A.; Versaci, M.; La Foresta, F. Development of an Integrated System of sEMG Signal Acquisition, Processing, and Analysis with AI Techniques. Signals 2024, 5, 476–493. [Google Scholar] [CrossRef]
- Gardner, N.; Tekes, C.; Weinberg, N.; Ray, N.; Duran, J.; Housley, S.N.; Wu, D.; Hung, C.C. EMG based simultaneous wrist motion prediction using reinforcement learning. In Proceedings of the 2020 IEEE 20th International Conference on Bioinformatics and Bioengineering (BIBE), Chncinnati, OH, USA, 26–28 October 2020; IEEE: New York, NY, USA, 2020; pp. 1016–1021. [Google Scholar]
- Yılmaz, A.; Büyükyılmaz, B.; Sert, H.C.; Uğuroğlu, O.; Algüner, A.E. Hand Movement Classification with Four Channel EMG Signals for Underactuated Hand Prosthesis Test Platform. In Proceedings of the 2024 32nd Signal Processing and Communications Applications Conference (SIU), Mersin, Turkey, 15–18 May 2024; IEEE: New York, NY, USA, 2024; pp. 1–4. [Google Scholar]
- Nguyen, A.T.; Drealan, M.W.; Luu, D.K.; Jiang, M.; Xu, J.; Cheng, J.; Zhao, Q.; Keefer, E.W.; Yang, Z. A portable, self-contained neuroprosthetic hand with deep learning-based finger control. J. Neural Eng. 2021, 18, 056051. [Google Scholar] [CrossRef]
- Yang, Z.; Clark, A.B.; Chappell, D.; Rojas, N. Instinctive real-time SEMG-based control of prosthetic hand with reduced data acquisition and embedded deep learning training. In Proceedings of the 2022 International Conference on Robotics and Automation (ICRA), Philadelphia, PA, USA, 23–27 May 2022; IEEE: New York, NY, USA, 2022; pp. 5666–5672. [Google Scholar]
- Yu, G.; Deng, Z.; Bao, Z.; Zhang, Y.; He, B. Gesture Classification in Electromyography Signals for Real-Time Prosthetic Hand Control Using a Convolutional Neural Network-Enhanced Channel Attention Model. Bioengineering 2023, 10, 1324. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lu, Y.; An, Q.; Chen, C.; Li, Y.; Wang, Y. Real-time multiple gesture recognition: Application of a lightweight individualized 1D CNN model to an edge computing system. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Donati, E.; Chen, B.; Ren, P.; Zheng, N.; Indiveri, G. Neuromorphic implementation of a recurrent neural network for EMG classification. In Proceedings of the 2020 2nd IEEE International Conference on Artificial Intelligence Circuits and Systems (AICAS), Genova, Italy, 31 August–2 September 2020; IEEE: New York, NY, USA, 2020; pp. 69–73. [Google Scholar]
- Ceolini, E.; Frenkel, C.; Shrestha, S.B.; Taverni, G.; Khacef, L.; Payvand, M.; Donati, E. Hand-gesture recognition based on EMG and event-based camera sensor fusion: A benchmark in neuromorphic computing. Front. Neurosci. 2020, 14, 637. [Google Scholar] [CrossRef]
- Donati, E.; Payvand, M.; Risi, N.; Krause, R.; Indiveri, G. Discrimination of EMG signals using a neuromorphic implementation of a spiking neural network. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 795–803. [Google Scholar] [CrossRef]
- Donati, E.; Payvand, M.; Risi, N.; Krause, R.; Burelo, K.; Indiveri, G.; Dalgaty, T.; Vianello, E. Processing EMG signals using reservoir computing on an event-based neuromorphic system. In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS), Cleveland, OH, USA, 17–19 October 2018; IEEE: New York, NY, USA, 2018; pp. 1–4. [Google Scholar]
- Behrenbeck, J.; Tayeb, Z.; Bhiri, C.; Richter, C.; Rhodes, O.; Kasabov, N.; Espinosa-Ramos, J.I.; Furber, S.; Cheng, G.; Conradt, J. Classification and regression of spatio-temporal signals using NeuCube and its realization on SpiNNaker neuromorphic hardware. J. Neural Eng. 2019, 16, 026014. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, B.; Ren, P.; Zheng, N.; Indiveri, G.; Donati, E. EMG-based gestures classification using a mixed-signal neuromorphic processing system. IEEE J. Emerg. Sel. Top. Circuits Syst. 2020, 10, 578–587. [Google Scholar] [CrossRef]
- Vitale, A.; Donati, E.; Germann, R.; Magno, M. Neuromorphic edge computing for biomedical applications: Gesture classification using emg signals. IEEE Sens. J. 2022, 22, 19490–19499. [Google Scholar] [CrossRef]
- Bezugam, S.S.; Shaban, A.; Suri, M. Neuromorphic recurrent spiking neural networks for emg gesture classification and low power implementation on loihi. In Proceedings of the 2023 IEEE International Symposium on Circuits and Systems (ISCAS), Monterey, CA, USA, 21–25 May 2023; IEEE: New York, NY, USA, 2023; pp. 1–5. [Google Scholar]
- George, J.A.; Radhakrishnan, S.; Brinton, M.; Clark, G.A. Inexpensive and portable system for dexterous high-density myoelectric control of multiarticulate prostheses. In Proceedings of the 2020 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Toronto, ON, Canada, 11–14 October 2020; IEEE: New York, NY, USA, 2020; pp. 3441–3446. [Google Scholar]
- Ahkami, B.; Ahmed, K.; Thesleff, A.; Hargrove, L.; Ortiz-Catalan, M. Electromyography-based control of lower limb prostheses: A systematic review. IEEE Trans. Med. Robot. Bionics 2023, 5, 547–562. [Google Scholar] [CrossRef]
- Gohel, V.; Mehendale, N. Review on electromyography signal acquisition and processing. Biophys. Rev. 2020, 12, 1361–1367. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).