3D Ultrasound and MRI in Assessing Resection Margins during Tongue Cancer Surgery: A Research Protocol for a Clinical Diagnostic Accuracy Study
Abstract
:1. Introduction
2. Research Question
3. Materials and Methods
3.1. Eligibility Criteria
3.2. Perioperative Assessment of Margins
3.2.1. Surgical Specimen Examination by the Surgeon
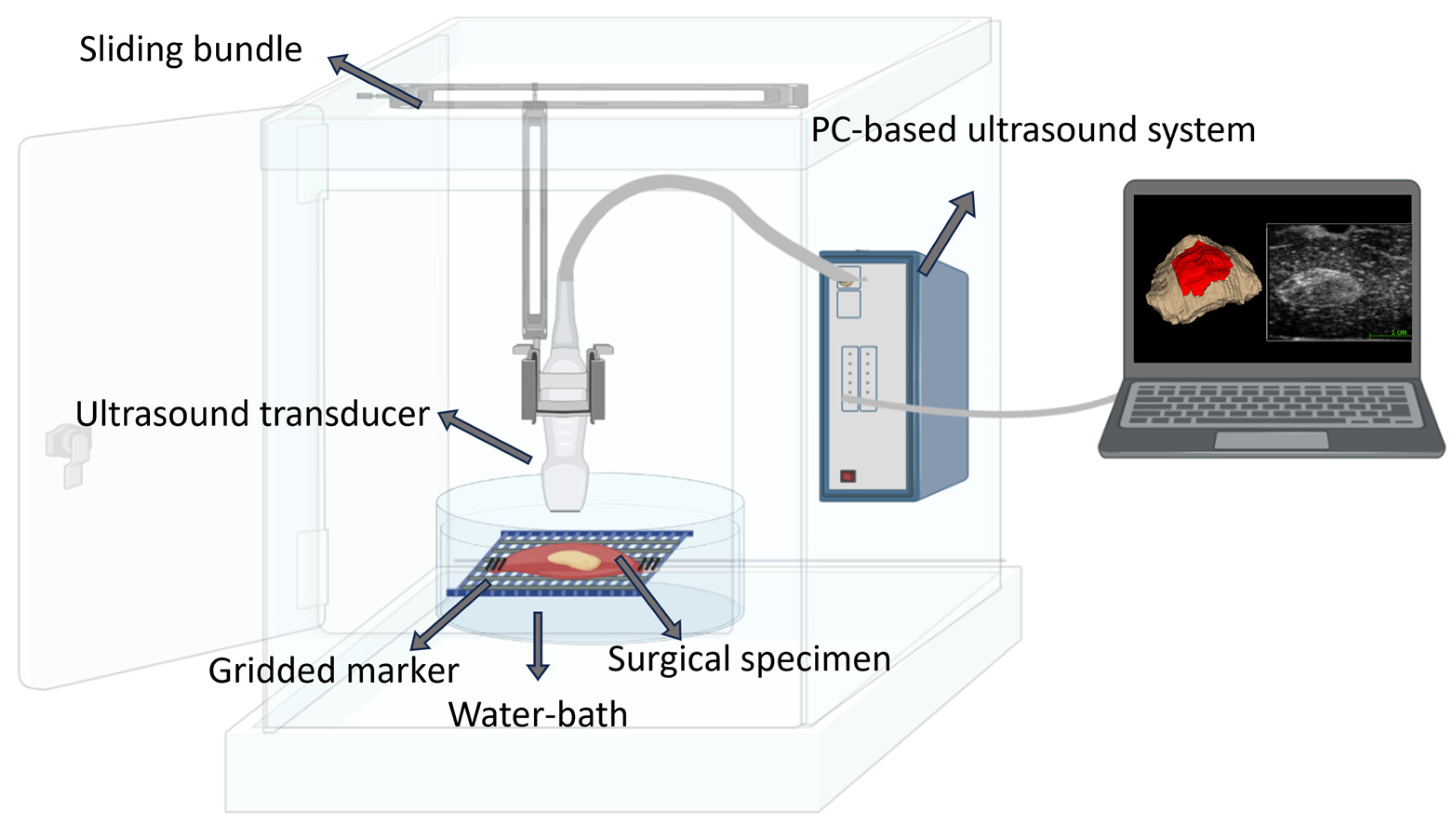
3.2.2. 3D Ultrasound Scan (3Sonics) of the Surgical Specimen on-Site
3.2.3. MRI Scan of the Surgical Specimen in the Radiology Department
3.2.4. Tissue Preparation and Histopathology
4. Clinical Outcome Definition
- The perioperative measurement of resection margins (mm) at five directions (Figure 1) with clinical exam and imaging compared to the post-surgical histopathology results (reference standard).
- The image-by-image comparison of the depth of invasion measurement (mm) from 3D ultrasound/MR imaging and histopathology slides.
- The depth of invasion (mm) comparison between in vivo and ex vivo ultrasound.
- The number of margins correctly classified as free (>5 mm), close (1–5 mm), or positive (<1 mm) margins by 3D ultrasound and MRI using histopathology findings as the reference.
- The number of cases requiring adjuvant treatments (surgery or chemo/radiotherapy) due to T-site residuals.
- A change in tumor volume and resection margin measurements with 3D ultrasound imaging before and after formalin fixation.
- The time usage (minutes) and cost estimation for perioperative 3D ultrasound and MRI.
5. Statistics
6. Power Calculation and Inclusion Period
7. Ethics and Data Management
8. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, J.H.; Iyer, N.G.; Tan, M.-H.; Edgren, G. Changing epidemiology of oral squamous cell carcinoma of the tongue: A global study. Head Neck 2016, 39, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Karnov, K.K.S.; Grønhøj, C.; Jensen, D.H.; Wessel, I.; Charabi, B.W.; Specht, L.; Kjaer, A.; von Buchwald, C. Increasing incidence and survival in oral cancer: A nationwide Danish study from 1980 to 2014. Acta Oncol. 2017, 56, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Chinn, S.B.; Myers, J.N. Oral Cavity Carcinoma: Current Management, Controversies, and Future Directions. J. Clin. Oncol. 2015, 33, 3269–3276. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, O.; Knutsson, J.; Landström, F.J.; Magnuson, A.; von Beckerath, M. Ultrasound accurately assesses depth of invasion in T1-T2 oral tongue cancer. Laryngoscope Investig. Otolaryngol. 2022, 7, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Pagedar, A.N. Better Visualization of Oral Cancer Margins—A Struggle of Cancer and Technology. JAMA Otolaryngol. Neck Surg. 2020, 146, 1156–1157. [Google Scholar] [CrossRef]
- Jensen, J.S.; Jakobsen, K.K.; Mirian, C.; Christensen, J.T.; Schneider, K.; Nahavandipour, A.; Wingstrand, V.L.L.; Wessel, I.; Tvedskov, J.F.; Frisch, T.; et al. The Copenhagen Oral Cavity Squamous Cell Carcinoma database: Protocol and report on establishing a comprehensive oral cavity cancer database. Clin. Epidemiol. 2019, 11, 733–741. [Google Scholar] [CrossRef]
- de Koning, K.J.; Koppes, S.A.; de Bree, R.; Dankbaar, J.W.; Willems, S.M.; van Es, R.J.; Noorlag, R. Feasibility study of ultrasound-guided resection of tongue cancer with immediate specimen examination to improve margin control—Comparison with conventional treatment. Oral Oncol. 2021, 116, 105249. [Google Scholar] [CrossRef]
- de Kleijn, B.J.; Heldens, G.T.N.; Herruer, J.M.; Sier, C.F.M.; Piazza, C.; de Bree, R.; Guntinas-Lichius, O.; Kowalski, L.P.; Poorten, V.V.; Rodrigo, J.P.; et al. Intraoperative Imaging Techniques to Improve Surgical Resection Margins of Oropharyngeal Squamous Cell Cancer: A Comprehensive Review of Current Literature. Cancers 2023, 15, 896. [Google Scholar] [CrossRef]
- Shah, A.K. Postoperative pathologic assessment of surgical margins in oral cancer: A contemporary review. J. Oral Maxillofac. Pathol. 2018, 22, 78–85. [Google Scholar] [CrossRef]
- Lee, Y.; Krishnan, G.; Nishio, N.; Berg, N.S.v.D.; Lu, G.; Martin, B.A.; Keulen, S.; Colevas, A.D.; Kapoor, S.; Liu, J.T.; et al. Intraoperative Fluorescence-Guided Surgery in Head and Neck Squamous Cell Carcinoma. Laryngoscope 2020, 131, 529–534. [Google Scholar] [CrossRef]
- Heidkamp, J.; Weijs, W.L.J.; Grunsven, A.C.H.v.E.; Vries, I.L.; Maas, M.C.; Rovers, M.M.; Fütterer, J.J.; Steens, S.C.A.; Takes, R.P. Assessment of surgical tumor-free resection margins in fresh squamous-cell carcinoma resection specimens of the tongue using a clinical MRI system. Head Neck 2020, 42, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Steens, S.C.A.; Bekers, E.M.; Weijs, W.L.J.; Litjens, G.J.S.; Veltien, A.; Maat, A.; Broek, G.B.v.D.; van der Laak, J.A.W.M.; Fütterer, J.J.; van der Kaa, C.A.H.; et al. Evaluation of tongue squamous cell carcinoma resection margins using ex-vivo MR. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zeng, Z. A Review on Real-Time 3D Ultrasound Imaging Technology. BioMed Res. Int. 2017, 2017, 1–20. [Google Scholar] [CrossRef]
- Narayana, H.M.; Panda, N.K.; Mann, S.B.S.; Katariya, S.; Vasishta, R.K. Ultrasound versus physical examination in staging carcinoma of the mobile tongue. J. Laryngol. Otol. 1996, 110, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Nulent, T.J.K.; Noorlag, R.; Van Cann, E.M.; Pameijer, F.A.; Willems, S.M.; Yesuratnam, A.; Rosenberg, A.J.; de Bree, R.; van Es, R.J. Intraoral ultrasonography to measure tumor thickness of oral cancer: A systematic review and meta-analysis. Oral Oncol. 2018, 77, 29–36. [Google Scholar] [CrossRef]
- Tarabichi, O.; Bulbul, M.G.; Kanumuri, V.V.; Faquin, W.C.; Juliano, A.F.; Cunnane, M.E.; Varvares, M.A. Utility of intraoral ultrasound in managing oral tongue squamous cell carcinoma: Systematic review. Laryngoscope 2018, 129, 662–670. [Google Scholar] [CrossRef]
- Yokoo, Y.; Yuasa, K.; Ohno, J.; Ikebe, T. Relationship between tumor thickness on images and cellular dissociation grading composing of tumor budding and cell nest size in early tongue cancer. Oral Sci. Int. 2023. [Google Scholar] [CrossRef]
- de Koning, K.J.; van Es, R.J.; Klijn, R.J.; Breimer, G.E.; Dankbaar, J.W.; Braunius, W.W.; Dieleman, F.J.; Rijken, J.A.; Tijink, B.M.; Noorlag, R.; et al. Application and accuracy of ultrasound-guided resections of tongue cancer. Oral. Oncol. 2022, 133, 106023. [Google Scholar] [CrossRef]
- Adriaansens, C.M.E.M.; de Koning, K.J.; de Bree, R.; Dankbaar, J.W.; Breimer, G.E.; van Es, R.J.J.; Noorlag, R. Ultrasound-guided resection for squamous cell carcinoma of the buccal mucosa: A feasibility study. Head Neck 2022, 45, 647–657. [Google Scholar] [CrossRef]
- Mozaffari, M.H.; Lee, W.-S. Freehand 3-D Ultrasound Imaging: A Systematic Review. Ultrasound Med. Biol. 2017, 43, 2099–2124. [Google Scholar] [CrossRef]
- Bekedam, N.; Smit, J.; de Koekkoek-Doll, P.K.; van Alphen, M.; van Veen, R.; Karssemakers, L.; Karakullukcu, M.; Smeele, L. Intra-operative resection margin model of tongue carcinoma using 3D reconstructed ultrasound. Adv. Oral Maxillofac. Surg. 2021, 4, 100154. [Google Scholar] [CrossRef]
- de Ruijter, J.; van Sambeek, M.; van de Vosse, F.; Lopata, R. Automated 3D geometry segmentation of the healthy and diseased carotid artery in free-hand, probe tracked ultrasound images. Med. Phys. 2020, 47, 1034–1047. [Google Scholar] [CrossRef] [PubMed]
- Pelz, J.O.; Weinreich, A.; Karlas, T.; Saur, D. Evaluation of Freehand B-Mode and Power-Mode 3D Ultrasound for Visualisation and Grading of Internal Carotid Artery Stenosis. PLoS ONE 2017, 12, e0167500. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Zheng, Y.-P.; Kot, B.C.-W.; Cheung, J.C.-W.; Cheng, S.C.-H.; Kwong, D.L.-W. Three-Dimensional Elastography for Cervical Lymph Node Volume Measurements: A Study to Investigate Feasibility, Accuracy and Reliability. Ultrasound Med. Biol. 2013, 39, 396–406. [Google Scholar] [CrossRef]
- Sabiniok, M.; Opieliński, K.J. Different Types of Ultrasound Probes Usage for Multi-Angle Conventional 3D Ultrasound Compound Imaging: A Breast Phantom Study. Appl. Sci. 2022, 12, 2689. [Google Scholar] [CrossRef]
- Yan, P.; Cheeseborough, J.C., 3rd; Chao, K.S. Automatic shape-based level set segmentation for needle tracking in 3-D TRUS-guided prostate brachytherapy. Ultrasound Med. Biol. 2012, 38, 1626–1636. [Google Scholar] [CrossRef]
- Cenni, F.; Monari, D.; Desloovere, K.; Aertbeliën, E.; Schless, S.-H.; Bruyninckx, H. The reliability and validity of a clinical 3D freehand ultrasound system. Comput. Methods Programs Biomed. 2016, 136, 179–187. [Google Scholar] [CrossRef]
- Fenster, A.; Downey, D.B.; Cardinal, H.N. Three-dimensional ultrasound imaging. Phys. Med. Biol. 2001, 46, R67–R99. [Google Scholar] [CrossRef]
- Chung, D.; Bandarkar, A.; Rana, S.; Tabrizi, P.R.; Preciado, D.; Jago, J.; Linguraru, M.G.; Reilly, B.K. Pilot study of the potential of 3D ultrasound to measure tonsillar volume and hypertrophy. Int. J. Pediatr. Otorhinolaryngol. 2019, 126, 109612. [Google Scholar] [CrossRef]
- Makouei, F.; Ewertsen, C.; Agander, T.K.; Olesen, M.V.; Pakkenberg, B.; Todsen, T. 3D Ultrasound versus Computed Tomography for Tumor Volume Measurement Compared to Gross Pathology—A Pilot Study on an Animal Model. J. Imaging 2022, 8, 329. [Google Scholar] [CrossRef]
- Zhao, L.; Prior, S.J.; Kampmann, M.; Sorkin, J.D.; Caldwell, K.; Braganza, M.; McEvoy, S.; Lal, B.K. Measurement of thrombus resolution using three-dimensional ultrasound assessment of deep vein thrombosis volume. J. Vasc. Surgery Venous Lymphat. Disord. 2014, 2, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Housden, R.J.; Gee, A.H.; Treece, G.M.; Prager, R.W. Sensorless Reconstruction of Freehand 3D Ultrasound Data. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2006; Springer: Berlin/Heidelberg, Germany, 2006; pp. 356–363. [Google Scholar]
- Li, P.-C.; Li, C.-Y.; Yeh, W.-C. Tissue motion and elevational speckle decorrelation in freehand 3D ultrasound. Ultrason. Imaging 2002, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Kim, J.-H.; Choi, S.H.; Yun, T.J.; Wi, J.Y.; Kim, S.A.; Sun, H.Y.; Ryoo, I.; Park, S.-W. Off-site evaluation of three-dimensional ultrasound for the diagnosis of thyroid nodules: Comparison with two-dimensional ultrasound. Eur. Radiol. 2016, 26, 3353–3360. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.; Carreira, J.; Thompson, R.; Morais, A.; Miller, C.; Wein, W.; Ghosh, J.; McCollum, C. An Ex Vivo Evaluation of Tomographic 3-D Ultrasound, B-Mode Ultrasound, CT And MR Imaging to Measure Artery Diameter, Length and Wall Volume. Ultrasound Med. Biol. 2019, 45, 2819–2829. [Google Scholar] [CrossRef] [PubMed]
- Scheipers, U.; Koptenko, S.; Remlinger, R.; Falco, T.; Lachaine, M. 3-D ultrasound volume reconstruction using the direct frame interpolation method. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2010, 57, 2460–2470. [Google Scholar] [CrossRef]
- Wein, W.; Pache, F.; Röper, B.; Navab, N. Backward-warping ultrasound reconstruction for improving diagnostic value and registration. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2006; Springer: Berlin/Heidelberg, Germany, 2006; pp. 750–757. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, S.-Y.; Liu, R.-C.; Luo, F.; Shu, D.-X. Vascularity Index of Laryngeal Cancer Derived from 3-D Ultrasound: A Predicting Factor for the in vivo Assessment of Cervical Lymph Node Status. Ultrasound Med. Biol. 2009, 35, 1596–1600. [Google Scholar] [CrossRef]
- Saß, B.; Pojskic, M.; Zivkovic, D.; Carl, B.; Nimsky, C.; Bopp, M.H.A. Utilizing Intraoperative Navigated 3D Color Doppler Ultrasound in Glioma Surgery. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Smits, R.W.H.; Koljenović, S.; Hardillo, J.A.; Hove, I.T.; Meeuwis, C.A.; Sewnaik, A.; Dronkers, E.A.; Schut, T.C.B.; Langeveld, T.P.M.; Molenaar, J.; et al. Resection margins in oral cancer surgery: Room for improvement. Head Neck 2016, 38, E2197–E2203. [Google Scholar] [CrossRef]
- Brouwer de Koning, S.G.; Karakullukcu, M.B.; Lange, C.A.H.; Ruers, T.J.M. The oral cavity tumor thickness: Measurement accuracy and consequences for tumor staging. Eur. J. Surg. Oncol. 2019, 45, 2131–2136. [Google Scholar] [CrossRef]
- Lodder, W.L.; Teertstra, H.J.; Tan, I.B.; Pameijer, F.A.; Smeele, L.E.; van Velthuysen, M.-L.F.; Brekel, M.W.M.v.D. Tumour thickness in oral cancer using an intra-oral ultrasound probe. Eur. Radiol. 2010, 21, 98–106. [Google Scholar] [CrossRef]
- Alsaffar, H.A.; Goldstein, D.P.; King, E.V.; de Almeida, J.R.; Brown, D.H.; Gilbert, R.W.; Gullane, P.J.; Espin-Garcia, O.; Xu, W.; Irish, J.C. Correlation between clinical and MRI assessment of depth of invasion in oral tongue squamous cell carcinoma. J. Otolaryngol. Head Neck Surg. 2016, 45, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gorpas, D.; Phipps, J.; Bec, J.; Ma, D.; Dochow, S.; Yankelevich, D.; Sorger, J.; Popp, J.; Bewley, A.; Gandour-Edwards, R.; et al. Autofluorescence lifetime augmented reality as a means for real-time robotic surgery guidance in human patients. Sci. Rep. 2019, 9, 1187. [Google Scholar] [CrossRef]
- Kahng, P.W.; Wu, X.; Ramesh, N.P.; Pastel, D.A.; Halter, R.J.; Paydarfar, J.A. Improving target localization during trans-oral surgery with use of intraoperative imaging. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.W.; Teraphongphom, N.T.; Berg, N.S.v.D.; Martin, B.A.; Oberhelman, N.J.; Divi, V.; Kaplan, M.J.; Hong, S.S.; Lu, G.; Ertsey, R.; et al. Determination of Tumor Margins with Surgical Specimen Mapping Using Near-Infrared Fluorescence. Cancer Res 2018, 78, 5144–5154. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, P.M.; Reitsma, J.B.; E Bruns, D.; A Gatsonis, C.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.W.; et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Jakobsen, K.K.; Grønhøj, C.; Jensen, D.H.; Karnov, K.K.S.; Agander, T.K.; Specht, L.; von Buchwald, C. Increasing incidence and survival of head and neck cancers in Denmark: A nation-wide study from 1980 to 2014. Acta Oncol. 2018, 57, 1143–1151. [Google Scholar] [CrossRef]
- McCoy, E. Understanding the Intention-to-treat Principle in Randomized Controlled Trials. West. J. Emerg. Med. 2017, 18, 1075–1078. [Google Scholar] [CrossRef]
- Tarabichi, O.; Kanumuri, V.; Juliano, A.F.; Faquin, W.C.; Cunnane, M.E.; Varvares, M.A. Intraoperative Ultrasound in Oral Tongue Cancer Resection: Feasibility Study and Early Outcomes. Otolaryngol. Neck Surg. 2017, 158, 645–648. [Google Scholar] [CrossRef]

| Tracking Technique | Year | Reference | Aim | Highlights of the Technique |
|---|---|---|---|---|
| Electromagnetic sensor | 2021 | Bekedam et al. [21] | Intraoperative tongue tumor margin assessment |
|
| 2020 | Ruijter et al. [22] | 3D geometry assessment of carotid artery | ||
| 2017 | Pelz et al. [23] | Direct visualization of internal carotid artery stenosis | ||
| 2013 | Ying et al. [24] | Cervical lymph node volume measurement | ||
| Mechanical arm | 2022 | Sabiniok et al. [25] | Breast phantom study |
|
| 2012 | Yan et al. [26] | Needle tracking in prostate brachytherapy | ||
| Optikal tracker | 2016 | Cenni et al. [27] | Phantom study |
|
| 3D probe | 2019 | Chung et al. [29] | Tonsillar volume measurement |
|
| 2022 | Makouei et al. [30] | Animal model | ||
| 2014 | Zhao et al. [31] | Acquiring and analyzing 3D ultrasound images of deep vein thrombosis | ||
| Sensorless | 2006 | Housden et al. [32] | Animal model |
|
| 2002 | Li et al. [33] | Simulation study |
| Imaging Technique | Year | Number of Cases | Reference | Diagnostic Conclusion |
|---|---|---|---|---|
| The use of 2D ultrasound and MRI | 2019 | 83 | de Koning et al. [41] | For preoperative tumor staging in oral cancer, the tumor thickness is better estimated by the use of ultrasound compared to MRI. |
| The use of 2D ultrasound and MRI | 2011 | 65 | Lodder et al. [42] | Tumor thickness in oral cancer is an important predictive marker for lymph node metastases. |
| MRI and clinical examination | 2016 | 53 | Alsaffar et al. [43] | There is a high correlation between pathological, radiological, and clinical examinations in the measurement of tongue tumor thickness in deep tumors (≥5 mm). |
| Time-resolved fluorescence spectroscopy | 2019 | 4 | Gorpas et al. [44] | Label-free and real-time assessment and visualization of tissue biochemical features during oral tumor robotic surgery procedures have the potential to improve intraoperative decision making during transoral robotic surgery. |
| CT | 2019 | 4 | Kahng et al. [45] | Intraoperative imaging improves localization accuracy when targeting submucosal beads in cadaver heads during operative laryngoscopy. |
| The use of 2D ultrasound | 2021 | 10 | De Koning et al. [7] | The use of ultrasound-guided tongue SCC is feasible and improves margin control. |
| Fluorescence | 2018 | 21 | Gao et al. [46] | Fluorescence can be used as a sensitive method for guiding surgery in head and neck cancers, increasing the probability of complete resections and improving oncologic outcomes. |
| Criteria | Description |
|---|---|
| Inclusion |
|
| Exclusion |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makouei, F.; Agander, T.K.; Ewertsen, C.; Søndergaard Svendsen, M.B.; Norling, R.; Kaltoft, M.; Hansen, A.E.; Rasmussen, J.H.; Wessel, I.; Todsen, T. 3D Ultrasound and MRI in Assessing Resection Margins during Tongue Cancer Surgery: A Research Protocol for a Clinical Diagnostic Accuracy Study. J. Imaging 2023, 9, 174. https://doi.org/10.3390/jimaging9090174
Makouei F, Agander TK, Ewertsen C, Søndergaard Svendsen MB, Norling R, Kaltoft M, Hansen AE, Rasmussen JH, Wessel I, Todsen T. 3D Ultrasound and MRI in Assessing Resection Margins during Tongue Cancer Surgery: A Research Protocol for a Clinical Diagnostic Accuracy Study. Journal of Imaging. 2023; 9(9):174. https://doi.org/10.3390/jimaging9090174
Chicago/Turabian StyleMakouei, Fatemeh, Tina Klitmøller Agander, Caroline Ewertsen, Morten Bo Søndergaard Svendsen, Rikke Norling, Mikkel Kaltoft, Adam Espe Hansen, Jacob Høygaard Rasmussen, Irene Wessel, and Tobias Todsen. 2023. "3D Ultrasound and MRI in Assessing Resection Margins during Tongue Cancer Surgery: A Research Protocol for a Clinical Diagnostic Accuracy Study" Journal of Imaging 9, no. 9: 174. https://doi.org/10.3390/jimaging9090174
APA StyleMakouei, F., Agander, T. K., Ewertsen, C., Søndergaard Svendsen, M. B., Norling, R., Kaltoft, M., Hansen, A. E., Rasmussen, J. H., Wessel, I., & Todsen, T. (2023). 3D Ultrasound and MRI in Assessing Resection Margins during Tongue Cancer Surgery: A Research Protocol for a Clinical Diagnostic Accuracy Study. Journal of Imaging, 9(9), 174. https://doi.org/10.3390/jimaging9090174







