Muscle Activation Reduction During Walking with an Active Hip Exoskeleton
Abstract
1. Introduction
2. Methods
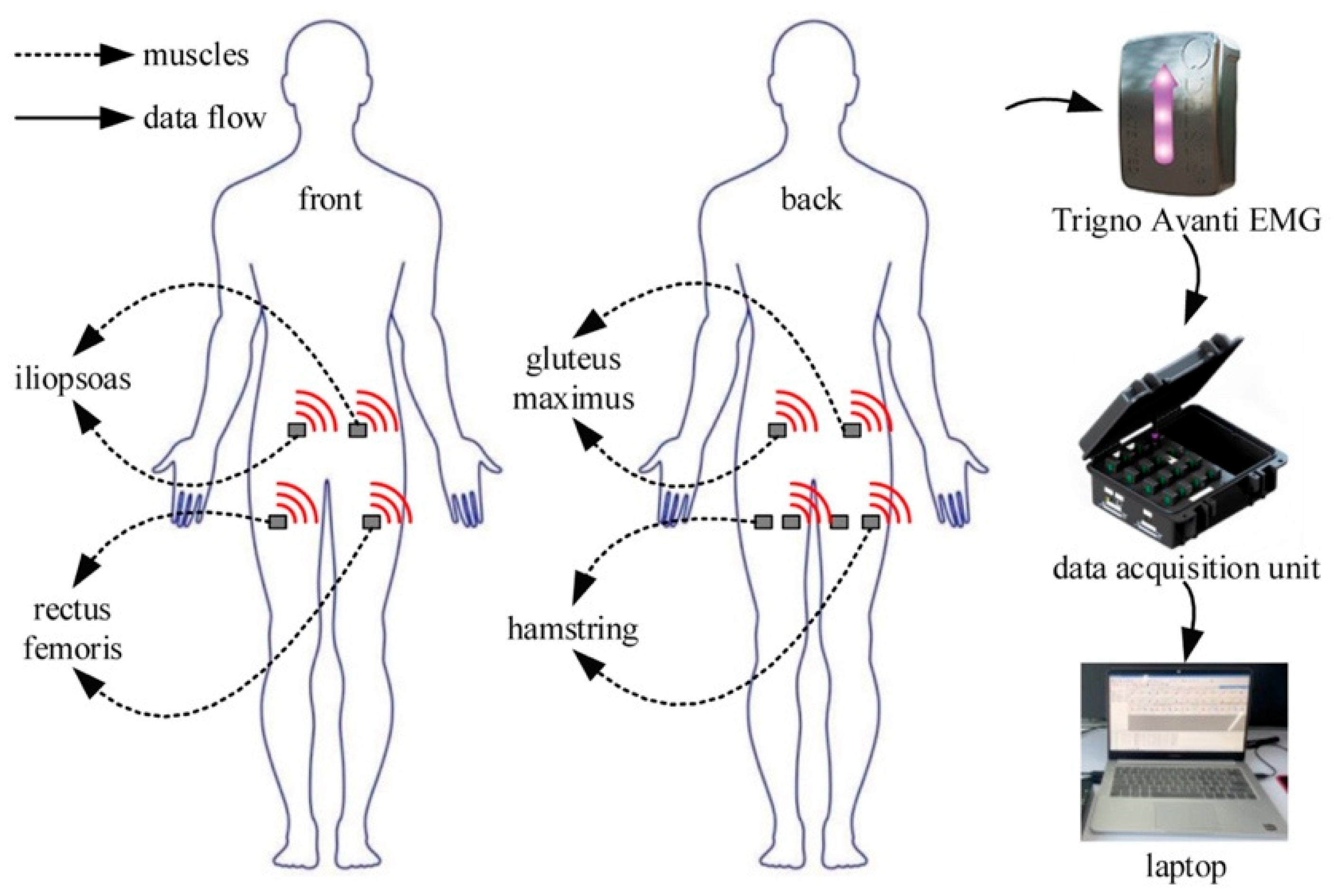
2.1. Data Acquisition and Process
2.2. Muscles’ Activation Characteristics
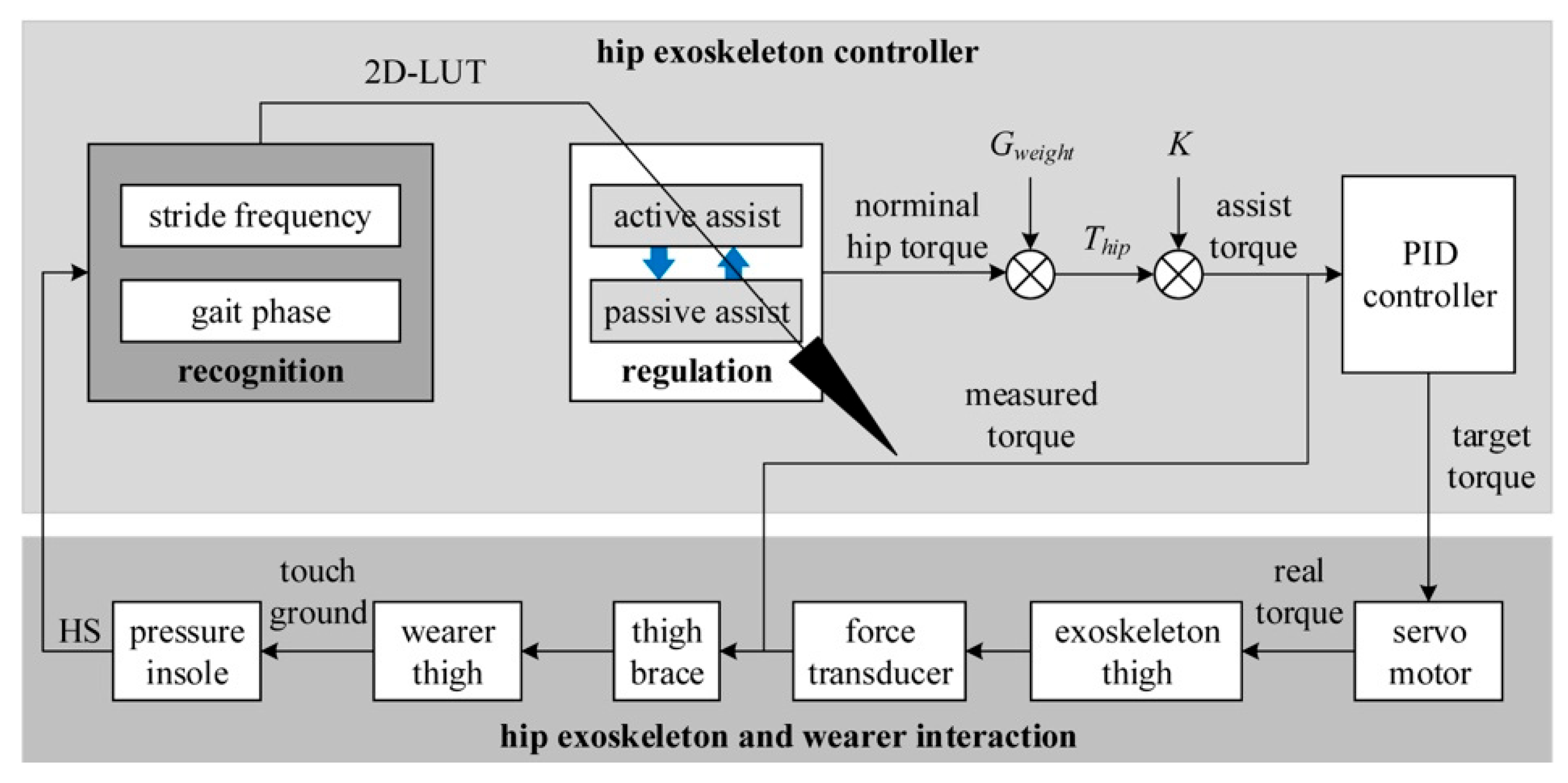
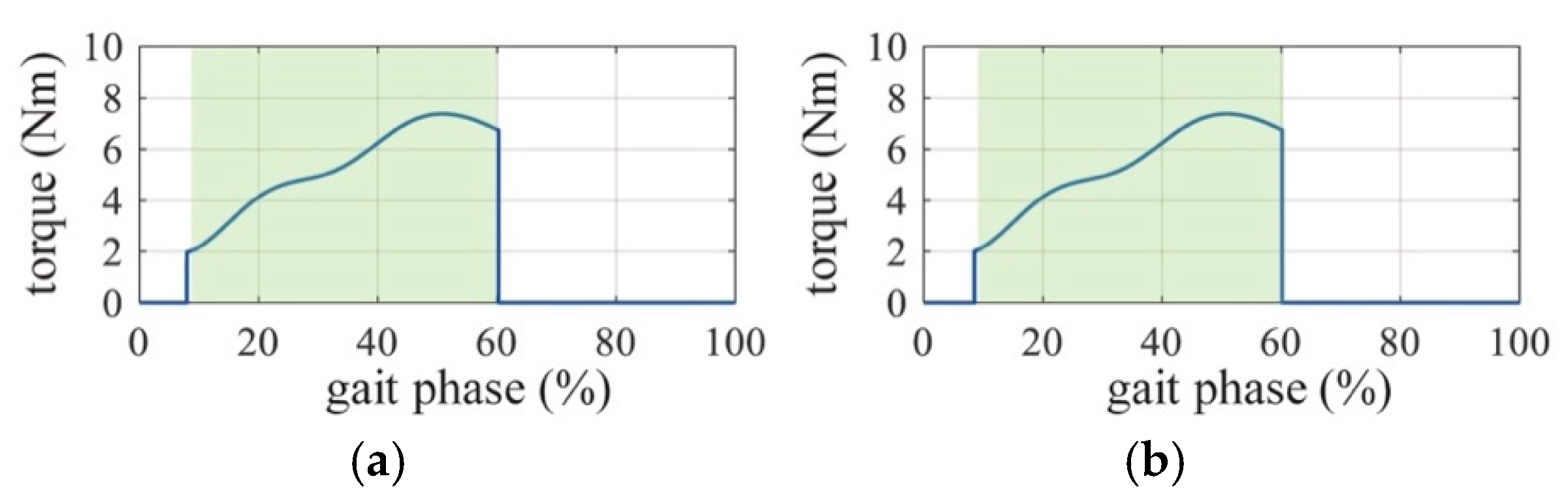
2.3. Active Assistance Strategy
3. Experiments and Results
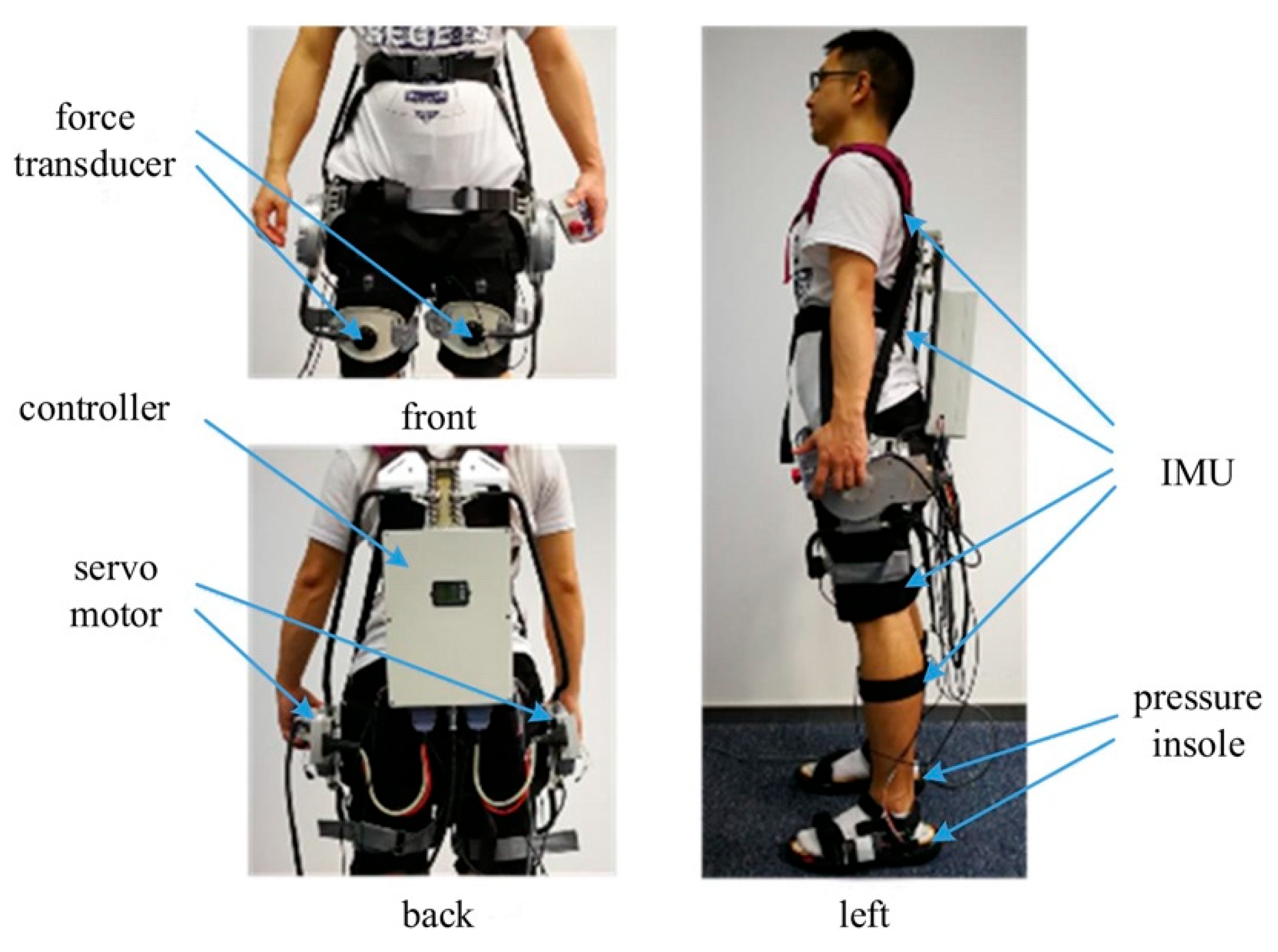
3.1. Verification Experiments
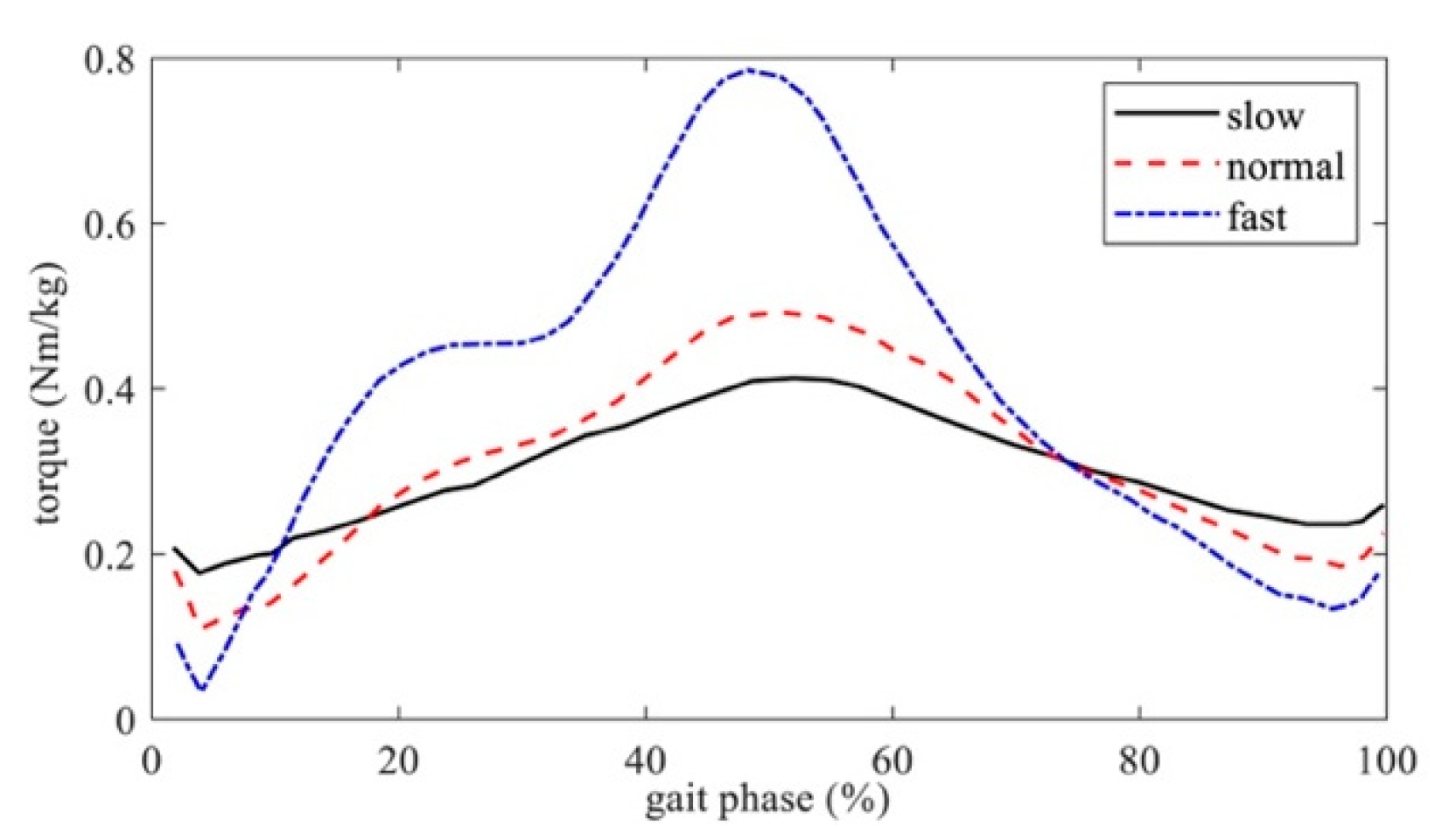
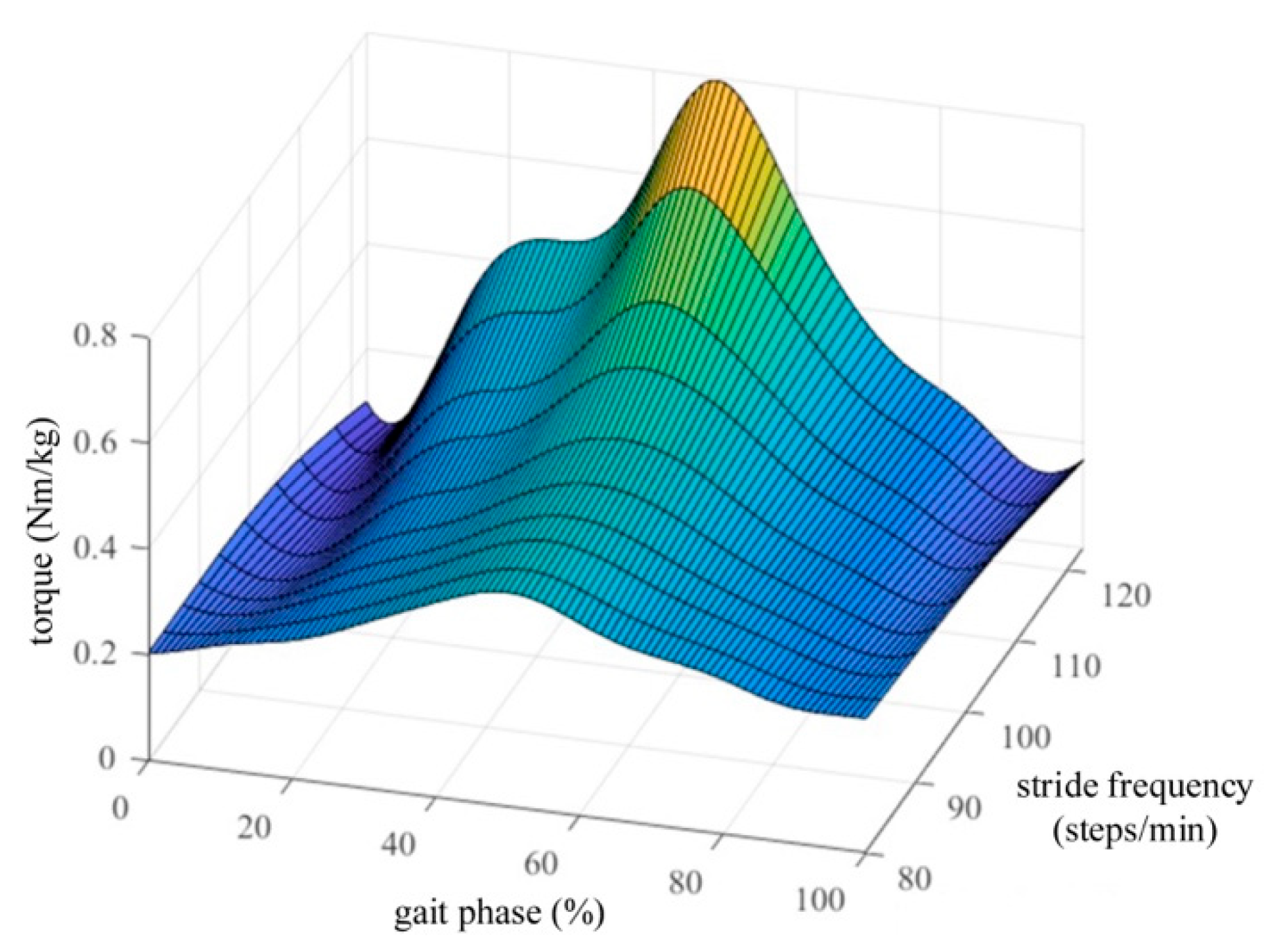
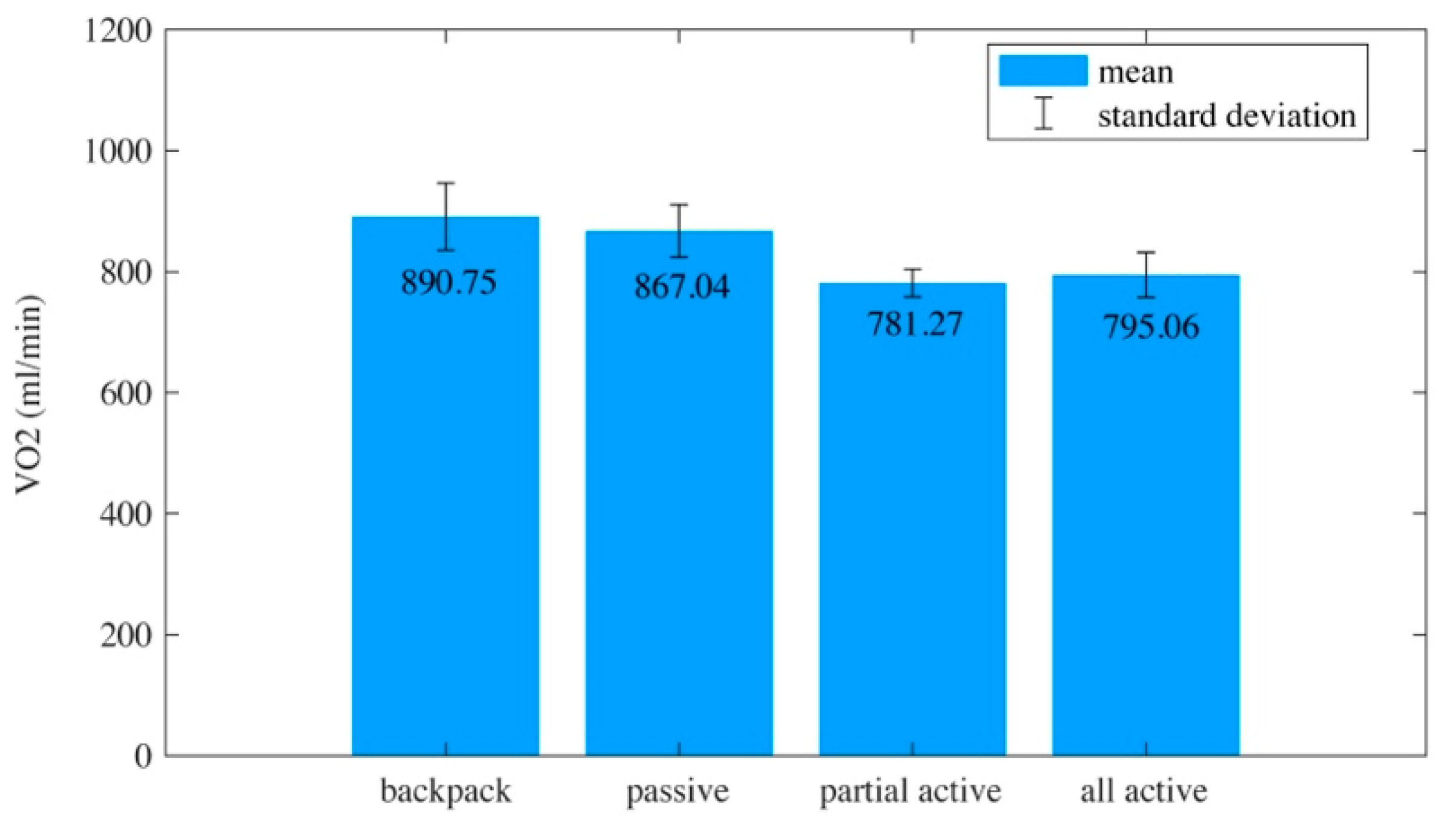
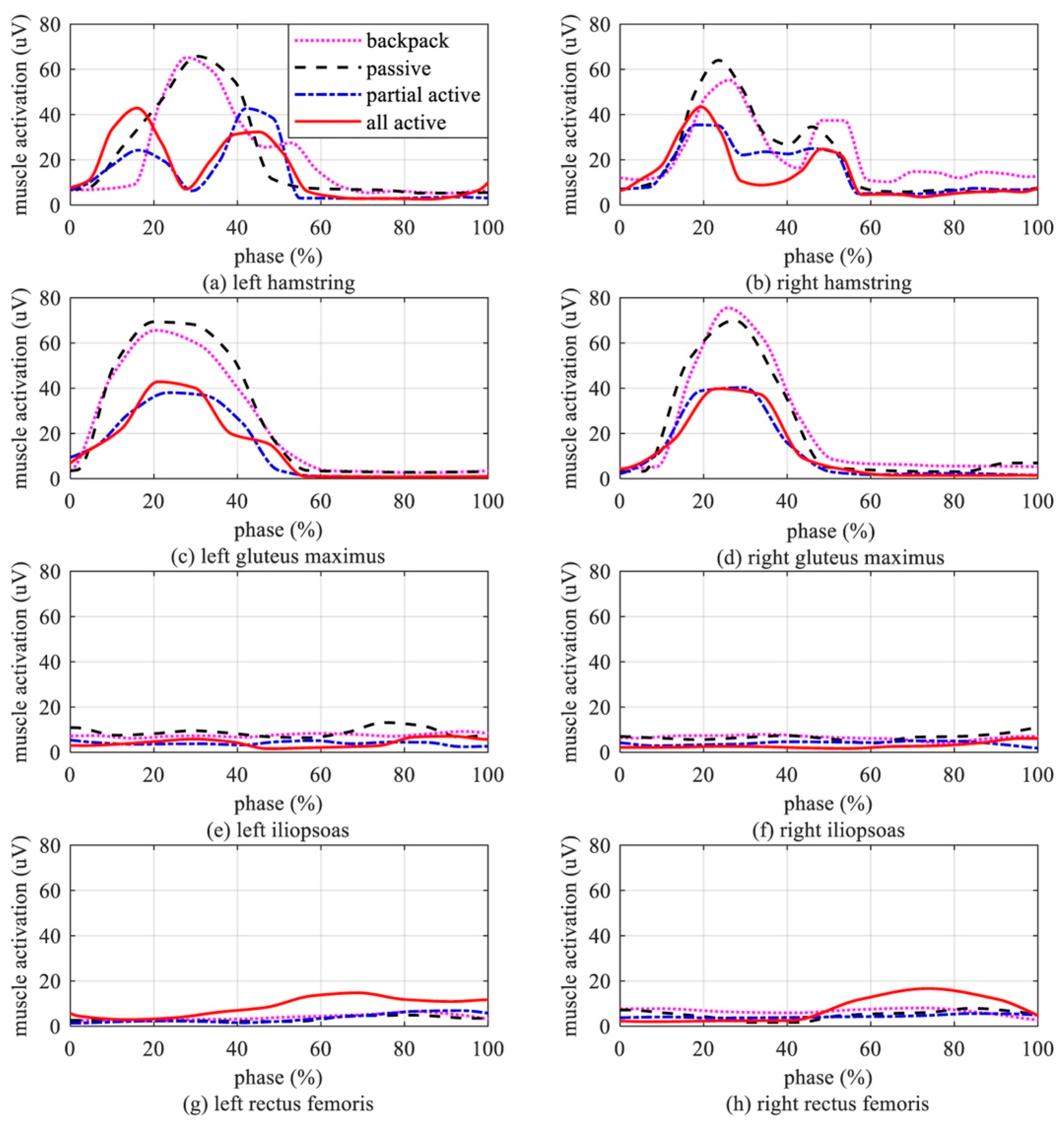
3.2. Experimental Results
4. Discussion
5. Conclusions
6. Key Points
- A maximum reduction in hip muscle activation was found when the active assistance phase was 9–60% of the gait cycle;
- Surface electromyography and oxygen consumption showed signs of muscle activation reduction using the proposed active assistance strategy;
- Since the findings may point towards applications for activities in the daily lives of elderly people, further research is needed to verify the relation between age and muscle activation.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gurriet, T.; Tucker, M.; Duburcq, A.; Boeris, G.; Ames, A.D. Towards variable assistance for lower body exoskeletons. IEEE Robot. Autom. Lett. 2020, 5, 266–273. [Google Scholar] [CrossRef]
- Hidayah, R.; Bishop, L.; Jin, X.; Chamarthy, S.; Stein, J.; Agrawal, S.K. Gait adaptation using a cable-driven active leg exoskeleton (C-ALEX) with post-stroke participants. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Li, G.T.; Liang, X.; Lu, H.J.; Su, T.T.; Hou, Z.G. Development and validation of a self-aligning knee exoskeleton with hip rotation capability. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Jiang, M.; Zhang, S.; Zhu, J.; Yu, S.; Silva, I.D.; Wang, T.; Rouse, E.; Zhou, B.; Yuk, H. Experiment-free exoskeleton assistance via learning in simulation. Nature 2024, 630, 353–359. [Google Scholar] [CrossRef]
- Plaza, A.; Hernandez, M.; Puyuelo, G.; Garces, E.; Garcia, E. Lower-limb medical and rehabilitation exoskeletons: A review of the current designs. IEEE Rev. Biomed. Eng. 2023, 16, 278–291. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, A.; Lobo-Prat, J.; Font-Llagunes, J.M. Systematic review on wearable lower-limb exoskeletons for gait training in neuromuscular impairments. J. Neuroeng. Rehabil. 2021, 18, 22. [Google Scholar] [CrossRef]
- Shushtari, M.; Nasiri, R.; Arami, A. Online reference trajectory adaptation: A personalized control strategy for lower limb exoskeletons. IEEE Robot. Autom. Lett. 2022, 7, 128–134. [Google Scholar] [CrossRef]
- Ali, A.; Fontanari, V.; Schmodelz, W.; Agrawal, S.K. Systematic review of back-support exoskeletons and soft robotic suits. Front. Bioeng. Biotechnol. 2021, 9, 765257. [Google Scholar] [CrossRef]
- Farris, D.J.; Harris, D.J.; Rice, H.M.; Campbell, J.; Weare, A.; Risius, D.; Armstrong, N.; Rayson, M.P. A systematic literature review of evidence for the use of assistive exoskeletons in defence and security use cases. Ergonomics 2023, 66, 61–87. [Google Scholar] [CrossRef]
- O’Connor, S. Exoskeletons in nursing and healthcare: A bionic future. Clin. Nurs. Res. 2021, 30, 1123–1126. [Google Scholar] [CrossRef]
- Bryan, G.M.; Franks, P.W.; Klein, S.C.; Peuchen, R.J.; Collines, S.H. A hip-knee-ankle exoskeleton emulator for studying gait assistance. Int. J. Robot. Res. 2021, 40, 722–746. [Google Scholar] [CrossRef]
- Khazoom, C.; Véronneau, C.; Bigué, J.P.L.; Grenier, J.; Girard, A.; Plante, J.S. Design and control of a multifunctional ankle exoskeleton powered by magnetorheological actuators to assist walking, jumping, and landing. IEEE Robot. Autom. Lett. 2019, 4, 3083–3090. [Google Scholar] [CrossRef]
- Zhu, Z.; Dutta, A.; Dai, F. Exoskeletons for manual material handling—A review and implication for construction applications. Autom. Constr. 2021, 122, 103493. [Google Scholar] [CrossRef]
- Tiboni, M.; Borboni, A.; Verite, F.; Bregoli, C.; Amici, C. Sensors and actuation technologies in exoskeletons: A review. Sensors 2022, 22, 884. [Google Scholar] [CrossRef]
- Brinkemper, A.; Grasmücke, D.; Yilmaz, Z.; Reinecke, F.; Schildhauer, T.A.; Aach, M. Influence of locomotion therapy with the wearable cyborg HAL on bladder and bowel function in acute and chronic SCI patients. Glob. Spine J. 2023, 13, 667–676. [Google Scholar] [CrossRef]
- Laubscher, C.A.; Goo, A.; Farris, R.J.; Sawicki, J.T. Hybrid impedance-sliding mode switching control of the Indego explorer lower—Limb exoskeleton in able-bodied walking. J. Intell. Robot. Syst. 2022, 104, 76. [Google Scholar] [CrossRef]
- Nakajima, T.; Sankai, Y.; Takata, S.; Kobayashi, Y.; Ando, Y.; Nakagawa, M.; Saito, T.; Saito, K.; Ishida, C.; Tamaoka, A.; et al. Cybernic treatment with wearable cyborg Hybrid Assistive Limb (HAL) improves ambulatory function in patients with slowly progressive rare neuromuscular diseases: A multicentre, randomised, controlled crossover trial for efficacy and safety (NCY-3001). Orphanet J. Rare Dis. 2021, 16, 304. [Google Scholar] [CrossRef]
- Christensen, S.; Rafique, S.; Bai, S.P. Design of a powered full-body exoskeleton for physical assistance of elderly people. Int. J. Adv. Robot. Syst. 2021, 18, 17298814211053534. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, Q.; Liu, Y.; Wang, T.; Wan, G. A review of the design of load-carrying exoskeletons. Sci. China-Technol. Sci. 2022, 65, 2051–2067. [Google Scholar] [CrossRef]
- Cenciarini, M.; Dollar, A.M. Biomechanical considerations in the design of lower limb exoskeletons. In Proceedings of the International Conference on Rehabilitation Robotics: Reaching Users & the Community, Zurich, Switzerland, 29 June–1 July 2011. [Google Scholar] [CrossRef]
- Kang, I.; Hsu, H.; Young, A. The effect of hip assistance levels on human energetic cost using robotic hip exoskeletons. IEEE Robot. Autom. Lett. 2019, 4, 430–437. [Google Scholar] [CrossRef]
- Orekhov, G.; Lerner, Z.F. Design and electromechanical performance evaluation of a powered parallel-elastic ankle exoskeleton. IEEE Robot. Autom. Lett. 2022, 7, 8092–8099. [Google Scholar] [CrossRef]
- Peng, X.; Acosta-Sojo, Y.; Wu, M.; Stirling, L. Actuation timing perception of a powered ankle exoskeleton and its associated ankle angle changes during walking. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; McLain, B.; Kang, I.; Young, A. Biomechanical comparison of assistance strategies using a bilateral robotic knee exoskeleton. IEEE Trans. Biomed. Eng. 2021, 68, 2870–2879. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Peng, Y. Design and control of a quasi-direct drive actuated knee exoskeleton. J. Bionic Eng. 2022, 19, 678–687. [Google Scholar] [CrossRef]
- Chen, B.; Zi, B.; Qin, L.; Pan, Q. State-of-the-art research in robotic hip exoskeletons: A general review. J. Orthop. Transl. 2020, 20, 4–13. [Google Scholar] [CrossRef]
- Lee, J.; Huber, M.E.; Hogan, N. Applying hip stiffness with an exoskeleton to compensate gait kinematics. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 2645–2654. [Google Scholar] [CrossRef]
- Gordon, D.F.N.; McGreavy, C.; Christou, A.; Vijayakumar, S. Human-in-the-loop optimization of exoskeleton assistance via online simulation of metabolic cost. IEEE Trans. Robot. 2022, 38, 1410–1429. [Google Scholar] [CrossRef]
- Cao, W.; Chen, C.; Wang, D.; Wu, X.; Chen, L.; Xu, T.; Liu, J. A lower limb exoskeleton with rigid and soft structure for loaded walking assistance. IEEE Robot. Autom. Lett. 2022, 7, 454–461. [Google Scholar] [CrossRef]
- Wei, W.; Zha, S.; Xia, Y.; Gu, J.; Lin, X. A hip active assisted exoskeleton that assists the semi-squat lifting. Appl. Sci. 2020, 10, 2424. [Google Scholar] [CrossRef]
- Lim, B.; Lee, J.; Jang, J.; Kim, K.; Park, Y.J.; Seo, K.; Shim, Y. Delayed output feedback control for gait assistance with a robotic hip exoskeleton. IEEE Trans. Robot. 2019, 35, 1055–1062. [Google Scholar] [CrossRef]
- Yang, W.; Xu, L.; Yu, L.; Chen, Y.; Yan, Z.; Yang, C. Hybrid oscillator-based no-delay hip exoskeleton control for free walking assistance. Ind. Robot-Int. J. Robot. Res. Appl. 2021, 48, 906–914. [Google Scholar] [CrossRef]
- Perry, J. Gait Analysis: Normal and Pathological Function, 2nd ed.; SLACK Books Press: Thorofare, NJ, USA, 2010. [Google Scholar]
- Brand, R.A. The biomechanics and motor control of human gait: Normal, elderly, and pathological. J. Biomech. 1992, 25, 949. [Google Scholar] [CrossRef]
- Zha, F.; Sheng, W.; Guo, W.; Qiu, S.; Deng, J.; Wang, X. Dynamic parameter identification of a lower extremity exoskeleton using RLS-PSO. Appl. Sci. 2019, 9, 324. [Google Scholar] [CrossRef]
- Ludlow, L.W.; Weyand, P.G. Energy expenditure during level human walking: Seeking a simple and accurate predictive solution. J. Appl. Physiol. 2016, 120, 481–494. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Smith, B.; Dahle, J.; Kennedy, S.; Fearnbach, N.; Thomas, D.M.; Bosy-Westphal, A.; Müller, M.J. Resting energy expenditure: From cellular to whole-body Level, a mechanistic historical perspective. Obesity 2021, 29, 500–511. [Google Scholar] [CrossRef]
- Mukherjee, S.D.; Koch, L.G.; Britton, S.L.; Novak, C.M. Aerobic capacity modulates adaptive thermogenesis: Contribution of non-resting energy expenditure. Physiol. Behav. 2020, 225, 113048. [Google Scholar] [CrossRef]
- Gouwanda, D.; Senanayake, S.M.N.A. Identifying gait asymmetry using gyroscopes—A cross-correlation and normalized symmetry index approach. J. Biomech. 2011, 44, 972–978. [Google Scholar] [CrossRef]
- Meijneke, C.; van Oort, G.; Sluiter, V.; van Asseldonk, E.; Tagliamonte, N.L.; Tamburella, F.; Pisotta, I.; Masciullo, M.; Arquilla, M.; Molinari, M.; et al. Symbitron exoskeleton: Design, control, and evaluation of a modular exoskeleton for incomplete and complete spinal cord injured individuals. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 330–339. [Google Scholar] [CrossRef]



| Subject | Gender | Age (years) | Height (cm) | Weight (kg) |
|---|---|---|---|---|
| 1 | Male | 25 | 174.9 | 68.3 |
| 2 | Male | 28 | 177.1 | 75.2 |
| 3 | Male | 22 | 173.6 | 62.7 |
| 4 | Male | 31 | 180.0 | 71.9 |
| 5 | Male | 28 | 172.3 | 55.7 |
| 6 | Male | 24 | 181.5 | 78.7 |
| 7 | Male | 35 | 168.9 | 57.6 |
| 8 | Male | 33 | 170.4 | 61.4 |
| 9 | Female | 27 | 161.5 | 49.2 |
| 10 | Female | 21 | 165.7 | 51.3 |
| 11 | Female | 20 | 170.5 | 54.9 |
| 12 | Female | 28 | 158.3 | 47.3 |
| 13 | Female | 32 | 155.7 | 48.9 |
| 14 | Female | 35 | 163.1 | 52.4 |
| 15 | Female | 34 | 161.9 | 61.3 |
| 16 | Female | 37 | 159.2 | 65.4 |
| Mean [SD] | - | 28.8 [5.4] | 168.4 [8.0] | 60.1 [9.7] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, W.; Ghalichi, F.; Ding, L.; Yu, C.; Lu, M.; Ye, X. Muscle Activation Reduction During Walking with an Active Hip Exoskeleton. Biomimetics 2025, 10, 24. https://doi.org/10.3390/biomimetics10010024
Sheng W, Ghalichi F, Ding L, Yu C, Lu M, Ye X. Muscle Activation Reduction During Walking with an Active Hip Exoskeleton. Biomimetics. 2025; 10(1):24. https://doi.org/10.3390/biomimetics10010024
Chicago/Turabian StyleSheng, Wentao, Farzan Ghalichi, Li Ding, Chengtao Yu, Mingyue Lu, and Xia Ye. 2025. "Muscle Activation Reduction During Walking with an Active Hip Exoskeleton" Biomimetics 10, no. 1: 24. https://doi.org/10.3390/biomimetics10010024
APA StyleSheng, W., Ghalichi, F., Ding, L., Yu, C., Lu, M., & Ye, X. (2025). Muscle Activation Reduction During Walking with an Active Hip Exoskeleton. Biomimetics, 10(1), 24. https://doi.org/10.3390/biomimetics10010024







