Comparison of Image Quality and Quantification Parameters between Q.Clear and OSEM Reconstruction Methods on FDG-PET/CT Images in Patients with Metastatic Breast Cancer
Abstract
1. Introduction
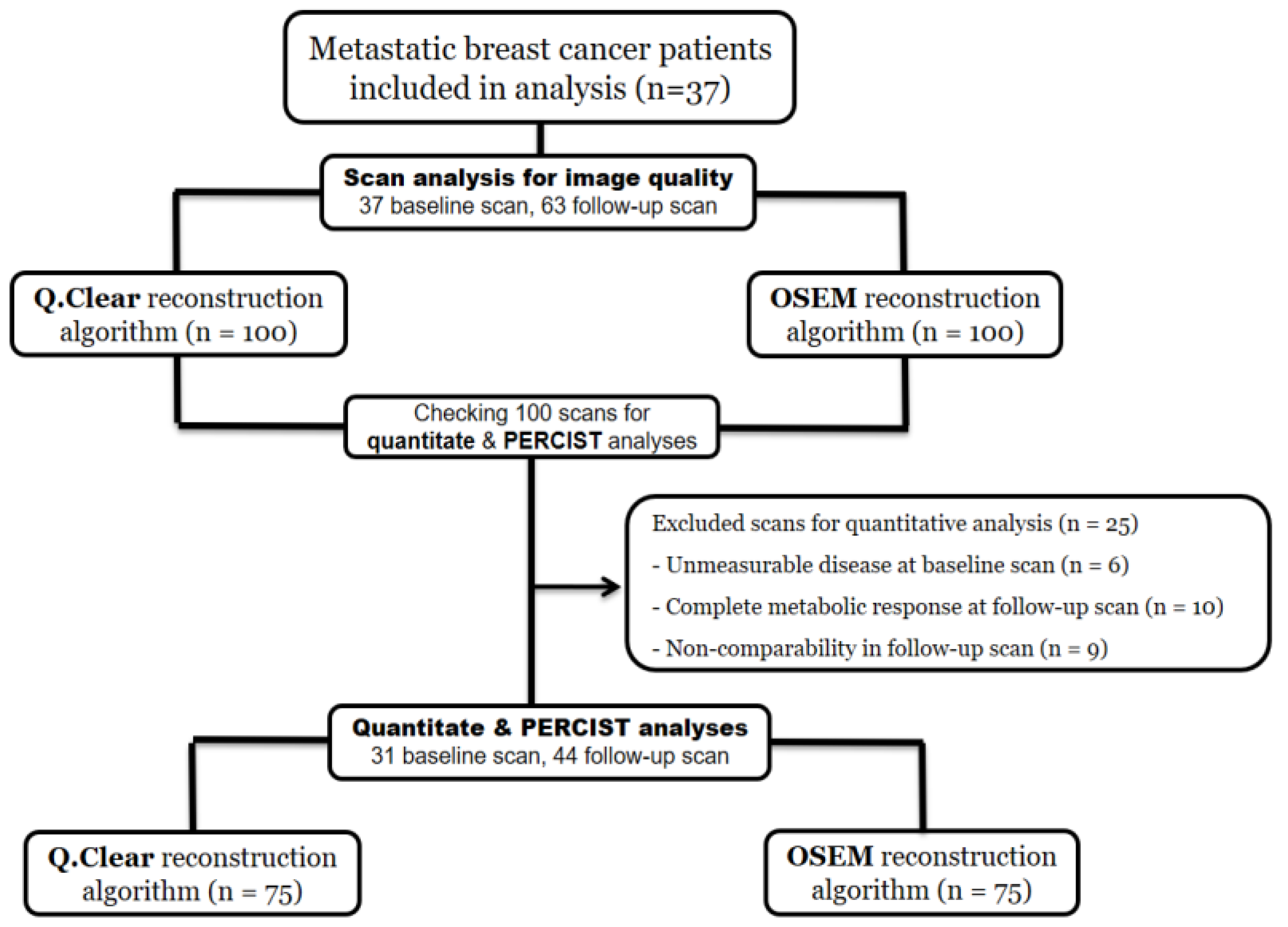
2. Materials and Methods
2.1. Patients
2.2. PET/CT Imaging Protocol
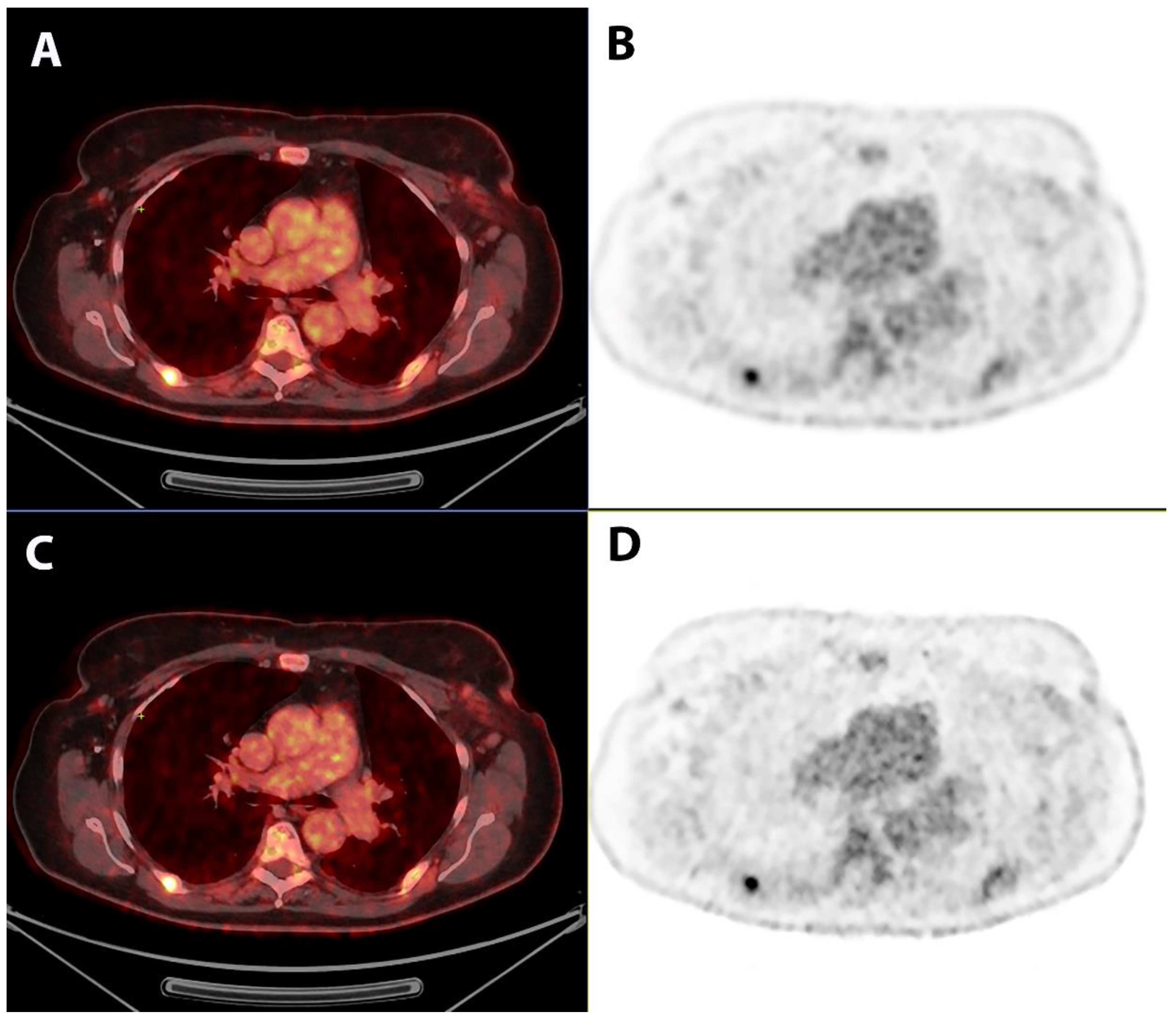
2.3. Qualitative Image Analysis
2.4. Quantitative Image Analysis
2.5. Outcome Measure and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ben-Haim, S.; Ell, P. 18F-FDG PET and PET/CT in the evaluation of cancer treatment response. J. Nucl. Med. 2009, 50, 88–99. [Google Scholar] [CrossRef]
- Tian, D.; Yang, H.; Li, Y.; Cui, B.; Lu, J. The effect of Q.Clear reconstruction on quantification and spatial resolution of 18F-FDG PET in simultaneous PET/MR. EJNMMI Phys. 2022, 9, 1. [Google Scholar] [CrossRef]
- Fuentes-Ocampo, F.; López-Mora, D.A.; Flotats, A.; Camacho, V.; Sizova, M.; Abouzian, S.; Duch, J.; Fernández, A.; Estorch, M.; Carrió, I. Digital versus analog PET/CT in patients with known or suspected liver metastases. Nucl. Med. Commun. 2021, 42, 421–428. [Google Scholar] [CrossRef] [PubMed]
- López-Mora, D.A.; Flotats, A.; Fuentes-Ocampo, F.; Camacho, V.; Fernández, A.; Ruiz, A.; Duch, J.; Sizova, M.; Domènech, A.; Estorch, M.; et al. Comparison of image quality and lesion detection between digital and analog PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Maniawski, P.; Knopp, M.V. Performance evaluation of the next generation solid-state digital photon counting PET/CT system. EJNMMI Res. 2018, 8, 97. [Google Scholar] [CrossRef]
- Salvadori, J.; Imbert, L.; Perrin, M.; Karcher, G.; Lamiral, Z.; Marie, P.Y.; Verger, A. Head-to-head comparison of image quality between brain (18)F-FDG images recorded with a fully digital versus a last-generation analog PET camera. EJNMMI Res. 2019, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.C.; Vercher-Conejero, J.L.; Sattar, A.; Miller, M.A.; Maniawski, P.J.; Jordan, D.W.; Muzic, R.F., Jr.; Su, K.H.; O’Donnell, J.K.; Faulhaber, P.F. Image Quality and Diagnostic Performance of a Digital PET Prototype in Patients with Oncologic Diseases: Initial Experience and Comparison with Analog PET. J. Nucl. Med. 2015, 56, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Ocampo, F.; López-Mora, D.A.; Flotats, A.; Paillahueque, G.; Camacho, V.; Duch, J.; Fernández, A.; Domènech, A.; Estorch, M.; Carrió, I. Digital vs. analog PET/CT: Intra-subject comparison of the SUVmax in target lesions and reference regions. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1745–1750. [Google Scholar] [CrossRef]
- Texte, E.; Gouel, P.; Thureau, S.; Lequesne, J.; Barres, B.; Edet-Sanson, A.; Decazes, P.; Vera, P.; Hapdey, S. Impact of the Bayesian penalized likelihood algorithm (Q.Clear®) in comparison with the OSEM reconstruction on low contrast PET hypoxic images. EJNMMI Phys. 2020, 7, 28. [Google Scholar] [CrossRef]
- Wyrzykowski, M.; Siminiak, N.; Kaźmierczak, M.; Ruchała, M.; Czepczyński, R. Impact of the Q.Clear reconstruction algorithm on the interpretation of PET/CT images in patients with lymphoma. EJNMMI Res. 2020, 10, 99. [Google Scholar] [CrossRef]
- Sun, Z.; Yi, Y.L.; Liu, Y.; Xiong, J.P.; He, C.Z. Comparison of whole-body PET/PET-CT and conventional imaging procedures for distant metastasis staging in patients with breast cancer: A meta-analysis. Eur. J. Gynaecol. Oncol. 2015, 36, 672–676. [Google Scholar] [CrossRef]
- Hildebrandt, M.G.; Naghavi-Behzad, M.; Vogsen, M. A role of FDG-PET/CT for response evaluation in metastatic breast cancer? Semin. Nucl. Med. 2022, 52, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Riedl, C.C.; Pinker, K.; Ulaner, G.A.; Ong, L.T.; Baltzer, P.; Jochelson, M.S.; McArthur, H.L.; Gönen, M.; Dickler, M.; Weber, W.A. Comparison of FDG-PET/CT and contrast-enhanced CT for monitoring therapy response in patients with metastatic breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Naghavi-Behzad, M.; Vogsen, M.; Vester, R.M.; Olsen, M.M.B.; Oltmann, H.; Braad, P.E.; Asmussen, J.T.; Gerke, O.; Vach, W.; Kidholm, K.; et al. Response monitoring in metastatic breast cancer: A comparison of survival times between FDG-PET/CT and CE-CT. Br. J. Cancer 2022, 126, 1271–1279. [Google Scholar] [CrossRef]
- Depardon, E.; Kanoun, S.; Humbert, O.; Bertaut, A.; Riedinger, J.M.; Tal, I.; Vrigneaud, J.M.; Lasserre, M.; Toubeau, M.; Berriolo-Riedinger, A.; et al. FDG PET/CT for prognostic stratification of patients with metastatic breast cancer treated with first line systemic therapy: Comparison of EORTC criteria and PERCIST. PLoS ONE 2018, 13, e0199529. [Google Scholar] [CrossRef] [PubMed]
- Vogsen, M.; Bülow, J.L.; Ljungstrøm, L.; Oltmann, H.R.; Alamdari, T.A.; Naghavi-Behzad, M.; Braad, P.E.; Gerke, O.; Hildebrandt, M.G. FDG-PET/CT for Response Monitoring in Metastatic Breast Cancer: The Feasibility and Benefits of Applying PERCIST. Diagnostics 2021, 11, 723. [Google Scholar] [CrossRef] [PubMed]
- Hyun, O.J.; Lodge, M.A.; Wahl, R.L. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology 2016, 280, 576–584. [Google Scholar] [CrossRef]
- Ribeiro, D.; Hallett, W.; Tavares, A.A.S. Performance evaluation of the Q.Clear reconstruction framework versus conventional reconstruction algorithms for quantitative brain PET-MR studies. EJNMMI Phys. 2021, 8, 41. [Google Scholar] [CrossRef]
- Te Riet, J.; Rijnsdorp, S.; Roef, M.J.; Arends, A.J. Evaluation of a Bayesian penalized likelihood reconstruction algorithm for low-count clinical (18)F-FDG PET/CT. EJNMMI Phys. 2019, 6, 32. [Google Scholar] [CrossRef]
- Teoh, E.J.; McGowan, D.R.; Schuster, D.M.; Tsakok, M.T.; Gleeson, F.V.; Bradley, K.M. Bayesian penalised likelihood reconstruction (Q.Clear) of (18)F-fluciclovine PET for imaging of recurrent prostate cancer: Semi-quantitative and clinical evaluation. Br. J. Radiol. 2018, 91, 20170727. [Google Scholar] [CrossRef]
- Barrington, S.F.; Sulkin, T.; Forbes, A.; Johnson, P.W.M. All that glitters is not gold—New reconstruction methods using Deauville criteria for patient reporting. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 316–317. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef] [PubMed]
- Vogsen, M.; Harbo, F.; Jakobsen, N.M.; Nissen, H.J.; Dahlsgaard-Wallenius, S.E.; Gerke, O.; Jensen, J.D.; Asmussen, J.T.; Jylling, A.M.B.; Braad, P.E.; et al. Response monitoring in metastatic breast cancer—A prospective study comparing (18)F-FDG PET/CT with conventional CT. J. Nucl. Med. 2022, 64, 263358. [Google Scholar] [CrossRef]
- Arapakis, I.; Efstathopoulos, E.; Tsitsia, V.; Kordolaimi, S.; Economopoulos, N.; Argentos, S.; Ploussi, A.; Alexopoulou, E. Using “iDose4” iterative reconstruction algorithm in adults’ chest-abdomen-pelvis CT examinations: Effect on image quality in relation to patient radiation exposure. Br. J. Radiol. 2014, 87, 20130613. [Google Scholar] [CrossRef] [PubMed]
- Baun, C.; Falch, K.; Gerke, O.; Hansen, J.; Nguyen, T.; Alavi, A.; Høilund-Carlsen, P.F.; Hildebrandt, M.G. Quantification of FDG-PET/CT with delayed imaging in patients with newly diagnosed recurrent breast cancer. BMC Med. Imaging 2018, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Hosono, M.; Kanagaki, M.; Onishi, Y.; Matsubara, N.; Kawabata, K.; Kimura, H. Evaluation and Optimization of a New PET Reconstruction Algorithm, Bayesian Penalized Likelihood Reconstruction, for Lung Cancer Assessment According to Lesion Size. AJR Am. J. Roentgenol. 2019, 213, W50–W56. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, T.; Miwa, K.; Yamaguchi, M.; Shimada, K.; Wagatsuma, K.; Yamao, T.; Kamitaka, Y.; Hiratsuka, S.; Kobayashi, R.; Ichikawa, H.; et al. Optimization of a Bayesian penalized likelihood algorithm (Q.Clear) for (18)F-NaF bone PET/CT images acquired over shorter durations using a custom-designed phantom. EJNMMI Phys. 2020, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, L.; Argalia, G.; Fortunati, E.; Malizia, C.; Allegri, V.; Calabrò, D.; Civollani, S.; Campana, D.; Fanti, S.; Ambrosini, V. Can Q.Clear reconstruction be used to improve [68 Ga]Ga-DOTANOC PET/CT image quality in overweight NEN patients? Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Rijnsdorp, S.; Roef, M.J.; Arends, A.J. Impact of the Noise Penalty Factor on Quantification in Bayesian Penalized Likelihood (Q.Clear) Reconstructions of (68)Ga-PSMA PET/CT Scans. Diagnostics 2021, 11, 847. [Google Scholar] [CrossRef] [PubMed]
- Ter Voert, E.; Muehlematter, U.J.; Delso, G.; Pizzuto, D.A.; Müller, J.; Nagel, H.W.; Burger, I.A. Quantitative performance and optimal regularization parameter in block sequential regularized expectation maximization reconstructions in clinical (68)Ga-PSMA PET/MR. EJNMMI Res. 2018, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Chilcott, A.K.; Bradley, K.M.; McGowan, D.R. Effect of a Bayesian Penalized Likelihood PET Reconstruction Compared With Ordered Subset Expectation Maximization on Clinical Image Quality Over a Wide Range of Patient Weights. AJR Am. J. Roentgenol. 2018, 210, 153–157. [Google Scholar] [CrossRef]
- Economou Lundeberg, J.; Oddstig, J.; Bitzén, U.; Trägårdh, E. Comparison between silicon photomultiplier-based and conventional PET/CT in patients with suspected lung cancer-a pilot study. EJNMMI Res. 2019, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Devriese, J.; Beels, L.; Maes, A.; Van de Wiele, C.; Pottel, H. Impact of PET reconstruction protocols on quantification of lesions that fulfil the PERCIST lesion inclusion criteria. EJNMMI Phys. 2018, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, J.S.; Vilstrup, M.H.; Holm, J.; Vogsen, M.; Bülow, J.L.; Ljungstrøm, L.; Braad, P.E.; Gerke, O.; Hildebrandt, M.G. Interrater Agreement and Reliability of PERCIST and Visual Assessment When Using 18F-FDG-PET/CT for Response Monitoring of Metastatic Breast Cancer. Diagnostics 2020, 10, 1001. [Google Scholar] [CrossRef]
- Fledelius, J.; Khalil, A.; Hjorthaug, K.; Frøkiær, J. Inter-observer agreement improves with PERCIST 1.0 as opposed to qualitative evaluation in non-small cell lung cancer patients evaluated with F-18-FDG PET/CT early in the course of chemo-radiotherapy. EJNMMI Res. 2016, 6, 71. [Google Scholar] [CrossRef]
- Hildebrandt, M.G.; Lauridsen, J.F.; Vogsen, M.; Holm, J.; Vilstrup, M.H.; Braad, P.E.; Gerke, O.; Thomassen, M.; Ewertz, M.; Høilund-Carlsen, P.F. FDG-PET/CT for Response Monitoring in Metastatic Breast Cancer: Today, Tomorrow, and Beyond. Cancers 2019, 11, 1190. [Google Scholar] [CrossRef]
- Min, S.J.; Jang, H.J.; Kim, J.H. Comparison of the RECIST and PERCIST criteria in solid tumors: A pooled analysis and review. Oncotarget 2016, 7, 27848–27854. [Google Scholar] [CrossRef]
- Wu, Z.; Guo, B.; Huang, B.; Hao, X.; Wu, P.; Zhao, B.; Qin, Z.; Xie, J.; Li, S. Phantom and clinical assessment of small pulmonary nodules using Q.Clear reconstruction on a silicon-photomultiplier-based time-of-flight PET/CT system. Sci. Rep. 2021, 11, 10328. [Google Scholar] [CrossRef]
| Parameters | 5 | 4 | 3 | 2 | 1 |
|---|---|---|---|---|---|
| Noise | Minimal or no noise | No significant noise | Noisy diagnostic | Significant noise (affects diagnosis) | High-level noise (nondiagnostic) |
| Sharpness | Excellent sharpness | Good sharpness | Moderate sharpness | Poor sharpness (bad visibility) | Zero visibility (nondiagnostic) |
| Contrast | Excellent contrast | Very good contrast | Good contrast | Poor contrast (unsatisfactory visualization) | Image similar to use of no contrast (nondiagnostic) |
| Diagnostic confidence | Completely confidence | High confidence | Good confidence | Poor confidence | No diagnostic confidence (unacceptable) |
| Artefacts | No artefacts | Insignificant artefacts | Minor artefacts | Major artefacts (diagnosis still possible) | Artefacts affecting diagnostic information |
| Blotchy appearance | Absent | Mild | Moderate | Significant (diagnosis still possible) | Intense (affecting diagnosis) |
| Characteristics | Results * | |
|---|---|---|
| Age (years) | 71.9 (45.9–91.1) | |
| Primary cancer treatment | Postoperative adjuvant treatment | 24 (64.7) |
| Adjuvant and neoadjuvant treatments | 3 (8.1) | |
| No treatment/unknown | 10 (27.0) | |
| History of radiotherapy | 24 (64.7) | |
| Primary disseminated cancer | 12 (32.4) | |
| Histopathology | Adenocarcinoma | 28 (75.7) |
| Invasive ductal carcinoma | 5 (13.5) | |
| Invasive lobular carcinoma | 4 (10.8) | |
| Positive estrogen receptor | 32 (86.5) | |
| Negative Herceptin receptor | 34 (91.9) | |
| Origin of biopsy ** | Bone | 13 (35.1) |
| Liver | 7 (18.9) | |
| Lung | 1 (2.7) | |
| Lymph nodes | 6 (16.2) | |
| Breast | 10 (27.0) | |
| First-line treatment | Endocrine therapy | 5 (13.5) |
| Endocrine therapy + CDK4/6 inhibitor | 24 (64.9) | |
| Chemotherapy | 4 (10.8) | |
| Others | 4 (10.8) | |
| Characteristics | OSEM * | Q.Clear * | Mean Difference (95% CI) | p-Value |
|---|---|---|---|---|
| Noise | 4.41 ± 0.55 | 4.42 ± 0.54 | 0.01 (−0.16–0.14) | 0.88 |
| Sharpness | 3.91 ± 0.49 | 4.65 ± 0.59 | −0.74 (−0.83–−0.65) | <0.001 |
| Contrast | 4.1 ± 0.66 | 4.23 ± 0.74 | −0.13 (−0.22–−0.04) | 0.001 |
| Diagnostic confidence | 4.52 ± 0.70 | 4.52 ± 0.69 | 0 (−0.28–0.28) | 0.99 |
| Artifacts | 4.37 ± 0.68 | 4.38 ± 0.66 | −0.01 (−0.3–0.01) | 0.32 |
| Blotchy appearance | 4.57 ± 0.57 | 4.34 ± 0.59 | 0.23 (0.12–0.34) | <0.001 |
| Characteristics | OSEM * | Q.Clear * | Mean Difference (95% CI) | p-Value | |
|---|---|---|---|---|---|
| SULpeak | Baseline scans | 5.82 (1.4–12.12) | 6.84 (1.61–12.95) | −0.6 (−0.82–−0.39) | <0.001 |
| Follow-up scans | 3.01 (1.65–11.01) | 3.47 (1.79–12.82) | −0.39 (−0.52–−0.26) | 0.001 | |
| All scans | 4.3 (1.4–12.12) | 4.63 (1.61–12.95) | −0.47 (−0.59–−0.36) | <0.001 | |
| Change to 1st follow-up | 1.94 (0.07–5.71) | 1.95 (0–5.86) | 0.04 (−0.24–0.33) | 0.75 | |
| Change to 2nd follow-up | 0.53 (0.02–4) | 0.8 (0.05–4.16) | 0.12 (−0.23–0.26) | 0.1 | |
| SUVmax | Baseline scans | 8.12 (2.0–18.42) | 9.46 (2.37–24.86) | −1.49 (−1.97–1.01) | <0.001 |
| Follow-up scans | 4.61 (2.22–18.42) | 5.48 (2.47–24.86) | −1.25 (−1.73–−0.77) | 0.005 | |
| All scans | 6.16 (2.0–18.42) | 7.15 (2.37–24.86) | −1.35 (−1.69–−1.01) | <0.001 | |
| Change to 1st follow-up | 2.34 (0–8.3) | 2.39 (0–9.5) | 0.45 (0.10–0.79) | 0.01 | |
| Change to 2nd follow-up | 1.1 (0.05–4.92) | 1.5 (0.26–11.2) | 0.89 (0.20–1.58) | 0.04 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naghavi-Behzad, M.; Vogsen, M.; Gerke, O.; Dahlsgaard-Wallenius, S.E.; Nissen, H.J.; Jakobsen, N.M.; Braad, P.-E.; Vilstrup, M.H.; Deak, P.; Hildebrandt, M.G.; et al. Comparison of Image Quality and Quantification Parameters between Q.Clear and OSEM Reconstruction Methods on FDG-PET/CT Images in Patients with Metastatic Breast Cancer. J. Imaging 2023, 9, 65. https://doi.org/10.3390/jimaging9030065
Naghavi-Behzad M, Vogsen M, Gerke O, Dahlsgaard-Wallenius SE, Nissen HJ, Jakobsen NM, Braad P-E, Vilstrup MH, Deak P, Hildebrandt MG, et al. Comparison of Image Quality and Quantification Parameters between Q.Clear and OSEM Reconstruction Methods on FDG-PET/CT Images in Patients with Metastatic Breast Cancer. Journal of Imaging. 2023; 9(3):65. https://doi.org/10.3390/jimaging9030065
Chicago/Turabian StyleNaghavi-Behzad, Mohammad, Marianne Vogsen, Oke Gerke, Sara Elisabeth Dahlsgaard-Wallenius, Henriette Juel Nissen, Nick Møldrup Jakobsen, Poul-Erik Braad, Mie Holm Vilstrup, Paul Deak, Malene Grubbe Hildebrandt, and et al. 2023. "Comparison of Image Quality and Quantification Parameters between Q.Clear and OSEM Reconstruction Methods on FDG-PET/CT Images in Patients with Metastatic Breast Cancer" Journal of Imaging 9, no. 3: 65. https://doi.org/10.3390/jimaging9030065
APA StyleNaghavi-Behzad, M., Vogsen, M., Gerke, O., Dahlsgaard-Wallenius, S. E., Nissen, H. J., Jakobsen, N. M., Braad, P.-E., Vilstrup, M. H., Deak, P., Hildebrandt, M. G., & Andersen, T. L. (2023). Comparison of Image Quality and Quantification Parameters between Q.Clear and OSEM Reconstruction Methods on FDG-PET/CT Images in Patients with Metastatic Breast Cancer. Journal of Imaging, 9(3), 65. https://doi.org/10.3390/jimaging9030065







