Integrating New Approach Methodologies (NAMs) into Preclinical Regulatory Evaluation of Oncology Drugs
Abstract
1. Introduction
2. Why Oncology Needs NAMs
2.1. Poor Clinical Translation of Animal Models in Cancer
2.2. Tumor Heterogeneity, Resistance, and Immune Dynamics Poorly Modeled In Vivo
2.3. NAMs as Tools for Improving Relevance, Efficiency, and Patient-Centric Evaluation
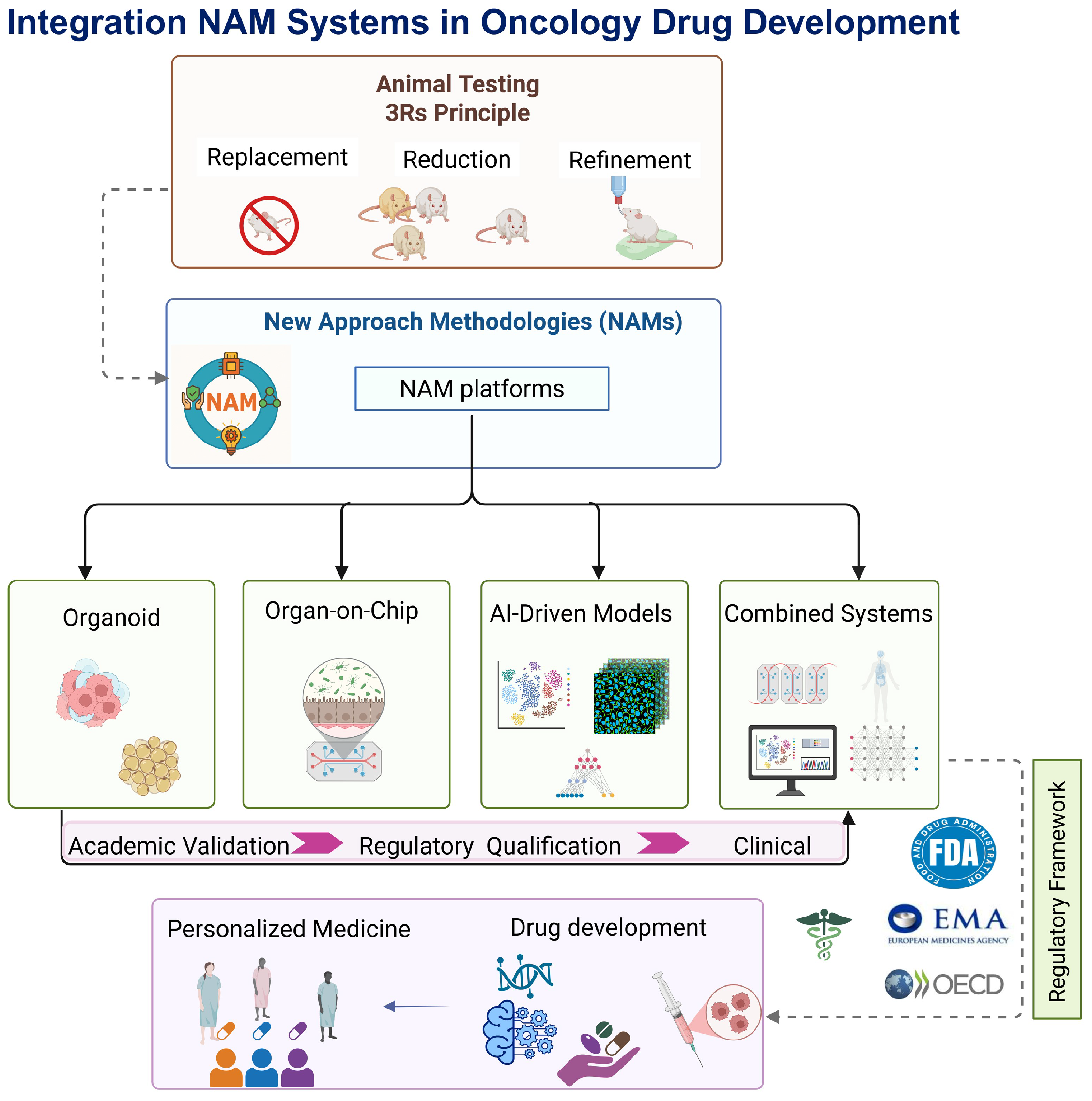
3. Comparative Overview of Key NAM Platforms
3.1. Organoids
3.2. Organ-on-Chip
3.3. AI-Driven Models
3.4. Combined Systems (AI + Biological NAMs)
4. Regulatory and Policy Context
4.1. Recent Shifts: FDA Modernization Act, NIH’s ARIVA, EMA Initiatives
4.2. Oncology-Specific Flexibility
4.3. Challenges: Lack of Harmonized Criteria, Context-of-Use Validation Still Evolving
4.4. Implementation Challenges for NAM Integration
4.5. Validation Frameworks and Fit-for-Purpose Qualification of NAMs
- (i)
- Scientific benchmarking, comparison of NAM outputs against established “gold-standard” in vivo or clinical reference data for efficacy, toxicity, or pharmacokinetics [79].
- (ii)
- Inter-laboratory reproducibility testing, multi-site ring studies following OECD GD 211 and ICCVAM templates to verify robustness across settings.
- (iii)
- Performance metrics, quantitative evaluation of reproducibility, sensitivity, specificity, and concordance with reference methods, with acceptance thresholds typically >80% agreement for regulatory qualification [80].
- (iv)
- Clinical correlation, mapping NAM-derived endpoints to early-phase human trial biomarkers to confirm translational validity.
5. Future Outlook
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Guo, H.; Xu, X.; Zhang, J.; Du, Y.; Yang, X.; He, Z.; Zhao, L.; Liang, T.; Guo, L. The pivotal role of preclinical animal models in anti-cancer drug discovery and personalized cancer therapy strategies. Pharmaceuticals 2024, 17, 1048. [Google Scholar] [CrossRef] [PubMed]
- Van Norman, G.A. Limitations of animal studies for predicting toxicity in clinical trials: Is it time to rethink our current approach? JACC Basic Transl. Sci. 2019, 4, 845–854. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, A.; Yuan, Y.; Zhu, B.; Long, H. Heterogeneity of the tumor immune microenvironment and its clinical relevance. Exp. Hematol. Oncol. 2022, 11, 24. [Google Scholar] [CrossRef]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef] [PubMed]
- Hartung, T. The (misleading) role of animal models in drug development. Front. Drug Discov. 2024, 4, 1355044. [Google Scholar] [CrossRef]
- Haeussler, C.; Assmus, A. Bridging the gap between invention and innovation: Increasing success rates in publicly and industry-funded clinical trials. Res. Policy 2021, 50, 104155. [Google Scholar] [CrossRef]
- Sewell, F.; Alexander-White, C.; Brescia, S.; Currie, R.A.; Roberts, R.; Roper, C.; Vickers, C.; Westmoreland, C.; Kimber, I. New approach methodologies (NAMs): Identifying and overcoming hurdles to accelerated adoption. Toxicol. Res. 2024, 13, tfae044. [Google Scholar] [CrossRef]
- Roberts, R.A. New approach methodologies (NAMs) in drug safety assessment: A vision of the future. Curr. Opin. Toxicol. 2024, 40, 100502. [Google Scholar] [CrossRef]
- Avula, L.R.; Grodzinski, P. How organ-on-a-chip is advancing cancer research and oncology—A cancer hallmarks’ perspective. Front. Lab Chip Technol. 2024, 3, 1487377. [Google Scholar] [CrossRef]
- Turner, J.; Pound, P.; Owen, C.; Hutchinson, I.; Hop, M.; Chau, D.Y.S.; Barrios Silva, L.V.; Coleman, M.; Dubourg, A.; Harries, L.W.; et al. Incorporating new approach methodologies into regulatory nonclinical pharmaceutical safety assessment. ALTEX-Altern. Anim. Exp. 2023, 40, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Han, J.J. FDA Modernization Act 2.0 allows for alternatives to animal testing. Artif. Organs 2023, 47, 449–450. [Google Scholar] [CrossRef]
- Tagle, D. Prioritizing human tissue research: Q&A with Danilo Tagle. Cell Stem Cell 2025, 32, 870–872. [Google Scholar] [CrossRef]
- Audebert, M.; Assmann, A.-S.; Azqueta, A.; Babica, P.; Benfenati, E.; Bortoli, S.; Bouwman, P.; Braeuning, A.; Burgdorf, T.; Coumoul, X. New approach methodologies to facilitate and improve the hazard assessment of non-genotoxic carcinogens—A PARC project. Front. Toxicol. 2023, 5, 1220998. [Google Scholar] [CrossRef]
- Wong, C.C.; Cheng, K.-W.; Rigas, B. Preclinical predictors of anticancer drug efficacy: Critical assessment with emphasis on whether nanomolar potency should be required of candidate agents. J. Pharmacol. Exp. Ther. 2012, 341, 572–578. [Google Scholar] [CrossRef]
- Wong, C.H.; Siah, K.W.; Lo, A.W. Estimation of clinical trial success rates and related parameters. Biostatistics 2019, 20, 273–286. [Google Scholar] [CrossRef]
- Fang, Y.; Eglen, R.M. Three-dimensional cell cultures in drug discovery and development. Slas Discov. Adv. Life Sci. RD 2017, 22, 456–472. [Google Scholar]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning from 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Pasquali, S.; Moura, D.S.; Danks, M.R.; Manasterski, P.J.; Zaffaroni, N.; Stacchiotti, S.; Mondaza-Hernandez, J.L.; Kerrison, W.G.; Martin-Broto, J.; Huang, P.H. Preclinical models of soft tissue sarcomas–generation and applications to enhance translational research. Crit. Rev. Oncol./Hematol. 2025, 207, 104621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, C.; Tu, J.; Tang, M.; Ashrafizadeh, M.; Nabavi, N.; Sethi, G.; Zhao, P.; Liu, S. Advances in cancer immunotherapy: Historical perspectives, current developments, and future directions. Mol. Cancer 2025, 24, 136. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Yoshimura, K.; Sewastjanow-Silva, M.; Song, S.; Ajani, J.A. Challenges and prospects of patient-derived xenografts for cancer research. Cancers 2023, 15, 4352. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, X. Patient-derived xenograft models: Current status, challenges, and innovations in cancer research. Genes Dis. 2025, 12, 101520. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Hughes, C.C. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef]
- Peng, Z.; Lv, X.; Sun, H.; Zhao, L.; Huang, S. 3D tumor cultures for drug resistance and screening development in clinical applications. Mol. Cancer 2025, 24, 93. [Google Scholar] [CrossRef]
- Seyfoori, A.; Liu, K.; Caruncho, H.J.; Walter, P.B.; Akbari, M. Tumoroid-On-a-Plate (ToP): Physiologically Relevant Cancer Model Generation and Therapeutic Screening. Adv. Healthc. Mater. 2025, 14, e2402060. [Google Scholar] [CrossRef]
- Tong, L.; Cui, W.; Zhang, B.; Fonseca, P.; Zhao, Q.; Zhang, P.; Xu, B.; Zhang, Q.; Li, Z.; Seashore-Ludlow, B.; et al. Patient-derived organoids in precision cancer medicine. Med 2024, 5, 1351–1377. [Google Scholar] [CrossRef]
- Moresi, F.; Noto, F.; Vasudevan, J.; Bisteau, X.; Adriani, G.; Pavesi, A. Microphysiological Systems in Cancer Research: Advancing Immunotherapy through Tumor Microenvironment-Integrated Organ-On-Chip Models. Adv. Ther. 2025, 8, e00098. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Chen, R.; Song, Y.; Wei, W.; Baek, D.; Gillin, M.; Kurabayashi, K.; Chen, W. Cancer-on-a-chip for precision cancer medicine. Lab Chip 2025, 25, 3314–3347. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.-Y.; Zhu, J.; Gao, Y.-Q.; Jiang, M.; Yin, H. Narrative review of 3D bioprinting for the construction of in vitro tumor models: Present and prospects. Transl. Cancer Res. 2025, 14, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Huang, S. Advances in the application of 3D tumor models in precision oncology and drug screening. Front. Bioeng. Biotechnol. 2022, 10, 1021966. [Google Scholar] [CrossRef]
- Goldrick, C.; Guri, I.; Herrera-Oropeza, G.; O’Brien-Gore, C.; Roy, E.; Wojtynska, M.; Spagnoli, F.M. 3D multicellular systems in disease modelling: From organoids to organ-on-chip. Front. Cell Dev. Biol. 2023, 11, 1083175. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- van der Zalm, A.J.; Barroso, J.; Browne, P.; Casey, W.; Gordon, J.; Henry, T.R.; Kleinstreuer, N.C.; Lowit, A.B.; Perron, M.; Clippinger, A.J. A framework for establishing scientific confidence in new approach methodologies. Arch. Toxicol. 2022, 96, 2865–2879. [Google Scholar] [CrossRef]
- Lee, S.H.; Hu, W.; Matulay, J.T.; Silva, M.V.; Owczarek, T.B.; Kim, K.; Chua, C.W.; Barlow, L.J.; Kandoth, C.; Williams, A.B.; et al. Tumor Evolution and Drug Response in Patient-Derived Organoid Models of Bladder Cancer. Cell 2018, 173, 515–528.e517. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Wang, P.; Zhang, N.; Wang, P. Tumor organoids for cancer research and personalized medicine. Cancer Biol. Med. 2021, 19, 319–332. [Google Scholar] [CrossRef]
- Heo, J.H.; Kang, D.; Seo, S.J.; Jin, Y. Engineering the Extracellular Matrix for Organoid Culture. Int. J. Stem Cells 2022, 15, 60–69. [Google Scholar] [CrossRef]
- Hu, J.W.; Pan, Y.Z.; Zhang, X.X.; Li, J.T.; Jin, Y. Applications and challenges of patient-derived organoids in hepatobiliary and pancreatic cancers. World J. Gastroenterol. 2025, 31, 106747. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Alam, K.; Roy, N.S.; Kaur, K.; Kaity, S.; Ravichandiran, V.; Roy, S. Exploring the interaction between extracellular matrix components in a 3D organoid disease model to replicate the pathophysiology of breast cancer. J. Exp. Clin. Cancer Res. 2023, 42, 343. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Lee, J.; Ha, J.; Kim, G.; Kim, H.-J.; Lee, S.; Koo, B.-K.; Park, Y. Long-term three-dimensional high-resolution imaging of live unlabeled small intestinal organoids via low-coherence holotomography. Exp. Mol. Med. 2024, 56, 2162–2170. [Google Scholar] [CrossRef]
- Ooft, S.N.; Weeber, F.; Dijkstra, K.K.; McLean, C.M.; Kaing, S.; van Werkhoven, E.; Schipper, L.; Hoes, L.; Vis, D.J.; van de Haar, J.; et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci. Transl. Med. 2019, 11, 513. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, J.; Li, C.; Gu, Q.; Li, G.; Li, Z.A.; Xu, J.; Zhou, J.; Tuan, R.S. Organoids and organs-on-chips: Recent advances, applications in drug development, and regulatory challenges. Med 2025, 6, 100667. [Google Scholar] [CrossRef]
- Nasiri, R.; Sankaranthi, A.; Pratx, G. Organ-on-a-chip systems for modeling tumor and normal tissue microenvironments in radiotherapy research. Trends Biotechnol. 2025. [Google Scholar] [CrossRef]
- Amereh, M.; Seyfoori, A.; Shojaei, S.; Lane, S.; Zhao, T.; Shokrollahi Barough, M.; Lum, J.J.; Walter, P.B.; Akbari, M. Tumoroid Model Reveals Synergistic Impairment of Metabolism by Iron Chelators and Temozolomide in Chemo-Resistant Patient-derived Glioblastoma Cells. Adv. Sci. 2025, 12, 2412505. [Google Scholar] [CrossRef]
- Jiang, X.-Y.; Wang, Y.-R.; Di, P.-R.; Qian, S.-Y.; Jiang, H.-T. Organoids in cancer therapies: A comprehensive review. Front. Bioeng. Biotechnol. 2025, 13, 1607488. [Google Scholar] [CrossRef]
- Nejati, B.; Shahhosseini, R.; Hajiabbasi, M.; Ardabili, N.S.; Baktash, K.B.; Alivirdiloo, V.; Moradi, S.; Rad, M.F.; Rahimi, F.; Farani, M.R.; et al. Cancer-on-chip: A breakthrough organ-on-a-chip technology in cancer cell modeling. Med. Biol. Eng. Comput. 2025, 63, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Mattei, F.; Andreone, S.; Mencattini, A.; De Ninno, A.; Businaro, L.; Martinelli, E.; Schiavoni, G. Oncoimmunology Meets Organs-on-Chip. Front. Mol. Biosci. 2021, 8, 627454. [Google Scholar] [CrossRef] [PubMed]
- Sartori, F.; Codicè, F.; Caranzano, I.; Rollo, C.; Birolo, G.; Fariselli, P.; Pancotti, C. A Comprehensive Review of Deep Learning Applications with Multi-Omics Data in Cancer Research. Genes 2025, 16, 648. [Google Scholar] [CrossRef]
- Salvati, A.; Melone, V.; Giordano, A.; Lamberti, J.; Palumbo, D.; Palo, L.; Rea, D.; Memoli, D.; Simonis, V.; Alexandrova, E.; et al. Multi-omics based and AI-driven drug repositioning for epigenetic therapy in female malignancies. J. Transl. Med. 2025, 23, 837. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.T.; Karatas, E.; Poquillon, T.; Grenci, G.; Furlan, A.; Dilasser, F.; Mohamad Raffi, S.B.; Blanc, D.; Drimaracci, E.; Mikec, D.; et al. Digitalized organoids: Integrated pipeline for high-speed 3D analysis of organoid structures using multilevel segmentation and cellular topology. Nat. Methods 2025, 22, 1343–1354. [Google Scholar] [CrossRef]
- Shi, H.; Kowalczewski, A.; Vu, D.; Liu, X.; Salekin, A.; Yang, H.; Ma, Z. Organoid intelligence: Integration of organoid technology and artificial intelligence in the new era of in vitro models. Med. Nov. Technol. Devices 2024, 21, 100276. [Google Scholar] [CrossRef]
- Maramraju, S.; Kowalczewski, A.; Kaza, A.; Liu, X.; Singaraju, J.P.; Albert, M.V.; Ma, Z.; Yang, H. AI-organoid integrated systems for biomedical studies and applications. Bioeng. Transl. Med. 2024, 9, e10641. [Google Scholar] [CrossRef]
- Bai, L.; Wu, Y.; Li, G.; Zhang, W.; Zhang, H.; Su, J. AI-enabled organoids: Construction, analysis, and application. Bioact. Mater. 2024, 31, 525–548. [Google Scholar] [CrossRef]
- Madan, A.; Saini, R.; Dhiman, N.; Juan, S.-H.; Satapathy, M.K. Organoids as Next-Generation Models for Tumor Heterogeneity, Personalized Therapy, and Cancer Research: Advancements, Applications, and Future Directions. Organoids 2025, 4, 23. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Shivnaraine, R.V.; Wu, J.C. FDA Modernization Act 2.0 Paves the Way to Computational Biology and Clinical Trials in a Dish. Circulation 2023, 148, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Bhinder, B.; Gilvary, C.; Madhukar, N.S.; Elemento, O. Artificial Intelligence in Cancer Research and Precision Medicine. Cancer Discov. 2021, 11, 900–915. [Google Scholar] [CrossRef]
- Albani, F.G.; Alghamdi, S.S.; Almutairi, M.M.; Alqahtani, T. Artificial Intelligence-Driven Innovations in Oncology Drug Discovery: Transforming Traditional Pipelines and Enhancing Drug Design. Drug Des. Devel. Ther. 2025, 19, 5685–5707. [Google Scholar] [CrossRef]
- Zushin, P.H.; Mukherjee, S.; Wu, J.C. FDA Modernization Act 2.0: Transitioning beyond animal models with human cells, organoids, and AI/ML-based approaches. J. Clin. Investig. 2023, 133, e175824. [Google Scholar] [CrossRef] [PubMed]
- Walrath, R. FDA’s shift from animal testing opens doors for organoid makers. In Chemical & Engineering News; American Chemical Society: Washington, DC, USA, 2025; Volume 103, Issue 13. [Google Scholar]
- Azkona, G. 3Rs (replacement, reduction, and refinement) alternatives. In Sustainability in the Manufacturing of Pharmaceuticals; Elsevier: Amsterdam, The Netherlands, 2025; pp. 281–309. [Google Scholar]
- Westmoreland, C.; Bender, H.J.; Doe, J.E.; Jacobs, M.N.; Kass, G.E.; Madia, F.; Mahony, C.; Manou, I.; Maxwell, G.; Prieto, P. Use of New Approach Methodologies (NAMs) in regulatory decisions for chemical safety: Report from an EPAA Deep Dive Workshop. Regul. Toxicol. Pharmacol. 2022, 135, 105261. [Google Scholar] [CrossRef]
- Stucki, A.O.; Barton-Maclaren, T.S.; Bhuller, Y.; Henriquez, J.E.; Henry, T.R.; Hirn, C.; Miller-Holt, J.; Nagy, E.G.; Perron, M.M.; Ratzlaff, D.E. Use of new approach methodologies (NAMs) to meet regulatory requirements for the assessment of industrial chemicals and pesticides for effects on human health. Front. Toxicol. 2022, 4, 964553. [Google Scholar] [CrossRef]
- Yordas Ltd.; Carramusa, L.; Mune, W.; Hunt, N.; Browne, L.; Osborne, O. Claire Potter New Approach Methodologies (NAMs) to Support Regulatory Decisions for Chemical Safety. FSA Res. Evid. 2024. [Google Scholar] [CrossRef]
- Gan, J.; Bolon, B.; Van Vleet, T.; Wood, C. Alternative models in biomedical research: In silico, in vitro, ex vivo, and nontraditional in vivo approaches. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 925–966. [Google Scholar]
- McPhail, M.; Weiss, E.; Bubela, T. Conditional drug approval as a path to market for oncology drugs in Canada: Challenges and recommendations for assessing eligibility and regulatory responsiveness. Front. Med. 2022, 8, 818647. [Google Scholar] [CrossRef]
- Alucozai, M.; Kulshreshtha, A.; Sperry, M.; Romero, K. Beyond Animals: Revolutionizing Drug Discovery with Human-Relevant Models. 2025. Available online: https://wyss.harvard.edu/news/beyond-animals-revolutionizing-drug-discovery-with-human-relevant-models/ (accessed on 2 April 2025).
- Hou, X.; Liu, S.; Zeng, Z.; Wang, Z.; Ding, J.; Chen, Y.; Gao, X.; Wang, J.; Xiao, G.; Li, B.; et al. Preclinical imaging evaluation of a bispecific antibody targeting hPD1/CTLA4 using humanized mice. Biomed. Pharmacother. 2024, 175, 116669. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Jiao, P. Anti-PD-L1-Based Bispecific Antibodies Targeting Co-Inhibitory and Co-Stimulatory Molecules for Cancer Immunotherapy. Molecules 2024, 29, 454. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.; Mazcko, C.; London, C.; Hergenrother, P.J.; Fan, T.M. Comparative oncology in action: Vignettes on small molecule development. Vet. Oncol. 2025, 2, 8. [Google Scholar] [CrossRef]
- Regan, D.; Garcia, K.; Thamm, D. Clinical, Pathological, and Ethical Considerations for the Conduct of Clinical Trials in Dogs with Naturally Occurring Cancer: A Comparative Approach to Accelerate Translational Drug Development. Ilar. J. 2018, 59, 99–110. [Google Scholar] [CrossRef]
- Mestrinho, L.A.; Santos, R.R. Translational oncotargets for immunotherapy: From pet dogs to humans. Adv. Drug Deliv. Rev. 2021, 172, 296–313. [Google Scholar] [CrossRef]
- European Medicines Agency. Regulatory Acceptance of New Approach Methodologies (NAMs) to Reduce Animal Use Testing. 2024. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/research-development/ethical-use-animals-medicine-testing/regulatory-acceptance-new-approach-methodologies-nams-reduce-animal-use-testing/ (accessed on 2 April 2025).
- Harrill, A.; Carstens, K.; Sipes, N.; Noyes, P.; Lowit, A.; Lynn, S.; Perron, M.; Gordon, J.; Fitzpatrick, S.; Kleinstreuer, N. Validation, Qualification, and Regulatory Acceptance of New Approach Methodologies; U.S. Environmental Protection Agency: Washington, DC, USA, 2024.
- National Institute for Public Health and the Environment (RIVM). Landscape New Approach Methodologies (NAMs) Safety Assessment Pharmaceutical Products; National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2024. [Google Scholar]
- Gravanis, I.; Berntgen, M.; Vamvakas, S.; Demolis, P.; Foggi, P. Challenges and ongoing initiatives towards better integrated EU scientific advice. Front. Med. 2025, 12, 1473346. [Google Scholar] [CrossRef]
- Kaplan, B.L.; Hoberman, A.M.; Slikker, W., Jr.; Smith, M.A.; Corsini, E.; Knudsen, T.B.; Marty, M.S.; Sobrian, S.K.; Fitzpatrick, S.C.; Ratner, M.H. Protecting human and animal health: The road from animal models to new approach methods. Pharmacol. Rev. 2024, 76, 251–266. [Google Scholar] [CrossRef]
- Carlson, J.M.; Janulewicz, P.A.; Kleinstreuer, N.C.; Heiger-Bernays, W. Impact of high-throughput model parameterization and data uncertainty on thyroid-based toxicological estimates for pesticide chemicals. Environ. Sci. Technol. 2022, 56, 5620–5631. [Google Scholar] [CrossRef] [PubMed]
- Ford, K.A. Refinement, Reduction, and Replacement of Animal Toxicity Tests by Computational Methods. ILAR J. 2016, 57, 226–233. [Google Scholar] [CrossRef]
- Hawkins, P.; Armstrong, R.; Boden, T.; Garside, P.; Knight, K.; Lilley, E.; Seed, M.; Wilkinson, M.; Williams, R.O. Applying refinement to the use of mice and rats in rheumatoid arthritis research. Inflammopharmacology 2015, 23, 131–150. [Google Scholar] [CrossRef]
- Stripecke, R.; Münz, C.; Schuringa, J.J.; Bissig, K.D.; Soper, B.; Meeham, T.; Yao, L.C.; Di Santo, J.P.; Brehm, M.; Rodriguez, E.; et al. Innovations, challenges, and minimal information for standardization of humanized mice. EMBO Mol. Med. 2020, 12, e8662. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Division on Earth and Life Studies; Board on Environmental Studies and Toxicology; Committee on Incorporating 21st Century Science into Risk-Based Evaluations. Using 21st Century Science to Improve Risk-Related Evaluations; National Academies Press: Washington, DC, USA, 2017. [Google Scholar]
- OECD. Guidance Document for Describing Non-Guideline In Vitro Test Methods; OECD Series on Testing and Assessment, No. 211; OECD Publishing: Paris, France, 2017. [Google Scholar]
- Food and Drug Administration. Potential Approaches to Drive Future Integration of New Alternative Methods for Regulatory Decision-Making; Food and Drug Administration: Washington, DC, USA, 2024.
- OECD. Overview of Concepts and Available Guidance Related to Integrated Approaches to Testing and Assessment (IATA); OECD Series on Testing and Assessment, No. 329; OECD Publishing: Paris, France, 2020. [Google Scholar]
- Food and Drug Administration. Roadmap to Reducing Animal Testing in Preclinical Safety Studies; Food and Drug Administration: Washington, DC, USA, 2025.

| Feature | Conventional 2D/3D Cell Cultures | Animal-Based Models (Xenografts, GEMMs) | New Approach Methodologies (NAMs) |
|---|---|---|---|
| Biological relevance to humans | Limited; simplified monolayers lack systemic and microenvironmental context. | Moderate; capture systemic interactions but differ genetically and immunologically. | High; built on human-derived tissues or computational data. |
| Tumor heterogeneity | Very low; clonal and homogeneous. | Partial; species-specific tumor evolution. | High; patient-derived organoids and CoC systems retain clonal diversity. |
| Immune–tumor interactions | Absent. | Species-specific and incomplete. | Human immune components can be incorporated. |
| Microenvironment complexity | Minimal; lacks ECM architecture and mechanical cues. | Moderate; stromal and vascular components present but non-human. | Advanced; recreate flow, shear stress, and ECM via microfluidics or bioprinting. |
| Throughput and scalability | High; inexpensive and suitable for screening. | Low; costly and time-consuming. | Moderate; platform-dependent. |
| Ethical considerations | Non-animal; ethically acceptable. | Involves animal use. | Fully aligned with 3Rs principle. |
| Regulatory acceptance | Informal use for early discovery; limited for safety evaluation. | Historically accepted standard. | Increasing acceptance through FDA Modernization Act 2.0 and EMA frameworks. |
| Cost and time efficiency | Very low cost; rapid. | High cost; long development. | Moderate; initial investment, then lower cost per assay. |
| Reproducibility | High under standardized conditions. | Variable; inter-animal and inter-lab differences. | Improving with standardization; still evolving. |
| Translational predictability | Low; oversimplified systems. | Limited; ~90% failure in translation. | High; human-relevant and mechanistic. |
| Ref | [14,23] | [4,5,6] | [7,8,10,24] |
| NAM Platform | Description | Advantages | Limitations | Ref |
|---|---|---|---|---|
| Advanced 2D/3D Human Cell Systems (Pre-NAM bridge) | Human cell lines or spheroids are used for preliminary mechanistic and toxicity screens. | Low-cost, high-throughput entry point; useful for initial pharmacological profiling. | Lack systemic integration; limited immune and vascular mimicry. | [14,17] |
| Patient-Derived Organoids (PDOs) | 3D cultures derived from patient tumors retaining histological and genetic fidelity. | Preserve intratumoral heterogeneity; suitable for drug sensitivity testing, functional genomics, and biomarker discovery. | Lack vasculature and systemic immune components; batch-to-batch variability due to matrix or culture conditions. | [25,34] |
| Organ-on-Chip (OoC)/Cancer-on-Chip (CoC) | Microfluidic devices mimicking physiological microenvironments and tissue-tissue interfaces. | Simulate vascular flow, mechanical stress, and immune infiltration; improve prediction of pharmacokinetic and immunotherapy responses. | Technically complex; limited scalability; requires standardization and automation for widespread use. | [40,41,43] |
| 3D Bioprinting Models | Biofabrication of tumor tissues using hydrogels and patient-derived cells. | High spatial control; replicate matrix stiffness, geometry, and tumor microenvironment for mechanistic and invasion studies. | Limited reproducibility across laboratories; still under validation for regulatory acceptance. | [28,29] |
| AI-Driven Computational Models | Machine-learning algorithms integrating omics, imaging, and pharmacological datasets. | Predict efficacy/toxicity; support in silico clinical trials; accelerate target identification. | Depending on dataset diversity, quality, and explainability, interpretability challenges for regulators. | [46,47,49] |
| Hybrid Systems (AI + Biological NAMs) | Integration of computational and experimental NAMs to enhance translational predictability. | Combine mechanistic insights with predictive analytics; accelerate drug prioritization; reduce reliance on animal models. | Require harmonized data governance, algorithmic transparency, and validation frameworks. | [53,54,56] |
| Validation Element | Description | Practical Example | Regulatory Alignment | Ref |
|---|---|---|---|---|
| Context-of-use definition | Defines NAM purpose (replacement, supplement, refinement) and target decision context | OoC for vascular permeability | EMA qualification advice; FDA context-of-use guidance | [81] |
| Benchmarking | Quantitative comparison of NAM outputs against gold-standard animal or clinical endpoints | OoC toxicity vs. animal LD50 and clinical data | ICCVAM, OECD validation principles | [71] |
| Inter-laboratory reproducibility | Replication of results across sites with standardized protocols | Multi-site PDO drug-response panel | OECD validation templates; ICCVAM study designs | [82] |
| Performance metrics | Sensitivity, specificity, reproducibility, uncertainty analysis, predictive value | ROC/AUC comparing NAM vs. clinical response | OECD, ICCVAM, FDA expectations | [83] |
| Clinical correlation | Alignment of NAM outputs with early-phase clinical trial data | PDO response concordance with patient outcomes | FDA/EMA scientific advice and qualification | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirlohi, M.S.; Yousefi, T.; Aref, A.R.; Seyfoori, A. Integrating New Approach Methodologies (NAMs) into Preclinical Regulatory Evaluation of Oncology Drugs. Biomimetics 2025, 10, 796. https://doi.org/10.3390/biomimetics10120796
Mirlohi MS, Yousefi T, Aref AR, Seyfoori A. Integrating New Approach Methodologies (NAMs) into Preclinical Regulatory Evaluation of Oncology Drugs. Biomimetics. 2025; 10(12):796. https://doi.org/10.3390/biomimetics10120796
Chicago/Turabian StyleMirlohi, Maryam Sadat, Tooba Yousefi, Amir Reza Aref, and Amir Seyfoori. 2025. "Integrating New Approach Methodologies (NAMs) into Preclinical Regulatory Evaluation of Oncology Drugs" Biomimetics 10, no. 12: 796. https://doi.org/10.3390/biomimetics10120796
APA StyleMirlohi, M. S., Yousefi, T., Aref, A. R., & Seyfoori, A. (2025). Integrating New Approach Methodologies (NAMs) into Preclinical Regulatory Evaluation of Oncology Drugs. Biomimetics, 10(12), 796. https://doi.org/10.3390/biomimetics10120796







