Development of an Allergic Rhinitis Diagnosis Application Using the Total Tear IgE Detection Kit for Examining Nasal Fluid: Comparison and Combination with the Conventional Nasal Smear Examination for Eosinophils
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Detection of Total IgE in Nasal Fluid
2.3. Nasal Smear Examination for Eosinophils
2.4. Severity of Symptoms and Quality of Life
2.5. Statistical Analysis
3. Results
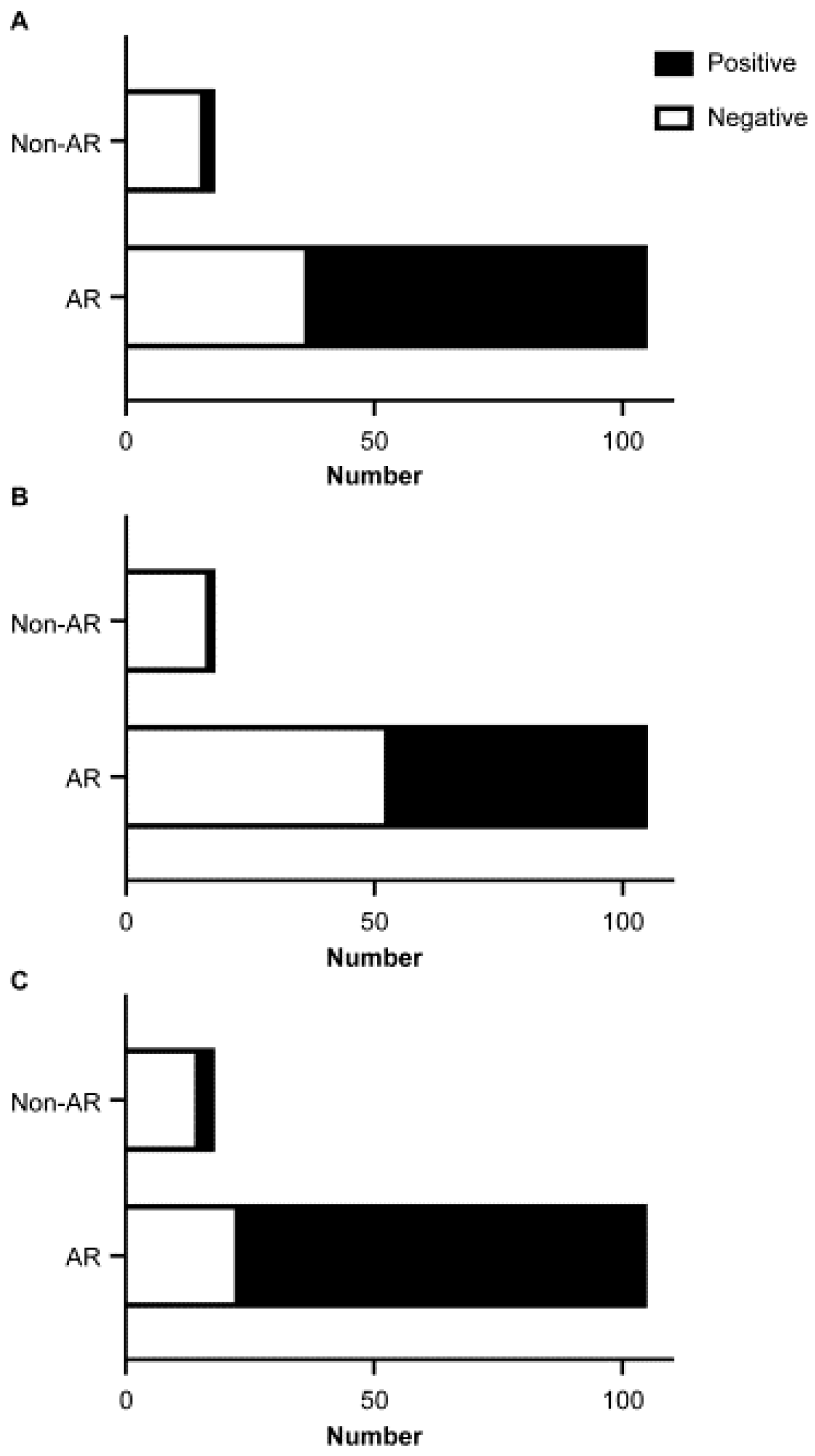
3.1. Detection Rate of Total IgE in Nasal Fluid
3.2. Relationship between the Detection Rates of Total IgE in Nasal Fluid and Eosinophils in Nasal Smear
3.3. Role of Nasal Total IgE in the Severity of Symptoms and QOL in AR
3.4. Association between Total IgE in Nasal Fluid and AR Phenotypes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okubo, K.; Kurono, Y.; Ichimura, K.; Enomoto, T.; Okamoto, Y.; Kawauchi, H.; Suzaki, H.; Fujieda, S.; Masuyama, K. Japanese guidelines for allergic rhinitis 2020. Allergol. Int. 2020, 69, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.K.; Lin, S.Y.; Toskala, E.; Orlandi, R.R.; Akdis, C.A.; Alt, J.A.; Azar, A.; Baroody, F.M.; Bachert, C.; Canonica, G.; et al. International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis: ICAR: Allergic Rhinitis. Int. Forum. Allergy Rhinol. 2018, 8, 108–352. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.; Park, K.T.; Yarlagadda, B.; Davis, E.M.; Platt, M. The significance of serum total immunoglobulin E for in vitro diagnosis of allergic rhinitis. Int. Forum. Allergy Rhinol. 2014, 4, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Karli, R.; Balbaloglu, E.; Uzun, L.; Çınar, F.; Uğur, M.B. Correlation of Symptoms with Total IgE and Specific IgE Levels in Patients Presenting with Allergic Rhinitis. Ther. Adv. Respir. Dis. 2013, 7, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Suzuki, N.; Nakano, Y.; Ohtake, T. Rapid test for conjunctival diseases. Hitachi Chem. Tech. Rep. 2009, 52, 31–34. (In Japanese). Available online: https://paperzz.com/doc/6689850/%E3%83%86%E3%82%AF%E3%83%8B%E3%82%AB%E3%83%AB%E3%83%AC%E3%83%9D%E3%83%BC%E3%83%88---%E6%97%A5%E7%AB%8B%E5%8C%96%E6%88%90%E6%A0%AA%E5%BC%8F%E4%BC%9A%E7%A4%BE (accessed on 11 December 2021).
- Miyazaki, D.; Takamura, E.; Uchio, E.; Ebihara, N.; Ohno, S.; Ohashi, Y.; Okamoto, S.; Satake, Y.; Shoji, J.; Namba, K.; et al. Japanese Guidelines for Allergic Conjunctival Diseases 2020. Allergol. Int. 2020, 69, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Takamura, E. Tear IgE Concentrations in Allergic Conjunctivitis. Eye 1998, 12, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Usui, T.; Yamagami, S.; Miyai, T.; Amano, S. Relation Between Total Tear IgE and Severity of Acute Seasonal Allergic Conjunctivitis. Currt. Eye Res. 2012, 37, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A. Allergy and Allergic Mediators in Tears. Exp. Eye Res. 2013, 117, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Takeuchi, H.; Morizane, R.; Kitano, H.; Sakoda, T.; Enomoto, T. Nonspecific IgE Potential in Nasal Discharge for Diagnosing Allergic Rhinitis. Nippon Jibiinkoka Gakkai Kaiho. 2011, 114, 774–779. (In Japanese) [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Tanimoto, M.; Ohtake, T.; Higuchi, M. Tear total IgE detection Kit. Hitachi Chem. Tech. Rep. 2014, 56, 20–21. Available online: https://www.mc.showadenko.com/english/report/056/56_tr05.pdf (accessed on 11 December 2021).
- Okano, M.; Satoskar, A.R.; Abe, M.; Harn, D.A.; Okano, M.; Nishizaki, K.; Takeda, Y.; Yoshino, T.; Brombacher, F.; Satoskar, A.A. Interleukin-4-Independent Production of Th2 Cytokines by Nasal Lymphocytes and Nasal Eosinophilia in Murine Allergic Rhinitis. Allergy 2000, 55, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Dykewicz, M.S.; Wallace, D.V.; Amrol, D.J.; Baroody, F.M.; Bernstein, J.A.; Craig, T.J.; Dinakar, C.; Ellis, A.K.; Finegold, I.; Golden, D.B.K.; et al. Rhinitis 2020: A Practice Parameter Update. J. Allergy Clin. Immunol. 2020, 146, 721–767. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.; Freedman, P.; Boswell, R. Nonallergic Rhinitis with Eosinophilia (NARES Syndrome). Clinical and Immunologic Presentation. J. Allergy Clin. Immunol. 1981, 67, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Rondón, C.; Fernandez, J.; Canto, G.; Blanca, M. Local Allergic Rhinitis: Concept, Clinical Manifestations, and Diagnostic Approach. J. Investig. Allergol. Clin. Immunol. 2010, 20, 364–371, quiz 2 p following 371. [Google Scholar] [PubMed]
- Testera-Montes, A.; Jurado, R.; Salas, M.; Eguiluz-Gracia, I.; Mayorga, C. Diagnostic Tools in Allergic Rhinitis. Front. Allergy 2021, 2, 721851. [Google Scholar] [CrossRef] [PubMed]
- Yamana, Y.; Fukuda, K.; Ko, R.; Uchio, E. Local Allergic Conjunctivitis: A Phenotype of Allergic Conjunctivitis. Int. Ophthalmol. 2019, 39, 2539–2544. [Google Scholar] [CrossRef] [PubMed]



| Nasal Smear Examination for Eosinophils | |||
|---|---|---|---|
| Positive | Negative | ||
| detection of total IgE in nasal fluid using the AW test | Positive | 38 | 30 |
| Negative | 14 | 23 | |
| Nasal Smear Examination for Eosinophils | |||
|---|---|---|---|
| Positive | Negative | ||
| detection of total IgE in nasal fluid using the AW test | Positive | 0 | 2 |
| Negative | 1 | 15 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumanomidou, H.; Okano, M. Development of an Allergic Rhinitis Diagnosis Application Using the Total Tear IgE Detection Kit for Examining Nasal Fluid: Comparison and Combination with the Conventional Nasal Smear Examination for Eosinophils. Allergies 2022, 2, 146-153. https://doi.org/10.3390/allergies2040014
Kumanomidou H, Okano M. Development of an Allergic Rhinitis Diagnosis Application Using the Total Tear IgE Detection Kit for Examining Nasal Fluid: Comparison and Combination with the Conventional Nasal Smear Examination for Eosinophils. Allergies. 2022; 2(4):146-153. https://doi.org/10.3390/allergies2040014
Chicago/Turabian StyleKumanomidou, Hiroshi, and Mitsuhiro Okano. 2022. "Development of an Allergic Rhinitis Diagnosis Application Using the Total Tear IgE Detection Kit for Examining Nasal Fluid: Comparison and Combination with the Conventional Nasal Smear Examination for Eosinophils" Allergies 2, no. 4: 146-153. https://doi.org/10.3390/allergies2040014
APA StyleKumanomidou, H., & Okano, M. (2022). Development of an Allergic Rhinitis Diagnosis Application Using the Total Tear IgE Detection Kit for Examining Nasal Fluid: Comparison and Combination with the Conventional Nasal Smear Examination for Eosinophils. Allergies, 2(4), 146-153. https://doi.org/10.3390/allergies2040014







