Comparison of Long-Term Clinical Outcomes of Zirconia and Lithium Disilicate Prostheses: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
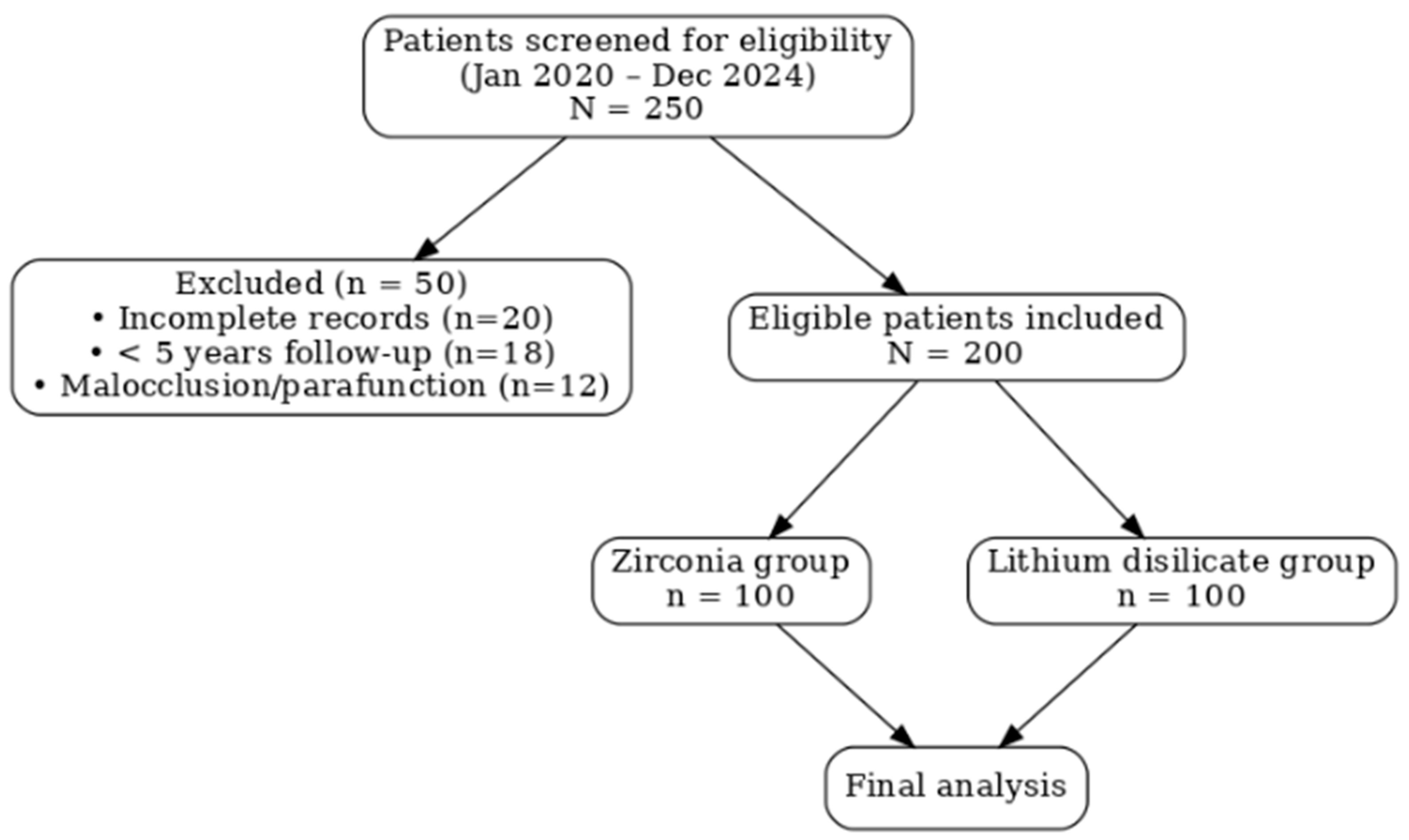
2.1. Study Design and Patient Selection
2.2. Data Collection and Variables
- ➢
- Biomechanical performance: Assessed by incidence of fractures and cracks.
- ➢
- Marginal adaptation: Evaluated in terms of cement dissolution, marginal discrepancies, and occurrence of secondary caries.
- ➢
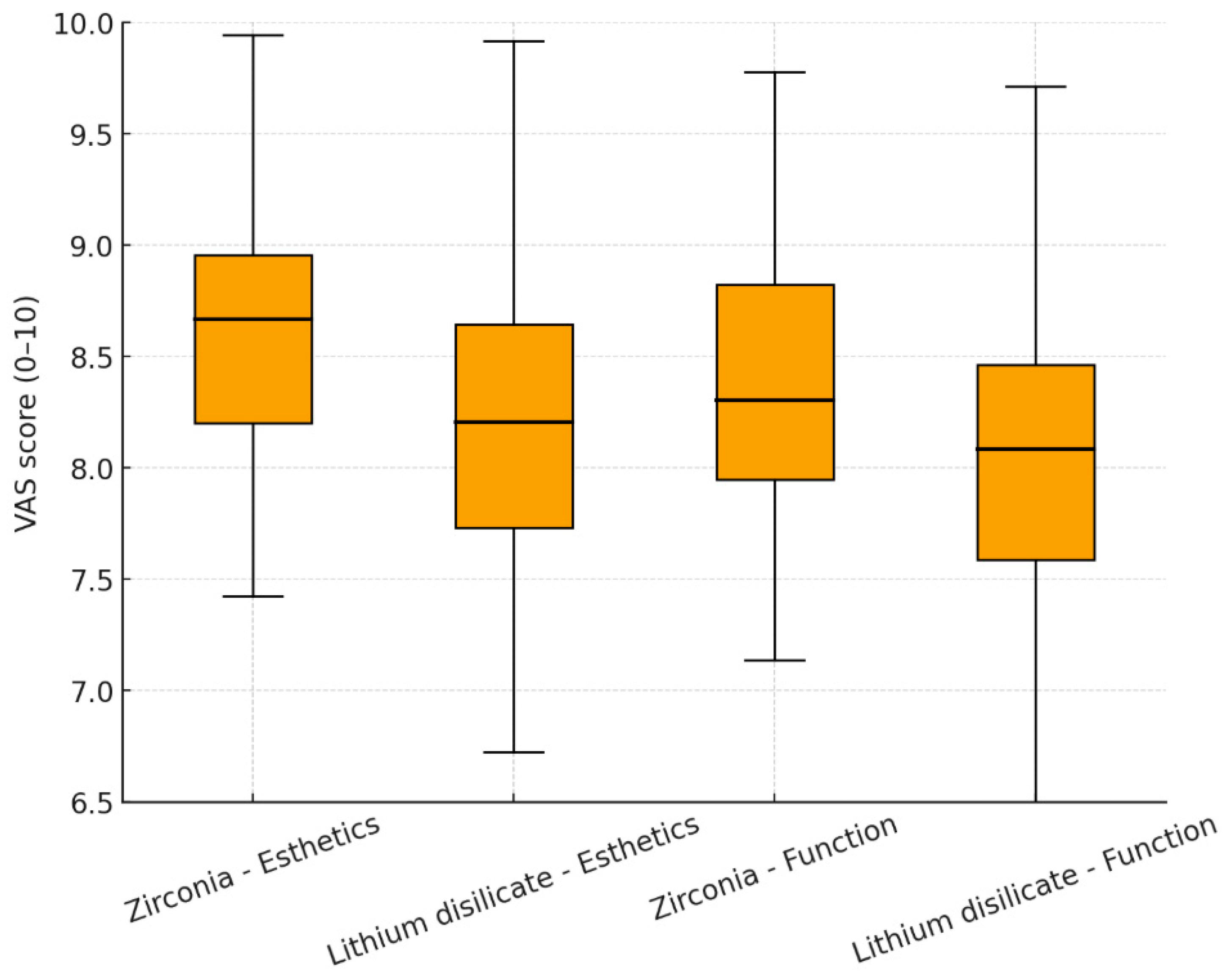
- Chewing function and patient satisfaction: Assessed using a Visual Analog Scale (VAS) ranging from 0 (not satisfied) to 10 (fully satisfied). The VAS method followed previously validated protocols for assessing esthetic and functional satisfaction in prosthodontic research, as described by Chander, Patil, and Tosun et al. [22,23,24].
- ➢
- Radiographic evaluation: Included assessment of peri-apical conditions for tooth-supported restorations and peri-implant bone stability for implant-supported restorations. In this study, periapical status in tooth-supported restorations was assessed to detect apical or periodontal pathology (not bone loss), as this parameter best represents the biological integrity of natural teeth. For implant-supported prostheses, marginal bone loss was measured as the vertical distance from the implant shoulder (IS) to the first bone-to-implant contact (BIC) on mesial and distal aspects. This distinction is anatomically justified and aligns with radiographic protocols cited in Rehberger Bescos et al. and Weigel et al. [25,26]. All implants were placed at the crestal level following a standard two-stage protocol, ensuring uniform bone reference points for radiographic assessment. Radiographic evaluations were performed using standardized periapical radiographs obtained with the paralleling technique and digital sensors (Planmeca ProX, Helsinki, Finland). Measurements of marginal bone levels and periapical status were conducted with ImageJ software (version 1.53, NIH, Bethesda, MD, USA). All radiographs were analyzed by two calibrated examiners, and intra-examiner reliability showed excellent agreement (ICC = 0.94). Identical imaging and measurement protocols were used for both tooth- and implant-supported prostheses, ensuring comparability between zirconia and lithium disilicate groups. For implant-supported prostheses, marginal bone loss was defined as the vertical distance between the implant shoulder and the most coronal bone-to-implant contact on the mesial and distal surfaces, following the standardized radiographic protocol described by Rehberger Bescos et al. [25]. For implant-supported restorations, marginal bone loss was measured as the vertical distance from the implant shoulder (IS) to the first bone-to-implant contact (BIC). For tooth-supported restorations, the vertical distance from the cementoenamel junction (CEJ) to the alveolar crest (AC) was measured to evaluate crestal bone integrity. All measurements were performed using ImageJ software (version 1.53, NIH, USA), calibrated according to sensor pixel dimensions. Standardized periapical radiographs were obtained with the paralleling technique using a fixed film holder to minimize distortion. Two experienced examiners performed all measurements, and intra- and inter-examiner reliability was excellent (ICC = 0.94 and 0.91, respectively). These protocols ensured reproducible and comparable radiographic assessments across zirconia and lithium disilicate groups. Radiographic evaluation protocols (measurement landmarks, calibration, distortion control) were adapted from Mailoa et al., who defined distances from the cementoenamel junction (CEJ), implant platform, and the first bone-to-implant contact [27]. In similar studies, CBCT-based measurements have validated periapical radiograph-based assessments of marginal bone loss [26,28].
- ➢
- Complications: biological and technical complications, including chipping, debonding, and loss of retention, were recorded.
2.3. Clinical Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ille, C.E.; Jivanescu, A.; Pop, D.; Stoica, E.T.; Flueras, R.; Talpos-Niculescu, I.C.; Cosoroaba, R.M.; Popovici, R.A.; Olariu, I. Exploring the Properties and Indications of Chairside CAD/CAM Materials in Restorative Dentistry. J. Funct. Biomater. 2025, 16, 46. [Google Scholar] [CrossRef]
- Kim, S.H.; Oh, K.C.; Moon, H.S. Effects of Surface-Etching Systems on the Shear Bond Strength of Dual-Polymerized Resin Cement and Zirconia. Materials 2024, 17, 3096. [Google Scholar] [CrossRef]
- Manziuc, M.; Kui, A.; Chisnoiu, A.; Labunet, A.; Negucioiu, M.; Ispas, A.; Buduru, S. Zirconia-Reinforced Lithium Silicate Ceramic in Digital Dentistry: A Comprehensive Literature Review of Our Current Understanding. Medicina 2023, 59, 2135. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, B.; Aktas, G.; Baris Guncu, M.; Deniz, D.; Muhtarogullari, M.; Al-Haj Husain, N.; Ozcan, M. Effects of Surface Treatments and Cement Type on Shear Bond Strength between Titanium Alloy and All-Ceramic Materials. Materials 2023, 16, 6240. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, H.; Haro Adanez, M.; Att, W. Current status of zirconia implants in dentistry: Preclinical tests. J. Prosthodont. Res. 2019, 63, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Oyague, R.; Sancho-Esper, R.; Lynch, C.D.; Suarez-Garcia, M.J. All-ceramic inlay-retained fixed dental prostheses for replacing posterior missing teeth: A systematic review. J. Prosthodont. Res. 2018, 62, 10–23. [Google Scholar] [CrossRef]
- Miyazaki, T.; Nakamura, T.; Matsumura, H.; Ban, S.; Kobayashi, T. Current status of zirconia restoration. J. Prosthodont. Res. 2013, 57, 236–261. [Google Scholar] [CrossRef]
- Zarone, F.; Russo, S.; Sorrentino, R. From porcelain-fused-to-metal to zirconia: Clinical and experimental considerations. Dent. Mater. 2011, 27, 83–96. [Google Scholar] [CrossRef]
- Aboushelib, M.N.; Kleverlaan, C.J.; Feilzer, A.J. Effect of zirconia type on its bond strength with different veneer ceramics. J. Prosthodont. 2008, 17, 401–408. [Google Scholar] [CrossRef]
- Aboushelib, M.N.; Kleverlaan, C.J.; Feilzer, A.J. Microtensile bond strength of different components of core veneered all-ceramic restorations. Part 3: Double veneer technique. J. Prosthodont. 2008, 17, 9–13. [Google Scholar] [CrossRef]
- Linkevicius, T. The Novel Design of Zirconium Oxide-Based Screw-Retained Restorations, Maximizing Exposure of Zirconia to Soft Peri-implant Tissues: Clinical Report After 3 Years of Follow-up. Int. J. Periodontics Restor. Dent. 2017, 37, 41–47. [Google Scholar] [CrossRef]
- Belli, R.; Petschelt, A.; Hofner, B.; Hajto, J.; Scherrer, S.S.; Lohbauer, U. Fracture Rates and Lifetime Estimations of CAD/CAM All-ceramic Restorations. J. Dent. Res. 2016, 95, 67–73. [Google Scholar] [CrossRef]
- Sulaiman, T.A. Materials in digital dentistry-A review. J. Esthet. Restor. Dent. 2020, 32, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Fasbinder, D.J. Materials for chairside CAD/CAM restorations. Compend. Contin. Educ. Dent. 2010, 31, 702–704, 706, 708–709. [Google Scholar]
- Floriani, F.; Abuhammoud, S.; Rojas-Rueda, S.; Unnadkat, A.; Fischer, N.G.; Fu, C.C.; Jurado, C.A. The Influence of Thickness on Light Transmission for Pre- and Fully Crystallized Chairside CAD/CAM Lithium Disilicate Ceramics. Materials 2024, 17, 2045. [Google Scholar] [CrossRef] [PubMed]
- Jurado, C.A.; Yeh, J.S.; Vidal, C.M.P.; Cho, S.H.; Abuhammoud, S. Fracture load of chairside CAD-CAM veneers fabricated with pre-and fully crystalized lithium disilicate ceramics. J. Prosthodont. 2025, 34, 429–435. [Google Scholar] [CrossRef]
- Gracis, S.; Thompson, V.P.; Ferencz, J.L.; Silva, N.R.; Bonfante, E.A. A new classification system for all-ceramic and ceramic-like restorative materials. Int. J. Prosthodont. 2015, 28, 227–235. [Google Scholar] [CrossRef]
- Heintze, S.D.; Rousson, V. Survival of zirconia- and metal-supported fixed dental prostheses: A systematic review. Int. J. Prosthodont. 2010, 23, 493–502. [Google Scholar]
- Edelhoff, D.; Liebermann, A.; Schubert, O.; Guth, J.F. Prospective Clinical Split-Mouth Study of Two-Wing-Retained Resin-Bonded Anterior Fixed Dental Prostheses with Metallic and Ceramic Frameworks: 5-year Results. Int. J. Prosthodont. 2023, 36, 253–261. [Google Scholar] [CrossRef]
- Oktay, F.; Yanikoglu, N.; Bayindir, F. Investigation of the Effect of Different Surface Treatments Applied to Titanium Dental Implant Abutments on the Retention of CAD/CAM Zirconium-Supported Ceramic Crowns. Niger. J. Clin. Pr. 2025, 28, 880–888. [Google Scholar] [CrossRef]
- Pai, R.; Shetty, K.H.S.; Nair, P.M.S.; Farookh, F.M.B.; Aphiya, A.; Kukkila, J. The effect of surface conditioning techniques on the shear bond strength of zirconia-reinforced lithium silicate ceramic following adhesive cementation—An in vitro study. J. Conserv. Dent. Endod. 2024, 27, 828–832. [Google Scholar] [CrossRef]
- Chander, N.G. Visual analog scale in prosthodontics. J. Indian Prosthodont. Soc. 2019, 19, 99–100. [Google Scholar] [CrossRef]
- Patil, P. Utility of the visual analog scale in dentistry. Indian J. Dent. Res. 2012, 23, 836. [Google Scholar] [CrossRef] [PubMed]
- Tosun, B.; Uysal, N. Examination of oral health quality of life and patient satisfaction in removable denture wearers with OHIP-14 scale and visual analog scale: A cross-sectional study. BMC Oral Heal. 2024, 24, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rehberger Bescos, F.; Salgado Peralvo, A.O.; Chamorro Petronacci, C.M.; Chele, D.; Camacho Alonso, F.; Penarrocha Oltra, D.; Lado Baleato, O.; Perez Sayans, M. Marginal bone loss and associated factors in immediate dental implants: A retrospective clinical study. Int. J. Implant. Dent. 2025, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Weigel, L.D.; Scherrer, A.; Schmid, L.; Stähli, A.; Imber, J.C.; Roccuzzo, A.; Salvi, G.E. Marginal bone level changes around dental implants with one or two adjacent teeth—A clinical and radiographic retrospective study with a follow-up of at least 10 years. Clin. Oral Implant. Res. 2023, 34, 872–880. [Google Scholar] [CrossRef]
- Mailoa, J.; Fu, J.-H.; Chan, H.-L.; Khoshkam, V.; Li, J.; Wang, H.-L. The Effect of Vertical Implant Position in Relation to Adjacent Teeth on Marginal Bone Loss in Posterior Arches: A Retrospective Study. Int. J. Oral Maxillofac. Implant. 2015, 30, 931–936. [Google Scholar] [CrossRef]
- Jalaluddin, M.; Rathod, A.; Mailankote, S.; Bipinchandra, L.N.; Raj, B.S.H.; Ahirwar, A. Radiographic Assessment of the Marginal Bone Loss Around the Implant Before and After the Prosthesis Placement—A Comparative Study. J. Pharm. Bioallied Sci. 2024, 16, S4693–S4696. [Google Scholar] [CrossRef]
- Srinivasan, G.; Manickam, A.; Sivakumar, S.; Murugan, J.; Elangomannan, S.; Mohan, S. A comprehensive review: Surface modification strategies to enhance corrosion resistance of zirconia-based biomaterials in implant applications. J. Mater. Sci. Mater. Eng. 2025, 20, 1–25. [Google Scholar] [CrossRef]
- Tu, Y.; Ren, H.; He, Y.; Ying, J.; Chen, Y. Interaction between microorganisms and dental material surfaces: General concepts and research progress. J. Oral Microbiol. 2023, 15, 2196897. [Google Scholar] [CrossRef]
- Ribeiro, G.d.A.; Oliveira, V.d.C.; Faria, A.C.L.; Macedo, A.P.; Maciel, C.R.d.O.; Silva, C.H.L.d.; Ribeiro, R.F.; Rodrigues, R.C.S. Influence of Polishing and Glazing on Surface Characteristics and Biofilm Formation on Zirconia: An In Vitro Study. Antibiotics 2025, 14, 739. [Google Scholar] [CrossRef]
- Topçu, S.; Tekçe, N.; Kopuz, D.; Özcelik, E.Y.; Kolaylı, F.; Tuncer, S.; Demirci, M. Effect of surface roughness and biofilm formation on the color properties of resin-infiltrated ceramic and lithium disilicate glass-ceramic CAD-CAM materials. J. Prosthet. Dent. 2024, 131, 935.e931–935.e938. [Google Scholar] [CrossRef] [PubMed]
- Aldhuwayhi, S. Zirconia in Dental Implantology: A Review of the Literature with Recent Updates. Bioengineering 2025, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Sailer, I.; Latyshev, A.; Rabel, K.; Kohal, R.J.; Karasan, D. A systematic review and meta-analysis evaluating the survival, the failure, and the complication rates of veneered and monolithic all-ceramic implant-supported single crowns. Clin. Oral Implant. Res. 2021, 32, 254–288. [Google Scholar] [CrossRef] [PubMed]
- Strasding, M.; Hicklin, S.P.; Todorovic, A.; Fehmer, V.; Mojon, P.; Sailer, I. A multicenter randomized controlled clinical pilot study of buccally micro-veneered lithium-disilicate and zirconia crowns supported by titanium base abutments: 1-year outcomes. Clin. Oral Implant. Res. 2022, 34, 56–65. [Google Scholar] [CrossRef]
- Shihabi, S.; Chrcanovic, B.R. Clinical outcomes of tooth-supported monolithic zirconia vs. porcelain-veneered zirconia fixed dental prosthesis, with an additional focus on the cement type: A systematic review and meta-analysis. Clin. Oral Investig. 2023, 27, 5755–5769. [Google Scholar] [CrossRef]
- Khijmatgar, S.; Tumedei, M.; Tartaglia, G.; Crescentini, M.; Isola, G.; Sidoti, E.; Sforza, C.; Del Fabbro, M.; Tartaglia, G.M. Fifteen-year recall period on zirconia-based single crowns and fixed dental prostheses. A prospective observational study. BDJ Open 2024, 10, 1–8. [Google Scholar] [CrossRef]
- Klein, P.; Spitznagel, F.A.; Zembic, A.; Prott, L.S.; Pieralli, S.; Bongaerts, B.; Metzendorf, M.I.; Langner, R.; Gierthmuehlen, P.C. Survival and Complication Rates of Feldspathic, Leucite-Reinforced, Lithium Disilicate and Zirconia Ceramic Laminate Veneers: A Systematic Review and Meta-Analysis. J. Esthet. Restor. Dent. 2024, 37, 601–619. [Google Scholar] [CrossRef]
- Rinke, S.; Zuck, T.; Hausdörfer, T.; Leha, A.; Wassmann, T.; Ziebolz, D. Prospective clinical evaluation of chairside-fabricated zirconia-reinforced lithium silicate ceramic partial crowns—5-year results. Clin. Oral Investig. 2021, 26, 1593–1603. [Google Scholar] [CrossRef]
- Rinke, S.; Reinermann, E.; Leha, A.; Roediger, M.; Ziebolz, D. Multicenter Prospective Clinical Study on Chairside-Fabricated Partial Crowns: 5-Year Results for Lithia-Zirconia Glass–Ceramic Restorations. J. Esthet. Restor. Dent. 2024, 37, 561–570. [Google Scholar] [CrossRef]
- Tajti, P.; Solyom, E.; Czumbel, L.M.; Szabó, B.; Fazekas, R.; Németh, O.; Hermann, P.; Gerber, G.; Hegyi, P.; Mikulás, K. Monolithic zirconia as a valid alternative to metal-ceramic for implant-supported single crowns in the posterior region: A systematic review and meta-analysis of randomized controlled trials. J. Prosthet. Dent. 2024, 132, 881–889. [Google Scholar] [CrossRef]
- Prott, L.S.; Pieralli, S.; Klein, P.; Spitznagel, F.A.; Ibrahim, F.; Metzendorf, M.I.; Carrasco-Labra, A.; Blatz, M.B.; Gierthmuehlen, P.C. Survival and Complications of Partial Coverage Restorations on Posterior Teeth—A Systematic Review and Meta-Analysis. J. Esthet. Restor. Dent. 2024, 37, 620–641. [Google Scholar] [CrossRef]
- AlMashaan, A.; Aldakheel, A. Survival of Complete Coverage Tooth-Retained Fixed Lithium Disilicate Prostheses: A Systematic Review. Medicina 2022, 59, 95. [Google Scholar] [CrossRef]
- Rauch, A.; Lorenz, L.; Reich, S.; Hahnel, S.; Schmutzler, A.; Schierz, O. Long-term survival of monolithic tooth-supported lithium disilicate crowns fabricated using a chairside approach: 15-year results. Clin. Oral Investig. 2023, 27, 3983–3989. [Google Scholar] [CrossRef]

| Characteristic | Zirconia (n = 100) | Lithium Disilicate (n = 100) | p-Value * |
|---|---|---|---|
| Age (years), mean ± SD | 51.8 ± 10.2 | 50.7 ± 9.8 | 0.420 |
| Sex, female, n (%) | 62 (62%) | 59 (59%) | 0.680 |
| Follow-up period (years), mean ± SD | 5.4 ± 0.6 | 5.3 ± 0.7 | 0.550 |
| Type of support, n (%) | |||
| —Tooth-supported | 58 (58%) | 61 (61%) | 0.700 |
| —Implant-supported | 42 (42%) | 39 (39%) | |
| Location, n (%) | |||
| —Anterior | 40 (40%) | 44 (44%) | 0.560 |
| —Posterior | 60 (60%) | 56 (56%) | |
| Type of restoration, n (%) | |||
| —Single crowns | 72 (72%) | 75 (75%) | 0.640 |
| —Fixed dental prostheses (FDP) | 28 (28%) | 25 (25%) |
| Complication Type | Zirconia (n = 100) | Lithium Disilicate (n = 100) | p-Value * |
|---|---|---|---|
| Any technical complication | 14 (14.0%) | 21 (21.0%) | 0.182 |
| Fracture/catastrophic failure | 6 (6.0%) | 12 (12.0%) | 0.126 |
| Veneer chipping | 5 (5.0%) | 7 (7.0%) | 0.552 |
| Loss of retention/debonding | 3 (3.0%) | 2 (2.0%) | 0.652 |
| Parameter | Zirconia (n = 100) | Lithium Disilicate (n = 100) | p-Value * |
|---|---|---|---|
| Tooth-supported restorations | |||
| Secondary caries, n (%) | 7 (7.0%) | 11 (11.0%) | 0.332 |
| Endodontic complications, n (%) | 4 (4.0%) | 6 (6.0%) | 0.516 |
| Implant-supported restorations | |||
| Peri-implant mucositis, n (%) | 9 (9.0%) | 12 (12.0%) | 0.495 |
| Peri-implantitis, n (%) | 3 (3.0%) | 5 (5.0%) | 0.470 |
| Radiographic outcomes | |||
| Stable periapical status, n (%) | 93 (93.0%) | 89 (89.0%) | 0.317 |
| Marginal bone loss (mm), mean ± SD | 0.46 ± 0.25 | 0.53 ± 0.30 | 0.148 |
| Subgroup | Zirconia (%) | Lithium Disilicate (%) | p-Value |
|---|---|---|---|
| Implant-supported | 93.0 | 88.0 | 0.224 |
| Tooth-supported | 95.0 | 90.0 | 0.210 |
| Posterior region | 93.0 | 87.0 | 0.198 |
| Anterior region | 95.0 | 91.0 | 0.245 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topdagi, B.; Kurum, M.; Guler, C.C.; Abo Haoran, M. Comparison of Long-Term Clinical Outcomes of Zirconia and Lithium Disilicate Prostheses: A Retrospective Cohort Study. Biomimetics 2025, 10, 740. https://doi.org/10.3390/biomimetics10110740
Topdagi B, Kurum M, Guler CC, Abo Haoran M. Comparison of Long-Term Clinical Outcomes of Zirconia and Lithium Disilicate Prostheses: A Retrospective Cohort Study. Biomimetics. 2025; 10(11):740. https://doi.org/10.3390/biomimetics10110740
Chicago/Turabian StyleTopdagi, Basak, Muhammed Kurum, Ceren Cakar Guler, and Mohammad Abo Haoran. 2025. "Comparison of Long-Term Clinical Outcomes of Zirconia and Lithium Disilicate Prostheses: A Retrospective Cohort Study" Biomimetics 10, no. 11: 740. https://doi.org/10.3390/biomimetics10110740
APA StyleTopdagi, B., Kurum, M., Guler, C. C., & Abo Haoran, M. (2025). Comparison of Long-Term Clinical Outcomes of Zirconia and Lithium Disilicate Prostheses: A Retrospective Cohort Study. Biomimetics, 10(11), 740. https://doi.org/10.3390/biomimetics10110740






