Enhanced Bladder Regeneration with Adipose-Derived Stem Cell-Seeded Silk Fibroin Scaffolds: A Comparative Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Fabrication of SF Scaffold
2.3. ADSCs Culture and Seeding
2.4. Surgical Technique for Rat Bladder Augmentation
2.5. Histological and Immunohistochemical Evaluation of Regenerative Processes in the Reconstructed Bladder Wall
2.6. Statistical Analysis
3. Results
3.1. Surgical Outcomes and Post-Operative Recovery
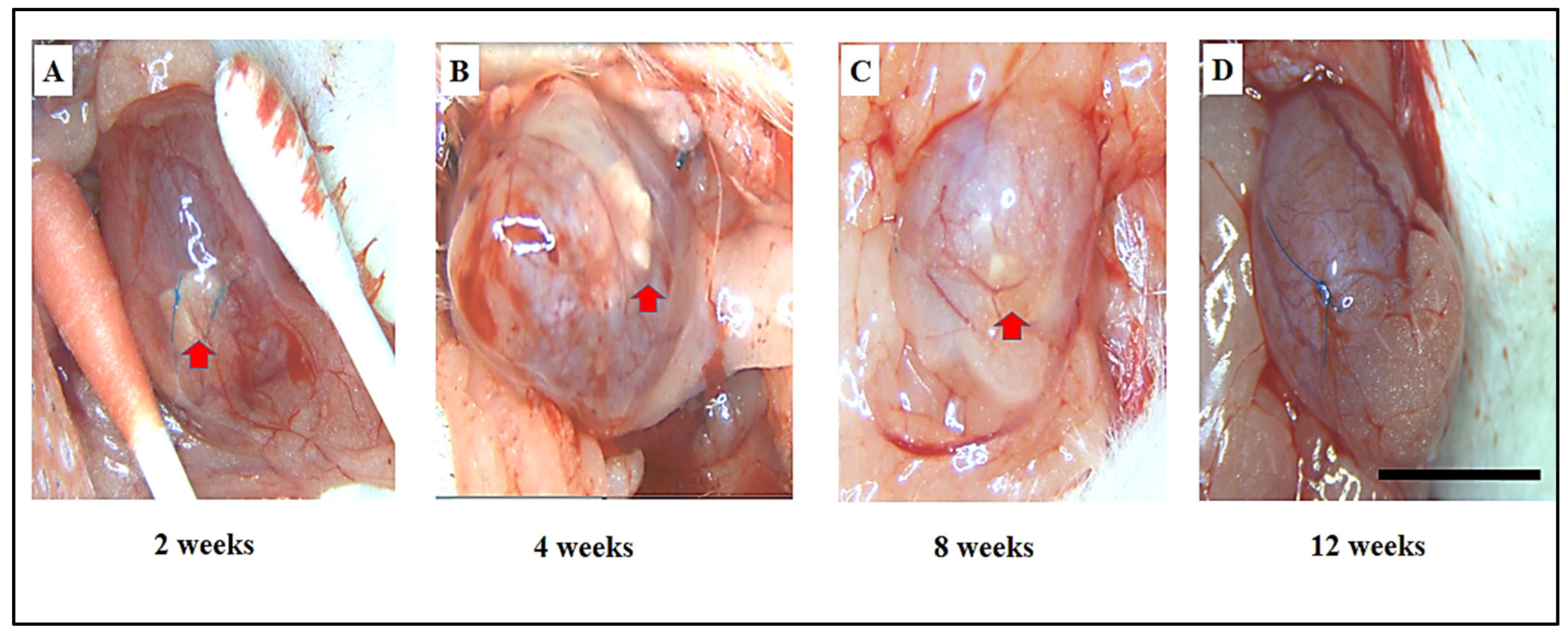
3.2. Assessment of Adhesions, Graft Shrinkage, and Morphological Changes in Bladder Regeneration Using SF and ADSCs-SF Scaffolds
3.3. Comparative Histological Analysis of Urothelium, Muscle Regeneration, Vessel Density, and Inflammatory Response in SF and ADSCs-SF Scaffold Implants
3.3.1. Urothelium
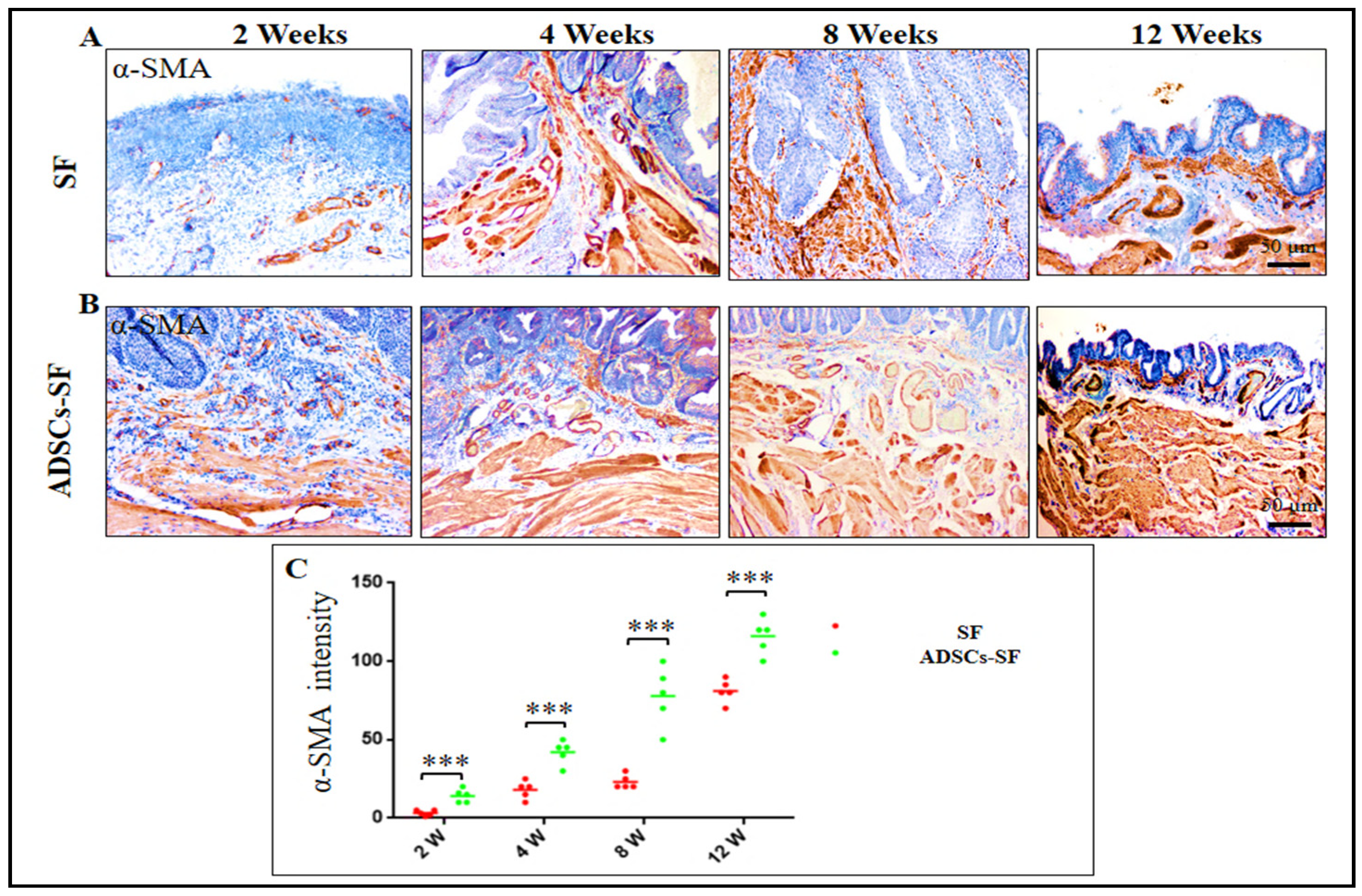
3.3.2. Smooth Muscle Cells
3.3.3. Vessel Density
3.3.4. Inflammatory Cells
3.4. Urothelial Regeneration and Uroplakin III Expression in ADSCs-SF and SF Scaffolds
3.5. Smooth Muscle Cell Regeneration in ADSCs-SF and SF Scaffolds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novak, T.E.; Salmasi, A.H.; Mathews, R.I.; Lakshmanan, Y.; Gearhart, J.P. Complications of complex lower urinary tract reconstruction in patients with neurogenic versus nonneurogenic bladder—Is there a difference? J. Urol. 2008, 180, 2629–2635. [Google Scholar] [CrossRef] [PubMed]
- Topoliova, K.; Harsanyi, S.; Danisovic, L.; Ziaran, S. Tissue engineering and stem cell therapy in neurogenic bladder dysfunction: Current and future perspectives. Medicina 2023, 59, 1416. [Google Scholar] [CrossRef]
- Ławkowska, K.; Rosenbaum, C.; Petrasz, P.; Kluth, L.; Koper, K.; Drewa, T.; Pokrywczynska, M.; Adamowicz, J.; Trauma and Reconstructive Urology Working Party of the European Association of Urology Young Academic Urologists. Tissue engineering in reconstructive urology—The current status and critical insights to set future directions-critical review. Front. Bioeng. Biotechnol. 2023, 10, 1040987. [Google Scholar] [CrossRef]
- Casarin, M.; Todesco, M.; Fontanella, C.G.; Morlacco, A.; Dal Moro, F.; Bagno, A. Hybrid materials for tissue repair and replacement: Another frontier in biomaterial exploitation focusing on cardiovascular and urological fields. Processes 2023, 11, 2013. [Google Scholar] [CrossRef]
- Horst, M.; Milleret, V.; Noetzli, S.; Gobet, R.; Sulser, T.; Eberli, D. Polyesterurethane and acellular matrix based hybrid biomaterial for bladder engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Ajalloueian, F.; Lemon, G.; Hilborn, J.; Chronakis, I.S.; Fossum, M. Bladder biomechanics and the use of scaffolds for regenerative medicine in the urinary bladder. Nat. Rev. Urol. 2018, 15, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Casarin, M.; Morlacco, A.; Dal Moro, F. Bladder substitution: The role of tissue engineering and biomaterials. Processes 2021, 9, 1643. [Google Scholar] [CrossRef]
- Li, G.; Sun, S. Silk fibroin-based biomaterials for tissue engineering applications. Molecules 2022, 27, 2757. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk fibroin as a functional biomaterial for tissue engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Yao, J.; Shao, Z.; Chen, X. Silk-Based 3D Porous Scaffolds for Tissue Engineering. ACS Biomater. Sci. Eng. 2024, 10, 2827–2840. [Google Scholar] [CrossRef] [PubMed]
- Gomez III, P.; Gil, E.S.; Lovett, M.L.; Rockwood, D.N.; Di Vizio, D.; Kaplan, D.L.; Adam, R.M.; Estrada, C.R., Jr.; Mauney, J.R. The effect of manipulation of silk scaffold fabrication parameters on matrix performance in a murine model of bladder augmentation. Biomaterials 2011, 32, 7562–7570. [Google Scholar] [CrossRef] [PubMed]
- Mauney, J.R.; Cannon, G.M.; Lovett, M.L.; Gong, E.M.; Di Vizio, D.; Gomez III, P.; Kaplan, D.L.; Adam, R.M.; Estrada, C.R., Jr. Evaluation of gel spun silk-based biomaterials in a murine model of bladder augmentation. Biomaterials 2011, 32, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qu, Q.; Ding, W.; Anatolyevich, L.S.; Pan, S. A Review on Silk Fibroin as a Biomaterial in Tissue Engineering. J. Biosci. Med. 2024, 12, 275–290. [Google Scholar]
- Horst, M.; Madduri, S.; Milleret, V.; Sulser, T.; Gobet, R.; Eberli, D. A bilayered hybrid microfibrous PLGA–acellular matrix scaffold for hollow organ tissue engineering. Biomaterials 2013, 34, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Hanczar, M.; Moazen, M.; Day, R. The significance of biomechanics and scaffold structure for bladder tissue engineering. Int. J. Mol. Sci. 2021, 22, 12657. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, H.K.; Frimberger, D.; Epstein, R.B.; Kropp, B.P. Growth of bone marrow stromal cells on small intestinal submucosa: An alternative cell source for tissue engineered bladder. BJU Int. 2005, 96, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Alberti, C. Whyever bladder tissue engineering clinical applications still remain unusual even though many intriguing technological advances have been reached? G. Chir.-J. Ital. Surg. Assoc. 2016, 37, 6–12. [Google Scholar] [CrossRef]
- Joseph, D.B.; Borer, J.G.; De Filippo, R.E.; Hodges, S.J.; McLorie, G.A. Autologous cell seeded biodegradable scaffold for augmentation cystoplasty: Phase II study in children and adolescents with spina bifida. J. Urol. 2014, 191, 1389–1395. [Google Scholar] [CrossRef]
- Hendawy, H.; Kaneda, M.; Metwally, E.; Shimada, K.; Tanaka, T.; Tanaka, R. A comparative study of the effect of anatomical site on multiple differentiation of adipose-derived stem cells in rats. Cells 2021, 10, 2469. [Google Scholar] [CrossRef]
- Hendawy, H.; Metwally, E.; Elfadadny, A.; Yoshida, T.; Ma, D.; Shimada, K.; Hamabe, L.; Sasaki, K.; Tanaka, R. Cultured versus freshly isolated adipose-derived stem cells in improvement of the histopathological outcomes in HCL-induced cystitis in a rat model. Biomed. Pharmacother. 2022, 153, 113422. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.; Chung, Y.G.; Gil, E.S.; Tu, D.; Franck, D.; Di Vizio, D.; Adam, R.M.; Kaplan, D.L.; Estrada, C.R., Jr.; Mauney, J.R. The performance of silk scaffolds in a rat model of augmentation cystoplasty. Biomaterials 2013, 34, 4758–4765. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Higuchi, A.; Kubo, R.; Murakami, T.; Nakazawa, Y.; Tanaka, R. The effect of a silk fibroin/polyurethane blend patch on rat vessels. Organogenesis 2017, 13, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Aytemiz, D.; Sakiyama, W.; Suzuki, Y.; Nakaizumi, N.; Tanaka, R.; Ogawa, Y.; Takagi, Y.; Nakazawa, Y.; Asakura, T. Small-diameter silk vascular grafts (3 mm diameter) with a double-raschel knitted silk tube coated with silk fibroin sponge. Adv. Healthc. Mater. 2013, 2, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, Y.; Zhao, Y.; Luo, J.; Shao, H.; Hu, X. Bio-inspired capillary dry spinning of regenerated silk fibroin aqueous solution. Mater. Sci. Eng. C 2011, 31, 1602–1608. [Google Scholar] [CrossRef]
- Zhu, J.; Shao, H.; Hu, X. Morphology and structure of electrospun mats from regenerated silk fibroin aqueous solutions with adjusting pH. Int. J. Biol. Macromol. 2007, 41, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Wang, Q.; Yan, H.; Lv, X.; Zhao, Y.; Zhou, Z.; Zhang, M.; Sun, Q.; Sun, K.; Li, W. Adipose-derived stem cells-seeded bladder acellular matrix graft-silk fibroin enhances bladder reconstruction in a rat model. Oncotarget 2017, 8, 86471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; He, Y.; Guo, J.-H.; Wu, J.-S.; Zhou, Z.; Zhang, M.; Li, W.; Zhou, J.; Xiao, D.-D.; Wang, Z. Time-dependent bladder tissue regeneration using bilayer bladder acellular matrix graft-silk fibroin scaffolds in a rat bladder augmentation model. Acta Biomater. 2015, 23, 91–102. [Google Scholar] [CrossRef]
- Williams, D.F. Challenges with the development of biomaterials for sustainable tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.E.; Farhat, W.A.; Ming, J.M.; McCammon, K.A. Review of clinical experience on biomaterials and tissue engineering of urinary bladder. World J. Urol. 2020, 38, 2081–2093. [Google Scholar] [CrossRef] [PubMed]
- Yudintceva, N.M.; Nashchekina, Y.A.; Blinova, M.I.; Orlova, N.V.; Muraviov, A.N.; Vinogradova, T.I.; Sheykhov, M.G.; Shapkova, E.Y.; Emeljannikov, D.V.; Yablonskii, P.K. Experimental bladder regeneration using a poly-l-lactide/silk fibroin scaffold seeded with nanoparticle-labeled allogenic bone marrow stromal cells. Int. J. Nanomed. 2016, 11, 4521–4533. [Google Scholar] [CrossRef]
- Sack, B.S.; Mauney, J.R.; Estrada, C.R. Silk fibroin scaffolds for urologic tissue engineering. Curr. Urol. Rep. 2016, 17, 1–10. [Google Scholar] [CrossRef]
- Khademolqorani, S.; Tavanai, H.; Ajalloueian, F. Mechanical properties of silk plain-weft knitted scaffolds for bladder tissue engineering applications. Polym. Adv. Technol. 2021, 32, 2367–2377. [Google Scholar] [CrossRef]
- Khademolqorani, S.; Tavanai, H.; Chronakis, I.S.; Boisen, A.; Ajalloueian, F. The determinant role of fabrication technique in final characteristics of scaffolds for tissue engineering applications: A focus on silk fibroin-based scaffolds. Mater. Sci. Eng. C 2021, 122, 111867. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, G.; Nguyen, T.; Hosseini Sharifi, S.H.; Starek, S.; Costa, K.; Jones, C.E.; Barham, D.; Gelman, J.; Clayman, R.V.; Mauney, J.R. Evaluation of silk fibroin-based urinary conduits in a porcine model of urinary diversion. Front. Bioeng. Biotechnol. 2023, 11, 1100507. [Google Scholar] [CrossRef] [PubMed]
- Badv, M.; Bayat, F.; Weitz, J.I.; Didar, T.F. Single and multi-functional coating strategies for enhancing the biocompatibility and tissue integration of blood-contacting medical implants. Biomaterials 2020, 258, 120291. [Google Scholar] [CrossRef] [PubMed]
- Lilly, J.D.; Parsons, C.L. Bladder surface glycosaminoglycans is a human epithelial permeability barrier. Surg. Gynecol. Obstet. 1990, 171, 493–496. [Google Scholar]
- Sutherland, R.S.; Baskin, L.S.; Hayward, S.W.; Cunha, G.R. Regeneration of bladder urothelium, smooth muscle, blood vessels and nerves into an acellular tissue matrix. J. Urol. 1996, 156, 571–577. [Google Scholar] [CrossRef]
- Jack, G.S.; Zhang, R.; Lee, M.; Xu, Y.; Wu, B.M.; Rodríguez, L.V. Urinary bladder smooth muscle engineered from adipose stem cells and a three dimensional synthetic composite. Biomaterials 2009, 30, 3259–3270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xia, J.; Qiu, X.; Wang, P.; Jia, R.; Chen, Y.; Yang, B.; Dai, Y. In vitro evaluation of endothelial progenitor cells from adipose tissue as potential angiogenic cell sources for bladder angiogenesis. PLoS ONE 2015, 10, e0117644. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, T.; Zhou, L.; Liu, J.; Mao, L.; Jia, R.; Zhao, F. Porous gelatin microspheres implanted with adipose mesenchymal stromal cells promote angiogenesis via protein kinase B/endothelial nitric oxide synthase signaling pathway in bladder reconstruction. Cytotherapy 2023, 25, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yu, X.; Wang, X.; He, Y.; Ding, J. Biomaterial–related cell microenvironment in tissue engineering and regenerative medicine. Engineering 2022, 13, 31–45. [Google Scholar] [CrossRef]
- Umuhoza, D.; Yang, F.; Long, D.; Hao, Z.; Dai, J.; Zhao, A. Strategies for tuning the biodegradation of silk fibroin-based materials for tissue engineering applications. ACS Biomater. Sci. Eng. 2020, 6, 1290–1310. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, G.; Lei, H.; Guan, R.; Yang, B.; Gao, Z.; Hui, Y.; Chen, F.; Xin, Z. Therapeutic potential of adipose-derived stem cell-based microtissues in a rat model of stress urinary incontinence. Urology 2016, 97, 277.e1–277.e7. [Google Scholar] [CrossRef] [PubMed]
- Anumanthan, G.; Makari, J.H.; Honea, L.; Thomas, J.C.; Wills, M.L.; Bhowmick, N.A.; Adams, M.C.; Hayward, S.W.; Matusik, R.J.; Brock, J.W. Directed differentiation of bone marrow derived mesenchymal stem cells into bladder urothelium. J. Urol. 2008, 180, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, S.; Yang, R.; Zou, Q.; Zhang, K.; Tian, Q.; Zhao, W.; Zong, L.; Fu, Q. Bioengineered bladder patches constructed from multilayered adipose-derived stem cell sheets for bladder regeneration. Acta Biomater. 2019, 85, 131–141. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendawy, H.; Farag, A.; Elhaieg, A.; Metwllay, E.; Shimada, K.; Elfadadny, A.; Tanaka, R. Enhanced Bladder Regeneration with Adipose-Derived Stem Cell-Seeded Silk Fibroin Scaffolds: A Comparative Analysis. Biomimetics 2025, 10, 93. https://doi.org/10.3390/biomimetics10020093
Hendawy H, Farag A, Elhaieg A, Metwllay E, Shimada K, Elfadadny A, Tanaka R. Enhanced Bladder Regeneration with Adipose-Derived Stem Cell-Seeded Silk Fibroin Scaffolds: A Comparative Analysis. Biomimetics. 2025; 10(2):93. https://doi.org/10.3390/biomimetics10020093
Chicago/Turabian StyleHendawy, Hanan, Ahmed Farag, Asmaa Elhaieg, Elsayed Metwllay, Kazumi Shimada, Ahmed Elfadadny, and Ryou Tanaka. 2025. "Enhanced Bladder Regeneration with Adipose-Derived Stem Cell-Seeded Silk Fibroin Scaffolds: A Comparative Analysis" Biomimetics 10, no. 2: 93. https://doi.org/10.3390/biomimetics10020093
APA StyleHendawy, H., Farag, A., Elhaieg, A., Metwllay, E., Shimada, K., Elfadadny, A., & Tanaka, R. (2025). Enhanced Bladder Regeneration with Adipose-Derived Stem Cell-Seeded Silk Fibroin Scaffolds: A Comparative Analysis. Biomimetics, 10(2), 93. https://doi.org/10.3390/biomimetics10020093








